Abstract
Background
The role of staging laparoscopy (SL) in patients with incidental gallbladder cancer (IGBC) is ill defined. This study evaluates the utility of SL with the aim of identifying variables associated with disseminated disease (DD).
Methods
Consecutive patients with IGBC who underwent re-exploration between 1998 and 2009 were identified from a prospective database. The yield and accuracy of SL were calculated. Demographics, tumour- and treatment-related variables were correlated with findings of DD.
Results
Of the 136 patients submitted to re-exploration for possible definitive resection, 19 (14.0%) had DD. Staging laparoscopy was carried out in 46 (33.8%) patients, of whom 10 (21.8%) had DD (peritoneal disease [n = 6], liver metastases [n = 3], retroperitoneal disease [n = 1]). Disseminated disease was identified by SL in two patients (yield = 4.3%), whereas eight were diagnosed after conversion to laparotomy (accuracy = 20.0%). The likelihood of DD correlated closely with T-stage (T1b, n = 0; T2, n = 5 [7.0%], T3, n = 14 [26.0%]; P = 0.004). A positive margin at initial cholecystectomy (odds ratio [OR] 5.44, 95% confidence interval [CI] 1.51–24.37; P = 0.004) and tumour differentiation (OR 7.64, 95% CI 1.1–NA; P = 0.006) were independent predictors of DD on multivariate analysis.
Discussion
Disseminated disease is relatively uncommon in patients with IGBC and SL provides a very low yield. However, patients with poorly differentiated, T3 or positive-margin gallbladder tumours are at high risk for DD and targeting these patients may increase the yield of SL.
Keywords: incidental gallbladder cancer, laparoscopic staging, re-exploration
Introduction
Gallbladder cancer (GBC) diagnosed after elective cholecystectomy for presumed benign disease is defined as incidental gallbladder cancer (IGBC).1 Over the past several years, the incidence of IGBC has increased, probably as a result of the increase in the number of elective cholecystectomies.2 For selected patients with invasive tumours in whom restaging does not show disseminated disease (DD), re-exploration and definitive resection are indicated and are associated with improved survival.3,4 However, despite negative findings on high-quality, preoperative imaging studies, a sizeable proportion (10–57%) of patients will have advanced disease at re-exploration.2,5
Laparoscopy has been shown to be an important staging tool in the management of gastrointestinal malignancies because it provides the ability to identify radiographically occult DD before proceeding to laparotomy for an attempt at resection.6–8 In the subgroup of patients with primary and secondary hepatobiliary cancers, the incidence of occult unresectable disease is high (25–75%).6 Given these high rates, staging laparoscopy (SL) is frequently utilized in order to decrease lengths of stay and overall hospital charges in the subgroup of patients with advanced disease that is not amenable to resection.9–13
Some authors have recommended using SL to identify DD in all patients with IGBC undergoing re-operation for planned definitive resection.2 As imaging technology improves, however, an increasingly larger proportion of patients are identified during preoperative staging examinations as having unresectable cancer. Thus, the rationale and potential benefits of SL must be weighed against the added cost and increased operating time incurred by using it in patients who are at low risk for DD. The role of SL in patients with IGBC has not been clearly defined because most studies have included patients with primary gallbladder tumours, which appear to have a higher risk for metastatic disease, or those with findings suspicious for GBC on preoperative imaging.14,15 The heterogeneity of patients included in these studies brings into question the objective evaluation of patients with IGBC and prevents the drawing of definitive conclusions.
The objectives of this study were to analyse all patients with IGBC who underwent re-operation at Memorial Sloan-Kettering Cancer Center (MSKCC). The correlation of operative findings with various clinical and histopathologic data may help to identify variables associated with a high likelihood of occult DD and may thus support the selective targeting of SL towards the patients who are at greatest risk.
Materials and methods
Subjects and data collection
The study protocol was approved by the Institutional Review Board at MSKCC, after which records of all patients with IGBC who underwent re-exploration between 1998 and 2009 were collected and analysed. Incidental GBC was defined as unsuspected GBC diagnosed histologically after cholecystectomy performed for presumed benign disease. Data were extracted from a prospective database and supplemented by the review of individual medical records. Recorded data included patient demographics, number and type of preoperative imaging studies, extent of laparoscopic examination, surgical findings and resectability, operative procedures performed, operative time, perioperative outcomes, length of hospital stay, tumour histopathology and staging. Before any re-exploration for possible definitive surgical treatment was conducted, all cases of IGBC were histopathologically confirmed at MSKCC by re-examining the primary tumour.
The authors' approach to patient selection and evaluation has been reported previously.4,16,17 All patients were re-staged according to physical examination and imaging studies (thoracic and abdominal/pelvic computed tomography [CT], magnetic resonance imaging [MRI] and/or 18F-fluorodeoxyglucose positron emission tomography [18FDG PET-CT]). Imaging studies included those performed at referring hospitals and at MSKCC. The number and type of preoperative staging studies was noted; findings suggesting residual disease were recorded. All patients with tumour invasion to at least the muscularis propria layer (T1b) and without evidence of stage IV disease were advised to undergo re-exploration and definitive resection.
Special attention was focused on clinical and pathologic features related to the initial cholecystectomy and the following factors were analysed: reason for cholecystectomy; findings on preoperative abdominal imaging; presence of acute cholecystitis; emergency vs. elective surgery; type of cholecystectomy (open, converted to laparoscopic, laparoscopic); disruption of the gallbladder during surgery, and use of a specimen bag to extract the gallbladder (in patients undergoing laparoscopic cholecystectomy). Pathologic factors evaluated were: depth of tumour invasion; presence of inflammation; presence of gallstones and/or polyps; histology and grade of differentiation; presence of perineural and/or lymphovascular invasion; margin status, and lymph node involvement.
Final disease staging was based on the sixth edition of the American Joint Committee on Cancer (AJCC) manual.18
Operative approach
The authors' operative approach to the resection of GBC has been documented previously.4,16,17 At the discretion of the attending surgeon, SL was performed just prior to laparotomy to exclude metastatic disease. A complete laparoscopic examination of the abdominal cavity included inspection of the liver, gastrohepatic ligament, porta hepatis, pelvis and peritoneal cavity. If these areas could not be visualized at least partially, laparoscopy was considered incomplete. In selected patients, laparoscopic exploration was complemented with laparoscopic ultrasound (US), using an Aloka US imaging system with a 7.5-MHz flexible laparoscopic probe (UST-5536; Aloka Co. Ltd, Tokyo, Japan). Laparoscopic US was used to assess the liver for evidence of metastatic disease and tumour extent within the porta hepatis. Patients who appeared to have localized, resectable disease at laparoscopy underwent a laparotomy. Open exploration included mobilization and palpation of the liver, duodenum, head of the pancreas and retroperitoneum, and ultrasonography of the liver. Biopsies were taken if any suspicious hepatic or extrahepatic lesions were identified and were evaluated with frozen-section pathology.
Tumours were considered unresectable, at either laparoscopy or laparotomy, if any one of the following findings of DD was identified: peritoneal metastases; intrahepatic metastases (outside the gallbladder fossa), and involved lymph nodes outside the porta hepatis basin (i.e. paraduodenal, retropancreatic, common hepatic artery/coeliac artery). Involvement of proximal porta hepatis lymph nodes did not necessarily contraindicate resection. In addition, locally advanced disease with extensive infiltration into the porta hepatis generally precluded resection, although this finding is uncommon in IGBC. Disseminated disease was chosen as the primary endpoint of the study because it is the most common reason for unresectability in patients with IGBC and it can be straightforward to identify laparoscopically.
The degree of liver resection selected was based on the extent of disease and possible involvement of major inflow pedicle structures. Major hepatectomy was defined as a right or left hepatectomy or extended hepatectomy. Minor hepatectomy was defined as resection of segments IVB and V or less. Major resection was performed if there was definite tumour or inflammatory tissue indistinguishable from tumour, involving major inflow vascular structures. Common bile duct resection was performed when there was definite or suspected involvement that mandated this extent of resection in order to achieve clear margins. The lymphadenectomy included nodal tissue in the porta hepatis and portocaval basins and along the common hepatic artery.
Operative time (total and laparoscopic), blood loss, perioperative outcomes and length of hospital stay were recorded. Surgical mortality was defined as death resulting from postoperative complications at any time after surgery.
Pathologic examination
In all patients, the surgical pathology material obtained at the initial cholecystectomy was re-reviewed to confirm the final diagnosis. For this analysis, special emphasis was placed on the depth of tumour invasion, presence of inflammation, factors associated with cancer (gallstones and/or polyps), tumour histology and grade of differentiation, and the presence of perineural and/or lymphovascular invasion. A positive margin was defined as a margin of <1 mm and lymph node involvement (N1 disease) as tumoral involvement in at least one lymph node resected. Depth of tumour invasion was defined according to the AJCC staging manual.18 Histologic types were categorized as adenocarcinoma, squamous cell carcinoma, adenosquamous cell carcinoma, neuroendocrine tumour and undifferentiated. Histologic differentiation was categorized as well, moderate or poorly differentiated.
Statistical analysis
Variables were summarized using proportions (categorical) or mean ± standard deviation and median (range [continuous]). Characteristics of patients with disseminated vs. non-disseminated disease were compared using Fisher's exact test for categorical variables and Wilcoxon's signed rank test for continuous variables. The laparoscopic yield was calculated by dividing the number of patients with unresectable or disseminated disease by the number of patients undergoing laparoscopy. The accuracy of SL was calculated by dividing the number of patients with findings of unresectable disease at laparoscopy by the number of all patients with unresectable disease. The relationship between T-stage and DD was calculated using the chi-squared test, the relationship between T-stage and laparoscopic yield was calculated using the Cochran–Armitage test, and the relationship between T-stage and laparoscopic accuracy was calculated using Fisher's exact test.
A univariate Cox proportional hazards regression model was used to identify factors individually predictive of DD in all patients (n = 136) who submitted to re-operation. All variables that were significant at the 10% level on univariate analysis were entered into a multivariate model using logistic regression. All tests were two-sided and statistical significance was defined as P < 0.05. Statistical analysis was performed using sas Version 9.2 (SAS Institute, Inc., Cary, NC, USA).
Results
Clinical presentation
Between 1998 and 2009, 136 patients with IGBC underwent re-exploration at MSKCC (Fig. 1). Re-staging was performed by laparotomy in 90 patients and by laparoscopy in 46. Nineteen patients (14.0%) were found to have DD at the time of re-exploration. Nine of the patients (10.0%) restaged with laparotomy (i.e. no laparoscopy) and 10 of the patients (21.8%) initially explored laparoscopically had DD (P = 0.07) (Table 1). In the entire cohort of 136 patients with IGBC, demographic and pathologic differences emerged between patients explored by laparotomy and those explored laparoscopically. The laparotomy group (median age 63 years, range 28–85 years) was younger than the laparoscopy group (median age 66 years, range 41–90 years) (P = 0.01) and had a higher incidence of gallbladder inflammation (n = 135) (77.5% [n = 69] vs. 56.5% [n = 26]; P = 0.01) and perineural invasion (n = 100) (55.6% [n = 30] vs. 34.8% [n = 16]; P = 0.04) in the initial cholecystectomy specimen.
Figure 1.
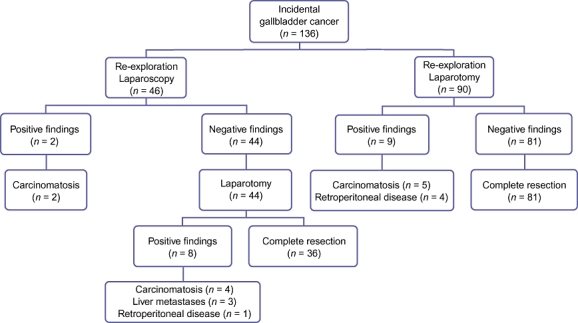
Type of re-exploration and findings in patients with incidental gallbladder treated at Memorial Sloan-Kettering Cancer Center
Table 1.
Comparison between patients with incidental gallbladder cancer and disseminated disease based on type of re-exploration
| All patients | Open procedure group | Laparoscopy group | P-value | |
|---|---|---|---|---|
| Gender, n (%) | 0.1 | |||
| Female | 11 (57.9) | 7 (77.8) | 4 (40.0) | |
| Male | 8 (42.1) | 2 (22.2) | 6 (60.0) | |
| Age, years, median (range) | 63 (40–90) | 61 (40–77) | 66 (56–90) | 0.3 |
| Reason for cholecystectomy, n (%) | 1 | |||
| Abdominal pain | 18 (94.7) | 9 (100) | 9 (90.0) | |
| Wall thickening | 1 (5.3) | 0 | 1 (10.0) | |
| Abdominal ultrasound findings, n (%) | 0.5 | |||
| Gallstones | 9 (47.4) | 5 (55.6) | 4 (40.0) | |
| Wall thickening | 9 (47.4) | 4 (44.4) | 5 (50.0) | |
| Sludge | 1 (5.3) | 0 | 1 (10.0) | |
| Cholecystectomy, n (%) | ||||
| Acute cholecystitis | 14 (73.7) | 7 (77.8) | 7 (70.0) | 1 |
| Emergency surgery | 10 (52.6) | 3 (33.3) | 7 (70.0) | 0.1 |
| Type of surgery, n (%) | 0.5 | |||
| Laparoscopic | 10 (52.6) | 4 (44.4) | 6 (60.0) | |
| Laparoscopic converted to open | 8 (42.1) | 4 (44.4) | 4 (40.0) | |
| Open | 1 (5.3) | 1 (11.1) | 0 | |
| Disruption of gallbladder wall, n (%) | 10 (52.6) | 5 (55.6) | 5 (50.0) | 1 |
| Pathologic examination, n (%) | ||||
| T-stagea | 0.3 | |||
| T2 | 5 (26.3) | 1 (11.1) | 4 (40.0) | |
| T3 | 14 (74.7) | 8 (88.9) | 6 (60.0) | |
| Inflammation | 16 (84.2) | 9 (100) | 7 (70.0) | 0.2 |
| Gallstones associated | 18 (94.7) | 9 (100) | 9 (9.0) | 1 |
| Tumour type | 0.3 | |||
| Adenocarcinoma | 16 (84.2) | 7 (77.8) | 9 (90.0) | |
| Adenosquamous | 1 (5.3) | 1 (11.1) | 0 | |
| Squamous | 1 (5.3) | 1 (11.1) | 0 | |
| Endocrine | 1 (5.3) | 0 | 1 (1.0) | |
| Grade | 0.1 | |||
| Moderated | 5 (26.3) | 4 (44.4) | 1 (10.0) | |
| Poor | 14 (74.7) | 5 (55.6) | 9 (90.0) | |
| Lymph nodes status | 0.2 | |||
| Negative | 2 (10.5) | 2 (22.2) | 0 | |
| Positive | 5 (26.3) | 2 (22.2) | 3 (30.0) | |
| Unknown | 12 (63.2) | 5 (55.6) | 7 (70.0) | |
| Lymphovascular invasion (n = 18) | 11 (61.1) | 4 (50.0) | 7 (70.0) | 0.6 |
| Perineural invasion (n = 16) | 11 (68.8) | 4 (66.7) | 7 (70.0) | 1 |
| Positive cholecystectomy margin | 15 (78.9) | 8 (88.9) | 7 (70.0) | 0.5 |
| Liver | 6 (40.0) | 5 (62.5) | 1 (14.3) | 0.1 |
| Cystic duct | 6 (40.0) | 2 (25.0) | 4 (57.1) | 0.1 |
| Liver + cystic duct | 3 (20.0) | 1 (12.5) | 2 (28.6) | 0.1 |
| Preoperative imaging studies (staging), median (range) | 1 (1–3) | 1 (1–3) | 1 (1–2) | 0.5 |
| Studies suggesting residual disease, median (range) | 1 (0–2) | 1 (0–2) | 0 (0–2) | 0.2 |
| Time between surgeries, months, median (range) | 2 (1–5) | 2 (1–5) | 2 (1–3) | 0.6 |
| Re-exploration | ||||
| Disseminated disease, n (%) | 19 (14.0) | 9 (10.0) | 10 (21.8) | 0.07 |
| Length of procedure, min, median (range) | 120 (60–480) | 120 (90–480) | 139 (60–186) | 0.6 |
| Blood loss, ml, median (range) | 50 (40–1200) | 50 (40–1200) | 50 (50–200) | 0.9 |
| Complications, n (%) | 3 (15.8) | 0 | 3 (30.0) | 1 |
| Atrial fibrillation, n | 2 | 0 | 2 | |
| Wound infection, n | 1 | 0 | 1 | |
| Length of hospital stay, days, median (range) | 5 (1–12) | 4 (3–12) | 5 (1–9) | 0.8 |
| Definition of disseminated disease, n (%) | 0.08 | |||
| Carcinomatosis | 11 (57.9) | 5 (55.5) | 6 (60.0) | |
| Liver metastases | 3 (15.8) | 0 | 3 (30.0) | |
| N3 disease | 5 (26.3) | 4 (44.4) | 1 (10.0) | |
None of the patients with T1b tumours had disseminated disease.
The 46 patients in the laparoscopy group included 29 women and 17 men. Before cholecystectomy, the majority of patients had abdominal pain (n = 42), three were asymptomatic and one presented with jaundice. The most frequent finding in preoperative US was gallstones (n = 32). Thickening of the gallbladder wall was observed in nine patients, a gallbladder polyp in two, gallbladder sludge in two and a mass in one. The most common preoperative diagnosis was acute cholecystitis (n = 29) and 25 patients underwent emergency surgery. Complete laparoscopic cholecystectomy was performed in 38 patients, laparoscopic cholecystectomy was converted to open surgery in seven cases and one patient underwent an open procedure with no laparoscopy. Thirteen patients suffered disruption of the gallbladder wall during cholecystectomy. The gallbladder was extracted in a bag in 17 of 25 patients who underwent a total laparoscopic procedure and for whom this information was available.
Pathology
The initial cholecystectomy specimen was re-examined in all 136 patients with IGBC, revealing compromise of the muscularis propria layer (T1b) in 14 (10.3%) patients, the perimuscular connective tissue (T2) in 68 (50.0%), and the serosal layer or invasion of the liver tissue (T3) in 54 (39.7%). A total of 125 (91.9%) patients were found to have gallstones and 12 (8.8%) had gallbladder polyps. Acute or chronic inflammation was seen in 95 (70.0%) patients. In total, 124 (91.2%) patients had an adenocarcinoma, eight (5.9%) patients had an adenosquamous cell carcinoma, two (1.5%) patients had a squamous cell carcinoma and two (1.5%) patients had an endocrine tumour. Tumour grading was well differentiated in 15 patients, moderately differentiated in 67, poorly differentiated in 52 and unknown in two patients. Lymphovascular invasion was evaluated in 105 patients, in 53 of whom it was found to be positive. Perineural invasion was observed in 46 of 100 patients evaluated. Positive margins were found in 61 (44.8%) patients after cholecystectomy; the gallbladder bed was the most common site (32 patients), followed by the cystic duct (25 patients), and both the gallbladder bed and cystic duct (four patients). The cystic duct lymph node was analysed in 34 (25.0%) patients and was involved by tumour in 19 of them.
In the subgroup of 46 patients who underwent SL, re-examination of the initial cholecystectomy specimen showed compromise of the muscularis propria layer (T1b) in six patients, the perimuscular connective tissue (T2) in 26 patients, and the serosal layer or invasion of the liver tissue (T3) in 14 patients. Forty patients had gallstones and six had gallbladder polyps. Acute or chronic inflammation was seen in 26 patients. Forty-four patients had an adenocarcinoma, one had a squamous cell carcinoma and another had an endocrine tumour. Three patients had well-differentiated tumours, 20 patients had moderately differentiated tumours and 23 had poorly differentiated tumours. Lymphovascular invasion was present in 20 patients and perineural invasion was observed in 16. A positive margin after cholecystectomy was noted in 20 patients. The most common site of involvement was the gallbladder bed (10 patients), the cystic duct (eight patients) and both the gallbladder bed and cystic duct (two patients). The cystic duct lymph node was analysed in 12 patients and was involved by tumour in eight of these.
Imaging studies before re-exploration
The type of staging imaging studies utilized prior to re-exploration was evaluated for the entire cohort of 136 patients with IGBC. All patients were restaged with at least one cross-sectional imaging study and external imaging studies were reviewed at MSKCC. Abdominal CT was used in 95 patients and indicated possible residual disease in 29 of them. Abdominal MRI/cholangio-MRI was used in 57 patients and indicated possible residual disease in 11; PET-CT was used in 32 patients and indicated possible residual disease in 10. Abdominal US was used in 13 patients and indicated possible residual disease in three. Overall, 79 patients were restaged with one modality, 52 with two modalities and four with three modalities. The median number of imaging studies (CT, MRI/cholangio-MRI, PET-CT, abdominal US) analysed before re-exploration was one (range: 1–3 images) and the median number of studies suspicious of intra- or extra-abdominal disease was zero (range: 0–2 images).
Re-exploration
The median time between cholecystectomy and surgical re-exploration was 2 months (range: 1–13 months). Staging laparoscopy was performed as a separate procedure in one of 46 patients. This patient was returned to the operating room for laparotomy at a later date. In the remaining 45 patients, laparoscopy was performed immediately prior to planned laparotomy and definitive resection. Laparoscopy was considered complete in all patients and identified DD (carcinomatosis) that precluded laparotomy and re-resection in two patients. The remaining 44 patients underwent laparotomy, which identified DD that precluded resection in eight patients, of whom four had peritoneal disease, three had liver metastases, and one had retroperitoneal nodal disease. Thus, the yield of laparoscopy was 4.3% (two of 46 patients explored) and its accuracy was 20% (two of 10 patients with DD). The yield and accuracy of laparoscopy increased from 0% to 14.3% (P = 0.06) and from 0% to 33.3% (P = 0.47), respectively, when correlated with the depth of tumour invasion in the gallbladder wall (Table 2).
Table 2.
Relationships between T-stage and yield and accuracy of laparoscopy
| T-stage | All patients (n = 136) n (%) | SL group (n = 46) n (%) | DDa All patients n (%) | DD at laparoscopy, n | DD after SL, n | Yieldbn (%) | Accuracycn (%) |
|---|---|---|---|---|---|---|---|
| 1b | 14 (10.3) | 6 (13.0) | 0 | 0 | 0 | 0/6 | – |
| 2 | 68 (50.0) | 26 (56.5) | 5 (7.4) | 0 | 4 | 0/26 | 0/4 |
| 3 | 54 (39.7) | 14 (30.4) | 14 (25.9) | 2 | 6 | 2/14 (14.3) | 2/6 (33.3) |
P = 0.004, chi-squared test
P = 0.06, Cochran–Armitage test
P = 0.47, Fisher's exact test.
SL, staging laparoscopy; DD, disseminated or unresectable disease.
Comparison of patients with disseminated and localized disease
Of the 136 patients with IGBC, 19 (14.0%) were found to have DD during surgical exploration. There were no differences in gender, age or symptoms before cholecystectomy among patients with disseminated (n = 19) and localized (n = 117) disease. However, patients with DD had a higher incidence of gallbladder wall thickening on pre-cholecystectomy US (47.4% [n = 9] vs. 19.7% [n = 23]; P = 0.016), a higher incidence of conversion from laparoscopic to open cholecystectomy (42.1% [n = 8] vs. 17.1% [n = 20]; P = 0.04), a higher proportion of stage T3 tumours (73.7% [n = 14] vs. 34.2% [n = 40]; P = 0.02), more poorly differentiated tumours (73.7% [n = 14] vs. 32.5% [n = 38]; P = 0.01), a positive cholecystectomy margin (78.9% [n = 15] vs. 39.7% [n = 46]; P = 0.002), and a higher number of re-staging imaging studies suggesting residual cancer before re-exploration (median = 1 [range: 0–2] vs. median = 0 [range: 0–2]; P = 0.02) than patients with localized disease. The likelihood of DD after cholecystectomy was closely related to the T-stage of the cholecystectomy specimen (Table 2).
Univariate and multivariate analyses
Univariate analysis of the entire cohort (n = 136) showed that thickening of the gallbladder wall observed on pre-cholecystectomy US, the conversion of laparoscopic to open cholecystectomy, T3 and poorly differentiated tumours, positive cholecystectomy margin, and a greater number of re-staging imaging studies suggesting residual disease were associated with DD at re-exploration. Multivariate analysis showed that only a positive margin at cholecystectomy (odds ratio [OR] 5.44, 95% confidence interval [CI] 1.51–24.37) and a poorly differentiated tumour (OR 7.64, 95% CI 1.1–NA) were independent predictors associated with disseminated or unresectable disease during re-exploration of IGBC (Table 3).
Table 3.
Univariate and multivariate analysis of variables associated with disseminated or unresectable disease in patients with incidental gallbladder cancer submitted to re-exploration
| Variable | All patients (n = 136) | Non-DD group (n = 117) n (%) | DD group (n = 19) n (%) | P-value, univariate | P-value, multivariate | Hazard ratio | 95% CI |
|---|---|---|---|---|---|---|---|
| Gender | 0.53 | ||||||
| Female | 93 | 82 (88.2) | 11 (11.8) | ||||
| Male | 43 | 35 (81.4) | 8 (18.6) | ||||
| Age, years | 0.2 | ||||||
| Mean ± standard deviation | 64 ± 11 | 64 ± 10 | 67 ± 14 | ||||
| Median (range) | 65 (28–90) | 65 (28–85) | 63 (40–90) | ||||
| Presentation of disease | 0.97 | ||||||
| Abdominal pain | 126 | 108 (85.7) | 18 (14.3) | ||||
| Abnormal study | 7 | 6 (87.7) | 1 (12.3) | ||||
| Jaundice | 1 | 1 (100) | 0 | ||||
| Nausea | 1 | 1 (100) | 0 | ||||
| Pancreatitis | 1 | 1 (100) | 0 | ||||
| Gallstones on pre-cholecystectomy US | 0.066 | ||||||
| Yes | 91 | 82 (90.1) | 9 (9.9) | ||||
| No | 45 | 35 (77.8) | 10 (22.2) | ||||
| GB wall thickening on pre-cholecystectomy US | 0.016 | ||||||
| Yes | 32 | 23 (71.9) | 9 (28.1) | ||||
| No | 104 | 94 (89.6) | 10 (10.4) | ||||
| Acute cholecystitis | 0.4 | ||||||
| Yes | 87 | 73 (83.9) | 14 (16.1) | ||||
| No | 48 | 43 (89.6) | 5 (10.4) | ||||
| Emergency cholecystectomy | 1 | ||||||
| Yes | 73 | 63 (86.3) | 10 (13.7) | ||||
| No | 62 | 53 (85.5) | 9 (14.5) | ||||
| Type of cholecystectomy | 0.044 | ||||||
| Open | 11 | 10 (90.9) | 1 (9.1) | ||||
| Laparoscopic converted to open | 28 | 20 (71.4) | 8 (28.6) | ||||
| Laparoscopic | 97 | 87 (89.7) | 10 (10.3) | ||||
| Disruption of gallbladder wall | 0.2 | ||||||
| Yes | 47 | 37 (78.7) | 10 (21.3) | ||||
| No | 73 | 64 (87.7) | 9 (12.3) | ||||
| T-stage | 0.025 | ||||||
| 1b | 14 | 14 (100) | 0 | ||||
| 2 | 68 | 63 (92.6) | 5 (7.4) | ||||
| 3 | 54 | 40 (74.1) | 14 (25.9) | ||||
| Inflammation | 0.18 | ||||||
| Yes | 95 | 79 (83.2) | 16 (16.8) | ||||
| No | 40 | 37 (92.5) | 3 (7.5) | ||||
| Gallstones | 1 | ||||||
| Yes | 125 | 107 (85.6) | 18 (14.4) | ||||
| No | 11 | 10 (90.9) | 1 (9.1) | ||||
| Gallbladder polyp | 0.2 | ||||||
| Yes | 12 | 12 (100) | 0 | ||||
| No | 124 | 105 (84.7) | 19 (15.3) | ||||
| Tumour type | 0.2 | ||||||
| Adenocarcinoma | 124 | 108 (87.1) | 16 (12.9) | ||||
| Adenosquamous | 8 | 7 (87.5) | 1 (12.5) | ||||
| Squamous | 2 | 1 (50.0) | 1 (50.0) | ||||
| Neuroendocrine | 2 | 1 (50.0) | 1 (50.0) | ||||
| Grade of differentiation | 0.015 | 0.006 | |||||
| Good | 15 | 15 (100) | 0 | Ref. | |||
| Moderate | 67 | 62 (92.5) | 5 (7.5) | 1.77 | 0.22–NA | ||
| Poor | 52 | 38 (73.1) | 14 (26.9) | 7.64 | 1.10–NA | ||
| Lymph node status | 0.2 | ||||||
| Negative | 15 | 13 (86.7) | 2 (13.3) | ||||
| Positive | 19 | 14 (73.7) | 5 (26.3) | ||||
| Unknown | 102 | 90 (88.2) | 12 (11.8) | ||||
| Lymphovascular invasion | 0.4 | ||||||
| Present | 53 | 42 (79.2) | 11 (20.8) | ||||
| Absent | 52 | 45 (86.5) | 7 (13.5) | ||||
| Perineural invasion | 0.058 | ||||||
| Present | 46 | 35 (76.1) | 11 (23.9) | ||||
| Absent | 54 | 49 (90.7) | 5 (9.3) | ||||
| Positive cholecystectomy margin | 0.002 | 0.004 | |||||
| Yes | 61 | 46 (75.4) | 15 (24.6) | 5.44 | 1.51–24.37 | ||
| No | 74 | 70 (94.6) | 4 (5.4) | ||||
| Site of positive margin | <0.0001 | ||||||
| Gallbladder bed | 32 | 26 (81.3) | 6 (18.8) | ||||
| Cystic duct | 25 | 19 (76.0) | 4 (16.0) | ||||
| Gallbladder bed + cystic duct | 4 | 1 (25.0) | 3 (75.0) | ||||
| Negative margin | 74 | 70 (94.6) | 4 (5.4) | ||||
| Studies suggesting residual disease | 0.02 | ||||||
| Mean ± standard deviation | 0.38 ± 0.6 | 0.33 ± 0.6 | 0.68 ± 0.7 | ||||
| Median (range) | 0 (0–2) | 1 (1–3) | 1 (0–2) | ||||
DD, disseminated or unresectable disease; US, ultrasound; GB, gallbladder.
Discussion
Gallbladder cancer is an uncommon and aggressive malignancy, and a high proportion of patients have advanced-stage disease at presentation.19,20 Over the past several years, incidental diagnosis after cholecystectomy for presumed benign disease has increased and is now the most common reason for presentation. Re-exploration with complete resection is the treatment of choice in patients with localized disease;4,16,21–23 however, radiographically occult DD is found at re-exploration in a subgroup of these patients (24–46%).5,19,24 A number of prior studies have shown that laparoscopy is an effective staging tool for many abdominal malignancies, particularly those of hepatopancreatobiliary origin, which include a high proportion of occult metastatic disease that can be detected laparoscopically.9,11–13,25–27
The results of the present study show that the incidence of DD in patients undergoing re-exploration for IGBC was relatively low at 14.0%, and that the yield and accuracy of laparoscopic re-staging were equally low. Indeed, the results were inferior to those reported for SL in previous publications, although it must be emphasized that these studies included patients at greater risk for DD, specifically comprising patients with primary GBC and patients with symptoms caused by GBC.9,11,14,15,28 Thus, clinical presentation would appear to be a useful criterion in the selection of GBC patients for SL, but its utility in patients with IGBC is likely to be very low because symptoms are often absent in this subgroup. Other factors potentially contributing to the low yield of SL refer to the relatively small number of events (i.e. of DD) and bias related to patient selection for the procedure. The latter would seem less likely, however, given the absence of differences in the rate of DD, demographics, and pathology-, treatment- and outcome-related variables between the laparoscopic and open exploration groups.
It has previously been shown that, despite the utility of SL in GBC in general terms, a lower yield can be expected in patients with IGBC. In a study of 401 patients with hepatobiliary malignancies,11 the highest yield of SL was found in the subset of patients with GBC, nearly 50% of whom were spared a laparotomy; however, in a subsequent analysis focusing on patients with biliary cancer, the yield of SL decreased significantly in the subgroup of patients with IGBC (20%).28 Similar results were observed by Agrawal et al.9 in a study of laparoscopic staging in 91 patients with suspected GBC.
Given the history of recent abdominal exploration at the time of the original cholecystectomy procedure, the lower yield of SL in patients with IGBC, compared with those with primary GBC, is understandable. The original procedure was, however, performed for a diagnosis that was presumed to be benign and was not undertaken to specifically assess for metastatic cancer. Nevertheless, the procedure might have been expected to identify many patients in whom such findings were available. Whether evidence of advanced cancer was present at the time of the initial cholecystectomy and was not appreciated, or whether it arose as a direct result of the laparoscopic procedure is a matter of conjecture. The direct and significant correlation between T-stage and DD observed in this study may be cited in support of the latter hypothesis because violation of the tumour plane is much more likely to occur with T3 tumours.
The results of the present study showed the overall yield of SL to be low, but indicated the presence of a subset of patients with an elevated risk for metastatic disease who should potentially be offered this procedure. Analysis of the clinical presentation, findings related to the initial cholecystectomy, particularly a complete histopathologic review, and re-staging imaging studies may predict the risk for tumour dissemination and support the rational selection of patients for laparoscopic staging. Specifically, disruption of the gallbladder wall during cholecystectomy, T-stage, tumour grade or differentiation, lymphovascular and perineural invasion, a positive margin, and metastasis to the cystic duct lymph node have been associated with residual and/or disseminated disease, recurrence and longterm survival in prior studies of patients with IGBC.5,17,29–32 The current study showed that margin status at the initial cholecystectomy and a poorly differentiated tumour were independent factors associated with the occurrence of DD during re-exploration of IGBC. In addition, there was a strong and significant correlation between T-stage and the finding of DD. These variables may therefore be useful in identifying the subset of patients at the highest risk for DD for staging laparoscopy, thereby improving the yield.
It should be noted that eight of the 10 patients with DD who underwent SL were not identified during this procedure. Whether this represents a failure of the technique itself (i.e. the disease was not identifiable) or its application (i.e. the examination was not as thorough as it should have been) is impossible to know. Four patients had low-volume peritoneal disease, three had liver metastases, and another had retroperitoneal nodal disease. Adhesions to the gallbladder bed appeared to be the main cause of the failure to identify DD in the patients with peritoneal disease. By contrast, hepatic and retroperitoneal metastases were found only after conversion to laparotomy and palpation of the metastatic disease. Similar findings regarding the identification of lymph node or subcapsular hepatic metastases have been reported in prior studies.25,28,29 The results underscore the need for a thorough and rigorous laparoscopic staging procedure, particularly in high-risk patients.
In summary, the yield and accuracy of SL in this cohort of patients with IGBC were lower than those reported previously. Most other studies included symptomatic patients with preoperative suspicion of DD. The higher yield of laparoscopy in these studies suggests that the yield depends on symptoms at presentation. The present study showed that a positive cholecystectomy margin and poor tumour differentiation were independent factors associated with the finding of DD at re-exploration. Furthermore, the likelihood of DD correlated with T-stage and was present in over one-quarter of patients with T3 tumours. Staging laparoscopy has a limited role in patients with IGBC. The only patients for whom the procedure should be considered are those with adverse clinical factors such as high T-stage, a positive pathologic margin and high tumour grade.
Conflicts of interest
None declared.
References
- 1.Cucinotta E, Lorenzini C, Melita G, Iapichino G, Curro G. Incidental gallbladder carcinoma: does the surgical approach influence the outcome? ANZ J Surg. 2005;75:795–798. doi: 10.1111/j.1445-2197.2005.03528.x. [DOI] [PubMed] [Google Scholar]
- 2.Hueman MT, Vollmer CM, Jr, Pawlik TM. Evolving treatment strategies for gallbladder cancer. Ann Surg Oncol. 2009;16:2101–2115. doi: 10.1245/s10434-009-0538-x. [DOI] [PubMed] [Google Scholar]
- 3.Shoup M, Fong Y. Surgical indications and extent of resection in gallbladder cancer. Surg Oncol Clin N Am. 2002;11:985–994. doi: 10.1016/s1055-3207(02)00041-8. [DOI] [PubMed] [Google Scholar]
- 4.D'Angelica M, Dalal KM, DeMatteo RP, Fong Y, Blumgart LH, Jarnagin WR. Analysis of the extent of resection for adenocarcinoma of the gallbladder. Ann Surg Oncol. 2009;16:806–816. doi: 10.1245/s10434-008-0189-3. [DOI] [PubMed] [Google Scholar]
- 5.Butte JM, Waugh E, Meneses M, Parada H, De La Fuente HA. Incidental gallbladder cancer: analysis of surgical findings and survival. J Surg Oncol. 2010;102:620–625. doi: 10.1002/jso.21681. [DOI] [PubMed] [Google Scholar]
- 6.Kim HJ, D'Angelica M, Hiotis SP, Shoup M, Weber SM. Laparoscopic staging for liver, biliary, pancreas, and gastric cancer. Curr Probl Surg. 2007;44:228–269. doi: 10.1067/j.cpsurg.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Muntean V, Oniu T, Lungoci C, Fabian O, Munteanu D, Molnar G, et al. Staging laparoscopy in digestive cancers. J Gastrointestin Liver Dis. 2009;18:461–467. [PubMed] [Google Scholar]
- 8.Chang L, Stefanidis D, Richardson WS, Earle DB, Fanelli RD. The role of staging laparoscopy for intra-abdominal cancers: an evidence-based review. Surg Endosc. 2009;23:231–241. doi: 10.1007/s00464-008-0099-2. [DOI] [PubMed] [Google Scholar]
- 9.Agrawal S, Sonawane RN, Behari A, Kumar A, Sikora SS, Saxena R, et al. Laparoscopic staging in gallbladder cancer. Dig Surg. 2005;22:440–445. doi: 10.1159/000091447. [DOI] [PubMed] [Google Scholar]
- 10.Corvera CU, Weber SM, Jarnagin WR. Role of laparoscopy in the evaluation of biliary tract cancer. Surg Oncol Clin N Am. 2002;11:877–891. doi: 10.1016/s1055-3207(02)00033-9. [DOI] [PubMed] [Google Scholar]
- 11.D'Angelica M, Fong Y, Weber S, Gonen M, DeMatteo RP, Conlon K, et al. The role of staging laparoscopy in hepatobiliary malignancy: prospective analysis of 401 cases. Ann Surg Oncol. 2003;10:183–189. doi: 10.1245/aso.2003.03.091. [DOI] [PubMed] [Google Scholar]
- 12.Goere D, Wagholikar GD, Pessaux P, Carrere N, Sibert A, Vilgrain V, et al. Utility of staging laparoscopy in subsets of biliary cancers: laparoscopy is a powerful diagnostic tool in patients with intrahepatic and gallbladder carcinoma. Surg Endosc. 2006;20:721–725. doi: 10.1007/s00464-005-0583-x. [DOI] [PubMed] [Google Scholar]
- 13.Jarnagin WR, Bodniewicz J, Dougherty E, Conlon K, Blumgart LH, Fong Y. A prospective analysis of staging laparoscopy in patients with primary and secondary hepatobiliary malignancies. J Gastrointest Surg. 2000;4:34–43. doi: 10.1016/s1091-255x(00)80030-x. [DOI] [PubMed] [Google Scholar]
- 14.Dagnini G, Marin G, Patella M, Zotti S. Laparoscopy in the diagnosis of primary carcinoma of the gallbladder. A study of 98 cases. Gastrointest Endosc. 1984;30:289–291. doi: 10.1016/s0016-5107(84)72420-5. [DOI] [PubMed] [Google Scholar]
- 15.Kriplani AK, Jayant S, Kapur BM. Laparoscopy in primary carcinoma of the gallbladder. Gastrointest Endosc. 1992;38:326–329. doi: 10.1016/s0016-5107(92)70425-8. [DOI] [PubMed] [Google Scholar]
- 16.Fong Y, Jarnagin W, Blumgart LH. Gallbladder cancer: comparison of patients presenting initially for definitive operation with those presenting after prior non-curative intervention. Ann Surg. 2000;232:557–569. doi: 10.1097/00000658-200010000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jarnagin WR, Ruo L, Little SA, Klimstra D, D'Angelica M, DeMatteo RP, et al. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer. 2003;98:1689–1700. doi: 10.1002/cncr.11699. [DOI] [PubMed] [Google Scholar]
- 18.American Joint Committee on Cancer. AJCC Cancer Staging Manual. 6th edn. Chicago, IL: AJCC; 2002. Gallbladder; pp. 155–161. [Google Scholar]
- 19.Duffy A, Capanu M, Abou-Alfa GK, Huitzil D, Jarnagin W, Fong Y, et al. Gallbladder cancer (GBC): 10-year experience at Memorial Sloan-Kettering Cancer Centre (MSKCC) J Surg Oncol. 2008;98:485–489. doi: 10.1002/jso.21141. [DOI] [PubMed] [Google Scholar]
- 20.Butte JM, Matsuo K, Gonen M, D'Angelica MI, Waugh E, Allen PJ, et al. Gallbladder cancer: differences in presentation, surgical treatment, and survival in patients treated at centres in three countries. J Am Coll Surg. 2011;212:50–61. doi: 10.1016/j.jamcollsurg.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Corvera CU, Blumgart LH, Akhurst T, DeMatteo RP, D'Angelica M, Fong Y, et al. 18F-fluorodeoxyglucose positron emission tomography influences management decisions in patients with biliary cancer. J Am Coll Surg. 2008;206:57–65. doi: 10.1016/j.jamcollsurg.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Wakai T, Shirai Y, Hatakeyama K. Radical second resection provides survival benefit for patients with T2 gallbladder carcinoma first discovered after laparoscopic cholecystectomy. World J Surg. 2002;26:867–871. doi: 10.1007/s00268-002-6274-z. [DOI] [PubMed] [Google Scholar]
- 23.Shukla PJ, Barreto G, Kakade A, Shrikhande SV. Revision surgery for incidental gallbladder cancer: factors influencing operability and further evidence for T1b tumours. HPB (Oxford) 2008;10:43–47. doi: 10.1080/13651820701867794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pawlik TM, Gleisner AL, Vigano L, Kooby DA, Bauer TW, Frilling A, et al. Incidence of finding residual disease for incidental gallbladder carcinoma: implications for re-resection. J Gastrointest Surg. 2007;11:1478–1486. doi: 10.1007/s11605-007-0309-6. discussion 1486–1487. [DOI] [PubMed] [Google Scholar]
- 25.Callery MP, Strasberg SM, Doherty GM, Soper NJ, Norton JA. Staging laparoscopy with laparoscopic ultrasonography: optimizing resectability in hepatobiliary and pancreatic malignancy. J Am Coll Surg. 1997;185:33–39. [PubMed] [Google Scholar]
- 26.Cho A, Yamamoto H, Nagata M, Takiguchi N, Shimada H, Kainuma O, et al. Total laparoscopic resection of the gallbladder together with the gallbladder bed. J Hepatobiliary Pancreat Surg. 2008;15:585–588. doi: 10.1007/s00534-008-1363-5. [DOI] [PubMed] [Google Scholar]
- 27.Gouma DJ, Nieveen van Dijkum EJ, de Wit LT, Obertop H. Laparoscopic staging of biliopancreatic malignancy. Ann Oncol. 1999;10(Suppl. 4):33–36. [PubMed] [Google Scholar]
- 28.Weber SM, DeMatteo RP, Fong Y, Blumgart LH, Jarnagin WR. Staging laparoscopy in patients with extrahepatic biliary carcinoma. Analysis of 100 patients. Ann Surg. 2002;235:392–399. doi: 10.1097/00000658-200203000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fong Y, Brennan MF, Turnbull A, Colt DG, Blumgart LH. Gallbladder cancer discovered during laparoscopic surgery. Potential for iatrogenic tumour dissemination. Arch Surg. 1993;128:1054–1056. doi: 10.1001/archsurg.1993.01420210118016. [DOI] [PubMed] [Google Scholar]
- 30.Shibata K, Uchida H, Iwaki K, Kai S, Ohta M, Kitano S. Lymphatic invasion: an important prognostic factor for stages T1b–T3 gallbladder cancer and an indication for additional radical resection of incidental gallbladder cancer. World J Surg. 2009;33:1035–1041. doi: 10.1007/s00268-009-9950-4. [DOI] [PubMed] [Google Scholar]
- 31.Park JS, Yoon DS, Kim KS, Choi JS, Lee WJ, Chi HS, et al. Actual recurrence patterns and risk factors influencing recurrence after curative resection with stage II gallbladder carcinoma. J Gastrointest Surg. 2007;11:631–637. doi: 10.1007/s11605-007-0109-z. [DOI] [PubMed] [Google Scholar]
- 32.Goetze TO, Paolucci V. Use of retrieval bags in incidental gallbladder cancer cases. World J Surg. 2009;33:2161–2165. doi: 10.1007/s00268-009-0163-7. [DOI] [PubMed] [Google Scholar]


