Abstract
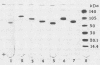
The interaction of the 56-kilodalton (kDa) proteinase from Serratia marcescens with human plasma activated C1 (C1) inhibitor, alpha 2-antiplasmin, and antithrombin III was investigated. The 56-kDa proteinase was not affected by these inhibitors; on the contrary, all the inhibitors were inactivated by the 56-kDa proteinase within 2 to 6 h. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis indicated that all three inhibitors showed decreases in molecular weight of approximately 8,000 to 10,000 as a result of proteolytic cleavage by the 56-kDa proteinase. The 56-kDa proteinase also inactivated serum complement within 2 to 6 h. The loss of inhibitory activity caused by the 56-kDa proteinase, together with the effects of endogenous serine proteinases, may facilitate tissue destruction and inflammation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Colman R. W. Activation of plasminogen by human plasma kallikrein. Biochem Biophys Res Commun. 1969 Apr 29;35(2):273–279. doi: 10.1016/0006-291x(69)90278-2. [DOI] [PubMed] [Google Scholar]
- Edy J., Collen D. The interaction in human plasma of antiplasmin, the fast-reacting plasmin inhibitor, with plasmin, thrombin, trypsin and chymotrypsin. Biochim Biophys Acta. 1977 Oct 13;484(2):423–432. doi: 10.1016/0005-2744(77)90098-5. [DOI] [PubMed] [Google Scholar]
- Griffith M. J. Measurement of the heparin enhanced-antithrombin III/thrombin reaction rate in the presence of synthetic substrate. Thromb Res. 1982 Feb 1;25(3):245–253. doi: 10.1016/0049-3848(82)90244-4. [DOI] [PubMed] [Google Scholar]
- Kamata R., Matsumoto K., Okamura R., Yamamoto T., Maeda H. The serratial 56K protease as a major pathogenic factor in serratial keratitis. Clinical and experimental study. Ophthalmology. 1985 Oct;92(10):1452–1459. doi: 10.1016/s0161-6420(85)33855-1. [DOI] [PubMed] [Google Scholar]
- Kamata R., Yamamoto T., Matsumoto K., Maeda H. A serratial protease causes vascular permeability reaction by activation of the Hageman factor-dependent pathway in guinea pigs. Infect Immun. 1985 Jun;48(3):747–753. doi: 10.1128/iai.48.3.747-753.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Kreger A. S. Importance of serratia protease in the pathogenesis of experimental Serratia marcescens pneumonia. Infect Immun. 1983 Apr;40(1):113–119. doi: 10.1128/iai.40.1.113-119.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda H. Assay of proteolytic enzymes by the fluorescence polarization technique. Anal Biochem. 1979 Jan 1;92(1):222–227. doi: 10.1016/0003-2697(79)90649-3. [DOI] [PubMed] [Google Scholar]
- Maeda H., Molla A., Oda T., Katsuki T. Internalization of serratial protease into cells as an enzyme-inhibitor complex with alpha 2-macroglobulin and regeneration of protease activity and cytotoxicity. J Biol Chem. 1987 Aug 15;262(23):10946–10950. [PubMed] [Google Scholar]
- Maki D. G., Hennekens C. G., Phillips C. W., Shaw W. V., Bennett J. V. Nosocomial urinary tract infection with Serratia marcescens: an epidemiologic study. J Infect Dis. 1973 Nov;128(5):579–587. doi: 10.1093/infdis/128.5.579. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Maeda H., Takata K., Kamata R., Okamura R. Purification and characterization of four proteases from a clinical isolate of Serratia marcescens kums 3958. J Bacteriol. 1984 Jan;157(1):225–232. doi: 10.1128/jb.157.1.225-232.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K., Yamamoto T., Kamata R., Maeda H. Enhancement of vascular permeability upon serratial infection: activation of Hageman factor--kallikrein--kinin cascade. Adv Exp Med Biol. 1986;198(Pt B):71–78. doi: 10.1007/978-1-4757-0154-8_9. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Yamamoto T., Kamata R., Maeda H. Pathogenesis of serratial infection: activation of the Hageman factor-prekallikrein cascade by serratial protease. J Biochem. 1984 Sep;96(3):739–749. doi: 10.1093/oxfordjournals.jbchem.a134892. [DOI] [PubMed] [Google Scholar]
- Miller R. L., Reichgott M. J., Melmon K. L. Biochemical mechanisms of generation of bradykinin by endotoxin. J Infect Dis. 1973 Jul;128(Suppl):144–156. doi: 10.1093/infdis/128.supplement_1.s144. [DOI] [PubMed] [Google Scholar]
- Molla A., Kagimoto T., Maeda H. Cleavage of immunoglobulin G (IgG) and IgA around the hinge region by proteases from Serratia marcescens. Infect Immun. 1988 Apr;56(4):916–920. doi: 10.1128/iai.56.4.916-920.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molla A., Matsumoto K., Oyamada I., Katsuki T., Maeda H. Degradation of protease inhibitors, immunoglobulins, and other serum proteins by Serratia protease and its toxicity to fibroblast in culture. Infect Immun. 1986 Sep;53(3):522–529. doi: 10.1128/iai.53.3.522-529.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molla A., Matsumura Y., Yamamoto T., Okamura R., Maeda H. Pathogenic capacity of proteases from Serratia marcescens and Pseudomonas aeruginosa and their suppression by chicken egg white ovomacroglobulin. Infect Immun. 1987 Oct;55(10):2509–2517. doi: 10.1128/iai.55.10.2509-2517.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molla A., Oda T., Maeda H. Different binding kinetics of Serratia 56K protease with plasma alpha 2-macroglobulin and chicken egg white ovomacroglobulin. J Biochem. 1987 Jan;101(1):199–205. doi: 10.1093/oxfordjournals.jbchem.a121892. [DOI] [PubMed] [Google Scholar]
- Molla A., Tanase S., Hong Y. M., Maeda H. Interdomain cleavage of plasma fibronectin by zinc-metalloproteinase from Serratia marcescens. Biochim Biophys Acta. 1988 Jun 29;955(1):77–85. doi: 10.1016/0167-4838(88)90181-1. [DOI] [PubMed] [Google Scholar]
- Pemberton P. A., Stein P. E., Pepys M. B., Potter J. M., Carrell R. W. Hormone binding globulins undergo serpin conformational change in inflammation. Nature. 1988 Nov 17;336(6196):257–258. doi: 10.1038/336257a0. [DOI] [PubMed] [Google Scholar]
- Potempa J., Shieh B. H., Travis J. Alpha-2-antiplasmin: a serpin with two separate but overlapping reactive sites. Science. 1988 Aug 5;241(4866):699–700. doi: 10.1126/science.2456616. [DOI] [PubMed] [Google Scholar]
- Saito H., Goldsmith G. H., Moroi M., Aoki N. Inhibitory spectrum of alpha 2-plasmin inhibitor. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2013–2017. doi: 10.1073/pnas.76.4.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvesen G. S., Catanese J. J., Kress L. F., Travis J. Primary structure of the reactive site of human C1-inhibitor. J Biol Chem. 1985 Feb 25;260(4):2432–2436. [PubMed] [Google Scholar]
- Virca G. D., Lyerly D., Kreger A., Travis J. Inactivation of human plasma alpha 1-proteinase inhibitor by a metalloproteinase from Serratia marcescens. Biochim Biophys Acta. 1982 Jun 4;704(2):267–271. doi: 10.1016/0167-4838(82)90155-8. [DOI] [PubMed] [Google Scholar]
- Vogel C. N., Kingdon H. S., Lundblad R. L. Correlation of in vivo and in vitro inhibition of thrombin by plasma inhibitors. J Lab Clin Med. 1979 Apr;93(4):661–673. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]