Abstract
Background
A growth in the utilization of high-risk allografts is reflective of a critical national shortage and the increasing waiting list mortality. Using risk-adjusted models, the aim of the present study was to determine whether a volume–outcome relationship existed among liver transplants at high risk for allograft failure.
Methods
From 2002 to 2008, the Scientific Registry of Transplant Recipients (SRTR) database for all adult deceased donor liver transplants (n = 31 587) was queried. Transplant centres (n = 102) were categorized by volume into tertiles: low (LVC; 31 cases/year), medium (MVC: 64 cases/year) and high (HVC: 102 cases/year). Donor risk comparison groups were stratified by quartiles of the Donor Risk Index (DRI) spectrum: low risk (DRI ≤ 1.63), moderate risk (1.64 > DRI > 1.90), high risk (1.91 > DRI > 2.26) and very high risk (DRI ≥ 2.27).
Results
HVC more frequently used higher-risk livers (median DRI: LVC: 1.82, MVC: 1.90, HVC: 1.97; P < 0.0001) and achieved better risk adjusted allograft survival outcomes compared with LVC (HR: 0.90, 95%CI: 0.85–0.95). For high and very high risk groups, transplantation at a HVC did contribute to improved graft survival [high risk: hazard ratio (HR): 0.85, 95% confidence interval (CI): 0.76–0.96; Very High Risk: HR: 0.88, 95%CI: 0.78–0.99].
Conclusion
While DRI remains an important aspect of allograft survival prediction models, liver transplantation at a HVC appears to result in improved allograft survival with high and very high risk DRI organs compared with LVC.
Keywords: donor risk index, marginal donors, MELD, outcomes, liver transplantation, organ allocation, deceased donation
Introduction
The volume–outcome relationship has been validated in surgery across numerous specialties and high-risk procedures by a growing body of literature over the past quarter of a century.1–4 However, as studies continue to demonstrate that high-volume institutions deliver improved outcomes, particularly among high-risk patient populations, the implication of these findings remains controversial.5,6 This is because many volume–outcomes studies are compromised by limited data, varying definitions of volume groups and problematic statistical methodology.7,8 However, nowhere is this debate more complex than in the field of liver transplantation, where procedures are influenced not only by recipient and transplantation centre factors, but also by a number of donor variables.9–13
Unique to orthotopic liver transplantation, the ideal or reference donor is defined as less than 40 years of age, without significant steatosis, chronic liver lesions or other transmissible diseases, who deceased as a result of traumatic brain injury, is donating after brain death and prior to haemodynamic instability.14 In the past, transplant surgeons were confronted with the challenge of evaluating the donor by qualitatively comparing multiple risk factors such as donor age, race, weight, cause of death, donations after cardiac death (DCD), length of hospital stay before death, use of vasopressors, cold ischaemia time and degree of steatosis.15 Feng et al. clarified the significance of donor variables by creating the Donor Risk Index (DRI), which identified the following risk factors for allograft failure: age (≥40 years old), split/partial grafts, DCD, ethnicity (African-American), cause of death as a result of a cerebrovascular accident (CVA), ‘other’ causes of death and short stature.16
As annual procedure volume appears to positively impact transplant outcomes, we aimed to determine whether this volume–outcomes relationship existed among liver transplants at high risk for allograft failure, as defined by DRI scores. Therefore, using a risk-adjusted model accounting for donor, recipient, regional and centre characteristics, we evaluated the combined effect of annual procedure volume and DRI on allograft survival.
Methods
Database
Observations were queried from the Scientific Registry of Transplant Recipients (SRTR), a nation-wide database that draws from of the Organ Procurement and Transplantation Network (OPTN). All adult recipients (≥18 years of age) of deceased donor liver transplants between 1 January 2002 and 31 December 2008 were compiled. Procedures involving partial liver transplants (reduced-liver, living donor and split-liver transplants) were excluded from the analysis of differences in organ allocation between these groups and the majority of patients with chronic liver disease awaiting liver transplantation as previously described.17
Volume groups
All observations were identified based on year and centre of transplantation. Each institution was coded with encrypted hospital identifiers, used to calculate centre-specific annual procedure volumes. Centres were then ranked based on annual prolificacy. Transplant centres with five or fewer cases in a year were excluded to reduce confounding variables in our analysis. Based on this order, centres were categorized into tertiles groups containing even fractions of the dataset: low volume centres (LVC), middle volume centres (MVC) and high volume centres (HVC). Because centre-specific procedure volumes varied from year to year, centre rank was re-calculated for each year studied as previously described.3,18
DRI groups
Donor risk comparison groups were similarly stratified by quartiles of the DRI spectrum, each containing an equal number of observations: low risk (DRI ≤ 1.63), moderate risk (DRI = 1.64–1.90), high risk (DRI = 1.91–2.26) and very high risk (DRI ≥ 2.27).
Demographics and variables
Donor demographics included age, gender, ethnicity (Caucasian, African American and all other minorities), cause of death (anoxia, CVA, head trauma or other), DCD, cold ischaemia time (in hours) and DRI. Recipient demographics included age, gender, ethnicity, time spent on the waiting list, region, year of transplantation and model for end-stage liver disease (MELD) score. Nominal variables included gender, ethnicity, cause of death and DCD status. Ordinal variables included year and region of transplantation. Continuous variables included age, time spent on the waitlist, cold ischaemia time, recipient MELD score and DRI. MELD scores were calculated for each recipient based on the United Network of Organ Sharing (UNOS) modification to the formulary described in.19 DRI was calculated for each donor as previously described.16
Analysis
Nominal and ordinal categorical variables were tested for statistical significance (P < 0.05) with Pearson's χ2-tests and the Mantel–Haenszel χ2-tests, respectively. Variation in central tendencies of continuous variables between centre volume groups was evaluated using Kruskal–Wallis non-parametric anova, because of non-normal distribution. Univariate analysis of all categorical variables was performed using the Log-Rank test of equality to evaluate for significance as predictors of endpoints, defined as allograft failure. Assessment results were visualized using Kaplan–Meier curves. Variables included in the calculations of DRI16 and MELD,19 which have already been shown to be significant, were excluded from univariate analysis.
Four separate multivariate Cox proportional hazards regression modelling were generated for each quartile of the DRI spectrum.20 These risk-adjusted models accounted for donor characteristics (DRI), recipient characteristics (age, ethnicity and MELD), centre volume groups (LVC, MVC and HVC) and location (Regions 1–11) shown to be significant on univariate analysis. Components of the DRI and MELD score were omitted from the Cox regression model to avoid over-adjustment. Each covariate was evaluated as a predictor for allograft failure by maximum likelihood estimates of hazard ratios (HR) and 95% confidence intervals (95% CI).
SAS version 9.2 (SAS Institute, Cary, NC) was used for all statistical analysis. The present study was reviewed by the University of Massachusetts Medical School Institutional Review Board (IRB) and deemed appropriate for exemption from IRB oversight as no personal identifiers were used among datasets.
Results
Cohort description
In all, 31 587 OLT were queried. Between 92 and 102 transplant centres actively contributed data to OPTN during the time period studied. Transplant centres were sorted into tertiles: LVC (33.64% of cases; 46.06% of centres), MVC (33.93% of cases; 32.10% of centres) and HVC (32.44% of cases; 21.84% of centres; P < 0.0001). Donor-risk comparison groups were stratified into quartiles: low risk (DRI ≤ 1.63; n = 7922), moderate risk (1.63 < DRI ≤ 1.90; n = 7918), high risk (1.90 ≤ DRI < 2.26; n = 7925) and very high risk (DRI > 2.26; n = 7924). Regional trends suggest that the largest contributions came from Region 3 (16.43% of cases), whereas the smallest contributions came from Region 1 (2.79% of cases), as described in Table 1.
Table 1.
Number of transplant centres per year and number of transplant cases per region per tertile group for all observations (n = 31 587)
| Variables | LVC (n = 10 621) | MVC (n = 10 713) | HVC (n = 10 242) | Total (n = 31 587) |
|---|---|---|---|---|
| 2002 | 67 centres | 18 centres | 7 centres | 92 centres |
| 2003 | 39 centres | 35 centres | 20 centres | 94 centres |
| 2004 | 39 centres | 32 centres | 24 centres | 95 centres |
| 2005 | 41 centres | 32 centres | 24 centres | 97 centres |
| 2006 | 39 centres | 33 centres | 24 centres | 96 centres |
| 2007 | 40 centres | 33 centres | 24 centres | 97 centres |
| 2008 | 45 centres | 33 centres | 24 centres | 102 centres |
| Region 1 | 880 cases | 0 cases | 0 cases | 880 cases |
| Region 2 | 1837 cases | 286 cases | 1698 cases | 3821 cases |
| Region 3 | 613 cases | 1599 cases | 2977 cases | 5189 cases |
| Region 4 | 1284 cases | 996 cases | 849 cases | 3129 cases |
| Region 5 | 2151 cases | 575 cases | 1598 cases | 4324 cases |
| Region 6 | 403 cases | 668 cases | 0 cases | 1071 cases |
| Region 7 | 905 cases | 1565 cases | 230 cases | 2700 cases |
| Region 8 | 417 cases | 1543 cases | 0 cases | 1960 cases |
| Region 9 | 0 cases | 1971 cases | 554 cases | 2525 cases |
| Region 10 | 559 cases | 458 cases | 1954 cases | 2971 cases |
| Region 11 | 1572 cases | 1052 cases | 382 cases | 3006 cases |
| % of All Centers | 46.06% | 32.10% | 21.84% | 100% |
| % of All Cases | 33.64% | 33.93% | 32.44% | 100% |
| Median# Cases (SD) | 31 cases (10.86) | 64 cases (9.76) | 102 cases (26.00) | 197 cases |
LVC, low volume centers; MVC, middle volume centres; HVC, high volume centres; %, per cent; #, number; SD, standard deviation.
Demographics
The majority of donors were male (59.54%) and Caucasians (69.42%; P < 0.0001). The median donor age was 43 years and 15.72% were ≥60 years of age. The primary cause of death was CVA (44.50%) and a minority were DCD donors (4.32%; P < 0.0001). The majority of recipients were also male (68.20%) and Caucasian (73.45%). The median recipient age was 54 years with a MELD score of 18.
Table 2 outlines the demographics of each tertile. The following donor characteristics had statistically significant (P < 0.05) differences between tertiles: age, ethnicity, cause of death, DCD status and DRI. The following recipient characteristics were also found to be different: age, ethnicity, MELD score, time spent on the waiting list and region. Evaluation of DRI groups showed that higher volume groups utilizing higher median DRI allografts (LVC: 1.82, MVC: 1.90, HVC: 1.97; P < 0.05), as depicted in Fig. 1. In contrast, median MELD scores (LVC: 19.0, MVC: 19.0, HVC: 17.0; P < 0.05) and median time spent on the waiting list (LVC: 92 days, MVC: 79 days, HVC: 55 days; P < 0.05) were inversely proportional to procedure volume.
Table 2.
Donor and recipient demographics for all observations (n = 31 587)
| Demographics | LVC (n = 10 621) | MVC (n = 10 713) | HVC (n = 10 242) | P-value |
|---|---|---|---|---|
| Recipient female | 31.17% | 32.19% | 32.07% | 0.223 |
| Donor female | 40.17% | 40.38% | 40.87% | 0.569 |
| Recipient ethnicity | 0.003 | |||
| Caucasian | 72.31% | 74.60% | 73.50% | |
| African American | 9.18% | 8.38% | 8.41% | |
| Other ethnicities | 18.51% | 17.02% | 18.09% | |
| Donor ethnicity | <0.0001 | |||
| Caucasian | 69.03% | 70.97% | 68.28% | |
| African American | 14.62% | 15.46% | 15.51% | |
| Other ethnicities | 16.34% | 13.57% | 16.21% | |
| Recipient age (≥18 years), Median | 53 years | 54 years | 54 years | <0.05 |
| Donor age, median | 41 years | 43 years | 45 years | <0.05 |
| ≥40 years of age | 51.29% | 55.03% | 58.37% | <0.0001 |
| ≥60 years of age | 12.39% | 16.85% | 18.02% | <0.0001 |
| Cold ischaemia time, median | 7.0 h | 7.0 h | 7.0 h | NA |
| Recipient wait time, median | 92 days | 79 days | 55 days | <0.05 |
| Donor cause of death | <0.0001 | |||
| Anoxia | 13.15% | 14.22% | 15.35% | |
| CVA | 43.36% | 3.71% | 46.53% | |
| Head trauma | 41.14% | 38.72% | 35.79% | |
| Other | 2.35% | 3.35% | 2.31% | |
| DCD | 3.51% | 4.45% | 5.04% | <0.0001 |
| Recipient MELD, Median (SD) | 19.0 (9.10) | 19.0 (8.84) | 17.0 (8.65) | <0.05 |
| DRI, Median (SD) | 1.82 (0.41) | 1.90 (0.48) | 1.97 (0.49) | <0.05 |
HVC, high volume centres; MVC, middle volume centres; LVC, low volume centres; %, per cent; other ethnicities, Hispanics, Asians and ‘others’; CVA, cerebrovascular accident or stroke; DCD, donation after cardiac death; DRI, donor risk index; SD, standard deviation; MELD, model for end-stage liver disease; NA, not applicable.
Figure 1.
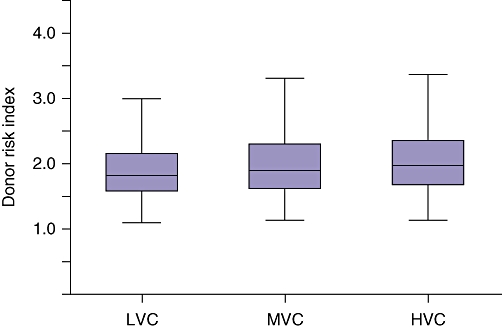
Box and whisker* plot of donor risk index (DRI) by transplant centre volume, P < 0.0001. *Whiskers calculated as data ≤1.5 inter-quartile range
Multivariate allograft survival outcomes
HVC achieved better overall risk-adjusted allograft survival outcomes compared with LVC (HR: 0.90, 95%CI: 0.85–0.95) for all patients who underwent liver transplantation. However, risk-adjusted models showed that for low- and moderate-risk groups, HVC did not confer significant graft survival benefits, as described in Table 3, and shown in Fig. 2a–b, respectively. However, for high and very high risk groups, HVC did significantly contribute to graft survival (high risk: HR: 0.85, 95%CI: 0.76–0.96; very high risk: HR: 0.88, 95%CI: 0.78–0.99), as described in Table 3, and shown in Fig. 2c–d, respectively.
Table 3.
Risk-adjusted analysis of allograft failure risk among DRI quartiles
| Centre volume groups | Donor risk groups | HR | 95% CI | P-value |
|---|---|---|---|---|
| LVC | Low risk | Reference | – | – |
| MVC | 1.06 | 0.93, 1.20 | 0.41 | |
| HVC | 0.99 | 0.86, 1.14 | 0.90 | |
| LVC | Moderate risk | Reference | – | – |
| MVC | 1.04 | 0.92, 1.19 | 0.50 | |
| HVC | 0.91 | 0.80, 1.04 | 0.16 | |
| LVC | High risk | Reference | – | – |
| MVC | 0.90 | 0.80, 1.02 | 0.10 | |
| HVC | 0.85 | 0.76, 0.96 | 0.007 | |
| LVC | Very high risk | Reference | – | – |
| MVC | 1.01 | 0.89, 1.14 | 0.92 | |
| HVC | 0.88 | 0.78, 0.99 | 0.03 | |
LVC, low volume centres; MVC, middle volume centre; HVC, high volume centre; MELD, model for end-stage liver disease; DRI, donor risk index; HR, hazard ratio; %, per cent; CI, confidence interval.
Figure 2.
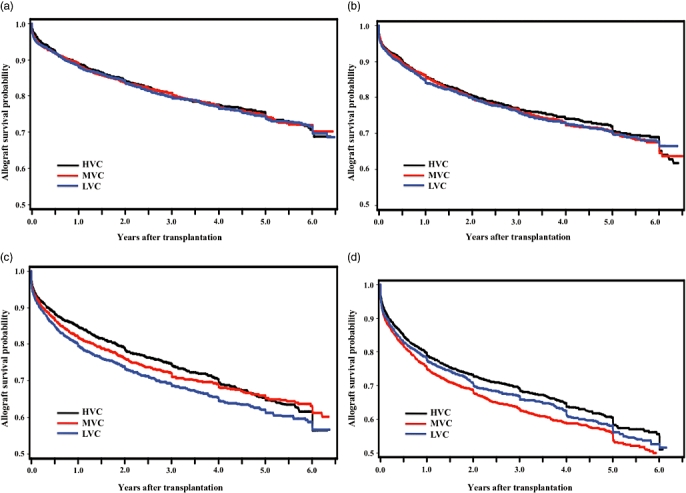
(a) Allograft survival by transplant Centre volume for low donor risk (DRI ≤ 1.63, n = 7922; P = 0.865). (b) Allograft survival by transplant centre volume for moderate donor risk (1.63 < DRI ≤ 1.90, n = 7918; P = 0.718). (c) Allograft survival by transplant centre volume for high donor risk (1.90 < DRI ≤ 2.26, n = 7925; P = 0.0006). (d) Allograft survival by transplant centre volume for very high donor risk (DRI > 2.26, n = 7924; P = 0.0002)
Discussion
The volume–outcome relationship in liver transplantation continues to be defined and its significance studied as a result of the current era of quality, cost containment and health care reform. In the present study, we have found that a centre volume benefit exists at HVC and in a risk-stratified model, the largest benefit appears to exist in the transplantation of the highest risk organs. The current results are present in spite of minimal differences in donor and recipient demographics. Risk-adjusted models showed that for low- and moderate-risk groups, HVC did not confer significant graft survival benefits. However, for high and very high risk groups, HVC did contribute to improved graft survival of 12–15% compared with transplantation at LVC. While DRI remains an important aspect of allograft survival prediction models, further understanding in its use as a predictor for graft failure is necessary.
Differences in centre volume were evident by groups, region as well as year. Certain regions do have HVC in our cohort based on tertiles for creating volume groups. Minor differences were seen in donor and recipient demographics between volume groups. HVC for instance, used organs with a higher DRI, but yet the median MELD score of the recipient was lower. Further understanding in these practices is needed. Should centre volume be a component in determining the true ‘risk index’ of use of an allograft?
In spite of its benefit as an addition to the donor pool, the use of high-risk or marginal donor livers have brought about much attention because of the potential for inferior outcomes. As no defined criteria exists for expanded donor liver allografts, the DRI as described by Feng, has gained acceptance in the liver transplant community for quantification of donor risk and potential for allograft failure.16,21,22 Use of these organs requires experience and expertise and appropriate allocation for optimal use. Studies have described its utility as well as its drawbacks with a comprehensive risk assessment for all organs.21,23,24 Bashes et al. described acceptable results with these high-risk organs by documenting reduced mortality on the waiting list.25 We used the DRI to create quartile gradients to assess if there is a volume impact on not only the use of these organs, but also in the results after transplantation. In these cohorts that were created based on quartiles of DRI, there was no volume benefit at low- and medium-risk organs while a significant advantage was noted in the higher risk organs. This is the first report to describe a volume relationship with gradients of DRI liver allografts.
The results of the present study are important because they suggest a volume advantage with these high-risk allografts. While further research is necessary to understand the implications of the results, use of these organs clearly requires experience for optimal results. Previous studies have shown conflicting data regarding the role of volume with improved transplant outcomes.9,12,17 Based on the data, it is unclear whether this is as a result of organ allocation, recipient selection or post-transplant care. Identifying this level of care and determining where the difference lies, if any, is imperative to determine how we can improve outcomes overall and consider the use of other high-risk organs such as hepatitis C positive livers, older organs or donors with malignancy. As a result of the retrospective nature, large database used and inherent biases present in the use of these organs, it is not possible to determine if there was a reduction in waiting list mortality with the use of these organs at HVC.
Several limitations must be considered. As this was a retrospective study, it has the associated constraints specific to the variables collected in the SRTR database. While the database is comprehensive and inclusive, it does not include significant clinical variables that may be important for organ selection. For example, data on steatosis or causes of allografts failure were not available and thus could not be included in our analysis. Furthermore, such standards may vary significantly from centre to centre or between regions. We tried to control for this by examining the results within regions. All centres that performed deceased donor liver transplantations and contributed data to the SRTR database during the evaluated time period were utilized in the present study. We excluded all centres that performed less than five liver transplants per year to reduce statistical variability and ensure the volume groups were appropriately represented.
A centre volume advantage exists in the use of increasingly higher risk donor allografts. Based on these results, we have shown that the potential for optimization for these high-risk organs may exist in organ allocation, recipient selection or regional variability. Further research and comparison in specific practices is imperative to understand how best to optimize results and the use of these allografts.
Acknowledgments
Supported by the ASTS Faculty Development Award (SAS).
Conflicts of interest
None declared.
References
- 1.Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? The empirical relation between surgical volume and mortality. N Engl J Med. 1979;301:1364–1369. doi: 10.1056/NEJM197912203012503. [DOI] [PubMed] [Google Scholar]
- 2.Birkmeyer JD, Siewers AE, Finlayson EV, Stukel TA, Lucas FL, Batista I, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 3.Singla A, Simons J, Li Y, Csikesz NG, Ng SC, Tseng JF, et al. Admission volume determines outcome for patients with acute pancreatitis. Gastroenterology. 2009;137:1995–2001. doi: 10.1053/j.gastro.2009.08.056. [DOI] [PubMed] [Google Scholar]
- 4.Singla A, Simons JP, Carroll JE, Li Y, Ng SC, Tseng JF, et al. Hospital volume as a surrogate for laparoscopically assisted colectomy. Surg Endosc. 2009;24:662–669. doi: 10.1007/s00464-009-0665-2. [DOI] [PubMed] [Google Scholar]
- 5.Birkmeyer JD, Sun Y, Goldfaden A, Birkmeyer NJ, Stukel TA. Volume and process of care in high-risk cancer surgery. Cancer. 2006;106:2476–2481. doi: 10.1002/cncr.21888. [DOI] [PubMed] [Google Scholar]
- 6.Khuri SF, Henderson WG. The case against volume as a measure of quality of surgical care. World J Surg. 2005;29:1222–1229. doi: 10.1007/s00268-005-7987-6. [DOI] [PubMed] [Google Scholar]
- 7.Kulkarni GS, Laupacis A, Urbach DR, Fleshner NE, Austin PC. Varied definitions of hospital volume did not alter the conclusions of volume-outcome analyses. J Clin Epidemiol. 2009;62:400–407. doi: 10.1016/j.jclinepi.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Finlayson SR. The volume-outcome debate revisited. Am Surg. 2006;72:1038–1042. [PubMed] [Google Scholar]
- 9.Edwards EB, Roberts JP, McBride MA, Schulak JA, Hunsicker LG. The effect of the volume of procedures at transplantation centers on mortality after liver transplantation. N Engl J Med. 1999;341:2049–2053. doi: 10.1056/NEJM199912303412703. [DOI] [PubMed] [Google Scholar]
- 10.Ahmad J, Bryce CL, Cacciarelli T, Roberts MS. Differences in access to liver transplantation: disease severity, waiting time, and transplantation center volume. Ann Intern Med. 2007;146:707–713. doi: 10.7326/0003-4819-146-10-200705150-00004. [DOI] [PubMed] [Google Scholar]
- 11.Bryce CL, Angus DC, Arnold RM, Chang CC, Farrell MH, Manzarbeitia C, et al. Sociodemographic differences in early access to liver transplantation services. Am J Transplant. 2009;9:2092–2101. doi: 10.1111/j.1600-6143.2009.02737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Axelrod DA, Guidinger MK, McCullough KP, Leichtman AB, Punch JD, Merion RM. Association of center volume with outcome after liver and kidney transplantation. Am J Transplant. 2004;4:920–927. doi: 10.1111/j.1600-6143.2004.00462.x. [DOI] [PubMed] [Google Scholar]
- 13.Axelrod DA, Kalbfleisch JD, Sun RJ, Guidinger MK, Biswas P, Levine GN, et al. Innovations in the assessment of transplant center performance: implications for quality improvement. Am J Transplant. 2009;9:959–969. doi: 10.1111/j.1600-6143.2009.02570.x. [DOI] [PubMed] [Google Scholar]
- 14.New York State Department of Health Workgroup. Workgroup on expanded criteria organs for liver transplantation. Liver Transpl. 2005;11:1184–1192. doi: 10.1002/lt.20569. [DOI] [PubMed] [Google Scholar]
- 15.Cameron AM, Ghobrial RM, Yersiz H, Farmer DG, Lipshutz GS, Gordon SA, et al. Optimal utilization of donor grafts with extended criteria: a single-center experience in over 1000 liver transplants. Ann Surg. 2006;243:748–753. doi: 10.1097/01.sla.0000219669.84192.b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783–790. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 17.Northup PG, Pruett TL, Stukenborg GJ, Berg CL. Survival after adult liver transplantation does not correlate with transplant center case volume in the MELD era. Am J Transplant. 2006;6:2455–2462. doi: 10.1111/j.1600-6143.2006.01501.x. [DOI] [PubMed] [Google Scholar]
- 18.Singla A, Li Y, Ng SC, Csikesz NG, Tseng JF, Shah SA. Is the growth in laparoscopic surgery reproducible with more complex procedures? Surgery. 2009;146:367–374. doi: 10.1016/j.surg.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 20.Harrell FE, Jr, Lee KL, Califf RM, Pryor DB, Rosati RA. Regression modelling strategies for improved prognostic prediction. Stat Med. 1984;3:143–152. doi: 10.1002/sim.4780030207. [DOI] [PubMed] [Google Scholar]
- 21.Axelrod DA, Schnitzler M, Salvalaggio PR, Swindle J, Abecassis MM. The economic impact of the utilization of liver allografts with high donor risk index. Am J Transplant. 2007;7:990–997. doi: 10.1111/j.1600-6143.2006.01724.x. [DOI] [PubMed] [Google Scholar]
- 22.Alkofer B, Samstein B, Guarrera JV, Kin C, Jan D, Bellemare S, et al. Extended-donor criteria liver allografts. Semin Liver Dis. 2006;26:221–233. doi: 10.1055/s-2006-947292. [DOI] [PubMed] [Google Scholar]
- 23.Northup PG, Pruett TL, Kashmer DM, Argo CK, Berg CL, Schmitt TM. Donor factors predicting recipient survival after liver retransplantation: the retransplant donor risk index. Am J Transplant. 2007;7:1984–1988. doi: 10.1111/j.1600-6143.2007.01887.x. [DOI] [PubMed] [Google Scholar]
- 24.Segev DL, Maley WR, Simpkins CE, Locke JE, Nguyen GC, Montgomery RA, et al. Minimizing risk associated with elderly liver donors by matching to preferred recipients. Hepatology. 2007;46:1907–1918. doi: 10.1002/hep.21888. [DOI] [PubMed] [Google Scholar]
- 25.Barshes NR, Horwitz IB, Franzini L, Vierling JM, Goss JA. Waitlist mortality decreases with increased use of extended criteria donor liver grafts at adult liver transplant centers. Am J Transplant. 2007;7:1265–1270. doi: 10.1111/j.1600-6143.2007.01758.x. [DOI] [PubMed] [Google Scholar]


