Abstract
Background
In selected patients with chronic pancreatitis, extensive pancreatectomy can be effective for the treatment of intractable pain. The resultant morbid diabetes can be ameliorated with islet autotransplantation (IAT). Conventionally, islet infusion occurs intraoperatively after islet processing. A percutaneous transhepatic route in the immediate postoperative period is an alternative approach.
Methods
A prospectively collected database of patients undergoing pancreatectomy with percutaneous IAT (P-IAT) was reviewed. Hospital billing data were obtained and median charges determined and compared with estimated charges for an intraoperative infusion method of IAT (I-IAT).
Results
Thirty-six patients (28 women; median age 48 years) underwent pancreatectomy with P-IAT. Median operative time was 232 min (range: 98–395 min) and median estimated blood loss was 500 cc (range: 75–3000 cc). Median time from pancreatic resection to islet transplantation was 269 min (range: 145–361 min). A median of 208 248 IEq (2298 IEq/kg) were harvested. Median peak portal venous pressure during islet infusion was 13 mmHg (range: 5–37 mmHg). Postoperative complications occurred in 15 patients (42%) and included hepatic artery pseudoaneurysm and portal vein thrombosis; the latter occurred in two patients with portal pressures during infusion > 30 mmHg. At a median follow-up of 10.7 months, eight patients (22%) were insulin-free. Median pertinent charges for P-IAT were US$36 318 and estimated median charges for I-IAT were US$56 440. Surgeon time freed by P-IAT facilitated an additional 66 procedures, charges for which amounted to US$463 375.
Conclusions
Percutaneous transhepatic IAT is feasible and safe. Islet infusion in the immediate postoperative period is cost-effective. Further follow-up is needed to assess longterm results.
Keywords: surgery, chronic pancreatitis, interventional radiology, investigations
Introduction
Chronic pancreatitis is a devastating and debilitating disease when marked by severe and intractable abdominal pain. Frontline management strategies for patients with this disease target medical and endoscopic intervention. However, non-operative treatment is ineffective in nearly 50% of patients and thus surgical therapy represents an important option. Surgical strategies for the management of chronic pancreatitis fall broadly into three categories comprising the resection of diseased tissue, the drainage of obstructed ducts, and a combination of both of these. The cardinal aims of pancreatic surgery are the preservation of pancreatic function, the delivery of durable pain relief, and the avoidance of major morbidity and mortality. For most patients, these goals can be achieved with partial resection and bypass procedures. However, when traditional surgical management fails and intractable pain and nausea make life unbearable for selected patients, there are sound surgical principles which indicate that total pancreatectomy can be an appropriate and successful therapeutic intervention.
For many years, surgeons have avoided total pancreatectomy in order to evade the resultant labile diabetes of the apancreatic state. The loss of endogenous insulin production, as well as that of the counter-regulatory hormones (glucagon, pancreatic polypeptide), often results in a difficult form of diabetes characterized by wide swings in blood glucose levels and an inconsistent response to therapeutic insulin. In addition, patients develop hypoglycaemic unawareness, which heightens the morbidity of this condition. Islet autotransplantation (IAT), however, has engendered new enthusiasm for total pancreatectomy because it represents a method of ameliorating this brittle diabetes in the patient post-pancreatectomy. Moreover, earlier total pancreatic resection has the potential to impede the development of post-pancreatectomy neuropathic pain syndromes.
Islet autotransplantation involves taking the pancreas immediately after resection and transporting it to a clean cell laboratory for processing. In the laboratory, it undergoes a process of mechanical and enzymatic digestion to separate the islets from the remainder of the gland. This islet harvesting procedure takes several hours to accomplish.
In most centres, the isolated islets are then returned to the operating room (OR), where the patient has been waiting under anaesthesia, for autotransplantation. The cells are infused by gravity through a catheter placed into an immediate branch of the portal vein. In our programme, however, we have chosen to end the operation and move the patient to the intensive care unit (ICU) for stabilization and to await the islet harvest. When the islets are prepared, the patient is transported to the interventional radiology suite (IR), where the portal vein is accessed percutaneously through the liver under fluoroscopic guidance (Fig. 1). The islets are then infused into the liver. This approach is modelled after a similar technique used for cadaveric islet allotransplantation.
Figure 1.
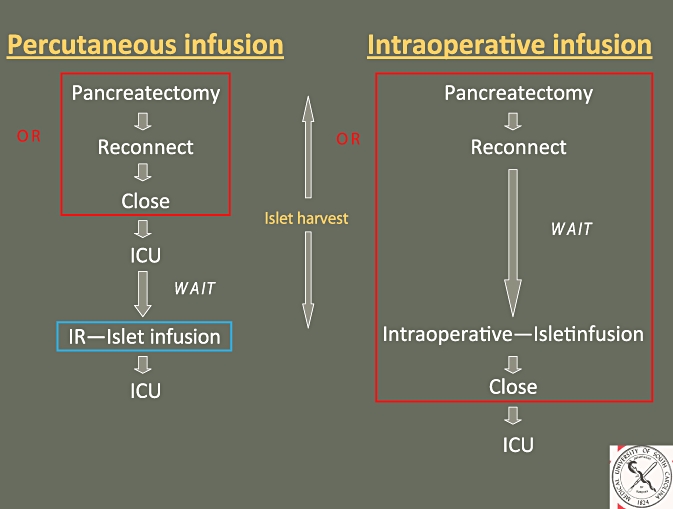
Schematic diagram comparing percutaneous transhepatic islet autotransplantation with the more common method of intraoperative islet autotransplantation. OR, operating room; ICU, intensive care unit; IR, interventional radiology suite
An evaluation of outcomes was undertaken in order to assess the feasibility and safety of this novel approach to IAT. In addition, cost modelling was performed to appraise the cost efficiency of this method compared with that of the more common intraoperative infusion method.
Materials and methods
Islet harvest
The pancreas is prepared on the back table in the OR by perfusion with cold balanced electrolyte solution. Non-pancreatic tissue is separated and the main pancreatic duct is cannulated. The pancreas is then transported on ice to the clean cell laboratory. There, the prepared pancreas is hand-injected through the main pancreatic duct with Liberase HI and placed in a temperature-controlled circuit and subjected to mechanical agitation to allow optimal enzymatic activity. The progress of digestion is periodically evaluated; when the islets are found to be optimally separated, the circuit is cooled and diluted. The islets, now largely separated from the exocrine and connective tissue, are recovered and assessed. They are placed in albumin solution with heparin (70 U per kg of patient weight) and antibiotic (cefazolin, 1 g).
Islet autotransplantation
Percutaneous transhepatic access to the portal vein is obtained in the IR suite under fluoroscopic guidance. A Seldinger technique is utilized to place a 5-F catheter into the main portal vein below the confluence. The islets are infused by gravity through the catheter. Portal venous pressures are measured initially, at the midpoint of transplant and at the completion of infusion. At the end of the procedure, the intraparenchymal access catheter tract is ablated with gel foam to obtain haemostasis.
Outcomes assessment
A prospectively collected database of patients who underwent pancreatectomy with percutaneous IAT (P-IAT) between March 2009 and May 2010 was reviewed. Clinical parameters including demographics, perioperative course and outpatient follow-up data were assessed. Islet harvest and transplant information was evaluated.
Hospital billing data were obtained and pertinent charges were determined as a direct correlate to costs. Specifically, all patient charges for the relevant patient encounters were itemized and reviewed. All potential differential charges between a percutaneous transhepatic IAT approach and the more common intraoperative IAT route were delineated. Time-dependent OR and IR charges were identified and defined for the P-IAT route and the median value for the 36 patients was calculated. Surgeons' professional fees were excluded from the analysis.
A model for calculated expected charges based on time-dependent OR charges was established. To validate this method, the expected charges for percutaneous transhepatic islet infusion were calculated. These were compared with the actual median charges to the patient.
Expected charges for a hypothetical intraoperative infusion case at our institution were estimated based on the collected financial data. Per minute OR charges (including anaesthesia charges) were utilized and an additional 240 min of OR time was assumed to be required in the intraoperative infusion method (I-IAT). This additional 240 min was calculated as follows: the median islet harvest time (time from pancreas removal to islet infusion) was 270 min; 60 min was subtracted from this for anastomosis (choledochojejunostomy, gastrojejunostomy); 30 min was subtracted for closure time but then re-added as closure occurred after islet infusion in the intraoperative infusion group, and an additional 30 min was added for islet infusion in the OR, thus bringing the total to 240 min (Fig. 2).
Figure 2.
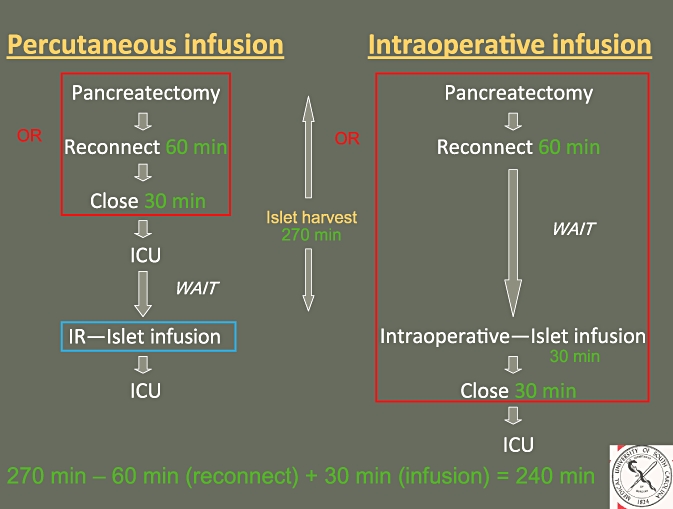
Schematic diagram demonstrating the additional 240 min of operating room time required for the intraoperative infusion of islet autotransplantation. OR, operating room; ICU, intensive care unit; IR, interventional radiology suite
Expected charges for the hypothetical case of I-IAT were then calculated and compared with expected and real charges for P-IAT.
To determine surgeon opportunity costs, surgeons' professional fees for other cases performed on the same days as islet transplant cases were determined.
Results
During the 14-month period of the study (March 2009 to May 2010), 36 patients underwent pancreatectomy with P-IAT. Twenty-eight (78%) of the patients were women. Median patient age was 48 years (range: 19–62 years) and median body mass index was 27 kg/m2 (range: 17.4–37.9 kg/m2) (Table 1). The aetiology of pancreatitis was sphincter of Oddi dysfunction (n = 16), idiopathic (n = 9), pancreas divisum (n = 4), alcohol-related (n = 4) and familial (n = 3). Seven patients had diabetes preoperatively.
Table 1.
Demographics of patients undergoing pancreatectomy with islet autotransplantation (n = 36)
| Factors | |
|---|---|
| Women, n | 28 |
| Median age, years | 48 |
| Median body mass index, kg/m2 | 27 |
| Operating time, min, median (range) | 232 (98–395) |
| Operations performed, n | |
| Total pancreatectomy | 21 |
| Completion pancreatectomy | 15 |
| Median estimated blood loss, cc, median (range) | 500 (75–3000) |
Twenty-one patients underwent total pancreatectomy and 15 underwent partial (completion) pancreatectomy. Median operative time was 232 min (range: 98–395 min); median estimated blood loss was 500 cc (range: 75–3000 cc). Median time from pancreatic resection to islet transplantation (islet harvest time) was 269 min (range: 145–361 min). A median of 208 248 IEq (2298 IEq/kg) were harvested (range: 3667–1 168 725 IEq). Median time of islet infusion was 30 min (range: 18–57 min). Median peak portal venous pressure during islet infusion was 13 mmHg (range: 5–37 mmHg). Median change in portal venous pressure during transplant was 3 mmHg (range: 0–33 mmHg). Postoperative complications occurred in 15 patients (42%) (Table 2) and included requirements for two re-operations, one for bleeding and one for persistent lactic acidosis. Minor complications included wound infection (n = 4), pneumonia (n = 4), intra-abdominal abscess (n = 3), urinary tract infection (n = 3), deep vein thrombosis (n = 2) and acute renal failure (n = 1). One patient had the significant complication (>Clavien grade III) of prolonged ventilation. Complications related specifically to IAT included partial portal vein thrombosis (PVT), which occurred in two patients, in both of whom portal venous pressures during infusion rose to >30 mmHg. One patient was diagnosed during her completion portal venogram after transplant, was treated with systemic heparin i.v. and thrombosis resolved within 24 h without clinical consequence. The other was diagnosed remotely, 3 months post-transplant, incidentally on computed tomography (CT). She was treated with heparin and then warfarin, and thrombosis resolved. One patient had both a hepatic artery pseudoaneurysm and a hepatic abscess. Median postoperative length of stay was 10 days (range: 5–33 days).
Table 2.
Complications in 15 of 36 patients undergoing pancreatectomy with islet autotransplantation
| Complication | n |
|---|---|
| Re-operation | 2 |
| Wound infection | 4 |
| Intra-abdominal abscess | 3 |
| Pneumonia | 4 |
| Prolonged ventilation | 1 |
| Acute renal failure | 1 |
| Urinary tract infection | 3 |
| Deep vein thrombosis | 2 |
| Portal vein thrombosisa | 2 |
| Hepatic artery pseudoaneurysm | 1 |
| Hepatic abscess | 1 |
Portal vein thrombosis occurred in two patients with peak infusion pressures >30 mmHg
At a median follow-up of 10.7 months, eight patients (22%) were insulin-free and an additional 10 (28%) had minimal insulin requirements (<10 U insulin/day).
Median actual pertinent charges for the P-IAT method were US$36 318, calculated as an expected charge of US$38 955. Estimated median charges for hypothetical I-IAT were US$56 440 (Fig. 3). Freeing the OR for other procedures to be performed on other patients by the operative surgeon was demonstrated to allow an additional 66 procedures to be performed on the days of the 36 IAT procedures. These procedures collectively represented US$463 375 in surgeons' professional fees.
Figure 3.
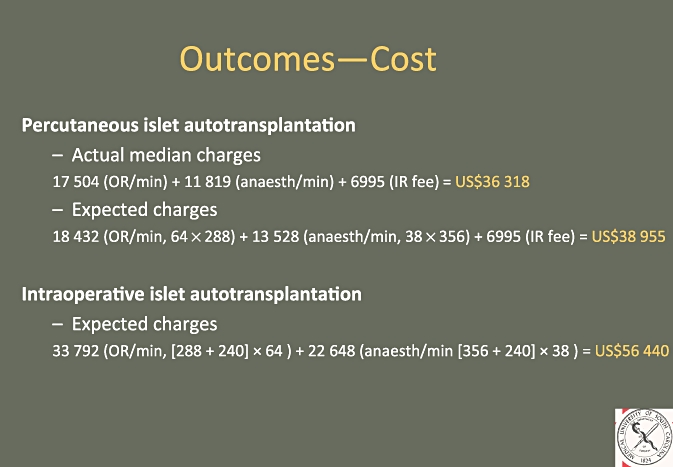
Cost calculations for patients undergoing percutaneous islet autotransplantation compared with estimated charges for intraoperative islet autotransplantation. OR, operating room; IR, interventional radiology suite
Discussion
Selected patients suffering intractable debilitating pain caused by chronic pancreatitis may benefit from total pancreatectomy. Patients with diffuse small duct pancreatitis, without a dilated main pancreatic duct or an inflammatory mass, obtain suboptimal analgesic results from drainage1 or targeted resection procedures, and thus total pancreatectomy potentially represents their best option for surgical pain relief. After surgery for chronic pancreatitis, at least 15% of patients have persistent or recurrent pain, regardless of the aetiology of pancreatitis, the procedure undertaken, or the centre at which it is conducted.2–8 Salvage pancreatectomy in selected cases, particularly in patients in whom disease progression is a suspected mechanism for failure, can be an effective means of pain control.2,9 Patients with hereditary pancreatitis who demonstrate progressive disease have a risk for pancreatic cancer estimated to be 50 times greater than that of the healthy population and thus are good candidates for total pancreatectomy.10
Islet autotransplantation, pioneered at the University of Minnesota, has been performed at several centres with good outcomes.11–17 The most common method of islet infusion involves intraoperative infusion through a direct branch of the portal vein or superior mesenteric vein. There are clear advantages to this approach. Exposure is already obtained through the course of surgery and direct visualization of the operative field during the systemic heparinization that accompanies islet infusion allows the opportunity to ensure haemostasis. The disadvantage of this approach is that the islet harvest takes several hours (a median of 269 min in this series), and the patient is maintained under anaesthesia in the OR during this time.
An alternative method of infusion involves accessing the portal vein via a percutaneous transhepatic route for islet infusion in the IR suite in the immediate postoperative period. The wait for islet harvest is then spent in the ICU rather than the OR and thus this method optimizes the efficient use of OR time, a finite resource.
The percutaneous transhepatic method has been utilized in cadaveric islet allotransplantation with minimal morbidity and acceptable short-term outcomes.18–20 This study shows similarly acceptable results for IAT performed using this method. In our series, we observed four occurrences in three of 36 patients (8%) of procedure-specific complications.
The two cases of PVT were partial, incidentally noted and easily treated without apparent negative clinical consequence. Portal vein thrombosis is of significant concern as a potentially life-threatening complication. The islets as prepared for autoinfusion are not purified as they are for allotransplant, as the purification process is time-consuming and lends itself to islet instability and islet loss. The remaining exocrine cells in the preparation are a source of tissue thromboplastin and thus are potentially thrombogenic. Elevated portal venous pressure during infusion has been found to be a risk factor for PVT in islet allotransplantation.20 A portal vein pressure of >30 mmHg occurred only in the two patients who developed PVT in this study, supporting this condition as a risk factor. Measurement of portal venous pressure during infusion seems prudent; in patients with pressures >30 mmHg, the infusion should be stopped and the pressure allowed to decrease prior to completion of the transplant. This elevated pressure in most cases seems to be a phenomenon of volume rather than occlusion as the pressure typically returns to a normal level promptly. Anticoagulation is also routinely employed to minimize the occurrence of thrombosis.
A hepatic artery pseudoaneurysm was incidentally discovered in one patient on CT in the postoperative period. This was managed with percutaneous embolization. The patient developed some surrounding fluid near the pseudoaneurysm with a small amount of air in it after the embolization, in the setting of fever and leukocytosis. This abscess was drained percutaneously with image guidance and the patient was treated with antibiotics.
Transplanted islet function as measured by the need for exogenous insulin was acceptable: 22% of patients were insulin-free. It is important to note that seven of the patients required insulin preoperatively. This percentage is lower than those reported by some groups,11–13,15,16 but greater than those reported by others.14,17 Presumably the overall functional outcomes of the transplanted islets are related not only to technique of isolation and transplant, but also very closely to preoperative patient selection.
The method employed to estimate expected charges for a procedure was validated by using the formula to estimate the expected charges for the percutaneous transhepatic method of islet infusion. These estimated charges were comparable with the actual charges made to patients. Using this model, the estimated charges for an intraoperative infusion were notably higher than those for the percutaneous method as a result of the variance in the utilization of OR time. In addition, the provision of surgeon time to perform other procedures in other patients represented significant charges.
Conclusions
Total pancreatectomy with IAT offers a promising intervention for pain relief in a select group of patients with chronic pancreatitis. A percutaneous transhepatic method of IAT is feasible and safe. Acceptable islet function is achieved. According to our modelling, this novel approach is cost-efficient when compared with the more common intraoperative infusion route. Longterm outcomes are needed to fully assess the role of total pancreatectomy with IAT in the management of pain caused by chronic pancreatitis.
Conflicts of interest
None declared.
References
- 1.Rios GA, Adams DB, Yeoh KG, Tarnasky PR, Cunningham JT, Hawes RH. Outcome of lateral pancreaticojejunostomy in the management of chronic pancreatitis with non-dilated pancreatic ducts. J Gastrointest Surg. 1998;2:223–229. doi: 10.1016/s1091-255x(98)80016-4. [DOI] [PubMed] [Google Scholar]
- 2.Howard TJ, Browne JS, Zyromski NJ, Lavu H, Baker MS, Shen C, et al. Mechanisms of primary operative failure and results of remedial operation in patients with chronic pancreatitis. J Gastrointest Surg. 2008;12:2087–2096. doi: 10.1007/s11605-008-0713-6. [DOI] [PubMed] [Google Scholar]
- 3.Schnelldorfer T, Lewin DN, Adams DB. Operative management of chronic pancreatitis. Longterm results in 372 patients. J Am Coll Surg. 2007;204:1039–1045. doi: 10.1016/j.jamcollsurg.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 4.Adams DB, Ford MC, Anderson MC. Outcome after lateral pancreaticojejunostomy for chronic pancreatitis. Ann Surg. 1994;219:481–489. doi: 10.1097/00000658-199405000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakorafas GH, Sarr MG, Rowland CM, Farnell MB. Postobstructive chronic pancreatitis: results with distal resection. Arch Surg. 2001;136:643–648. doi: 10.1001/archsurg.136.6.643. [DOI] [PubMed] [Google Scholar]
- 6.Traverso LW, Kozarek RA. Pancreatoduodenectomy for chronic pancreatitis: anatomic selection criteria and subsequent long-term outcome analysis. Ann Surg. 1997;226:429–438. doi: 10.1097/00000658-199710000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muller MW, Friess H, Leitzbach S, Michalski CW, Berberat P, Ceyhan GO, et al. Perioperative and follow up results after central pancreatic head resection in a consecutive series of patients with chronic pancreatitis. Am J Surg. 2008;196:364–372. doi: 10.1016/j.amjsurg.2007.08.065. [DOI] [PubMed] [Google Scholar]
- 8.Strate T, Taherpour Z, Bloechle C, Mann O, Bruhn J, Schneider C, et al. Longterm follow up of a randomized trial comparing Beger and Frey procedures for patients suffering from chornic pancreatitis. Ann Surg. 2005;241:591–598. doi: 10.1097/01.sla.0000157268.78543.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgan KA, Fontenot BB, Harvey NR, Adams DB. Revision of anastomosis after pancreatic head resection for chronic pancreatitis: is it futile. HPB. 2010;12:211–215. doi: 10.1111/j.1477-2574.2009.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitcomb DC, Gorry MC, Preston RA, Furey W, Sossenheimer MJ, Ulrich CD, et al. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet. 1996;14:141–145. doi: 10.1038/ng1096-141. [DOI] [PubMed] [Google Scholar]
- 11.Gruessner RWG, Sutherland DER, Dunn DL, Najarian JS. Transplant options for patients undergoing total pancreatectomy for chronic pancreatitis. J Am Coll Surg. 2004;198:559–567. doi: 10.1016/j.jamcollsurg.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 12.Jie T, Hering B, Ansite J, Gilmore T. Pancreatectomy and auto islet transplantation in patients with chronic pancreatitis. J Am Coll Surg. 2005;201:S14. [Google Scholar]
- 13.Blondet JJ, Carlson AM, Kobayashi T, Jie T, Bellin M, Hering BJ, et al. The role of total pancreatectomy and islet autotransplantation in chronic pancreatitis. Surg Clin N Am. 2007;87:1477–1501. doi: 10.1016/j.suc.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 14.Argo JL, Contreras JL, Wesley MM, Christein JD. Pancreatic resection with islet cell autotransplant for the treatment of severe chronic pancreatitis. Am Surg. 2008;74:530–536. [PubMed] [Google Scholar]
- 15.Rilo HR, Ahmad SA, D'Alessio D. Total pancreatectomy and autologous islet cell transplant as a means to treat severe chronic pancreatitis. J Gastrointest Surg. 2003;7:978–989. doi: 10.1016/j.gassur.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Ahmad SA, Lowy AM, Wray CJ, D'Alessio D, Choe KA, James LE, et al. Factors associated with insulin and narcotic independence after islet autotransplantation in patients with severe chronic pancreatitis. J Am Coll Surg. 2005;201:680–687. doi: 10.1016/j.jamcollsurg.2005.06.268. [DOI] [PubMed] [Google Scholar]
- 17.Webb MA, Illouz SC, Pollard CA, Gregory R, Mayberry JF, Tordoff SG, et al. Islet auto transplantation following total pancreatectomy: a longterm assessment of graft function. Pancreas. 2008;37:282–287. doi: 10.1097/mpa.0b013e31816fd7b6. [DOI] [PubMed] [Google Scholar]
- 18.Goss JA, Soltes G, Goodpastor SE, Barth M, Lam R, Brunicardi FC, et al. Pancreatic islet transplantation. The radiographic approach. Transplantation. 2003;76:199–203. doi: 10.1097/01.TP.0000073976.26604.96. [DOI] [PubMed] [Google Scholar]
- 19.Low G, Hussein N, Owen RJT, Toso C, Patel VH, Bhargava R, et al. Role of imaging in clinical islet transplantation. Radiographics. 2010;30:353–366. doi: 10.1148/rg.302095741. [DOI] [PubMed] [Google Scholar]
- 20.Kawahara T, Kin T, Kashkoush S, Bigam D, Kneteman N, Shapiro J. The interaction of low standard liver volume, high packed cell volume, portal hypertension, and risk of portal vein thrombosis in clinical islet allotransplantation. 2011. Meeting of the American Hepatopancreatobiliary Association, 11–13 March 2011, Miami, FL.


