Abstract
Herpes simplex virus type 1 (HSV-1) is an important human pathogen and a leading cause of infectious blindness in the developed world. HSV-1 exploits heparan sulfate proteoglycans (HSPG) for attachment to cells. While the significance of heparan sulphate (HS) moieties in HSV-1 infection is well established, the role of specific proteoglycan core proteins in the infection process remains poorly understood. The objective of this study was to assess the roles of syndecan-1 and syndecan-2 core proteins in HSV-1 infection, both of which are expressed by many HSV-1 target cell types. Our results demonstrate that syndecan-1 and syndecan-2 gene silencing by RNA interference reduces HSV-1 entry, plaque formation and facilitates cell survival. Furthermore, HSV-1 infection increases syndecan-1 and syndecan-2 protein synthesis and a resultant increase in cell surface expression of HS. Our observations suggest that changes in syndecan-1 and syndecan-2 expression levels may be related to active viral infection. Taken together, our findings provide new insights into HSPG functions during HSV-1 entry and spread.
INTRODUCTION
Herpes simplex virus type 1 (HSV-1) is a clinically important pathogen and a leading cause of infectious blindness in the developed world. HSV-1 productively infects epithelial cells and establishes latent infection in sensory ganglia for the life of the host (Kumaraguru & Rouse, 2002; Terasaka et al., 2010). Currently, no cure exists against HSV-1, which can be transmitted via asymptomatic shedding by latently infected individuals (Hill & Clement, 2009). Prevention of virus transmission to uninfected people is a real challenge compounded by our limited understanding of HSV-1–host cell interactions including virus entry, which is the first essential step for the establishment of an acute and/or latent infection.
Enveloped viruses including HSV-1 penetrate host cells by inducing fusion between the virus envelope and the host cell membrane. HSV-1 entry is a stepwise process, which starts when HSV-1 envelope glycoproteins gB and gC attach to cell surface heparan sulfate proteoglycans (HSPGs) (Herold et al., 1991; Nicola et al., 2003; Trybala et al., 2000). This initial interaction enables HSV-1 glycoprotein D (gD) to bind to one of the known gD entry co-receptors. There are three classes of gD co-receptors that have been characterized: nectin-1 (HveC) and nectin-2 (HveB), which are both members of the immunoglobulin superfamily (Geraghty et al., 1998), herpesvirus entry mediator (HVEM) that belongs to the tumour necrosis factor receptor family (Montgomery et al., 1996), and 3-O-sulfated heparan sulfate (3-OS HS) which is a specifically modified form of heparan sulfate (HS) (Shukla et al., 1999b; O'Donnell et al., 2010). The binding of gD to one of its receptors leads to conformational changes in gD that allows it to activate a multi-glycoprotein complex involving gB, gD, gH and gL that triggers the viral fusion with the host cell membrane (Atanasiu et al., 2007; Spear et al., 2000). This fusion mechanism is utilized by HSV-1 when it enters the host cell by fusion with the plasma membrane, or when using different entry pathways, including endocytosis and phagocytosis (Clement et al., 2006; Nicola et al., 2003; Reske et al., 2007; Shukla & Spear, 2001).
The HS is a glycosaminoglycan (GAG) that is present in almost all mammalian tissues on cell surfaces and in the extracellular matrix (Esko & Lindahl, 2001; Lindahl et al., 1998). HS commonly occurs as proteoglycans, where HS GAG chains are attached to a core protein via a trisaccharide linkage on a serine residue forming the HSPG (O'Donnell & Shukla, 2008). The syndecan family is one of the most abundant HSPGs expressed on mammalian cells (Muto et al., 2007; Schofield et al., 1999; Tumova et al., 2000). There are four members in the syndecan family (syndecan-1, 2, 3 and 4) composed of a single membrane-spanning protein, a conserved transmembrane domain, and an extracellular domain that is specific for each syndecan (Beauvais et al., 2004). The divergent ectodomains share conserved attachment sites for GAG chains. These GAGs are predominantly heparan sulfated (Carey, 1997; Lopes et al., 2006) but syndecan-1 and syndecan-4 can also contain chondroitin sulfate (CS) GAGs in addition to HS-GAGs (Deepa et al., 2004; Shworak et al., 1994). HSV-1 commonly infects epithelial cells, which express detectable amounts of syndecan-1 and syndecan-2 (Bobardt et al., 2007; Cheshenko et al., 2007). In addition, syndecan-1 (CD138; NCBI reference sequence: NP_001006947) is expressed by many cell types including plasma cells. Syndecan-2 (fibroglycan; NCBI reference sequence: NP_002989) shows somewhat restricted expression, limited mainly to fibroblasts and neurons (Ethell et al., 2001; Shimabukuro et al., 2008; Su et al., 2007). The latter is an important site for HSV-1 latency and reactivation (Shukla & Spear, 2001).
While studies have shown that HS plays an important role in HSV-1 entry as an attachment receptor (Shukla & Spear, 2001), the role of specific proteoglycan core proteins in the infection process remains poorly understood. Since syndecan-1 and syndecan-2 are relatively common HSPGs found on the target cell types for HSV-1 infection, we aimed to explore the role of their core proteins in HSV-1 infection. In this paper, we performed in vitro studies using HeLa cells, which are known to express both HSPGs (Brule et al., 2006; Kubo et al., 2006) to determine the effect of syndecan-1 and syndecan-2 gene silencing on viral entry, plaque formation and cell survival using small interfering RNAs (siRNAs) specific for HSPGs. Since several microbial pathogens can alter syndecan-1 expression (Anastasiadou et al., 2009; Freissler et al., 2000; Wang et al., 2006), we also tested whether HSV-1 infection has an effect on syndecan-1 and syndecan-2 expression in HeLa cells. Our results provide new insights into HSPG core protein function during HSV-1 entry and spread.
RESULTS
Syndecan-1 and syndecan-2 downregulation in HeLa cells
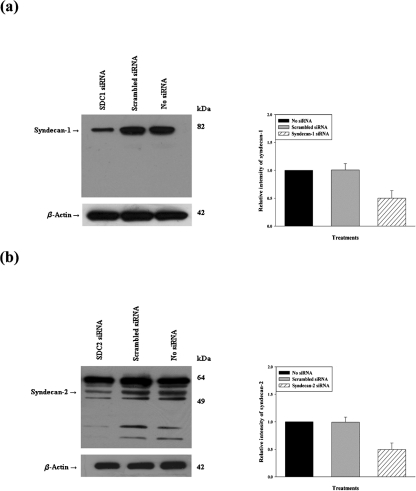
The significance of syndecan-1 and syndecan-2 on HSV-1 infection was examined by selective gene silencing of syndecan-1 and syndecan-2 in HeLa cells using siRNA expression constructs (Beauvais et al., 2004). Cells were treated with either syndecan-1- or syndecan-2-specific siRNA to downregulate syndecan-1 and syndecan-2 gene expression, respectively. Gene silencing of syndecan-1 (Fig. 1a) and syndecan-2 (Fig. 1b) was detected at the protein level by using Western blot analysis. Densitometric analysis showed that treatment with syndecan-1- and syndecan-2-specific siRNA resulted in a significant (about 50 %) reduction in syndecan-1 and syndecan-2 protein expression, respectively. The 50 % decrease in expression corresponded well with 45–50 % transfection efficacy seen with HeLa cells in our experiments. The effect of siRNA was specific since scrambled siRNA failed to bring down syndecan expression and likewise, neither scrambled nor syndecan-specific siRNAs had any effects on β-actin expression. Additional experiments (data not shown) demonstrated that siRNA against syndecan-1 was specific to its subtype and did not interfere with the expression of syndecan-2 and vice versa syndecan-2 siRNA also demonstrated high specificity.
Fig. 1.
Western blot analysis of syndecan-1 and syndecan-2 protein expression after siRNA downregulation. Protein expression of syndecan-1 and syndecan-2 measured in a sample of HeLa cells mock treated (no transfection) or treated with scrambled siRNA, syndecan-1 siRNA or syndecan-2 siRNA for 48 h. Representative Western blots showed knockdown of syndecan-1 (a) or syndecan-2 (b) after siRNA downregulation. Densitometric analysis revealed 50 % reduction in the signal intensity of both syndecan-1 and syndecan-2. β-Actin protein expression was measured as the loading control. Representative blots from three independent experiments are shown. The results are expressed as means±sd values from three independent experiments.
Downregulation of syndecan-1 and syndecan-2 inhibits HSV-1 entry
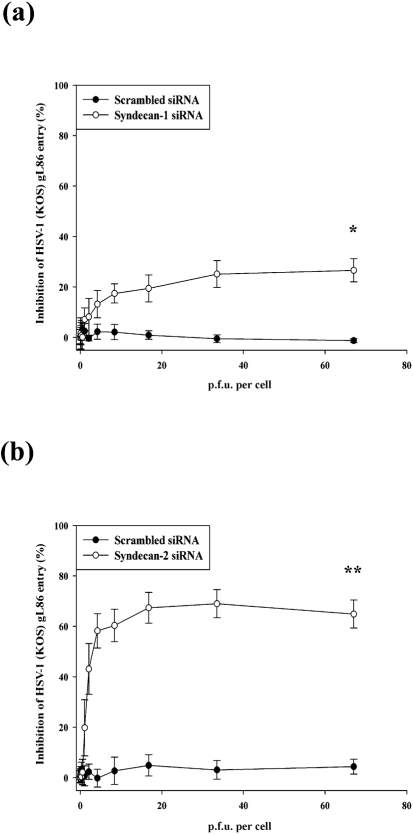
After verifying syndecan-1 and syndecan-2 downregulation by siRNA transfection, the effect of reduced syndecan-1 and syndecan-2 protein levels on HSV-1 entry into HeLa cells was examined. A previously described HSV-1 entry assay (Akhtar et al., 2008; Tiwari et al., 2006) was used to compare viral entry into cells treated with syndecan-1 or syndecan-2 siRNA with those treated with scrambled siRNA or mock treated. HeLa cells were infected with a recombinant β-galactosidase expressing HSV-1 (KOS) gL86 reporter virus. The entry of HSV-1 was measured after 6 h of viral infection. As indicated in Fig. 2(a), a statistically significant, 26.6±4.6 % inhibition of HSV-1 entry was observed in cells transfected with syndecan-1 siRNA (P<0.05). Transfected cells with syndecan-2 siRNA resulted in even more significant, 64.9±5.5 % inhibition of HSV-1 entry (P<0.0001) (Fig. 2b).
Fig. 2.
Downregulation of syndecan-1 and syndecan-2 inhibits HSV-1 entry into HeLa cells. HSV-1 entry was analysed in HeLa cells mock treated (no transfection) or transfected with scrambled siRNA, syndecan-1 siRNA or syndecan-2 siRNA. After siRNA transfection (48 h), cells were inoculated with a serial dilution of β-galactosidase-expressing recombinant HSV-1 (KOS) gL86 virus for 6 h. The soluble substrate ONPG was added and enzymic activity was measured. Downregulation of both syndecan-1 (Fig. 2a) and syndecan-2 (Fig. 2b) inhibits HSV-1 (KOS) gL86 entry into HeLa cells although syndecan-2 siRNA has more significant effect. Per cent inhibition of HSV-1(KOS) gL86 entry by syndecan-1 siRNA or syndecan-2 siRNA treatment was calculated relative to mock-treated (no transfection) cells. Scrambled siRNA-transfected cells were used as negative control. Each value shown is the mean of six determinations (±sd) (*P<0.05, **P<0.0001).
Anti-syndecan-1 and anti-syndecan-2 polyclonal antibodies (pAbs) block HSV-1 entry
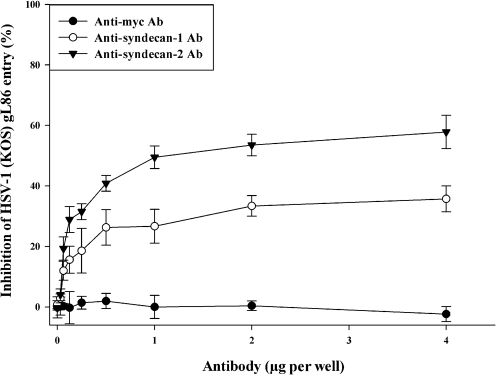
To confirm the role of syndecan-1 and syndecan-2 in HSV-1 entry and to determine whether reduced HSV-1 entry after syndecan-1 and syndecan-2 knockdown is specific to syndecan ectodomains, a previously described antibody blocking assay (Shukla et al., 1999a) and pAbs blocking syndecan-1 and syndecan-2 ectodomains were utilized. As shown in Fig. 3, pAbs specific for the extracellular region of syndecan-1 or syndecan-2 were able to block HSV-1 entry into HeLa cells in a dose-dependent manner. At the maximum pAb concentration (4 μg per well) the observed inhibitions were 35.7±4.2 and 57.84±6.5 %, respectively, compared with HeLa cells that were treated with a control, anti-myc mAb. Although both syndecan-1 and syndecan-2 pAbs reduced HSV-1 entry, syndecan-2 pAbs blockade had a statistically significant reducing effect (P<0.05) that was more substantial than that caused by syndecan-1 pAbs treatment. The effect of the pAbs was not additive since a cocktail containing both antibodies did not enhance inhibition beyond what was seen with syndecan-2 pAbs (data not shown).
Fig. 3.
Syndecan-1- or syndecan-2-specific antibody treatment blocked HSV-1(KOS) gL86 entry into HeLa cells. Cells were incubated with a serial dilution of syndecan-1-specific pAbs, syndecan-2-specific pAbs, or control anti-myc mAb for 30 min. Cells were then inoculated with a constant dose of β-galactosidase-expressing recombinant HSV-1(KOS) gL86 (m.o.i. of 10) for 2 h. The soluble substrate ONPG was added and enzymic activity was measured. Per cent inhibition of HSV-1(KOS) gL86 entry by syndecan-1 pAbs or syndecan-2 pAbs treatment was calculated relative to anti-myc mAb-treated cells. Each value shown is the mean of four determinations (±sd) (P<0.05).
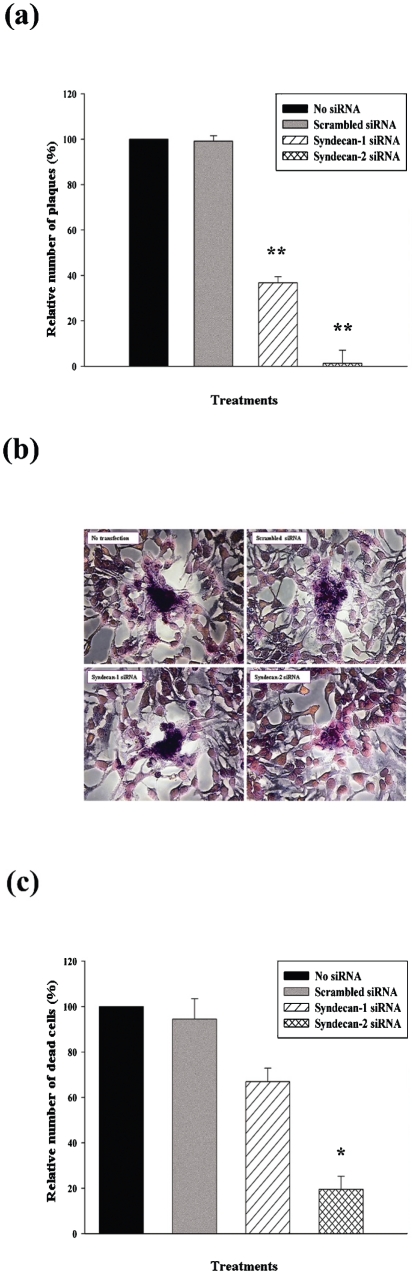
Downregulation of syndecan-1 and syndecan-2 inhibits plaque formation, reduces the size of HSV-1 plaques and enhances cell survival
The ability of HSV-1 to form plaques reflects its ability to enter cells, replicate and spread to infect neighbouring uninfected cells. Since the downregulation of syndecan-1 and syndecan-2 reduced HSV-1 entry, we sought to determine whether a similar decrease in HSV-1 plaque formation and spread in HeLa cells is also observed after syndecan-1 and syndecan-2 downregulation after siRNA transfection. A standard plaque assay (Akhtar et al., 2008) was performed. As shown in Fig. 4(a), there was a clear and significant reduction in the number of plaques formed in HeLa cells transfected with syndecan-1- and syndecan-2-specific siRNAs compared with mock treated or scrambled siRNA-transfected cells. Transfection with syndecan-1-specific siRNA reduced plaque number by 63.22±2.65 % (P<0.0001), while transfection with syndecan-2-specific siRNA reduced plaque number by 98.73±5.78 % (P<0.0001). Images taken of the plaques formed, show that the downregulation of syndecan-1 and syndecan-2 resulted in smaller plaques compared with the plaques formed in mock-treated or scrambled siRNA-treated cells (Fig. 4b).
Fig. 4.
Downregulation of syndecan-1 and syndecan-2 affects HSV-1 plaque formation, size and enhances cell survival in HeLa cells. Cells were either mock treated (no transfection), or treated with scrambled siRNA, syndecan-1 siRNA or syndecan-2 siRNA. Post-transfection cells (48 h) were infected with HSV-1(KOS) (m.o.i. of 0.01). (a) Post-infection infectivity at 72 h was measured by the number of p.f.u. Relative number of plaques was computed relative to mock-treated (no transfection) samples. Significant decreases of number of plaques were seen in both syndecan-1 siRNA- and syndecan-2 siRNA-transfected HeLa cells. Plaques that consisted of 15 or more nuclei were counted. Results are means±sd of four independent experiments conducted in triplicate (**P<0.0001). (b) Morphological appearance of Giemsa-stained HSV-1 (KOS) plaques 72 h post-infection. In syndecan-1 siRNA- and syndecan-2 siRNA-treated HeLa cells smaller plaques were observed compared with the plaques in mock-treated or scrambled siRNA-treated cells. Magnification, ×40. (c) Cytotoxicity was measured after 120 h HSV-1 (KOS) infection. Relative number of dead cells was calculated relative to mock-treated samples. Significant decreases of cytotoxicity were observed in both syndecan-1 siRNA- and syndecan-2 siRNA-treated cells; however, only the effect of syndecan-2 siRNA treatment was statistically significant. Results are means±sd of four independent experiments conducted in triplicate (*P<0.05).
To prove that the reduction in entry and plaque formation is syndecan downregulation specific, and not a result of more cell death, dead cells in each condition were counted 120 h after HSV-1 infection and the numbers were normalized to those observed with mock-treated cells. As shown in Fig. 4(c), downregulation of syndecan-1 reduced the percentage of dead cells after HSV-1 infection (by 33.06±5.89 %), and downregulation of syndecan-2 resulted in a statistically significant decline in the percentage of dead cells (by 80.45±5.68 %, P<0.05).
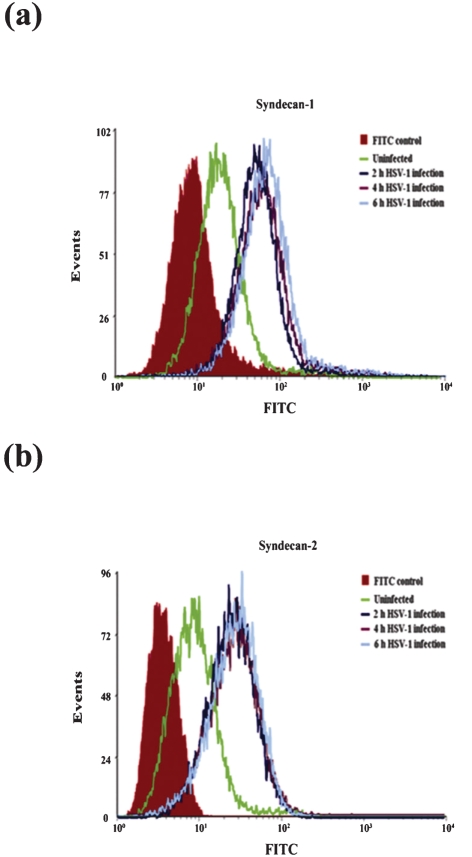
HSV-1 infection in HeLa cells enhances syndecan-1 and syndecan-2 cell surface expression
Earlier reports have established that infection with microbial pathogens can result in remarkable changes in syndecan expression (Magalhães et al., 2009). To determine whether HSV-1 infection affects cell surface expression of syndecan-1 and syndecan-2, the expression level of syndecan-1 and syndecan-2 on HeLa cell surface was analysed at various times after HSV-1(KOS) infection by flow cytometry. Cell surface syndecan-1 and syndecan-2 in uninfected HeLa cells were used as controls. As shown in Fig. 5, both syndecan-1 and syndecan-2 cell surface expressions were significantly upregulated as soon as 2 h after HSV-1 infection. The increases were also observed at 4 and 6 h post-infection in HeLa and CHO-K1 cells.
Fig. 5.
HSV-1 infection of host cell enhances syndecan-1 and syndecan-2 cell surface expression in HeLa cells. Cells were infected with a constant dose of HSV-1 (KOS) (m.o.i. of 10) for 2, 4 or 6 h. Syndecan-1 and syndecan-2 cell surface expression was then detected by FACS analysis. Enhanced syndecan-1 (a) and syndecan-2 (b) cell surface expression was detected when HeLa cells were infected with HSV-1. Mock-infected FITC stained cells were used as background control. Results are representative of three independent experiments.
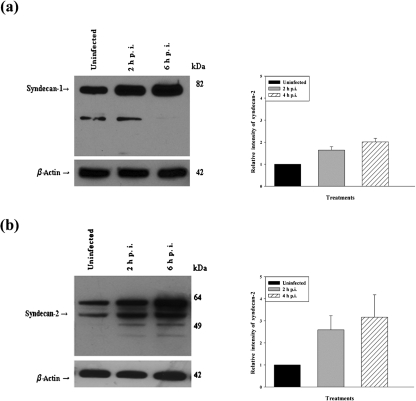
HSV-1 infection in HeLa cells enhances syndecan-1 and syndecan-2 protein synthesis
Since HSV-1 infection enhances syndecan-1 and syndecan-2 cell surface expression, this enhancement could be a result of more protein synthesis or a higher redistribution of HSPGs on the cell surface. To determine if HSV-1 infection modulated syndecan-1 and syndecan-2 expression at the protein level, Western blot analysis was performed on HeLa cells. Mock-infected cells were used as control. Densitometric analysis showed that the syndecan-1 protein expression level was increased by 1.64±0.16-fold at 2 h and 2.01±0.16-fold at 6 h after HSV-1 infection (Fig. 6a). Syndecan-2 protein expression level was also increased by 2.59±0.64-fold at 2 h and 3.16±1.02-fold at 6 h after HSV-1 infection (Fig. 6b). These results demonstrate that HSV-1 not only enhances the cell surface distribution of syndecans, but also induces syndecan-1 and syndecan-2 protein synthesis.
Fig. 6.
Western blot analysis of syndecan-1 and syndecan-2 protein expression after HSV-1 (KOS) infection. HeLa cells were infected with a constant dose of HSV-1 (KOS) (m.o.i. of 10) for 2 and 6 h. Cell lysates were then blotted against syndecan-1 and syndecan-2. According to the densitometric analysis syndecan-1 (a) and syndecan-2 (b) were more strongly expressed at 2 and 6 h post-infection compared with the uninfected cells in HeLa cells. Relative intensity of HSV-1(KOS) virus-infected bands expressed as a ratio relative to the mock-infected sample is shown. β-Actin protein expression was measured as loading control. The results are expressed as means±sd values from three independent experiments.
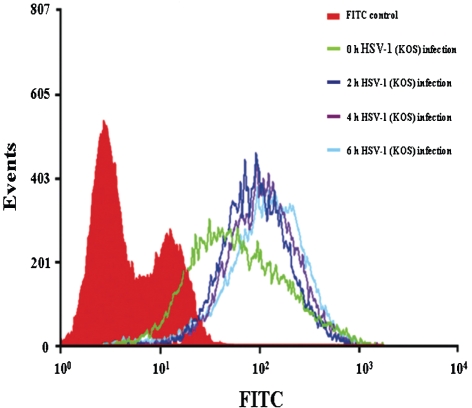
HSV-1 infection causes an increase of HS expression on cell surface
Finally, since HSV-1 infection of host cell results in an upregulation of syndecan cell surface and protein expression, we wanted to determine whether this may lead to an upregulation of HS as well. To investigate if HSV-1 infection affects HS cell surface expression, flow cytometry analysis was performed. At 2 h post-infection, HS surface expression increased in cells treated with HSV-1(KOS) compared to those that are mock treated (Fig. 7). However, at later time points, HS surface expression increased at a slower rate compared with the pattern that was observed for syndecan-1 and syndecan-2 upregulation after HSV-1 treatment. It is therefore likely that the core proteins may be synthesized at a higher rate than HS and this result may suggest an HS-independent function of the syndecan core proteins in the infection process.
Fig. 7.
Flow cytometry analysis of HS expression on HeLa cells infected with HSV-1. HeLa cells were infected with HSV-1(KOS) (m.o.i. of 10) at 37 °C for 2, 4 or 6 h. HS cell surface expression was then detected by FACS analysis. In HSV-1-infected cells, HS cell surface expression was enhanced compared with uninfected cells. Untreated FITC stained HeLa cells were used as background control.
DISCUSSION
HS is expressed on the cell surface of most mammalian cell types as HSPGs, and can serve as a receptor for a wide range of microbial pathogens, including viruses and bacteria (Barth et al., 2003; Giroglou et al., 2001; Menozzi et al., 2006; Smith et al., 2006). Although the role of HS is well studied as an attachment receptor for HSV-1, the role of HSPG core proteins in HSV-1 infection is poorly understood. This study is the first of its kind that directly implicates two members of the syndecan family of HSPGs, syndecan-1 and syndecan-2, as important mediators of HSV-1 infection. We demonstrate that both of them contribute significantly to viral entry and spread. Knocking down of either syndecan-1 or syndecan-2 shows detectable effects on HSV-1 entry and plaque formation. Our results also suggest that syndecan-2 may have a distinctly larger role in HSV-1 infection than syndecan-1.
In addition, we provide evidence to directly implicate the HSPG core protein in viral entry. Many known protein receptors for HSV-1 entry can be blocked by antibodies, which in turn, blocks viral entry (Akhtar et al., 2008; Shukla et al. 2009). Similar to those receptors, we also found that pAbs against synedecan-1 and syndecan-2 block entry. While it is quite possible that antibodies may act by producing steric hinderance to virus binding via HS, it is also possible that the core protein may directly interact with HSV-1 glycoproteins and that interaction is blocked by the antibodies. For unclear reasons the syndecan pAbs when combined together did not produce an additive effect. Future studies will determine whether the syndecan pAbs (especially syndecan-2 pAbs) may cross-react to block certain conserved syndecan epitopes shared for entry. In this case, one group of pAbs (e.g. syndecan-2 pAbs) may be able to block all the epitopes (whether on syndecan-1 or syndecan-2) and therefore, pAbs to another protein may not be able to show any additional effects. Alternatively, a second possibility is that the pAbs may be able to block low affinity interactions (or create steric hindrance) that do not involve conformational changes. However, high affinity interactions (accompanied by conformation changes) may not be blocked by antibodies and therefore, the net effect by combining the antibodies may not be significantly higher than individual effects. This may be a reason why a near complete blocking of HSV-1 infection by antibodies has been extremely rare even when all gD receptors were blocked (Akhtar et al., 2008; Shukla et al. 2009). Our results, nevertheless, highlight that syndecan-1 and syndecan-2 play a critical role during HSV-1 entry and that the two HSPGs show detectable differences in their abilities to facilitate infection.
A related interesting finding was that the effect of syndecan-2 knockdown was even more severe at the plaque formation level. Unlike entry, the downregulation of syndecan-2 expression almost completely inhibited plaque formation in HeLa cells. A reduction in plaque number was expected since we found that downregulation of syndecan-1 and syndecan-2 reduces HSV-1 entry. However, the observed dramatic reduction in plaque formation raises the possibility that reduced virus entry may not be the only reason for reduced plaque formation and that an additional role for syndecan-2 in HSV-1 replication or spread could not be ruled out. Since syndecans participate in endocytosis, they may affect virus transport as well (Fuki et al., 2000).
One possible way to explain the fact that syndecan-2 has a more significant role in HSV-1 entry is related to the differences in GAG distribution on the ectodomains of the HSPGs. While the syndecan-1 ectodomain carries HS and chondroitin sulfate (CS) chains, syndecan-2 carries solely HS chains on its ectodomain (Rapraeger et al., 1985; Shworak et al., 1994; Su et al., 2007). It is possible that the presence of HS alone may help reduce any non-specific virus binding generated by similarly charged but less effective CS. A second way to explain the observable differences relies on HSPG cytoplasmic domain that has been shown to interact with a variety of signalling and structural proteins, suggesting its involvement in various regulatory phenomena. The cytoplasmic domain consists of two conserved regions, a membrane proximal common region (C1) and C-terminal common region (C2). C2 mediates binding to cytoskeletal proteins and to PDZ-containing proteins (Couchman, 2003; Grootjans et al., 1997; Lopes et al., 2006). The C1 and C2 conserved regions are separated by a variable region (V) that is unique for each of the four syndecan family members (Couchman, 2003; Lopes et al., 2006). The difference in the variable region in syndecan-1 and syndecan-2 might explain how these two syndecans might have different regulatory roles during HSV-1 infection through activating different cellular pathways.
Previous studies have shown that the infection with various microbial pathogens can modulate the expression levels of different syndecan family members. For example, alterations in syndecan-1 expression level are observed during the infection with Pneumocystis jiroveci and Neisseria gonorrhoeae (Freissler et al., 2000; Wang et al., 2006). Epstein–Barr virus infection results in the downregulation of syndecan-1 (Anastasiadou et al., 2009). Our study is the first report of HSV-1 infection modulating the expression levels of syndecan-1 and syndecan-2 in infected cells. We demonstrated here that HSV-1-induced syndecan-1 and syndecan-2 expression enhancements occur both at the cell surface level and also at the protein synthesis level. Interestingly, our findings suggest that an increase in syndecan-1 and syndecan-2 expression levels, although important for many reasons, may also be used as a marker for active HSV-1 infection. Further experiments are needed to understand mechanisms by which HSV-1 upregulates syndecan-1 and syndecan-2 expression and to determine whether this upregulation is a result of specific signalling pathway activation by the virus. Further understanding of the role of the syndecans in HSV-1 infection could identify novel antiviral targets and lead to the development of improved antiviral strategies.
METHODS
Cells and viruses.
African green monkey kidney cells (Vero), gL-expressing Vero cells (79B4) and Chinese hamster ovarian-K1 (CHO-K1) cells were provided by P. G. Spear (North-western University, Chicago, IL, USA). Human cervical (HeLa) cells were obtained from B. P. Prabhakar (University of Illinois at Chicago, Chicago, IL, USA). HeLa, Vero and gL-expressing Vero cells (79B4) were grown in Dulbecco's modified Eagle's medium (DMEM; Gibco-BRL) supplemented with 10 % FBS and 100 mg l−1 of penicillin/streptomycin (P/S). CHO-K1 cells were grown in Ham's F-12 medium (Gibco-BRL) supplemented with 10 % FBS and P/S. The β-galactosidase expressing recombinant HSV-1 (KOS) gL86 and wild-type HSV-1 (KOS) viruses were provided by P. G. Spear (North-western University, Chicago, IL, USA). HSV-1 (KOS) K26GFP was provided by P. Desai (Johns Hopkins University, Baltimore, MD, USA). Jellyfish GFP was fused in-frame with the UL35 ORF generating K26GFP virus whose capsids express GFP (Desai & Person, 1998). Virus stocks were propagated in complementing cell lines and stored at −80 °C. Yields of infectious virus titre (p.f.u. ml−1) were determined by plaque assay in Vero cells.
Antibodies and enzymes.
Antibodies and their dilutions were as follows: anti-syndecan-1 mouse mAbs diluted at 1 μg per 1×106 cells (Santa Cruz Biotechnology); anti-syndecan-2 rat mAbs 1.25 μg per 1×106 cells (R&D Systems); anti-heparan sulfate mouse mAb (10E4 epitope) diluted 1 : 50 (US Biological); anti-syndecan-1 rabbit pAb diluted 1 : 500 (Santa Cruz Biotechnology); anti-syndecan-2 rabbit pAb diluted 1 : 500 (Santa Cruz Biotechnology); anti-β-actin mouse mAb diluted 1 : 1000 (Sigma-Aldrich); and anti-myc mouse mAb (Invitrogen). The secondary antibodies for flow cytometry were FITC-conjugated anti-mouse secondary antibody diluted 1 : 100 (Sigma-Aldrich); FITC-conjugated anti-rat secondary antibodies diluted 1 : 100 (Sigma-Aldrich); and FITC-conjugated anti-mouse IgM diluted 1 : 100 (Sigma-Aldrich). The secondary antibodies for Western blots were HRP-conjugated anti-rabbit IgG diluted 1 : 20 000 (Jackson ImmunoResearch Laboratories); and HRP-conjugated anti-mouse IgG diluted 1 : 25 000 (Jackson ImmunoResearch Laboratories). Heparinase I, II and III enzymes were provided by Jian Liu (University of North Carolina, Chapel Hill, NC, USA) and used at 12 μg per sample (170 μg ml−1 final concentration). Chondroitinase ABC used at 0.005 U per sample (0.1 U ml−1 final concentration) (Sigma-Aldrich).
siRNA transfections.
HeLa and CHO-K1 cells were transfected at 80 % confluence with a concentration of 200 nM per well syndecan-1 siRNA (5′-CCAUUCUGACUCGGUUUCU[dT][dT]-3′, and 5′-GCCAAGGUUUUAUAAGGCU[dT][dT]-3′; Sigma-Aldrich), syndecan-2 siRNA (SASI_Hs01_00195372, SASI_Hs01_00195365; Sigma-Aldrich), or non-specific, scrambled siRNA (5′-GAUCAUACGUGCGAUCAGA[dT][dT]-3′; Sigma-Aldrich). Transfection was done using Lipofectamine 2000 reagent (Invitrogen). At 6 h after transfection, each well was supplemented with 3 ml complete growth medium (DMEM supplemented with 10 % FBS and P/S). Cells were analysed for protein expression and cell surface expression 48 h after transfection by preparing Western blots and by flow cytometry. Transfection efficacy was measured by using a plasmid that encoded enhanced GFP (eGFP) (Invitrogen) by counting eGFP-positive cells by fluorescence microscopy.
Viral entry assay.
Standard entry assay was used as described previously (Shukla et al., 1999b). Briefly, HeLa cells and HSV-1 entry receptor lacking CHO-K1 cells were plated in a 96-well tissue culture dish at a density of 8×103 cells per well. Cells were transfected with syndecan-1, syndecan-2 or scrambled siRNA as described above. After 48 h, cells were infected with the β-galactosidase expressing recombinant HSV-1(KOS) gL86 in a twofold serial dilution for 6 h at 37 °C. β-Galactosidase expression can be induced by early viral protein synthesis upon HSV infection (Copeland et al., 2008). At 6 h post-infection, cells were washed twice with PBS and the soluble substrate O-nitrophenyl-β-d-galactopyranoside (ONPG; Pierce) was added. Enzymic activity was measured at 410 nm using a micro-plate reader (Spectra Max 190 Molecular Devices).
Antibody blocking assay.
Standard antibody blocking assay was used as described previously (Shukla et al., 1999a). Briefly, confluent HeLa cells in 96-well tissue culture dish were washed with PBS and incubated with serial dilutions of rabbit pAbs to sydecan-1, syndecan-2 (Santa Cruz Biotechnology) or control anti-myc mAb (Invitrogen) for 30 min at 37 °C. A constant dose of HSV-1(KOS) gL86 (m.o.i. of 10) was then added to all wells for 2 h at 37 °C. After 2 h, cells were washed and bound virus was removed by 20 s treatment with 100 mM citrate buffer (pH 3.0). Incubation was then continued for another 3 h. Cells were then washed twice with PBS, and β-galactosidase activity was measured by adding its substrate ONPG as described in virus entry assay.
Plaque assay.
Viral replication upon syndecan-1 and syndecan-2 knockdown was assessed by a plaque assay (Akhtar et al., 2008; Tiwari et al., 2006). In brief, monolayers of HeLa cells plated in six-well tissue culture dishes were transfected with syndecan-1, syndecan-2 or scrambled siRNA as described above. At 48 h post-transfection, cells were infected (m.o.i. of 0.01) with HSV-1 (KOS) or mock infected in PBS for 90 min at 37 °C. Cells were then washed with PBS, and fresh medium (DMEM supplemented with 10 % FBS and P/S) was added. Cells were incubated for 72 h at 37 °C. After 72 h, cells were washed with PBS, fixed with 100 % methanol for 5 min, and stained with the Giemsa stain (Sigma-Aldrich) for 20 min. Infectivity was measured by counting plaques formed using ×10 objective lens of an inverted light microscope (Zeiss Axiovert 200).
Cytotoxicity assay.
Confluent monolayer of HeLa cells was transfected with syndecan-1, syndecan-2 or scrambled siRNA as described above. In addition to the non-specific, scrambled siRNA-transfected cells, non-transfected cells were used as a control. After 48 h post-transfection, cells were infected with HSV-1(KOS) (m.o.i. of 0.01) for 90 min at 37 °C. The inoculum was then removed by washing the cells with PBS and fresh medium was added. Cells were incubated at 37 °C for 120 h, then fixed with 100 % methanol and stained with Giemsa stain. The number of dead cells was counted using NIH Image J software (version: 1.43) at twenty high power fields (×40 objective).
Flow cytometry.
Syndecan-1 and syndecan-2, and HS cell surface expression was detected after HSV-1(KOS) infection. For syndecan-1 and syndecan-2 cell surface expression, confluent monolayer of HeLa cells were infected with HSV-1 (KOS) (m.o.i. of 10) for 0, 2, 4 and 6 h. Cells were then washed with PBS, harvested, and incubated with the respective primary antibody (syndecan-1 at 1 μg per 1×106 cells, or syndecan-2 at 1.25 μg per 1×106 cells) diluted in PBS with 1 % BSA for 1 h. After primary antibody incubation, cells were washed and incubated for 45 min with anti-mouse or anti-rat-FITC-conjugated secondary anti-IgGs (1 : 100). Cells stained only with anti-mouse-FITC or anti-rat-FITC was used as background control. For HS cell surface expression, HeLa cells were infected with HSV-1 (KOS) (m.o.i. of 10) for 0, 2, 4 and 6 h. Cells were then washed with PBS, harvested and incubated with mouse anti-human HS mAb 10E4 diluted 1 : 50 (US Biological) for 20 min at 4 °C. After that the cells were washed and incubated for 30 min at 4 °C with FITC-conjugated anti-mouse IgM diluted to 1 : 100. Cells stained only with FITC-conjugated anti-mouse IgM were used as background controls.
Immunoblotting.
After 48 h post-transfection and 0, 2 and 6 h after HSV-1(KOS) infection (m.o.i. of 10), syndecan-1 and syndecan-2 protein expression was determined by Western blot analysis. The Western blot assay was performed according to the protocol described previously (Burbach et al., 2003; Shukla et al., 2009). Briefly, approximately 150–200 μg of total cell protein in lysis buffer was incubated with 2.5 volumes of −20 °C 100 % methanol overnight at −20 °C. After treatment of 500 μl 100 % acetone for 5 min, the protein pellet was redissolved in 100 μl heparinase buffer (0.1 M NaOAc+0.1 mM CaOAc, pH 7.0). GAGs were digested with heparinase I, II, III (12 μg per sample) and chondroitinase ABC (0.005 U per sample) twice for 2.5 h to remove all GAGs. Samples were then denatured in Laemmli Sample Buffer (Bio-Rad) with 5 % (v/v) β-mercaptoethanol and heated to 96 °C for 10 min before loading onto a gel. After that the samples were electrophoretically separated on 10 % SDS-gel, transferred to nitrocellulose membrane, blocked for 2 h at room temperature in 0.1 % TTBS (0.1 % Tween 20 in TBS) containing 5 % milk, and incubated with primary rabbit pAbs against sydecan-1 and syndecan-2 at 1 : 500 dilutions overnight at 4 °C. The blots were rinsed five times with 0.1 % TTBS (0.1 % Tween 20 in TBS) for 5 min and incubated for 1 h at room temperature with HRP-conjugated anti-rabbit IgG at 1 : 20 000 dilutions. Anti-β-actin mouse mAb as the primary antibody at 1 : 1000 dilution and HRP-conjugated anti-mouse IgG as the secondary antibody at 1 : 25 000 dilution were used for detecting β-actin loading control. The signal was visualized with SuperSignal West Femto maximum sensitivity substrate (Pierce) and the blots were exposed to X-ray film (Kodak) for 2 min. Developed films were scanned and protein bands were quantified using NIH Image J software (version: 1.42) in order to generate statistical data for specific bands. Syndecan-1 and syndecan-2 protein expression was quantified by calculating the relative intensity of each syndecan-1 and syndecan-2 band relative to the mock-treated bands.
Statistical analyses.
Statistical analyses were performed with the statistica software (version 8.0) for windows. Normality was tested using the Kolmogorov–Smirnov test. All variables were distributed normally. Homogeneity of variance was determined using Levene's test and was considered violated when this test yielded P<0.05. All variances were homogeneous. Data were assessed using ANOVA followed by Scheffé's post-hoc test to evaluate the effects of gene silencing of syndecan-1 and syndecan-2 on HSV-1 viral entry, plaque formation and cytotoxicity. *P<0.05 and **P<0.0001 were regarded as significant differences between treated and mock-treated groups. The results are expressed as means±sd values. Each experiment was repeated at least three times and representative results are shown in the figures.
Acknowledgments
This work was supported by NIH grants AI057860 (D. Shukla), AI081869 (D. Shukla), AI050050 (J. Liu) and a Core Grant EY01792. S. Bacsa and S. Dosa were supported by fellowship awards from Rosztoczy Foundation. We thank Patricia G. Spear (North-western University, Chicago, IL, USA) and Prashant Desai (Johns Hopkins University, Baltimore, MD, USA) for providing cell lines, viruses and enzymes used in this study and Myung-Jin Oh (University of Illinois at Chicago, Chicago, IL, USA) for excellent technical assistance.
References
- Akhtar, J., Tiwari, V., Oh, M. J., Kovacs, M., Jani, A., Kovacs, S. K., Valyi-Nagy, T. & Shukla, D. (2008). HVEM and nectin-1 are the major mediators of herpes simplex virus 1 (HSV-1) entry into human conjunctival epithelium. Invest Ophthalmol Vis Sci 49, 4026–4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasiadou, E., Vaeth, S., Cuomo, L., Boccellato, F., Vincenti, S., Cirone, M., Presutti, C., Junker, S., Winberg, G. & Frati, L. (2009). Epstein–Barr virus infection leads to partial phenotypic reversion of terminally differentiated malignant B cells. Cancer Lett 284, 165–174. [DOI] [PubMed] [Google Scholar]
- Atanasiu, D., Whitbeck, J. C., Cairns, T. M., Reilly, B., Cohen, G. H. & Eisenberg, R. J. (2007). Bimolecular complementation reveals that glycoproteins gB and gH/gL of herpes simplex virus interact with each other during cell fusion. Proc Natl Acad Sci U S A 104, 18718–18723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth, H., Schafer, C., Adah, M. I., Zhang, F., Linhardt, R. J., Toyoda, H., Kinoshita-Toyoda, A., Toida, T., Van Kuppevelt, T. H. & other authors (2003). Cellular binding of hepatitis C virus envelope glycoprotein E2 requires cell surface heparan sulfate. J Biol Chem 278, 41003–41012. [DOI] [PubMed] [Google Scholar]
- Beauvais, D. M., Burbach, B. J. & Rapraeger, A. C. (2004). The syndecan-1 ectodomain regulates αvβ3 integrin activity in human mammary carcinoma cells. J Cell Biol 167, 171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobardt, M. D., Chatterji, U., Selvarajah, S., Van der Schueren, B., David, G., Kahn, B. & Gallay, P. A. (2007). Cell-free human immunodeficiency virus type 1 transcytosis through primary genital epithelial cells. J Virol 81, 395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brule, S., Charnaux, N., Sutton, A., Ledoux, D., Chaigneau, T., Saffar, L. & Gattegno, L. (2006). The shedding of syndecan-4 and syndecan-1 from HeLa cells and human primary macrophages is accelerated by SDF-1/CXCL12 and mediated by the matrix metalloproteinase-9. Glycobiology 16, 488–501. [DOI] [PubMed] [Google Scholar]
- Burbach, B. J., Friedl, A., Mundhenke, C. & Rapraeger, A. C. (2003). Syndecan-1 accumulates in lysosomes of poorly differentiated breast carcinoma cells. Matrix Biol 22, 163–177. [DOI] [PubMed] [Google Scholar]
- Carey, D. J. (1997). Syndecans: multifunctional cell-surface co-receptors. Biochem J 327, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheshenko, N., Liu, W., Satlin, L. M. & Herold, B. C. (2007). Multiple receptor interactions trigger release of membrane and intracellular calcium stores critical for herpes simplex virus entry. Mol Biol Cell 18, 3119–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement, C., Tiwari, V., Scanlan, P. M., Valyi-Nagy, T., Yue, B. Y. & Shukla, D. (2006). A novel role for phagocytosis-like uptake in herpes simplex virus entry. J Cell Biol 174, 1009–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland, R., Balasubramaniam, A., Tiwari, V., Zhang, F., Bridges, A., Linhardt, R. J., Shukla, D. & Liu, J. (2008). Using a 3-O-sulfated heparin octasaccharide to inhibit the entry of herpes simplex virus type 1. Biochemistry 47, 5774–5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couchman, J. R. (2003). Syndecans: proteoglycan regulators of cell-surface microdomains? Nat Rev Mol Cell Biol 4, 926–938,. [DOI] [PubMed] [Google Scholar]
- Deepa, S. S., Yamada, S., Zako, M., Goldberger, O. & Sugahara, K. (2004). Chondroitin sulfate chains on syndecan-1 and syndecan-4 from normal murine mammary gland epithelial cells are structurally and functionally distinct and cooperate with heparan sulfate chains to bind growth factors. A novel function to control binding of midkine, pleiotrophin, and basic fibroblast growth factor. J Biol Chem 279, 37368–37376. [DOI] [PubMed] [Google Scholar]
- Desai, P. & Person, S. (1998). Incorporation of the green fluorescent protein into the herpes simplex virus type 1 capsid. J Virol 72, 7563–7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esko, J. D. & Lindahl, U. (2001). Molecular diversity of heparan sulfate. J Clin Invest 108, 169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethell, I. M., Irie, F., Kalo, M. S., Couchman, J. R., Pasquale, E. B. & Yamaguchi, Y. (2001). EphB/syndecan-2 signaling in dendritic spine morphogenesis. Neuron 31, 1001–1013. [DOI] [PubMed] [Google Scholar]
- Freissler, E., Meyer auf der Heyde, A., David, G., Meyer, T. F. & Dehio, C. (2000). Syndecan-1 and syndecan-4 can mediate the invasion of OpaHSPG-expressing Neisseria gonorrhoeae into epithelial cells. Cell Microbiol 2, 69–82. [DOI] [PubMed] [Google Scholar]
- Fuki, I. V., Meyer, M. E. & Williams, K. J. (2000). Transmembrane and cytoplasmic domains of syndecan mediate a multi-step endocytic pathway involving detergent-insoluble membrane rafts. Biochem J 351, 607–612. [PMC free article] [PubMed] [Google Scholar]
- Geraghty, R. J., Krummenacher, C., Cohen, G. H., Eisenberg, R. J. & Spear, P. G. (1998). Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science 280, 1618–1620. [DOI] [PubMed] [Google Scholar]
- Giroglou, T., Florin, L., Schäfer, F., Streeck, R. E. & Sapp, M. (2001). Human papillomavirus infection requires cell surface heparan sulfate. J Virol 75, 1565–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grootjans, J. J., Zimmermann, P., Reekmans, G., Smets, A., Degeest, G., Dürr, J. & David, G. (1997). Syntenin, a PDZ protein that binds syndecan cytoplasmic domains. Proc Natl Acad Sci U S A 94, 13683–13688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold, B. C., WuDunn, D., Soltys, N. & Spear, P. G. (1991). Glycoprotein C of herpes simplex virus type 1 plays a principal role in the adsorption of virus to cells and in infectivity. J Virol 65, 1090–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, J. M. & Clement, C. (2009). Herpes simplex virus type 1 DNA in human corneas: what are the virological and clinical implications? J Infect Dis 200, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo, T., Wada, T., Yamaguchi, Y., Shimizu, A. & Handa, H. (2006). Knock-down of 25 kDa subunit of cleavage factor Im in HeLa cells alters alternative polyadenylation within 3′-UTRs. Nucleic Acids Res 34, 6264–6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaraguru, U. & Rouse, B. T. (2002). The IL-12 response to herpes simplex virus is mainly a paracrine response of reactive inflammatory cells. J Leukoc Biol 72, 564–570. [PubMed] [Google Scholar]
- Lindahl, U., Kusche-Gullberg, M. & Kjellén, L. (1998). Regulated diversity of heparan sulfate. J Biol Chem 273, 24979–24982. [DOI] [PubMed] [Google Scholar]
- Lopes, C. C., Dietrich, C. P. & Nader, H. B. (2006). Specific structural features of syndecans and heparan sulfate chains are needed for cell signaling. Braz J Med Biol Res 39, 157–167. [DOI] [PubMed] [Google Scholar]
- Magalhães, A., Marcos, N. T., Carvalho, A. S., David, L., Figueiredo, C., Bastos, J., David, G. & Reis, C. A. (2009). Helicobacter pylori cag pathogenicity island-positive strains induce syndecan-4 expression in gastric epithelial cells. FEMS Immunol Med Microbiol 56, 223–232. [DOI] [PubMed] [Google Scholar]
- Menozzi, F. D., Reddy, V. M., Cayet, D., Raze, D., Debrie, A. S., Dehouck, M. P., Cecchelli, R. & Locht, C. (2006). Mycobacterium tuberculosis heparin-binding haemagglutinin adhesin (HBHA) triggers receptor-mediated transcytosis without altering the integrity of tight junctions. Microbes Infect 8, 1–9. [DOI] [PubMed] [Google Scholar]
- Montgomery, R. I., Warner, M. S., Lum, B. J. & Spear, P. G. (1996). Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 87, 427–436. [DOI] [PubMed] [Google Scholar]
- Muto, T., Miyoshi, K., Munesue, S., Nakada, H., Okayama, M., Matsuo, T. & Noma, T. (2007). Differential expression of syndecan isoforms during mouse incisor amelogenesis. J Med Invest 54, 331–339. [DOI] [PubMed] [Google Scholar]
- Nicola, A. V., McEvoy, A. M. & Straus, S. E. (2003). Roles for endocytosis and low pH in herpes simplex virus entry into HeLa and Chinese hamster ovary cells. J Virol 77, 5324–5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell, C. D. & Shukla, D. (2008). The importance of heparan sulfate in herpesvirus infection. Virol Sin 23, 383–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell, C. D., Kovacs, M., Akhtar, J., Valyi-Nagy, T. & Shukla, D. (2010). Expanding the role of 3-O sulfated heparan sulfate in herpes simplex virus type-1 entry. Virology 397, 389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapraeger, A., Jalkanen, M., Endo, E., Koda, J. & Bernfield, M. (1985). The cell surface proteoglycan from mouse mammary epithelial cells bears chondroitin sulfate and heparan sulfate glycosaminoglycans. J Biol Chem 260, 11046–11052. [PubMed] [Google Scholar]
- Reske, A., Pollara, G., Krummenacher, C., Chain, B. M. & Katz, D. R. (2007). Understanding HSV-1 entry glycoproteins. Rev Med Virol 17, 205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield, K. P., Gallagher, J. T. & David, G. (1999). Expression of proteoglycan core proteins in human bone marrow stroma. Biochem J 343, 663–668. [PMC free article] [PubMed] [Google Scholar]
- Shimabukuro, Y., Ichikawa, T., Terashima, Y., Iwayama, T., Oohara, H., Kajikawa, T., Kobayashi, R., Terashima, H., Takedachi, M. & Terakura, M. (2008). Basic fibroblast growth factor regulates expression of heparan sulfate in human periodontal ligament cells. Matrix Biol 27, 232–241. [DOI] [PubMed] [Google Scholar]
- Shukla, D. & Spear, P. G. (2001). Herpesviruses and heparan sulfate: an intimate relationship in aid of viral entry. J Clin Invest 108, 503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla, D., Rowe, C. L., Dong, Y., Racaniello, V. R. & Spear, P. G. (1999a). The murine homolog (Mph) of human herpesvirus entry protein B (HveB) mediates entry of pseudorabies virus but not herpes simplex virus types 1 and 2. J Virol 73, 4493–4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla, D., Liu, J., Blaiklock, P., Shworak, N. W., Bai, X., Esko, J. D., Cohen, G. H., Eisenberg, R. J., Rosenberg, R. D. & Spear, P. G. (1999b). A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell 99, 13–22. [DOI] [PubMed] [Google Scholar]
- Shukla, S. Y., Singh, Y. K. & Shukla, D. (2009). Role of nectin-1, HVEM, and PILR-α in HSV-2 entry into human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 50, 2878–2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shworak, N. W., Shirakawa, M., Mulligan, R. C. & Rosenberg, R. D. (1994). Characterization of ryudocan glycosaminoglycan acceptor sites. J Biol Chem 269, 21204–21214. [PubMed] [Google Scholar]
- Smith, M. F., Jr, Novotny, J., Carl, V. S. & Comeau, L. D. (2006). Helicobacter pylori and toll-like receptor agonists induce syndecan-4 expression in an NF-κB-dependent manner. Glycobiology 16, 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear, P. G., Eisenberg, R. J. & Cohen, G. H. (2000). Three classes of cell surface receptors for alphaherpesvirus entry. Virology 275, 1–8. [DOI] [PubMed] [Google Scholar]
- Su, G., Blaine, S. A., Qiao, D. & Friedl, A. (2007). Shedding of syndecan-1 by stromal fibroblasts stimulates human breast cancer cell proliferation via FGF2 activation. J Biol Chem 282, 14906–14915. [DOI] [PubMed] [Google Scholar]
- Terasaka, Y., Miyazaki, D., Yakura, K., Haruki, T. & Inoue, Y. (2010). Induction of IL-6 in transcriptional networks in corneal epithelial cells after herpes simplex virus type 1 infection. Invest Ophthalmol Vis Sci 51, 2441–2449. [DOI] [PubMed] [Google Scholar]
- Tiwari, V., Clement, C., Xu, D., Valyi-Nagy, T., Yue, B. Y., Liu, J. & Shukla, D. (2006). Role for 3-O-sulfated heparan sulfate as the receptor for herpes simplex virus type 1 entry into primary human corneal fibroblasts. J Virol 80, 8970–8980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trybala, E., Liljeqvist, J. A., Svennerholm, B. & Bergström, T. (2000). Herpes simplex virus types 1 and 2 differ in their interaction with heparan sulfate. J Virol 74, 9106–9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumova, S., Woods, A. & Couchman, J. R. (2000). Heparan sulfate proteoglycans on the cell surface: versatile coordinators of cellular functions. Int J Biochem Cell Biol 32, 269–288. [DOI] [PubMed] [Google Scholar]
- Wang, S. H., Zhang, C., Liao, C. P., Lasbury, M. E., Durant, P. J., Tschang, D. & Lee, C. H. (2006). Syndecan-1 expression in the lung during Pneumocystis infection. J Eukaryot Microbiol 53 (Suppl. 1), S122–S123. [DOI] [PubMed] [Google Scholar]