Abstract
A number of studies over the last two decades have demonstrated the critical importance of dopamine (DA) in the behavioral pharmacology and addictive properties of abused drugs. The DA transporter (DAT) is a major target for drugs of abuse in the category of psychostimulants, and for methylphenidate (MPH), a drug used for treating attention deficit hyperactivity disorder (ADHD), which can also be a psychostimulant drug of abuse. Other drugs of abuse such as nicotine, ethanol, heroin and morphine interact with the DAT in more indirect ways. Despite the different ways in which drugs of abuse can affect DAT function, one evolving theme in all cases is regulation of the DAT at the level of surface expression. DAT function is dynamically regulated by multiple intracellular and extracellular signaling pathways and several protein-protein interactions. In addition, DAT expression is regulated through the removal (internalization) and recycling of the protein from the cell surface. Furthermore, recent studies have demonstrated that individual differences in response to novel environments and psychostimulants can be predicted based on individual basal functional DAT expression. Although current knowledge of multiple factors regulating DAT activity has greatly expanded, many aspects of this regulation remain to be elucidated; these data will enable efforts to identify drugs that might be used therapeutically for drug dependence therapeutics.
Keywords: dopamine, dopamine transporter, trafficking, nicotine, amphetamine, cocaine, ethanol, opioid, individual differences, environmental enrichment, addiction
1. INTRODUCTION
Dopamine (DA) modulates many physiological processes including locomotor activity, cognitive processes, reward and addiction [1, 2]. Dysfunction of the dopaminergic system is thought to be attributable to the development of multiple neurological disorders such as schizophrenia, Parkinson’s disease, depression, attention deficit hyperactivity disorder (ADHD) and drug addiction [3-7]. Extracellular DA concentrations are the net result of release (exocytosis) and clearance (uptake) from the extracellular space. Uptake through the plasma membrane DA transporter (DAT) is the primary mechanism for regulating extracellular DA concentration, and thus, the most effective means of terminating DA actions at postsynaptic and presynaptic receptors [8, 9]. DAT knockout mice exhibit profound neurochemical and behavioral changes including elevated extracellular levels of DA, hyperactivity, cognitive deficits and altered psychostimulant response [10-14].
The DAT is a member of a larger family of the sodium- and chloride-dependent transporters, which includes GABA, noradrenaline, serotonin and glycine carriers [15-18]. In recent years, the molecular identification, expression cloning and genetic deletion of the DAT protein have contributed to more detailed understanding of DAT function not only in relation to the normal reuptake of DA but also to the reversal of transporter, leading to non-exocytotic DA release [19, 20]. The dynamic regulation of DAT function is controlled by complex processes involving phosphorylation, protein-protein interaction, substrate pretreatment, and interaction with presynaptic receptors [21-23]. The DAT is the principle target site for multiple psychostimulant drugs, including cocaine and amphetamine, likely mediating their high abuse potential and possibly neurotoxicity. Cocaine acts as a simple inhibitor that binds to the DAT and blocks its transport activity, while amphetamines not only competitively inhibit DA reuptake and thereby increase synaptic DA levels, but also reverse transport activity, resulting in efflux of DA via the DAT [21, 24-26]. The DAT is also a pharmacological target for methylphenidate (MPH) that is the most prescribed drug used to treat ADHD and addiction to substances such as nicotine [27-32]. In addition, recent studies have demonstrated that genetic and environmental factors can produce profound biological changes in DAT function and expression that have important behavioral associations, including vulnerability to drug abuse [33-36]. This review discusses the current knowledge of the mechanism and regulation of the DAT in relation to the ways drugs acting on the DAT to disturb (extra) synaptic dopaminergic transmission and the behavioral response. Understanding the mechanisms underlying the neurobiological effects of psychostimulant drugs on the regulation of the DAT protein may have the potential to facilitate the development of therapeutic programs for drug addiction.
2. REGULATION AND LOCALIZATION OF DAT
2.1. Regulation of DAT function and trafficking by phosphorylation
The function of the DAT is regulated by a number of cellular signaling pathways, including protein kinase A (PKA), protein kinase C (PKC), tyrosine kinases, phosphatases, calcium and calmodulin-dependent kinases, which tightly modulate DAT expression on the plasma membrane [22, 23, 37, 38]. It has been shown that activation of PKC causes a rapid downregulation of DAT activity, which is the result of removal of transporters from the plasma membrane to intracellular endosomal compartments [39, 40]. PKC-induced decrease in DAT cell surface expression is largely responsible for reduced DA uptake capacity [22, 36]. PKC also stimulates non-exocytotic DA efflux through transport reversal, which is thought to influence dopaminergic neuronal activity and drug neurotoxicity [41-43]. Evidence also indicates that DAT internalized by PKC is localized in the endosomal recycling compartment and is subsequently recycling back to the plasma membrane, suggesting that the DAT constitutively internalizes and recycles [40, 44]. PKC activation accelerates DAT internalization and attenuates the process of DAT recyling back to the cell surface [44]. For basal DAT internalization, PKC activation does not significantly change the endocytic rate of DAT when DAT is expressed at high levels but markedly increases DAT endocytosis when the DATs are expressed at low and moderate levels [44].
Several studies have shown that PKC-induced internalization of the DAT is blocked by inhibition of clathrin-mediated endocytosis [39, 45-47]. A recent study has reported that conananvalin A, a clathrin-mediated endocytosis inhibitor, blocks phorbol 12 myristate , 13-acetate (PMA, a PKC activator)-induced surface reduction of DAT but only partially inhibits DAT downregulation [48]. In addition, MßC, a cholesterol depleter/membrane raft disruptor, partially blocks DAT downregualtion but does not inhibit loss of the cell surface DAT [48]. These results demonstrate that PKC-induced DAT down-regulation is regulated by a combination of trafficking and non-trafficking processes. The latter study also suggests that DAT proteins are distributed approximately equally between Triton-insoluble, cholesterol-rich membrane rafts and Triton-soluble non-raft membranes [48]. PMA-induced loss of the cell surface DAT involves the non-raft population, whereas the non-trafficking component involves cholesterol-rich rafts, suggesting that distinct subcellular DAT populations are differentially regulated [48].
Tyrosine kinases, which are activated by insulin and insulin-like growth factor, also regulate DA clearance [49-51] and DAT cell surface expression [49, 52]. Inhibition of the tyrosine kinases has been shown to decrease DAT activity in striatal homegenates [49, 53] and human DAT in oocytes [49]. Tyrosine kinase inhibition also reduces DAT cell surface expression, measured by [3H]WIN 35,428 binding in human DAT (hDAT) [49]. Insulin signaling enhances tyrosine kinase activity via the activation of phosphatidylinositol 3-kinase (PI3K) [54]. Carvelli et al. have reported that insulin causes an increase in DA uptake in FLAG-hDAT cells and striatal synaptosomes [50]. Insulin-induced enhancement of DA uptake was accompanied by an increase in DAT cell surface expression [50]. LY294002, a pharmacological inhibitor of PI3K, reverses the insulin-evoked enhancement of DA uptake and causes internalization of the DAT, suggesting that tyrosine kinases-mediated PI3K activity regulates DAT function and trafficking [50]. Evidence also indicates that Akt, a protein kinase effector, decreases hDAT surface expression and DA uptake in a time-dependent manner [51]. Therefore, the basal activity of PI3K and Akt maintains basal cell surface DAT levels and thereby basal levels of DA uptake capacity. Additionally, activation of tropomysin-related kinase (TrkB) through the binding of brain-derived neurotrophic factor (BDNF) rapidly increases DA uptake and DAT cell surface expression in striatal synaptosomes [52]. The BDNF-mediated up-regulation of DA uptake requires both mitogen-activation protein kinase (MAPK) and PI3K signaling pathways [52]. Indeed, inhibition of the MAPK cascade decreases DAT transport capacity and DAT cell surface expression in HEK-293 cells expressing hDAT [55].
The basal phosphorylation of the DAT seems to be acutely regulated by other kinases such as cAMP dependent PKA, calcium calmodulin-dependent kinase II (CaMK II) and protein phosphatases [45, 56, 57]. Activation of cAMP was shown to increase DA uptake capacity in animal striatal synaptosomes, which was blocked by the PKA-selective inhibitor [56-58]. However, the effects of PKA on cAMP-mediated stimulation of DAT function were not observed in other studies [39, 59, 60]. It is important to note that although cAMP-mediated upregulation of DAT activity is robust and rapid, it occurs within 5 min and disappears after 15 min [56, 61], and therefore differences in timing in separate studies likely affect their outcomes. In addition, the 8-bromo-cAMP (membrane-permeable cAMP analogue)-induced upregulation of DA uptake capacity is CaMK II-dependent [56]. The second messenger cAMP binds to ion channels in particular to those conducting Ca2+, and thereby the bound Ca2+-calmodulin complex interacts with the CaMK II [56].
Collectively, the function and localization of the DAT can be regulated by the multiple second messenger pathways, including PKA, PKC, tyrosine kinases, phosphatases, and other kinases. Importantly, these second signaling pathways interact each other, thereby governing DAT cell surface expression and modulating endocytosis. Here, we provide an overview of DAT regulation and the trafficking criteria that are depended on different stimulations.
2.2. Regulation of DAT function by protein-protein interaction
DAT function is regulated by proteins that are associated with the transporter protein on the plasma membrane. These proteins fall in several categories: proteins that are part of signaling pathways, receptors acted upon by the same transmitter (DA) that is the substrate for the DAT, and DAT proteins themselves forming transporter oligomers. This review will focus primarily on protein-protein interactions for which there are indications that they may play a role in the DAT regulation in the context of drugs of abuse, but it is understood that many DAT-associated proteins could play as yet an unknown role in the action of drugs of abuse.
Signaling molecules that have been reported to regulate the DAT, and be associated with it, include the protein phosphatases PP2A and PP1; α-synuclein, a protein implicated in Parkinson’s disease; syntaxin 1A, an attachment protein required for transporter trafficking; PICK1, protein that interacts with C kinase; and Hic-5, the LIM homeodomain-containing protein [62]. The pharmacological relevance of these DAT-associated proteins in the action of drugs of abuse is an unexplored territory. As drugs of abuse can affect the DAT trafficking, a role is plausible for proteins directly related to trafficking such as syntaxin 1A. It is also possible that DAT activity triggers downstream signaling cascades through associated signaling molecules, but there is only limited evidence for DAT-mediated signaling events in the form of c-fos production in DAT-expressing HEK-293 cells lacking DA receptors for activation of the PKA cascade [63].
The spatial and temporal distribution of DA after its release into the extracellular space is determined by the DAT [64]. Like amphetamine (see section 3.2), DA itself can reduce the amount of DAT at the surface of the membrane [65, 66]. An added level of complexity is that the activity of the DAT is also regulated by DA acting on D2 DA receptors. Recent work by Bolan et al. (2007) strongly suggests that the DAT and D2 receptors are physically associated at the plasma membrane [67]. Although the DAT and D2 receptors appear to interact by protein-protein interaction, this interaction itself probably does not underlie the increase in DAT function that occurs as a result of D2 receptor activation. This is mainly so because DAT activation by D2 agonist occupation requires coupling to Gi/o proteins [68]. DAT regulation by D2 receptors has been known for more than a decade. The group of Justice [69] was the first to draw attention to the possibility of D2 receptors regulating DA uptake. The experimental design involved repeated cocaine administration (20 mg/kg i.p. for 10 days) previously shown by the same group [69, 70] to upregulate DA uptake when measured one day after the final cocaine injection. The effect of daily increased DA levels at the D2 receptors was counteracted in one group of animals by pretreatment with the dopamine D2 antagonist pimozide prior to cocaine injection [69]. DA uptake and release as measured in the nucleus accumbens (NAc) by microdialysis was enhanced by repeated cocaine administration, and these effects were blocked by pimozide pretreatment, suggesting a role for the D2 receptors in the cocaine-induced upregulation effect. Soon after the study of Parsons et al. (1993), more direct evidence supporting DAT regulation by D2 receptors was advanced by the group of Schenk [71]. With rotating disk voltammetry, DA uptake into striatal suspensions was found to be increased in the presence of the D2 agonist quinpirole, and this effect could be reversed with the D2 antagonist sulpiride. In addition, Meiergerd et al. (1993) measured DA uptake in vivo by voltammetry in the striatum, and found that D2 blockade by haloperidol decreased DA uptake, most likely via a reduction in the Vmax [71]. Another early study indicating a link between the D2 receptors and the DAT is by Cass and Gerhardt (1994), who measured in vivo DA uptake by voltammetry in the striatum, NAc, and medial prefrontal cortex (mPFC). Local application of raclopride, a D2 receptor antagonist, but not SCH-23390, a D1 receptor antagonist, inhibited DA transport [72]. Taken together, these data suggest that activation of the D2 receptors stimulates DA uptake, whereas the blockade counteracts this stimulation. Much of the more recent work fits in with this scenario. Thompson et al. (2001a, b) reported inhibition and potentiation by the D2 receptor antagonism and agonism, respectively, of [3H]DA uptake into NAc synaptosomes [73, 74]. Dickinson et al. (1999) found reduced DA clearance in striatum of the D2 receptor knockout mice; and their DA uptake was unchanged by D2 receptor antagonism, whereas it was decreased upon blockade of the D2 receptors in wild-type mice [75]. In oocytes coexpressing D2 receptors and DAT, D2 agonism increased DA uptake. This effect was voltage-independent and pertussis-toxin-sensitive suggesting the involvement of Gi/o signaling [68]. Evidence obtained in the same study also suggested an associated increase in the surface presence of the DAT. The lack of D2 regulation of [3H]DA uptake observed [76] in mesencephalic neuronal cultures suggests that D2 regulation is too transient to be detected with the slower [3H]DA accumulation measurement as compared with the voltammetric techniques, or that DA itself, when used as the substrate for the uptake measurement, activates the D2 receptors and thus complicates the interpretation of effects of exogenously added D2-selective compounds [67]. Another possibility is that D2 regulation of the DAT is only present in fully mature neurons. In a study with in vivo voltammetry to monitor DA uptake and release in rat striatum and NAc [77], we found that D2 antagonism reduced uptake at all frequencies used to stimulate the medial forebrain bundle, whereas release was enhanced only at lower frequencies (by blocking D2 release-modulating autoreceptors). Be that as it may, both processes, D2 regulation of DA uptake and release, serve as parallel feed-back mechanisms aimed at reducing extracellular DA levels when high DA levels activate D2 receptors. A recent study describes regulation of DAT function not only by D2, but also D3 DA receptors [78]. In HEK-293 cells expressing both the DAT and the D3 receptor, an increase in DAT function was observed upon short exposure to a DA receptor agonist, and a decrease upon prolonged agonist exposure. In addition, as opposed to DAT regulation by D2 receptors which involves ERK1/2 but not PI3K [67], both ERK1/2 and PI3K play a role in regulation of DAT function by D3 receptors [78]. It is not known whether that is a physical association between DAT and D3 receptors as that is for D2.
Finally, among proteins that can associate with the DAT, is the DAT itself, forming oligomeric transporter protein assemblies. It is becoming more and more clear that in the SLC6 family (Na+,Cl− -dependent neurotransmitter transporters encompassing DAT), transporters occur in oligomeric form [79-81]. In the DAT, TM4 (Cys243) and TM6 (Cys306 at the extracellular end) can form symmetrical interfaces [79-81]. Leucine heptad repeats in TM2, with a TM2-TM2 interface, have also been implicated in DAT’s quaternary structure [79-81]. Experiments with coexpression of different proportions of a transporters for serotonin (SERT) construct sensitive to methanethiosulfonate compounds (sulfhydryl reagents selective for cysteine) and another insensitive construct point to a functional interaction between SERT protomers [82]. Seidel et al. (2005) advance evidence for influx and efflux of substrate through coupled but separate protomers in monoamine transporter oligomers [83]. The GLUT1 [84] and LacS transporter [85] occur as oligomers (tetramers and dimers, respectively) in the plasma membrane, and in both cases it is thought that each subunit in the complex sustains co-transport of substrate and Na+. However, there is cooperativity between transporter units (dimers for GLUT1 and monomers for LacS) that is evident for ion gradient-driven uptake of substrate but not for substrate exchange. This can be explained if the reorientation of the empty carrier in one unit is coupled to translocation of a fully loaded carrier in another unit [84, 85]. All of this information points to an impact of transporter oligomerization on transporter function. However, no direct knowledge regarding the functional relevance of DAT oligomerization is available at this time.
3. ROLE OF DAT IN THE ACTIONS OF DRUGS OF ABUSE
3.1. Cocaine
Cocaine is a reinforcing and rewarding drug of abuse that confers substantial morbidity and mortality [19, 86]. The DAT is the key site of action for cocaine [87]. DAT knockout mice are profoundly hyperactive and fail to show any further stimulation of activity in response to cocaine or D-amphetamine [19]. Nevertheless, such animals continue to self-administer cocaine [88]. This could be interpreted to suggest that the rewarding properties of the drug cannot be explained entirely by its ability to inhibit DAT. In this context, it is important to note that cocaine is also a potent inhibitor of SERT and norepinephrine transporter (NET) [89]. However, at least two considerations need to be entertained. First, the DAT-knockout was not of the inducible type and therefore developmental compensatory changes may have occurred in these animals setting them apart from normally developed animals. Second, DA is an excellent substrate for the NET, and under conditions of reduced DAT expression, the NET in the NAc takes over the clearance of extracellular DA, which is blockable by cocaine acting at the NET [90]. Strong evidence in favor of the DAT mediating cocaine reward comes from a recent study from the group of Gu showing abolished cocaine reward as measured with conditioned place preference in transgenic mice carrying a cocaine-insensitive DAT [91].
Cocaine-induced blockade of the DAT prevents reuptake and leads to subsequent increases in extracellular DA levels in the (extra) synaptic space [92-94]. The ensuing extracellular increase in DA is generally accepted as the primary rewarding/reinforcing property underlying cocaine’s efficacy at multiple transporter sites, also increasing locomotor activity and inducing stereotypical behavior [95]. Chronically enhanced DA signaling is believed to trigger the plastic changes that are responsible for cocaine addiction [94]. Indeed, cocaine-induced long term alterations in the DAT have been reported in humans. For example, the density of DAT binding is increased in striatum from human cocaine users [96-98]. The increase in DAT binding is associated with an increase in DAT function, assessed by [3H]DA uptake, indicating functional upregulation of DAT following chronic cocaine abuse.
Recent studies demonstrate that acute exposure to cocaine increases DAT membrane expression. In cell line models, acute cocaine exposure increases [3H]DA uptake and DAT cell surface expression in HEK-293 cells expressing hDAT as measured with the cell surface biotinylation assay [99]. In consonance, in N2A neurons stably expressing the DAT, the cocaine-induced increased cell surface DAT expression has been found to be accompanied by an increase in [3H]WIN 35,428 binding [100]. It is possible that following removal of cocaine stimulation, the elevated DAT cell surface expression could then persistently present increased DAT function, which may cause a relative low level of basal DA concentration (extra) in the craft of synapse. Therefore, the cocaine-induced increase in cell surface DAT expression may contribute to neurobiological mechanism that underlies the cascade of changes that triggers relapse of cocaine abuse following withdrawal. It should be noted that the precise conditions enabling the effect of cocaine in enhancing surface DAT expression are not known, and therefore caution is required in drawing conclusions regarding the universality of the effect. Thus, in HEK-293 cells expressing DAT, we did not observe cocaine-induced changes in surface-resident DAT measured by biotinylation [101]. To our knowledge, there is only one study reporting a cocaine-induced increase in DA uptake in a relevant animal (rat) model (both in vitro and in vivo) [99] rather than heterologously expressing cell lines. Independent confirmation of this effect by other laboratories is needed.
Activation of PKC induces DAT down-regulation and increases the level of DAT phosphorylation [23, 38]. The PKC-induced down-regulation of the DAT is associated with DAT endocytosis, which moves the DAT protein from the plasma membrane into intracellular pool [39, 40]. Cocaine has been reported to inhibit PMA-induced DAT phosphorylation [102] or to show no effect on PMA-stimulated phosphorylation [103]. However, cocaine has been shown to prevent methamphetamine-induced increase in phosphorylation, suggesting that cocaine may possess an independent effect on DAT phosphorylation [104]. Cocaine, when administered with amphetamine or methamphetamine, blocked the reduction in the maximal rate of [3H]DA uptake and the (meth)amphetamine-induced down-regulation of DAT expression in the plasma membrane [65, 66, 105, 106]. In consonance, Kahlig et al. (2006) concluded that intracellularly build-up amphetamine is required to provide a signal for DAT internalization [147]. In preliminary experiments in our lab with suspensions of intact, hDAT-expressing HEK-293 cells, pretreatment with the substrate DA at room temperature reduced subsequent uptake of [3H]DA uptake as well as external binding of the cocaine analog [3H]CFT; replacing Na+ with NMDG+ (preventing DA translocation) did not reduce the substrate effect on apparent DAT internalization. This suggests DA translocation is not required for its effect in downregulating surface DAT. More work is needed to conclude the universality of the concept that substrates act solely from the inside to promote DAT internalization.
The cocaine analog MFZ 2-12 has been reported to decrease the DAT oligomerization as measured by cross-linking with Cu2+ or Hg2+ [81] In our recent study on the DAT oligomerization [101], cocaine itself increased, rather than decreased, cross-linking of the DAT. One reason for these divergent findings may be that we used the full cDNA sequence with the N-terminal Flag or Myc tag just after the translational start sequence, whereas Hastrup et al. (2003) used a DAT sequence from which the N-terminal 22 amino acids had been replaced by the Flag-hemagylutinin or Myc-His tag; an additional difference between the two studies is the detergent used for lysis. At this point, the pharmacological relevance of the effects of cocaine on cross-linking of DAT is not clear as we found no changes in DAT oligomerization measured by co-immunopricipation of one tag with another tag from cells co-expressing the two differential tags [101]. It is possible that in the cross-linking studies, cocaine affects the distance between cysteine residues in neighboring protomers, changing the susceptibility to cross-linking, without actually altering the degree of oligomerization.
3.1.1. Cocaine-related compounds: cocaine analogs, benztropine, and GBR 12909
Some compounds related to cocaine are also potent inhibitors of DAT. Benztropine contains the same N-methyl tropane portion that occurs in cocaine, but it lacks the carbomethoxy group on C2 while the C3 substituent is axial (and diphenylmethoxy) rather than equatorial (and phenylcarboxy) as in cocaine [107]. GBR 12909 shares its diphenylmethoxy portion with benztropine but has a piperazine rather than a tropane attached to it, with an N-linked phenylpropyl group rather than an N-linked methyl. Although cocaine, benztropine, and GBR 12909 are all inhibitors of the DAT, their behavioral profiles are dissimilar. The psychostimulant cocaine is well known for its locomotor stimulation action and rewarding effect [15, 19, 108], but GBR 12909 has been reported to show a different psychopharmacological profile [109, 110]. Thus, a long-acting decanoate formulation of GBR12909 can attenuate cocaine self-administration [111] and has been tested for human use in clinical trials. However, GBR 12909 had to be withdrawn from the trials because of electro cardiogram problems, consisting of QT interval prolongation [112]. Potentially, QT prolongation is considered a severe problem because it may induce ventricular tachyarrhythmia. This emphasizes the need to continue searching for other pharmacotherapeutics for cocaine dependence. Indeed, benztropine analogs are being pursued as potential substitution therapies for cocaine dependence [109, 113]. Preclinically, benztropine does not induce cocaine-like locomotor activity and self-administration, and this disparity seems unrelated to its anticholinergic activity [109]. The divergence between cocaine, GBR 12909, and benztropine in their behavioral profile may in part result from their different molecular actions at the DAT. Thus, as measured in vitro by rotating disk electrode voltammetry, cocaine inhibits DA uptake in a competitive manner and GBR12909 in a noncompetitive manner [114]. Moreover, binding of cocaine causes enhanced accessibility of C90 in the DAT to sulfhydryl reactive reagents [115, 116], whereas benztropine is inert in this respect [116]. A comparison of structure-activity relationship data indicates that benztropine analogs are more like GBR analogs in their mode of binding to the DAT than like cocaine analogs [117]. In agreement, photo-affinity labels based on GBR 12909 and benztropine have been found to be incorporated into a DAT region encompassing transmembrane domains (TMs) 1 and 2, whereas the cocaine-based label becomes attached to the region containing TMs 4-7 [118, 119]. A more recent study employing photoaffinity labeling of the DAT by a covalently-binding phenyazido-ester analogue of cocaine ([125I]RTI-82) implicates TM6 in cocaine binding [120]. However, a similar covalently-binding cocaine analogue possessing a phenylazido moiety affixed to the tropane nitrogen ([125I]MFZ 2-24), rather than the 2β-ester moiety, was shown to label a thirteen amino-acid long portion of TM1, from aspartate residue 68 to leucine residue 80 [121], attesting to the vicinity of TMs 1 and 6 (see Fig. 1 and the seminal report on the crystal structure of the bacterial homolog transporter LeuT [122]).
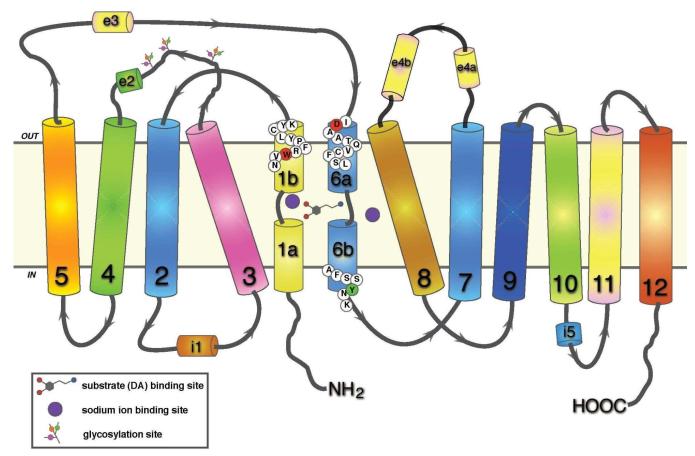
Fig. 1.
Two-dimensional representation of dopamine transporter topology based on LeuT structure. Twelve transmembrane domains are shown with helically unwound regions in the first and sixth domain; extracellular and intracellular loops include helical portions (e2, e3, e4a, e4b, and i1, 15, respectively) (see [122]). The residues mutation of which are discussed in the text are shown in colored circles: W84 in TM1b, red; D313 in TM6a, red; and Y335 in TM6b, green. The respective conformationally biased mutants are W84L and D313N (outward facing, [123]), and Y335A (inward facing, [126]).
We have used DAT mutants to explore different modes of binding of these blockers at the DAT [123]. Our previous mutagenesis studies [123, 124] have highlighted the importance of two particular residues in cocaine and cocaine-analogue binding: tryptophan 84 and aspartic acid 313, towards the extracellular face of TM1 and 6, respectively (Fig. 1). Mutation of tryptophan to leucine (W84L) and aspartate to asparagine (D313N) enhances the affinity of DAT for cocaine and the cocaine analogue 2β-carbomethoxy-3β-(4-fluorophenyl)-tropane (CFT, or WIN 35428). These two mutants are among the very small minority of the known DAT mutants with markedly enhancing cocaine and CFT binding. The binding affinity of the two mutants for cocaine was substantially enhanced, while for GBR12909 and benztropine was reduced or near normal [123]. In a recent study, we report on the interaction of various cocaine-, benztropine-, and GBR 12909-related compounds with W84L and D313N DAT [107]. The results indicate that inhibitors bearing a diphenylmethoxy functionality bind to the DAT in a different manner rather than cocaine and similar compounds. While the structural basis of this differential interaction is unknown, the response to W84L and D313N mutations gives us some insight into the nature of the respective binding sites. According to our previous studies, the W84L mutation appears to shift the conformational transition between outward- and inward-facing states towards the former, increasing the probability that the DAT substrate-binding site will be exposed to the extracellular space [123]. Cocaine-like molecules are presumed to interact with an extracellularly facing binding site formed by TM1 and TM6, in close proximity to the putative substrate site (see Fig. 1) [120, 121]. In this scenario, W84L DAT displays an increased affinity for cocaine analogues owing to an increased proportion of transporters with an exposed cocaine binding domain. The same likely applies to D313N, as this mutant displays a similar increase in affinity towards cocaine-like compounds. Evidence for the conformational effects of the W84L and D313N mutations is also provided by observations on Zn2+ interactions. Extracellular Zn2+, an ion shown to constrain the DAT in an outward-facing confirmation [125, 126], increases CFT binding at wild-type transporters, but is ineffective in enhancing CFT binding at W84L and D313N [123]. Presumably, the lack of Zn2+-mediated stimulation of CFT binding in the mutants is due to the fact that W84L and D313N are already stabilized in an outward-facing conformation. The decrease in affinity of diphenylmethoxy-based inhibitors for the W84L mutant may therefore reflect a preference of these inhibitors for the inward-facing transporter conformation.
In a recent study, another conformationally biased DAT mutant was used to probe the relationship between inhibitor binding mode and cocaine-like reinforcing effects [127], Y335A in which tyrosine 335 was mutated to alanine (see Fig. 1). In a mirror fashion to the W84L or D313N mutations, the Y335A mutation is thought to stabilize the inward-facing DAT conformational state [126]. A number of inhibitors were tested at Y335A, showing a decrease in efficacy of cocaine-like compounds but increased binding of benztropine analogues [127], giving further credence to the idea that benztropine-like inhibitors preferentially interact with the inward-facing conformational state of the DAT. Importantly, the authors also assessed the cocaine-like behavioral stimulus effects of the various inhibitors in a rat cocaine discrimination and locomotor test. Compounds that exhibited a cocaine-like loss of binding at the Y335A mutant more readily substituted for cocaine in the drug discrimination test and were more potent locomotor stimulants; analysis of quantified binding losses at Y335A and these behavioral measures showed a significant correlation. Substances with low sensitivity to the Y335A mutation included JHW 007, a benztropine analog which counteracts cocaine’s stimulant effect [128]. This raises the intriguing possibility that preferential interaction with the inward-facing conformation of DAT as determined in vitro, enables a compound to substitute for cocaine with reduced abuse liability in vivo. It has also been shown that JHW007 occupies DAT in vivo more slowly than cocaine [128]. Thus, interaction of compounds with inward-facing DAT, a closed conformation, could be contemplated to slow their on-rate; such a slower rate of onset of action links the role of distinct conformational states with the rate theory of reinforcement [129].
3.2. Amphetamine
Amphetamine and methamphetamine are two major amphetamine-like drugs that predominantly release monoamine including serotonin, norepinephrine and DA by reverse transport of DA through the DAT [24]. Although amphetamine has comparable effects in neurons containing monoamines, it is the action of DA that is mainly implicated in amphetamine-induced reinforcing and reward [130]. Several lines of evidences point to mechanisms involving the DAT underlying amphetamine-mediated DA release [130]. Amphetamine-induced DA release can be prevented by the DAT inhibitors such as nomifensine and cocaine [41, 131]. It has also been suggested that amphetamine-induced DA efflux results from the translocation of amphetamine into the cells followed by a counter movement of DA out to the extracellular compartment [41]. A typical model suggested by Sulzer et al. (1995) is that amphetamine acts by elevating the cytoplasmic DA concentration, and thereby alters the DA gradient across the plasma membrane, promoting reverse transport of DA and DA release [24, 43]. In addition, a study using in vivo voltammetry showed that the redistribution of DA from vesicles to the cytoplasm by the use of a reserpine-like compound, Ro4-1284, does not increase extracellular DA (DA efflux) in slices from wild-type animals; however, subsequent addition of amphetamine induces rapid release of DA [132]. Thus, this study suggests that the interaction of amphetamine with the DAT is required for the releasing action, but not the vesicle-depleting action, of amphetamine on DA neurons [132]. In addition to the ability of amphetamine to induce DA release through dopamine transporter reversal in a transporter-like pathway, it has also been proposed that amphetamine induces DA efflux through a fast channel-like pathway, releasing DA with timing and a magnitude akin to vesicular DA release [133].
Amphetamine acts as a substrate at the DAT through its structural similarity to DA [134], and causes DA release in a concentration-dependent dual mechanism [135]. At low concentration, amphetamine is transported by the DAT into the cytosol and increases the intracellular binding sites of the DAT for DA, resulting in the exchange of extracellular amphetamine by intracellular DA [94, 135]. When present at a higher extracellular concentration, amphetamine, a highly lipophilic compound, may diffuse into the nerve terminals through the plasmalemmal membrane [43, 133].
Amphetamine derivatives have common and distinct effects when compared with amphetamine. Although a subtly greater DA release is induced by amphetamine than methamphetamine in prefrontal cortex (PFC), no major differences for DA release in striatum are observed between amphetamine and methamphetamine [43]. Results from in vivo voltammetry studies show that cocaine and amphetamine decrease DAT function by reduce DA clearance [136, 137]. It has been shown that inactivation of PKC blocks the ability of amphetamine in induce DAT-mediated DA release [42]. Moreover, phosphorylation of the DAT was increased by in vitro treatment with methamphetamine in a cell line and in rat striatal tissue by in vitro application or in vivo injection of methamphetamine [104].
The function of the DAT has been shown to decrease even upon a single administration of methamphetamine, although it is likely that loss of function in this scenario is recovered within 24 hours. Multiple injections seem to down-regulate DAT function for an extended period of time [138]. The loss of DAT function has been demonstrated to persist for up to 1 month after cessation [139], and even after methamphetamine has been removed from the medium bathing synaptosomal preparations [140]. Western blot analyses indicated that the decrease in DAT activity may not be attributed to loss of the transporter protein per se [141], but to a modification of the protein or dysregulation of DAT function [138]. To this end, the Fleckenstein group has demonstrated a methamphetamine-induced DAT oligomerization as a result of the interaction between reactive quinones and cysteine containing the DAT. Extracellular DA overflow and unsequestered intracellular DA form reactive quinones that may further damage neuronal integrity, and further potentiate the inactivation of the DAT [138, 142-144]. It could be thought that such DAT complexes held together by cross-linked cysteine residues show reduced function or are non-functional, as opposed to the normally occurring, physiologically relevant DAT oligomers in which cysteines in neighboring promoters are not part of a disulfide bridge but are close enough to be cross-linked by exogenously added Cu2+ or Hg2+.
Several lines of evidences indicate that acute treatment with amphetamine and methamphetamine not only reduces DAT function, but also results in DAT redistribution away from the plasma membrane [65, 66, 105, 106, 145-147]. Amphetamine-induced down-regulation does not require DAT transport recycling, but requires the translocation of amphetamine to the intracellular pool [147]. Activation of insulin signaling, including PI3K and Art, is essential for maintaining DAT cell surface expression and activity [50, 51]. Activation of PI3K and Art via the insulin signaling pathway prevents the amphetamine-induced decrease in DAT cell surface expression [50, 51]. Moreover, stimulation of CaMKII function is essential for amphetamine-induced DAT cell surface redistribution [148]. Amphetamine-stimulated DA efflux through the DAT requires PKC activation [42, 102, 149]. More recent studies suggest that DAT residues 587-596 are required for PKC-mediated DAT downregulation [150] and for amphetamine-induced DAT sequestration [151]. However, amphetamine-induced losses from the plasma membrane are not dependent upon PKC activity, suggesting that amphetamine-mediated effects on DAT internalization are independent from those governing PKC-sensitive DAT endocytosis [151].
In what quaternary form is DAT internalized by the action of amphetamine? Is there a relationship between the oligomerization of DAT and its internalization? In hDAT expressing HEK-293 cells, we have observed that amphetamine dissociates DAT oligomers, shifting the distribution of the surface DAT towards a smaller ratio of oligomers to monomers [101]. This has been seen with both cross-linking and co-immuniprecipitation techniques. In conjuction with the reduction of the fraction of oligomerized DAT, the amount of surface DAT was decreased. Blocking endocytosis with phenylarsine oxide or sucrose counteracted these effects of amphetamine [101]. We entertain the model that the DAT at the cell surface is distributed between oligomers and monomers, and that monomers are internalized; amphetamine promotes the formation of monomers that then become internalized, reducing the DAT’s presence at the surface. When endocytosis is prevented by an exogenously added blocker, monomeric DAT formed by amphetamine remains at the surface and re-associates to form oligomers. This scenario links oligomerization and internalization in the effects of amphetamine. The resulting reduced surface density of DAT will impact amphetamine action (both influx and efflux), but there may be additional functional effects of amphetamine as a result of the changed distribution of the DAT between oligomers and monomers. It will be important to assess what the functional differences are between oligomerized and monomeric DAT.
3.3. Nicotine
Nicotine exerts diverse psychopharmacologic effects and is the key component that mediates tobacco smoking behavior [152-156]. Nicotine has been shown to modulate dopaminergic neurotransmission mainly by enhancing DA release through acting as an agonist at nicotinic acetylcholine receptors (nAChRs) located on dopaminergic cell bodies and terminals in both the mesocorticolimbic and nigrostriatal systems [157-159]. nAChRs are composed of α (α2- α10) and ß (ß2- ß4) subunits, which assemble into pentameric structures [160]; however, only α3-α7 and ß2-ß4 are expressed in DA neurons [161, 162]. The exact composition of nAChR subtypes mediating nicotine-evoked DA release is controversial, although α4ß2, α6ß2, α4α6ß2ß3, α6ß2ß3 and α4α5ß2-containing nAChRs may be involved [163-165]. Although there is no evidence that nicotine can act directly on the DAT protein, several lines of studies suggest a potential relationship.
In contrast to the stimulant drugs, nicotine induces an increase in DAT function by an indirect mechanism. By in vivo voltammetry measurements one can obtain estimates of the clearance rate of exogenously applied DA via the DAT [166, 167]. Using this technique, Hart and Ksir (1996) first reported that a single dose of nicotine enhanced DA clearance in rat NAc, suggesting that nicotine regulates extracellular DA concentration via the DAT [30]. Nicotine-induced increases in DA clearance would result in decreases in extracellular DA concentrations, thus tending to compensate for the nicotine-induced enhancement of DA release. Mecamylamine, a nonselective and noncompetitive nAChR antagonist, [168, 169] inhibited the nicotine-induced increase in DA clearance in the NAc [30], indicating nAChR mediation of the response. Nicotine dose-dependently increases DA clearance in mPFC and striatum [32]. Interestingly, in mPFC, a U-shaped nicotine dose-response relationship was observed for DA clearance, with a dose of 0.14 mg/kg of nicotine (base form) producing a maximal response in the range of nicotine doses tested from 0.03 to 0.3 mg/kg. However, the nicotine dose-response curve in striatum is monophasic, with a dose of 0.3 mg/kg producing a maximal response [32]. These results suggest a regional difference in nicotine dose-response relationship in striatum and mPFC.
nAChR modulation of DAT function is also supported by results showing that nAChR activation by acute and chronic nicotine augments amphetamine-induced reverse transport of DA by DAT (“DA release”) in slices of rat PFC, but not striatum [31, 170]. In an in vitro superfusion design, nicotine and other nicotinic receptor agonists, including epibatidine, cytisine, and A85380 enhanced 10 μM amphetamine-stimulated DA release in PFC slices in an assay buffer without added calcium [31]. The enhancement of amphetamine-induced DA release by nicotine was concentration-dependent and was fully reversed by nicotinic receptor antagonists DHßE and mecamylamine, suggesting that nAChRs modulate DAT function [31]. Thus, in addition to stimulating DA release from presynaptic terminals, nAChRs modulate DAT function to regulate extracellular DA concentration. DA release (exocytotic) stimulated by nicotine is calcium-dependent and transporter-mediated DA release is calcium-independent [171, 172]. The DAT, like other monoamine transporters, is a Na+/Cl−-dependent plasma membrane transporter. Activation of nAChRs is known to open voltage-dependent Na+ channels and causes depolarization of nerve terminals. Nicotine-mediated enhancement of amphetamine-stimulated [3H]DA release was blocked by tetrodotoxin, a sodium channel blocker [170]. DA uptake by the DAT is electrogenic (bringing negative charge into the cell) and therefore abolishing the internal positive charge at the plasma membrane by depolarization would counteract rather than promote DA uptake. However, depolarization by nicotine is followed by activation of several signaling pathways, which are thought to be involved in regulating DAT function [40]. Consonant with a role for signaling pathways, nicotine enhancement of amphetamine-stimulated [3H]DA release was abolished by chelation of endogenous calcium and inactivation of PKC, contributing to the modulation of the DAT [170].
Nicotine is not itself a competitor nor a substrate for the transporter, as in vitro exposure to nicotine does not directly alter [3H]DA uptake into striatal synaptosomes [173] and nicotine itself does not bind to a site on DAT protein [174]. However, acute systemic nicotine (0.8 mg/kg, base, s.c., 15 and 30 min post-injection) administration increased the maximal velocity of [3H]DA uptake into PFC synaptosomes with no change in Km [296]. The nicotine-induced increase in the [3H]DA uptake in PFC was due to an increase in DAT cell surface expression and a reduction of intracellular DAT protein [296]. Also, mecamylamine completely blocked the nicotine-induced increase in the [3H]DA uptake and DAT cell surface expression, suggesting that nAChR plays a role in the trafficking-dependent effect of nicotine on DAT function in PFC. In contrast, acute nicotine administration produced a small, but significant increase in the [3H]DA uptake in rat striatum, which was not accompanied by an increase in cell surface DAT expression [175]. In addition, a recent study reported that the α4 nAChR subunit knockout mice show a decrease in [3H]DA uptake into striatal synaptosomes compared to wild type mice, suggesting that α4 subunit is related to regulation of DAT function [176].
3.4. Others Drugs of Abuse
3.4.1. Methylphenidate
Methylphenidate (MPH), also known as Ritalin, is a widely used treatment for children and adolescents with ADHD [177]. MPH also has some abuse liability because of its stimulant properties [178, 179]. MPH is an indirect DA agonist that binds to the DAT [180] and, thereby, blocks the inward transport of DA into the presynaptic terminal which results in increased extracellular DA levels [177, 181, 182]. Clinical studies show a higher striatal DAT density in patients with ADHD [183] and a significantly decreased specific binding of [Tc99m]TRODAT-1 to hDAT in adults with ADHD after MPH treatment [184, 185]. MPH and cocaine have a similar in vitro affinity for the rat DAT (rDAT) [86], and clinical positron emission tomography studies report that MPH and cocaine also have similar in vivo affinity for the hDAT in human brain [181, 186, 187]. Nevertheless, the abuse of MPH in humans is substantially lower than that of cocaine [86]. In addition to pharmacokinetic characteristics contributing to rate of onset of action, and drug properties determining its pharmacological profile of transporter selectivity [188], other reasons, as described next, can be advanced to explain the differences between MPH and cocaine actions on the DAT.
Results from in vitro studies show that rat striatal transport of DA is inhibited in a competitive manner by MPH [189] and amphetamine [190, 191]. In the same brain region, the mechanism of inhibition for cocaine has been reported as uncompetitive [190, 191], or competitive [192]. Both the interaction of MPH and cocaine with the DAT may involve arginine residues [189, 193, 194]. Neither MPH nor cocaine affects DA release, suggesting that, similar to cocaine, MPH is not a substrate analogue at the DAT [189]. However, in contrast to observations for cocaine, no alterations in [3H]DA uptake into rat striatal synaptosomal preparation are observed upon in vitro exposure to MPH [145] or following systemic MPH administration [140]. Furthermore, in LLC-PK1 cells stably expressing rat DAT, MPH does not affect [3H]DA uptake and has no effect on PKC activator-induced down-regulation of DAT function, whereas mazindol, a DAT antagonist, as cocaine, inhibits both [3H]DA uptake and the PKC-mediated decrease in DAT function [103]. In addition to impacting the DAT, MPH indirectly affects DA transport by the vesicular monoamine transporter-2 (VMAT-2). For instance, MPH administration increases [3H]DA uptake into nonmembrane-associated vesicles purified from lysates of striatal synaptosomes [195, 196]. These phenomena are due to a redistribution of VMAT-2-containing vesicles within nerve terminals away from membranes and into the cytoplasm [195]. Importantly, a recent study shows that MPH increases DA transport into the membrane-associated vesicles rather than transport into cytoplasmic vesicles [197]. Therefore, MPH enhances DA transmission through its effects on both DAT and VMAT-2.
3.4.2. Ethanol
Ethanol has been shown to affect mesocorticolimbic dopaminergic neurotransmission through increasing the firing rate of DA neurons [198, 199], and thereby elevating DA release in the NAc [198, 200-203]. These effects of ethanol on the DA system, at least in part, mediate its rewarding and reinforcing activity [202, 204, 205]. A large number of evidence indicate that alcohol addiction are linked to DAT function and expression [206, 207]. For instance, increased [125I]RTI-55 binding was observed in ethanol-preferring monkeys, suggesting that DAT densities might be a phenotypic marker of alcohol preference in vulnerable monkeys [208]. Decreased ethanol preference and consumption were found in female DAT knockout mice [209]. Results from a site-directed mutagenesis study show that DAT mutants G130T or I137F display potentiation of the effect of ethanol on DAT function [210]. Since depressed patients are more prone to alcohol misuse, the Wistar-Kyoto rats were studied as an animal model of depressive behavior for effects of chronic ethanol consumption. Compared to Wistar control rats, there was increased [3H]GBR12935 binding in dopaminergic brain areas [211].
To date, acute and chronic ethanol studies have shown a potential link between DAT and ethanol; however, controversial results have been observed. Microdialysis studies found that acute systemic administration of ethanol increases DA levels [201, 212, 213]. Different studies have reported that a single injection of ethanol can cause an increase [214, 215], decrease [216, 217], or no change [200, 201, 218] on DA uptake capacity. Since ethanol is given systemically in these studies, it is not clear whether ethanol has the ability to directly alter DAT function. One study shows that in vitro exposure to ethanol (10-100 mM) enhances DAT-mediated [3H]DA uptake and transporter-associated currents Xenopus oocytes expressed hDAT in a time- and concentration-dependent manner [219]. This potentiation of DAT function is accompanied by an increase in the number of functional cell surface transporters, suggesting that ethanol alters DAT function and trafficking [219]. Recent studies show that acute systemic administration of ethanol does not alter DA uptake, measured by in vivo microdialysis and in vitro voltammetry in DAT knockout mice [220] and rats [221], suggesting that the acute ethanol dose not interact with the DAT to produce its effects. In contrast to acute effects of ethanol, chronic ethanol exposure enhanced DA uptake rates in monkey striatum [222]. Alcohol vapor inhalation animal models have been employed to characterize the effects of long-term alcohol exposure [222, 223]. Using such a rat model, Budygin et al. (2007) reported that ethanol vapors for 10 days enhanced DA uptake in the accumbal and striatal slices, measured by fast-scan cyclic voltammetry [224]. Since chronic alcohol exposure can lead to increased extracellular DA levels [203], the enhanced DA uptake could be a compensatory response of the DA system to increased DA signaling [224]. Thus, the enhanced DA uptake induced by chronic alcohol exposure may not be explained by a direct interaction of alcohol with the DAT, but rather by an increased number of the DATs.
3.4.3. Opioids
Several lines of studies have demonstrated that significant levels of kappa opioid receptors (KORs) are co-expressed in the NAc axons with DATs [225] and synaptic vesicles [226], suggesting that the KOR is directly involved in the presynaptic release and/or reuptake of DA. Acute administration of selective KOR agonists inhibits basal and drug-evoked increases in extracellular DA levels in the NAc, suggesting that KOR activation increases DA uptake [202, 227-229]. The selective KOR antagonist nor-binaltorphimine blocks the KOR agonist-induced increase in DA uptake and markedly increases basal extracellular DA levels [228, 230]. Furthermore, KOR knockout mice exhibit higher basal extracellular DA levels [231]. In contrast, repeated administration of U-69593, a selective KOR agonist, produces a dose-related decrease in DA uptake, whereas repeated cocaine increases DA uptake [73, 228]. These results demonstrate that KOR activation modulates DAT function in the NAc in a manner opposite to that of cocaine. Repeated U-69593 also decreased [125I]RTI-55 binding to DAT but not did not decrease total DAT immunoreactivity [228]. Chronic treatment with U-69593 significantly enhances the amphetamine- [229, 232] and cocaine-induced increase in DA extracellular levels [233].
The stimulatory effects induced by several psychostimulants are inhibited by co-administration of KOR agonists. For instance, pre-treatment or co-administration of KOR agonists blocks cocaine- and amphetamine-induced increased levels of DA in NAc and striatum [228, 234-236]. The KOR agonist stimulation in the NAc blocks the behavioral effects of psychostimulant drugs, such as cocaine and amphetamine [229, 236]. These effects of KOR on modulation of DAT function and DA release have been promoted to an implement that KOR agonists may be useful in treating drug addiction [237, 238]. The mechanism underlying the effects of repeated KOR agonists administration on the regulation of DAT function and DA release has been suggested to be related to adaptative changes in D2 autoreceptor function following repeated KOR agonist treatment [229, 232]. This interpretation is supported by previous results from in vivo voltammetry studies showing that blockade of D2 DA receptors by locally applied raclopride (a D2 DA receptor antagonist) decreased DA clearance in PFC [75] and by results from D2 receptor knockout mice showing a decreased DAT function in striatum [75] (see also section 2.2). Indeed, repeated administration of U-69593 induced alterations in D2 receptor expression and function in the NAc and striatum, but it was dependent on the schedule and timing of drug exposure [239]. Therefore, the adaptative changes in D2 receptors induced by repeated KOR agonists may alter the sensitization of dopaminergic neurons that lead to an augmented response to psychostimulants with an increase in DA transmission.
Morphine has been reported to increase DA release in the terminal areas of mesolimbic DA neurons [240]. The effect of morphine on the DA release is thought to be mediated via μ-opioid receptors on GABAergic neurons in the substantia nigra and ventral tegmental area [241, 242]. μ-opioid receptor agonists inhibit GABA release, and thus the GABAergic inhibition of DA ergic neurons is decreased, resulting in the enhancing firing of DAergic neurons. Systemic administration of morphine enhanced locomotor activity in mice [243] and conditioned place preference in rats [244].
4. INDIVIDUAL DIFFERENCES IN DAT FUNCTION IN RESPONSE TO PSYCHOSTIMULANTS
4.1. Individual differences in DAT function and expression
One focus of addiction research is the identification of neural substrates underlying individual differences in vulnerability to develop compulsive drug-seeking behaviour. In animal models, the locomotor response to a novel environment is predictive of individual differences in the behavioral effects of psychostimulants [245]. The high- and low-responder test in which rats are characterized as either high responders (HRs) or low responders (LRs) based on locomotor activity elicited in an inescapable novel environment is the most well studied [246]. Compared to LRs, HRs are more sensitive to amphetamine-induced increases in activity [246-250] and to acquisition of amphetamine self-administration [246, 250-252]. Since inescapable novelty involves a stress component independent of reward [253], it is important to use a novelty test that does not activate the stress axis. This may be accomplished by using a free-choice novelty test in which rats are given a choice between a novel object or place and a familiar object or place, and the amount of time spent with the novel stimulus is recorded. Rats classified as high in novelty place preference (Hi-NPP) show greater amphetamine-conditioned place preference compared to rats classified as low in novelty place preference (Lo-NPP) [251]; however, no difference in amphetamine self-administration is obtained [251, 252, 254]. This latter test may represent a better animal model of the novelty seeking trait that has been shown to be an important heritable trait in nonhuman primates and human drug abusers [255-257].
Individual differences in response to novelty based on inescapable or free-choice novelty tests have been shown to be predictive of the basal functional DAT expression [34, 258, 259]. Compared with LRs, HRs show greater velocity of exogenous DA uptake in NAc, assessed by using the rotating disk electrode voltammetry [258], suggesting that alternations in DA transmission in the NAc may partly mediate the locomotor response of an animal to a novel environment. A recent study extends these findings by showing that, in contrast to the increased DAT function in NAc, HRs show a decrease in the maximal velocity of [3H]DA uptake into PFC synaptosomes [34]. These contrasting findings indicate that there is a reciprocal relationship between DAT function in PFC and NAc in HRs and LRs. Interestingly, there was an inverse relationship between DAT function and the behavioral response to inescapable novelty and to novelty place preference, i.e. whereas activity in inescapable novelty was correlated negatively with DAT function, novelty preference was correlated positively [34]. These results suggest that individual differences in PFC DAT function are associated with individual differences in the behavioral response to novelty. In addition, DAT knock-down mice (expressing 10% of wild-type DAT levels) display hyperactivity and impaired response habituation activity in a novel environment, compared to wild-type mice [260]. This may provide insights into the mechanism by which an altered DAT function is associated with impaired fundamental behavioral process such as response habituation.
The clearance efficiency of the DAT is governed by the transport rate of individual transporters, the number of transporters on the neuronal cell surface, and the modulation of cell surface transporter expression [44, 47]. The alterations in DA function in NAc and PFC in the behavioral phenotypes were not accompanied by changes in total number of transporters as measured by [3H]WIN 35, 428 binding (i.e. the sum of nonfunctional transporters in intracellular membranes and functional transporters in the plasma membranes) [34, 259]. However, studies from biotinylation assay reveal that the decrease in PFC DAT function in HRs is associated with a decrease in cell surface expression of the DAT and an increase in intracellular DAT in the PFC [34]. Moreover, based on the novelty place preference test, the decrease in DAT function in PFC from Lo-NPP is associated with a decrease in cell surface expression of DAT in PFC. Thus, these findings demonstrate that there are inherent individual differences in DAT expression and localization that may underlie the differential behavioral responses to novelty.
Individual differences in the response to inescapable novelty also predicted drug effects in modulating DAT function. Compared with LRs, HRs show greater cocaine-induced inhibition of DA uptake in the NAc, assessed by quantitative microdialysis [259]. Acute in vivo nicotine administration has been shown to increase DA release and DAT function. In experiments applying inescapable novelty, nicotine increased Vmax for [3H]DA uptake in PFC from HRs but not from LRs [34]. The nicotine-induced enhancement of DAT function was accompanied by an increase in DAT cell surface expression in PFC. In contrast, in experiments testing novelty place preference, nicotine increased the Vmax for [3H]DA uptake in PFC from Lo-NPPs but not from Hi-NPPs, and was associated with an increase in DAR cell surface expression in PFC [34].
Another characterization of individual differences in the response to psychostimulants is the magnitude of an animal’s behavioral response to the initial drug exposure [261, 262]. A typical animal model in these studies is the classification of outbred male Spague-Dawley rats into low or high cocaine responders (LCRs or HCRs, respectively) based on the median split of their locomotor response to a single dose of cocaine (10 mg/kg, i.p) [263, 264]. The animals with activity scores below the median split were defined as LCRs, while those with scores above it are HCRs. Using in vivo electrochemical recording techniques, studies show greater basal DAT activity in NAc core and greater cocaine-induced inhibition of DA clearance in both NAc and striatum from HCRs compared to LCRs [263]. Throughout both LCRs and HCRs, the cocaine-induced inhibition of DA clearance in the NAc was positively correlated with cocaine-induced changes in locomotor activity [263]. Individual differences in cocaine- or amphetamine-induced behavioral stimulation could be due to individual variability in changes in DAT function and trafficking induced by the psychostimulants. In the case of cocaine, DAT function may reflect trafficking effects. In contrast, we speculate that individual differences in DAT responses to amphetamine involve differential effects on DAT function and trafficking in opposite direction. Thus, amphetamine (0.5, 1.0 and 5.0 mg/kg, i.p)-treated rats were again classified based on the median, and were described as ABs (amphetamine response below median) or AAs (amphetamine response above median) [265]. With this paradigm, individual differences in behavioral activation were not as pronounced as with cocaine. Also, no differences for the synaptosomal [3H]DA uptake and [3H]WIN 35,438 binding in striatum were observed between ABs and AAs, and individual amphetamine-induced locomotor activity and individual [3H]DA uptake were not correlated. It is possible that the lack of clear individual differences in DAT function and behavioral response to amphetamine are the result of an effect on transporter function counteracted by an opposite effect on trafficking.
The classification as LCRs and HCRs and differences in cocaine-induced behavior were no longer apparent 1 week after initial cocaine injection [266]. This finding may be result of differences in brain levels of cocaine mediating the behavioral differences observed between LCRs and HCRs following acute cocaine injection. However, no difference in brain cocaine levels in either NAc or striatum was found between LCRs and HCRs 30 min after cocaine administration, whereas the concentration of cocaine in the striatum was significantly higher than that measured in NAc [266]. Another possible explanation for this finding is the existence of difference in total number of transporters in LCRs, compared HCRs. The maximal binding sites of [3H]WIN 35,428 and DAT affinity for cocaine in the NAc was not different between LCRs and HCRs [265, 266]. However, the [3H]WIN 35,428 binding measures total cellular transporters and would not detect differences in cell surface DATs between LCRs and HCRs. Recently, DAT trafficking has been shown to be regulated by exposure to psychostimulant drug, including cocaine and amphetamine [65, 99, 100, 267]. Specifically, studies show that DAT function and expression can be altered in behavioral phenotypes following acute nicotine administration [34]. Thus, an inherent difference in basal DAT function and expression could exist in LCRs and HCRs, which may contribute to individual differences in the response to cocaine.
Repeated administration of psychostimulants is a widely used experimental paradigm to model the progression of behavioral and neurochemical changes leading to compulsive drug seeking behaviors [268-270]. It is important to determine whether long-lasting adaptations in DAT function contribute to cocaine-induced locomotor sensitization. With repeated administration of cocaine (10 mg/kg, daily), locomotor sensitization developed in LCRs, but not in HCRs, which was paralleled by an increased ability of cocaine to inhibit DA clearance in NAc from LCRs and by a lack of change in cocaine-induced inhibition of DA clearance [264]. These studies demonstrate that increased DAT inhibition by cocaine is associated with locomotor sensitization and that DAT serves as a primary cellular target for mediating both initial and sensitized locomotor responsiveness to cocaine.
4.2. Differences in DAT and environmental enrichment
Convergent evidence suggests that environmental factors contribute to individual vulnerability to drugs of abuse [271-274]. In an environmental animal model, rats are raised in one of two different conditions: an enriched condition (EC) containing novel objects and social partners, or an impoverished condition (IC) without objects and partners. This animal model can help identify environmental factors that may alter individual vulnerability to drug abuse. EC rats display less locomotor activity in an inescapable novel environment, compared to IC rats [275-282]. EC rats enter a novel compartment, manipulate novel objects and habituate to novel stimuli more rapidly than IC rats [281, 283, 284], suggesting inherent differences in basal locomotor activity and behavioral response to novel stimuli between EC and IC. Environmental enrichment contributes to individual differences in vulnerability and responsiveness to abused drugs. EC rats show reduced amphetamine-induced hyperactivity and decreased amphetamine self-administration [285], and exhibit less sensitivity to behavioral effects of nicotine compared to IC rats [286]. Thus, environmental enrichment may provide beneficial plays in reducing the psychostimulant effects of these drugs.
Environmental stimulus-induced alterations in the behavioral response to psychostimulants have been suggested to be mediated, at least in part, by differential modulation of dopaminergic neurotransmission [276, 287]. Within the dopaminergic system, the DAT is a potential candidate for regulation by environmental stimuli, possibly involved in on the observed increase in resistance to psychostimulant drugs. We first determined whether environmental enrichment differentially alters the effects of GBR 12935 administration, a selective DAT inhibitor on locomotor activity and sensitization [35]. Activity of EC rats following vehicle injection was lower than that for IC rats, which is in agreement with previous reports [275-282] showing that EC rats exhibit lower basal levels of activity compared to IC rats. Additionally, EC rats showed a greater, dose-dependent GBR 12935-induced increase in activity compared to IC rats, which is consistent with the previous results showing a greater activity in response to acute amphetamine in EC rats rather than in IC rats [276, 278]. Since GBR 12935 selectively inhibits DAT function, agreement between the result with GBR 12935 and the previous reports with amphetamine [276, 278] suggests that DAT is an important presynaptic target protein that may be modulated by environmental enrichment. Indeed, our in vivo and in vitro studies indicated that basal DAT function was diminished in mPFC from EC rats relative to IC rats [34, 35]. Therefore, environment-induced neuroadaptive changes in DAT function is the most likely attributable to the differences in the basal locomotor activity and the behavioral response to stimulant drugs between EC and IC rats.
Studies of drug-naïve rats found that the maximal velocity of [3H]DA uptake in synaptosomes prepared from the mPFC from EC rats was decreased compared to IC rats, with no differences between EC and IC rats for NAc and striatum [35]. Also, no differences between EC and IC rats were found in [3H]GBR12935 binding density, which reflects both cell surface and internalized DAT [35]. We therefore considered the possibility that there was a reduction in cell surface expression of DAT protein in EC compared to IC rats preferentially in the mPFC. Thus, we next employed cell surface biotinylation assay to determine cell surface expression of DAT in mPFC, NAc and striatum from EC and IC rats [33]. In this study, the cell surface expression of DAT in mPFC was decreased in EC compared to IC rats, without changes in total DAT immunoreactivity. This finding is consistent with the magnitude of the previously observed decrease in the maximal velocity for [3H]DA uptake in mPFC from EC rats. The mechanism by which environmental enrichment reduces DAT cell surface expression may involve regulation of intracellular signaling cascades. For example, activation of PKC by phorbol esters and inhibition of mitogen-activated protein kinase or PI3K have been shown to decrease cell surface DAT expression in cell systems and in brain [40]. Taken together, these results suggest the possibility that exposure to novelty during development activates neural systems in the mPFC that subsequently may alter cell signaling cascades leading to a decrease in cell surface DAT expression and function in this brain region.
Environmental enrichment-induced decreased cell surface expression of the DAT in mPFC could compensate for regulatory effects of psychostimulant drugs on DAT function and trafficking. Environmental enrichment has been shown to alter behavioral effects of nicotine [279, 286]. Nicotine has been shown to regulate DAT function and expression in vivo [30, 32] and in vitro [31]. Recent study using in vivo voltammetry shows that environmental enrichment decreases the basal DA clearance (dynamic DAT function) in mPFC, compared to IC rats [34]. This finding is consistent with the previous in vitro study showing decreased Vmax of [3H]DA uptake in the same brain region [35]. Furthermore, following acute systemic nicotine administration, nicotine-induced enhancement of DA clearance in the mPFC was increased in EC rats, but not in IC rats, suggesting that enrichment augments nicotine-induced increase in DAT function. Since DAT function and cell surface expression in mPFC was decreased in EC rats compared to IC rats, it appears that the effect of nicotine on cell surface DAT in mPFC was greatly increased in EC rats rather than in IC rats. Alternatively, the greater proportion of cell surface DAT relative to intracellular DAT in mPFC in IC under control conditions may have prohibited DAT trafficking to the cell surface in response to nicotine.
Differential expression or function of D2 DA receptors in mPFC in EC and IC may also contribute to enrichment-induced differences in DA clearance following nicotine or saline. Although there are some inconsistent reports, the majority of studies indicate that stimulation of D2 receptors increases DAT function [288]. Stimulation of D2 receptors with quinpirole increases DA clearance in in vivo voltammetry studies and increases DA uptake in in vitro studies [71, 73], whereas inhibition of D2 receptors by raclopride decreases DA clearance in voltammetry studies [72]. Nicotine increased DA release in mPFC [170, 289, 290], and increases in extracellular DA concentrations would be expected to stimulate D2 receptors, which could then augment DAT function indirectly. It remains to be determined if nicotine differentially augments DA release in mPFC in EC and IC, which could result in differential stimulation of D2 receptors in the mPFC or if enrichment alters mPFC D2 receptor function. Indeed, research from positron emission tomography imaging reported that the availability of D2 receptors was significantly increased in the monkeys in social housing group, but not in an individual housing condition [291]. This difference in the amount of D2 receptors was associated with differences in sensitivity to cocaine’s reinforcing effects.
Evidences from other studies using adult C57BL/6 mice raised in an enriched environment for only two months show significant decreases in DAT binding and DAT mRNA levels in the nigrostriatal system, compared to the mice raised in a standard condition [292, 293]. These studies also show that enriched mice display less sensitivity to novelty environment and are less responsive to cocaine when compared with the mice from the standard housing condition [292]. In addition, enriched mice show increased expression of BDNF mRNA levels in striatum compared to the standard mice [292]. The trophic factor activity of BDNF on DA neurons [294] may play a role in DAT downregulation and in subsequent resistance to cocaine.
5. CONCLUSIONS
Drug addiction presents a significant public health concern. It is now considered a brain disease, as predispositional brain conditions likely underlie the initial effects of early exposure to drugs of abuse, and because sequelae from repeated drug use are considered to precipitate long-term changes in brain processes that alter subsequent drug effects. A large number of studies over the last two decades have demonstrated the critical importance of DA in the behavioral pharmacology and addictive properties of abused drugs. In as far as there is co-morbidity between depression and drug abuse, it is relevant to note that there is a resurgence of interest in the DAT as a major target for treatment of depression [295]. The DAT is also a major target for drugs of abuse in the category of psychostimulants, playing a primary role in their reinforcing and behavioral-stimulant effects in animals and humans. MPH, a drug used for treating ADHD, can also be a psychostimulant drug of abuse, and interacts in important ways with the DAT. Other drugs of abuse such as nicotine, ethanol, and heroine or morphine, interact with the DAT in more indirect ways. Despite the different ways in which drugs of abuse can affect DAT function, one evolving theme in all cases is regulation of the DAT at the level of surface expression. Acute and long-term changes in the amount of the DAT at the cell surface emerge as the crucial mechanisms in the effects of drugs of abuse. It is therefore important to understand the precise mechanisms that underlie the regulation of DAT function and expression. An extensive literature demonstrates that DAT function is dynamically regulated by multiple intracellular and extracellular signaling pathways and several protein-protein interactions. Also, a growing literature indicates that DAT expression is regulated through the removal (internalization) and recycling of the protein from the cell surface. However, the precise interactions between these regulating factors still remain to be demonstrated. In addition to considering the pharmacology of drugs of abuse at the DAT, one needs to take into account that many abused drugs directly or indirectly affect DAT function and expression through different mechanisms. Furthermore, recent studies demonstrate that individual differences in response to novel environments and psychostimulants can be predicted based on individual basal functional DAT expression. Environmental enrichment appears to produce a degree of protection against drug abuse through altering DAT function and expression. Although current knowledge of multiple factors regulating DAT activity has greatly expanded, many aspects of this regulation remain to be elucidated. A better understanding of the effects of abused drugs in regulating DAT activity will enable efforts to (i) develop new medications for dependence on drugs for which there is no treatment available at this point in time, i.e. cocaine and amphetamine, (ii) improve existing medications for the treatment of dependence on drugs such as nicotine or opioids, or (iii) enhance medications targeting the DAT for smoking cessation (buproprion) or treatment of complex psychiatric diseases such as ADHD (MPH) and depression (buproprion, novel triple uptake inhibitors) [269].
Acknowledgments
The authors acknowledge the financial supports: Grants DA013261 and DA019676, USPHS grants DA05312, DA012964 and DA018372 from National Institute of Health.
ABBREVIATIONS
- AAs
Amphetamine response above median
- ABs
Amphetamine response below median
- ADHD
Attention deficit hyperactivity disorder
- BDNF
Brain-derived neurotrophic factor
- CaMK II
Calcium calmodulin-dependent kinase II
- cAMP
Cyclic adenosine-monophosphate
- CFT
2β-carbomethoxy-3β-(4-fluorophenyl)-tropane or WIN 35,428
- DA
Dopamine
- DAT
Dopamine transporter
- EC
Enriched condition
- ERK
Extracellular signal-regulated kinase
- GBR 12909
1-(2-[bis(4-Fluorophenyl)methoxy]ethyl)-4-(3-phenylpropyl)piperazine
- GBR12935
1-[2-(Diphenylmethoxy)ethyl]-4-(3-phenylpropyl)piperazine
- HCRs
High cocaine responders
- Hi-NPP
High novelty place preference
- HRs
High responders
- IC
Impoverished condition
- JHW007
N-(n-butyl)-(bis-fluorophenyl)methoxytropane
- KOR
Kappa opioid receptors
- Km
Michaelis-Menten constant for half saturation of uptake
- LCRs
Low cocaine responders
- Lo-NPP
Low novelty place preference
- LRs
Low responders
- LY294002
2-(4-Morpholinyl)-8-phenyl-4H-1-benzopyran-4-one
- MAPK
Mitogen-activation protein kinase
- MFZ 2-24
2beta-carbomethoxy-3beta-(4-chlorophenyl) tropane
- MPH
Methylphenidate
- mPFC
Medial prefrontal cortex
- NAc
Nucleus accumbens
- nAChRs
Nicotinic acetylcholine receptors
- NMDG+
N-methyl-d-glucamine
- PFC
Prefrontal cortex
- PI3K
Phosphatidylinositol 3-kinase
- PKA
Protein kinase A
- PKC
Protein kinase C
- PMA
12-O-Tetradecanoylphorbol 13-acetate
- PP
Protein phosphotase
- Ro 4-1284
2-hydroxy-2-ethyl-3-isobutyl-9,10-dimethoxy-1,2,3,4,6,7-hexahydrobenzo[alpha]chinolizin hydrochloride
- RTI-82
3bata-(p-chlorophenyl)tropane-2batacarboxylic acid, 4′-azido-3′-iodophenylethyl ester
- TM
Transmembrane
- U69593
[(+)-(5alpha,7alpha,8bata)-N-methyl-N-[7-(1-pyrrolidinyl)-1-oxaspiro[4.5]dec-8-yl]benzeneacetamide
- VMAT
Vesicular monoamine transporter
- Vmax
Maximal uptake velocity.
- WIN 35,428
2β-carbomethoxy-3β-(4-fluorophenyl)-tropane or CFT
REFERENCES
- 1.Giros B, Caron MG. Trends Pharmacol Sci. 1993;14(2):43. doi: 10.1016/0165-6147(93)90029-j. [DOI] [PubMed] [Google Scholar]
- 2.Dani JA, Zhou FM. Neuron. 2004;42(4):522. doi: 10.1016/j.neuron.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Koob GF, Bloom FE. Science. 1988;242(4879):715. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- 4.Seeman P, Niznik HB. Faseb J. 1990;4(10):2737. doi: 10.1096/fasebj.4.10.2197154. [DOI] [PubMed] [Google Scholar]
- 5.Fiorino DF, Coury A, Fibiger HC, Phillips AG. Behav Brain Res. 1993;55(2):131. doi: 10.1016/0166-4328(93)90109-4. [DOI] [PubMed] [Google Scholar]
- 6.Kugaya A, Fujita M, Innis RB. Ann Nucl Med. 2000;14(1):1. doi: 10.1007/BF02990472. [DOI] [PubMed] [Google Scholar]
- 7.Carlsson ML, Carlsson A, Nilsson M. Curr Med Chem. 2004;11(3):267. doi: 10.2174/0929867043456034. [DOI] [PubMed] [Google Scholar]
- 8.Gainetdinov RR, Jones SR, Fumagalli F, Wightman RM, Caron MG. Brain Res Brain Res Rev. 1998;26(2-3):148. doi: 10.1016/s0165-0173(97)00063-5. [DOI] [PubMed] [Google Scholar]
- 9.Benoit-Marand M, Jaber M, Gonon F. Eur J Neurosci. 2000;12(8):2985. doi: 10.1046/j.1460-9568.2000.00155.x. [DOI] [PubMed] [Google Scholar]
- 10.Gainetdinov RR, Jones SR, Caron MG. Biol Psychiatry. 1999;46(3):303. doi: 10.1016/s0006-3223(99)00122-5. [DOI] [PubMed] [Google Scholar]
- 11.Gainetdinov RR, Caron MG. Annu Rev Pharmacol Toxicol. 2003;43:261. doi: 10.1146/annurev.pharmtox.43.050802.112309. [DOI] [PubMed] [Google Scholar]
- 12.Powell SB, Lehmann-Masten VD, Paulus MP, Gainetdinov RR, Caron MG, Geyer MA. Psychopharmacology (Berl) 2004;173(3-4):310. doi: 10.1007/s00213-003-1765-7. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguiz RM, Chu R, Caron MG, Wetsel WC. Behav Brain Res. 2004;148(1-2):185. doi: 10.1016/s0166-4328(03)00187-6. [DOI] [PubMed] [Google Scholar]
- 14.Medvedev IO, Gainetdinov RR, Sotnikova TD, Bohn LM, Caron MG, Dykstra LA. Psychopharmacology (Berl) 2005;180(3):408. doi: 10.1007/s00213-005-2173-y. [DOI] [PubMed] [Google Scholar]
- 15.Amara SG, Arriza JL. Curr Opin Neurobiol. 1993;3(3):337. doi: 10.1016/0959-4388(93)90126-j. [DOI] [PubMed] [Google Scholar]
- 16.Uhl GR, Johnson PS. J Exp Biol. 1994;196:229. doi: 10.1242/jeb.196.1.229. [DOI] [PubMed] [Google Scholar]
- 17.Gether U, Andersen PH, Larsson OM, Schousboe A. Trends Pharmacol Sci. 2006;27(7):375. doi: 10.1016/j.tips.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Nelson N. J Neurochem. 1998;71(5):1785. doi: 10.1046/j.1471-4159.1998.71051785.x. [DOI] [PubMed] [Google Scholar]
- 19.Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Nature. 1996;379(6566):606. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 20.Gainetdinov RR, Sotnikova TD, Caron MG. Trends Pharmacol Sci. 2002;23(8):367. doi: 10.1016/s0165-6147(02)02044-8. [DOI] [PubMed] [Google Scholar]
- 21.Mortensen OV, Amara SG. European Journal of Pharmacology. 2003;479(1-3):159. doi: 10.1016/j.ejphar.2003.08.066. [DOI] [PubMed] [Google Scholar]
- 22.Melikian HE. Pharmacology & Therapeutics. 2004;104(1):17. doi: 10.1016/j.pharmthera.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Zahniser NR, Doolen S. Pharmacol Ther. 2001;92(1):21. doi: 10.1016/s0163-7258(01)00158-9. [DOI] [PubMed] [Google Scholar]
- 24.Sulzer D, Sonders MS, Poulsen NW, Galli A. Prog Neurobiol. 2005;75(6):406. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Kahlig KM, Galli A. European Journal of Pharmacology. 2003;479(1-3):153. doi: 10.1016/j.ejphar.2003.08.065. [DOI] [PubMed] [Google Scholar]
- 26.Howell LL, Kimmel HL. Biochem Pharmacol. 2008;75(1):196. doi: 10.1016/j.bcp.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Askenasy EP, Taber KH, Yang PB, Dafny N. Int J Neurosci. 2007;117(6):757. doi: 10.1080/00207450600910176. [DOI] [PubMed] [Google Scholar]
- 28.Dafny N, Yang PB. Brain Res Bull. 2006;68(6):393. doi: 10.1016/j.brainresbull.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Swanson JM, Lerner M, Williams L. N Engl J Med. 1995;333(14):944. doi: 10.1056/NEJM199510053331419. [DOI] [PubMed] [Google Scholar]
- 30.Hart C, Ksir C. J Neurochem. 1996;66(1):216. doi: 10.1046/j.1471-4159.1996.66010216.x. [DOI] [PubMed] [Google Scholar]
- 31.Drew AE, Derbez AE, Werling LL. Synapse. 2000;38(1):10. doi: 10.1002/1098-2396(200010)38:1<10::AID-SYN2>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 32.Middleton LS, Cass WA, Dwoskin LP. J Pharmacol Exp Ther. 2004;308(1):367. doi: 10.1124/jpet.103.055335. [DOI] [PubMed] [Google Scholar]
- 33.Zhu J, Apparsundaram S, Bardo MT, Dwoskin LP. J Neurochem. 2005;93(6):1434. doi: 10.1111/j.1471-4159.2005.03130.x. [DOI] [PubMed] [Google Scholar]
- 34.Zhu J, Bardo MT, Bruntz RC, Stairs DJ, Dwoskin LP. Eur J Neurosci. 2007;26(3):717. doi: 10.1111/j.1460-9568.2007.05690.x. [DOI] [PubMed] [Google Scholar]
- 35.Zhu J, Green T, Bardo MT, Dwoskin LP. Behav Brain Res. 2004;148(1-2):107. doi: 10.1016/s0166-4328(03)00190-6. [DOI] [PubMed] [Google Scholar]
- 36.Zahniser NR, Sorkin A. Neuropharmacology. 2004;47(Supplement 1):80. doi: 10.1016/j.neuropharm.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 37.Robinson MB. J Neurochem. 2002;80(1):1. doi: 10.1046/j.0022-3042.2001.00698.x. [DOI] [PubMed] [Google Scholar]
- 38.Vaughan RA. J Pharmacol Exp Ther. 2004;310(1):1. doi: 10.1124/jpet.103.052423. [DOI] [PubMed] [Google Scholar]
- 39.Daniels GM, Amara SG. J Biol Chem. 1999;274(50):35794. doi: 10.1074/jbc.274.50.35794. [DOI] [PubMed] [Google Scholar]
- 40.Melikian HE, Buckley KM. J Neurosci. 1999;19(18):7699. doi: 10.1523/JNEUROSCI.19-18-07699.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fischer JF, Cho AK. J Pharmacol Exp Ther. 1979;208(2):203. [PubMed] [Google Scholar]
- 42.Kantor L, Gnegy ME. J Pharmacol Exp Ther. 1998;284(2):592. [PubMed] [Google Scholar]
- 43.Sulzer D, Chen TK, Lau YY, Kristensen H, Rayport S, Ewing A. J Neurosci. 1995;15(5 Pt 2):4102. doi: 10.1523/JNEUROSCI.15-05-04102.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loder MK, Melikian HE. J Biol Chem. 2003 doi: 10.1074/jbc.M301845200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pristupa ZB, McConkey F, Liu F, Man HY, Lee FJ, Wang YT, Niznik HB. Synapse. 1998;30(1):79. doi: 10.1002/(SICI)1098-2396(199809)30:1<79::AID-SYN10>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 46.Granas C, Ferrer J, Loland CJ, Javitch JA, Gether U. J Biol Chem. 2003;278(7):4990. doi: 10.1074/jbc.M205058200. [DOI] [PubMed] [Google Scholar]
- 47.Sorkina T, Hoover BR, Zahniser NR, Sorkin A. Traffic. 2005;6(2):157. doi: 10.1111/j.1600-0854.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- 48.Foster JD, Adkins SD, Lever JR, Vaughan RA. J Neurochem. 2008;105(5):1683. doi: 10.1111/j.1471-4159.2008.05262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Doolen S, Zahniser NR. J Pharmacol Exp Ther. 2001;296(3):931. [PubMed] [Google Scholar]
- 50.Carvelli L, Moron JA, Kahlig KM, Ferrer JV, Sen N, Lechleiter JD, Leeb-Lundberg LM, Merrill G, Lafer EM, Ballou LM, Shippenberg TS, Javitch JA, Lin RZ, Galli A. J Neurochem. 2002;81(4):859. doi: 10.1046/j.1471-4159.2002.00892.x. [DOI] [PubMed] [Google Scholar]
- 51.Garcia BG, Wei Y, Moron JA, Lin RZ, Javitch JA, Galli A. Mol Pharmacol. 2005;68(1):102. doi: 10.1124/mol.104.009092. [DOI] [PubMed] [Google Scholar]
- 52.Hoover BR, Everett CV, Sorkin A, Zahniser NR. J Neurochem. 2007;101(5):1258. doi: 10.1111/j.1471-4159.2007.04522.x. [DOI] [PubMed] [Google Scholar]
- 53.Simon JR, Bare DJ, Ghetti B, Richter JA. Neurosci Lett. 1997;224(3):201. doi: 10.1016/S0304-3940(97)13479-6. [DOI] [PubMed] [Google Scholar]
- 54.Taha C, Klip A. J Membr Biol. 1999;169(1):1. doi: 10.1007/pl00005896. [DOI] [PubMed] [Google Scholar]
- 55.Moron JA, Zakharova I, Ferrer JV, Merrill GA, Hope B, Lafer EM, Lin ZC, Wang JB, Javitch JA, Galli A, Shippenberg TS. J Neurosci. 2003;23(24):8480. doi: 10.1523/JNEUROSCI.23-24-08480.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Page G, Barc-Pain S, Pontcharraud R, Cante A, Piriou A, Barrier L. Neurochem Int. 2004;45(5):627. doi: 10.1016/j.neuint.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 57.Batchelor M, Schenk JO. J. Neurosci. 1998;18(24):10304. doi: 10.1523/JNEUROSCI.18-24-10304.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kadowaki K, Hirota K, Koike K, Ohmichi M, Kiyama H, Miyake A, Tanizawa O. Neuroendocrinology. 1990;52(3):256. doi: 10.1159/000125595. [DOI] [PubMed] [Google Scholar]
- 59.Zhu SJ, Kavanaugh MP, Sonders MS, Amara SG, Zahniser NR. J Pharmacol Exp Ther. 1997;282(3):1358. [PubMed] [Google Scholar]
- 60.Copeland BJ, Vogelsberg V, Neff NH, Hadjiconstantinou M. J Pharmacol Exp Ther. 1996;277(3):1527. [PubMed] [Google Scholar]
- 61.Batchelor M, Schenk JO. J Neurosci. 1998;18(24):10304. doi: 10.1523/JNEUROSCI.18-24-10304.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Torres GE. J Neurochem. 2006;97(Suppl 1):3. doi: 10.1111/j.1471-4159.2006.03719.x. [DOI] [PubMed] [Google Scholar]
- 63.Yatin SM, Miller GM, Norton C, Madras BK. Synapse. 2002;45(1):52. doi: 10.1002/syn.10084. [DOI] [PubMed] [Google Scholar]
- 64.Cragg SJ, Rice ME. Trends Neurosci. 2004;27(5):270. doi: 10.1016/j.tins.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 65.Saunders C, Ferrer JV, Shi L, Chen J, Merrill G, Lamb ME, Leeb-Lundberg LM, Carvelli L, Javitch JA, Galli A. Proc Natl Acad Sci U S A. 2000;97(12):6850. doi: 10.1073/pnas.110035297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chi L, Reith ME. J Pharmacol Exp Ther. 2003 doi: 10.1124/jpet.103.055095. [DOI] [PubMed] [Google Scholar]
- 67.Bolan EA, Kivell B, Jaligam V, Oz M, Jayanthi LD, Han Y, Sen N, Urizar E, Gomes I, Devi LA, Ramamoorthy S, Javitch JA, Zapata A, Shippenberg TS. Mol Pharmacol. 2007;71(5):1222. doi: 10.1124/mol.106.027763. [DOI] [PubMed] [Google Scholar]
- 68.Mayfield RD, Zahniser NR. Mol Pharmacol. 2001;59(1):113. doi: 10.1124/mol.59.1.113. [DOI] [PubMed] [Google Scholar]
- 69.Parsons LH, Schad CA, Justice JB., Jr. J Neurochem. 1993;60(1):376. doi: 10.1111/j.1471-4159.1993.tb05864.x. [DOI] [PubMed] [Google Scholar]
- 70.Ng JP, Hubert GW, Justice JB., Jr. J Neurochem. 1991;56(5):1485. doi: 10.1111/j.1471-4159.1991.tb02042.x. [DOI] [PubMed] [Google Scholar]
- 71.Meiergerd SM, Patterson TA, Schenk JO. J Neurochem. 1993;61(2):764. doi: 10.1111/j.1471-4159.1993.tb02185.x. [DOI] [PubMed] [Google Scholar]
- 72.Cass WA, Gerhardt GA. Neurosci Lett. 1994;176(2):259. doi: 10.1016/0304-3940(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 73.Thompson TL, Bridges S, Miller C. Neurosci Lett. 2001;312(3):169. doi: 10.1016/s0304-3940(01)02209-1. [DOI] [PubMed] [Google Scholar]
- 74.Thompson TL, Bridges SR, Weirs WJ. Brain Res Bull. 2001;54(6):631. doi: 10.1016/s0361-9230(01)00472-5. [DOI] [PubMed] [Google Scholar]
- 75.Dickinson SD, Sabeti J, Larson GA, Giardina K, Rubinstein M, Kelly MA, Grandy DK, Low MJ, Gerhardt GA, Zahniser NR. J Neurochem. 1999;72(1):148. doi: 10.1046/j.1471-4159.1999.0720148.x. [DOI] [PubMed] [Google Scholar]
- 76.Prasad BM, Amara SG. J Neurosci. 2001;21(19):7561. doi: 10.1523/JNEUROSCI.21-19-07561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu Q, Reith ME, Walker QD, Kuhn CM, Carroll FI, Garris PA. J Neurosci. 2002;22(14):6272. doi: 10.1523/JNEUROSCI.22-14-06272.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zapata A, Kivell B, Han Y, Javitch JA, Bolan EA, Kuraguntla D, Jaligam V, Oz M, Jayanthi LD, Samuvel DJ, Ramamoorthy S, Shippenberg TS. J Biol Chem. 2007;282(49):35842. doi: 10.1074/jbc.M611758200. [DOI] [PubMed] [Google Scholar]
- 79.Hastrup H, Karlin A, Javitch JA. Proc Natl Acad Sci U S A. 2001;98(18):10055. doi: 10.1073/pnas.181344298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Torres GE, Carneiro A, Seamans K, Fiorentini C, Sweeney A, Yao WD, Caron MG. J Biol Chem. 2003;278(4):2731. doi: 10.1074/jbc.M201926200. [DOI] [PubMed] [Google Scholar]
- 81.Hastrup H, Sen N, Javitch JA. J Biol Chem. 2003;278(46):45045. doi: 10.1074/jbc.C300349200. [DOI] [PubMed] [Google Scholar]
- 82.Kilic F, Rudnick G. Proc Natl Acad Sci U S A. 2000;97(7):3106. doi: 10.1073/pnas.060408997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Seidel S, Singer EA, Just H, Farhan H, Scholze P, Kudlacek O, Holy M, Koppatz K, Krivanek P, Freissmuth M, Sitte HH. Mol Pharmacol. 2005;67(1):140. doi: 10.1124/mol.67.1.. [DOI] [PubMed] [Google Scholar]
- 84.Zottola RJ, Cloherty EK, Coderre PE, Hansen A, Hebert DN, Carruthers A. Biochemistry. 1995;34(30):9734. doi: 10.1021/bi00030a011. [DOI] [PubMed] [Google Scholar]
- 85.Veenhoff LM, Heuberger EH, Poolman B. Embo J. 2001;20(12):3056. doi: 10.1093/emboj/20.12.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Science. 1987;237(4819):1219. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- 87.Gonon F, Burie JB, Jaber M, Benoit-Marand M, Dumartin B, Bloch B. Prog Brain Res. 2000;125:291. doi: 10.1016/S0079-6123(00)25018-8. [DOI] [PubMed] [Google Scholar]
- 88.Rocha BA, Fumagalli F, Gainetdinov RR, Jones SR, Ator R, Giros B, Miller GW, Caron MG. Nat Neurosci. 1998;1(2):132. doi: 10.1038/381. [DOI] [PubMed] [Google Scholar]
- 89.Blakely RD, De Felice LJ, Hartzell HC. J Exp Biol. 1994;196(1):263. doi: 10.1242/jeb.196.1.263. [DOI] [PubMed] [Google Scholar]
- 90.Carboni E, Spielewoy C, Vacca C, Nosten-Bertrand M, Giros B, Di Chiara G. J Neurosci. 2001;21(9):RC141. doi: 10.1523/JNEUROSCI.21-09-j0001.2001. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen R, Tilley MR, Wei H, Zhou F, Zhou FM, Ching S, Quan N, Stephens RL, Hill ER, Nottoli T, Han DD, Gu HH. Proc Natl Acad Sci U S A. 2006;103(24):9333. doi: 10.1073/pnas.0600905103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kuhar MJ, Ritz MC, Boja JW. Trends Neurosci. 1991;14(7):299. doi: 10.1016/0166-2236(91)90141-g. [DOI] [PubMed] [Google Scholar]
- 93.Koob GF, Nestler EJ. J Neuropsychiatry Clin Neurosci. 1997;9(3):482. doi: 10.1176/jnp.9.3.482. [DOI] [PubMed] [Google Scholar]
- 94.Jones SR, Joseph JD, Barak LS, Caron MG, Wightman RM. J Neurochem. 1999;73(6):2406. doi: 10.1046/j.1471-4159.1999.0732406.x. [DOI] [PubMed] [Google Scholar]
- 95.Johanson CE, Fischman MW. Pharmacol Rev. 1989;41(1):3. [PubMed] [Google Scholar]
- 96.Little KY, Kirkman JA, Carroll FI, Clark TB, Duncan GE. Brain Res. 1993;628(1-2):17. doi: 10.1016/0006-8993(93)90932-d. [DOI] [PubMed] [Google Scholar]
- 97.Staley JK, Hearn WL, Ruttenber AJ, Wetli CV, Mash DC. J Pharmacol Exp Ther. 1994;271(3):1678. [PubMed] [Google Scholar]
- 98.Mash DC, Pablo J, Ouyang Q, Hearn WL, Izenwasser S. J Neurochem. 2002;81(2):292. doi: 10.1046/j.1471-4159.2002.00820.x. [DOI] [PubMed] [Google Scholar]
- 99.Daws LC, Callaghan PD, Moron JA, Kahlig KM, Shippenberg TS, Javitch JA, Galli A. Biochem Biophys Res Commun. 2002;290(5):1545. doi: 10.1006/bbrc.2002.6384. [DOI] [PubMed] [Google Scholar]
- 100.Little KY, Elmer LW, Zhong H, Scheys JO, Zhang L. Mol Pharmacol. 2002;61(2):436. doi: 10.1124/mol.61.2.436. [DOI] [PubMed] [Google Scholar]
- 101.Chen N, Reith ME. J Neurochem. 2008;105(3):910. doi: 10.1111/j.1471-4159.2007.05195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cowell RM, Kantor L, Hewlett GH, Frey KA, Gnegy ME. Eur J Pharmacol. 2000;389(1):59. doi: 10.1016/s0014-2999(99)00828-6. [DOI] [PubMed] [Google Scholar]
- 103.Gorentla BK, Vaughan RA. Neuropharmacology. 2005;49(6):759. doi: 10.1016/j.neuropharm.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 104.Cervinski MA, Foster JD, Vaughan RA. J Biol Chem. 2005;280(49):40442. doi: 10.1074/jbc.M501969200. [DOI] [PubMed] [Google Scholar]
- 105.Kahlig KM, Javitch JA, Galli A. J. Biol. Chem. 2004;279(10):8966. doi: 10.1074/jbc.M303976200. [DOI] [PubMed] [Google Scholar]
- 106.Sorkina T, Doolen S, Galperin E, Zahniser NR, Sorkin A. J Biol Chem. 2003;278(30):28274. doi: 10.1074/jbc.M210652200. [DOI] [PubMed] [Google Scholar]
- 107.Schmitt KC, Zhen J, Kharkar P, Mishra M, Chen N, Dutta AK, Reith ME. J Neurochem. 2008 Sep 11; doi: 10.1111/j.1471-4159.2008.05667.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wise RA, Bozarth MA. Psychol Rev. 1987;94(4):469. [PubMed] [Google Scholar]
- 109.Newman AH, Kulkarni S. Med Res Rev. 2002;22(5):429. doi: 10.1002/med.10014. [DOI] [PubMed] [Google Scholar]
- 110.Rothman RB, Glowa JR. Mol Neurobiol. 1995;11(1-3):1. doi: 10.1007/BF02740680. [DOI] [PubMed] [Google Scholar]
- 111.Glowa JR, Fantegrossi WE, Lewis DB, Matecka D, Rice KC, Rothman RB. J Med Chem. 1996;39(24):4689. doi: 10.1021/jm960551t. [DOI] [PubMed] [Google Scholar]
- 112.Vocci F, Ling W. Pharmacol Ther. 2005;108(1):94. doi: 10.1016/j.pharmthera.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 113.Zarbiv R, Grunewald M, Kavanaugh MP, Kanner BI. J Biol Chem. 1998;273(23):14231. doi: 10.1074/jbc.273.23.14231. [DOI] [PubMed] [Google Scholar]
- 114.Chen N, Trowbridge CG, Justice JB., Jr. J Pharmacol Exp Ther. 1999;290(3):940. [PubMed] [Google Scholar]
- 115.Ferrer JV, Javitch JA. Proc Natl Acad Sci U S A. 1998;95(16):9238. doi: 10.1073/pnas.95.16.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Reith ME, Berfield JL, Wang LC, Ferrer JV, Javitch JA. J Biol Chem. 2001;276(31):29012. doi: 10.1074/jbc.M011785200. [DOI] [PubMed] [Google Scholar]
- 117.Meltzer PC, Liang AY, Madras BK. J Med Chem. 1996;39(2):371. doi: 10.1021/jm950463t. [DOI] [PubMed] [Google Scholar]
- 118.Vaughan RA, Kuhar MJ. J Biol Chem. 1996;271(35):21672. doi: 10.1074/jbc.271.35.21672. [DOI] [PubMed] [Google Scholar]
- 119.Vaughan RA, Agoston GE, Lever JR, Newman AH. J Neurosci. 1999;19(2):630. doi: 10.1523/JNEUROSCI.19-02-00630.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vaughan RA, Sakrikar DS, Parnas ML, Adkins S, Foster JD, Duval RA, Lever JR, Kulkarni SS, Hauck-Newman A. J Biol Chem. 2007;282(12):8915. doi: 10.1074/jbc.M610633200. [DOI] [PubMed] [Google Scholar]
- 121.Parnas ML, Gaffaney JD, Zou MF, Lever JR, Newman AH, Vaughan RA. Mol Pharmacol. 2008;73(4):1141. doi: 10.1124/mol.107.043679. [DOI] [PubMed] [Google Scholar]
- 122.Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Nature. 2005;437(7056):215. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
- 123.Chen N, Zhen J, Reith ME. J Neurochem. 2004;89(4):853. doi: 10.1111/j.1471-4159.2004.02386.x. [DOI] [PubMed] [Google Scholar]
- 124.Chen N, Vaughan RA, Reith ME. J Neurochem. 2001;77(4):1116. doi: 10.1046/j.1471-4159.2001.00312.x. [DOI] [PubMed] [Google Scholar]
- 125.Loland CJ, Norregaard L, Gether U. J Biol Chem. 1999;274(52):36928. doi: 10.1074/jbc.274.52.36928. [DOI] [PubMed] [Google Scholar]
- 126.Loland CJ, Norregaard L, Litman T, Gether U. Proc Natl Acad Sci U S A. 2002;99(3):1683. doi: 10.1073/pnas.032386299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Loland CJ, Desai RI, Zou MF, Cao J, Grundt P, Gerstbrein K, Sitte HH, Newman AH, Katz JL, Gether U. Mol Pharmacol. 2008;73(3):813. doi: 10.1124/mol.107.039800. [DOI] [PubMed] [Google Scholar]
- 128.Desai RI, Kopajtic TA, Koffarnus M, Newman AH, Katz JL. J Neurosci. 2005;25(8):1889. doi: 10.1523/JNEUROSCI.4778-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wee S, Carroll FI, Woolverton WL. Neuropsychopharmacology. 2006;31(2):351. doi: 10.1038/sj.npp.1300795. [DOI] [PubMed] [Google Scholar]
- 130.Fleckenstein A, Volz TJ, Riddle EL, Gibb JW, Hanson GR. Annu Rev Pharmacol Toxicol. 2007;47:681. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- 131.Raiteri M, Cerrito F, Cervoni AM, Levi G. J Pharmacol Exp Ther. 1979;208(2):195. [PubMed] [Google Scholar]
- 132.Jones SR, Gainetdinov RR, Wightman RM, Caron MG. J Neurosci. 1998;18(6):1979. doi: 10.1523/JNEUROSCI.18-06-01979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kahlig KM, Binda F, Khoshbouei H, Blakely RD, McMahon DG, Javitch JA, Galli A. Proceedings of the National Academy of Sciences. 2005;102(9):3495. doi: 10.1073/pnas.0407737102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sitte HH, Huck S, Reither H, Boehm S, Singer EA, Pifl C. J Neurochem. 1998;71(3):1289. doi: 10.1046/j.1471-4159.1998.71031289.x. [DOI] [PubMed] [Google Scholar]
- 135.Liang NY, Rutledge CO. Biochem Pharmacol. 1982;31(6):983. doi: 10.1016/0006-2952(82)90332-x. [DOI] [PubMed] [Google Scholar]
- 136.Cass WA, Gerhardt GA, Gillespie K, Curella P, Mayfield RD, Zahniser NR. J Neurochem. 1993;61(1):273. doi: 10.1111/j.1471-4159.1993.tb03565.x. [DOI] [PubMed] [Google Scholar]
- 137.Zahniser NR, Larson GA, Gerhardt GA. J Pharmacol Exp Ther. 1999;289(1):266. [PubMed] [Google Scholar]
- 138.Fleckenstein AE, Gibb JW, Hanson GR. Eur J Pharmacol. 2000;406(1):1. doi: 10.1016/s0014-2999(00)00639-7. [DOI] [PubMed] [Google Scholar]
- 139.Eisch AJ, O’Dell SJ, Marshall JF. Synapse. 1996;22(3):217. doi: 10.1002/(SICI)1098-2396(199603)22:3<217::AID-SYN3>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 140.Fleckenstein AE, Haughey HM, Metzger RR, Kokoshka JM, Riddle EL, Hanson JE, Gibb JW, Hanson GR. Eur J Pharmacol. 1999;382(1):45. doi: 10.1016/s0014-2999(99)00588-9. [DOI] [PubMed] [Google Scholar]
- 141.Kokoshka JM, Vaughan RA, Hanson GR, Fleckenstein AE. Eur J Pharmacol. 1998;361(2-3):269. doi: 10.1016/s0014-2999(98)00741-9. [DOI] [PubMed] [Google Scholar]
- 142.Baucum AJ, 2nd, Rau KS, Riddle EL, Hanson GR, Fleckenstein AE. J Neurosci. 2004;24(13):3436. doi: 10.1523/JNEUROSCI.0387-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hanson GR, Rau KS, Fleckenstein AE. Neuropharmacology. 2004;47(Suppl 1):92. doi: 10.1016/j.neuropharm.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 144.Quinton MS, Yamamoto BK. Aaps J. 2006;8(2):E337. doi: 10.1007/BF02854904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sandoval V, Riddle EL, Ugarte YV, Hanson GR, Fleckenstein AE. J. Neurosci. 2001;21(4):1413. doi: 10.1523/JNEUROSCI.21-04-01413.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sandoval V, Hanson GR, Fleckenstein AE. Eur J Pharmacol. 2000;409(3):265. doi: 10.1016/s0014-2999(00)00871-2. [DOI] [PubMed] [Google Scholar]
- 147.Kahlig KM, Lute BJ, Wei Y, Loland CJ, Gether U, Javitch JA, Galli A. Mol Pharmacol. 2006;70(2):542. doi: 10.1124/mol.106.023952. [DOI] [PubMed] [Google Scholar]
- 148.Wei Y, Williams JM, Dipace C, Sung U, Javitch JA, Galli A, Saunders C. Mol Pharmacol. 2007;71(3):835. doi: 10.1124/mol.106.026351. [DOI] [PubMed] [Google Scholar]
- 149.Johnson LA, Guptaroy B, Lund D, Shamban S, Gnegy ME. J Biol Chem. 2005;280(12):10914. doi: 10.1074/jbc.M413887200. [DOI] [PubMed] [Google Scholar]
- 150.Holton KL, Loder MK, Melikian HE. Nat Neurosci. 2005;8(7):881. doi: 10.1038/nn1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Boudanova E, Navaroli DM, Melikian HE. Neuropharmacology. 2008;54(3):605. doi: 10.1016/j.neuropharm.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Stolerman IP, Jarvis MJ. Psychopharmacology (Berl) 1995;117(1):2. doi: 10.1007/BF02245088. [DOI] [PubMed] [Google Scholar]
- 153.Di Chiara G. European Journal of Pharmacology. 2000;393(1-3):295. doi: 10.1016/s0014-2999(00)00122-9. [DOI] [PubMed] [Google Scholar]
- 154.Balfour DJ. Curr Drug Targets CNS Neurol Disord. 2002;1(4):413. doi: 10.2174/1568007023339076. [DOI] [PubMed] [Google Scholar]
- 155.Mathieu-Kia AM, Kellogg SH, Butelman ER, Kreek MJ. Psychopharmacology (Berl) 2002;162(2):102. doi: 10.1007/s00213-002-1096-0. [DOI] [PubMed] [Google Scholar]
- 156.Balfour DJ. Respiration. 2002;69(1):7. doi: 10.1159/000049362. [DOI] [PubMed] [Google Scholar]
- 157.Clarke PB, Pert A. Brain Res. 1985;348(2):355. doi: 10.1016/0006-8993(85)90456-1. [DOI] [PubMed] [Google Scholar]
- 158.Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K, Changeux JP. Nature. 1998;391(6663):173. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- 159.Laviolette SR, van der Kooy D. Nat Rev Neurosci. 2004;5(1):55. doi: 10.1038/nrn1298. [DOI] [PubMed] [Google Scholar]
- 160.Anand R, Conroy WG, Schoepfer R, Whiting P, Lindstrom J. J Biol Chem. 1991;266(17):11192. [PubMed] [Google Scholar]
- 161.Klink R, de Kerchove d’Exaerde A, Zoli M, Changeux JP. J Neurosci. 2001;21(5):1452. doi: 10.1523/JNEUROSCI.21-05-01452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Azam L, McIntosh JM. J Pharmacol Exp Ther. 2005;312(1):231. doi: 10.1124/jpet.104.071456. [DOI] [PubMed] [Google Scholar]
- 163.Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC, Grady SR. Mol Pharmacol. 2004;65(6):1526. doi: 10.1124/mol.65.6.1526. [DOI] [PubMed] [Google Scholar]
- 164.Salminen O, Drapeau JA, McIntosh JM, Collins AC, Marks MJ, Grady SR. Mol Pharmacol. 2007;71(6):1563. doi: 10.1124/mol.106.031492. [DOI] [PubMed] [Google Scholar]
- 165.Scholze P, Orr-Urtreger A, Changeux JP, McIntosh JM, Huck S. Br J Pharmacol. 2007;151(3):414. doi: 10.1038/sj.bjp.0707236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gerhardt GA, Dwoskin LP, Zahniser NR. J Neurosci Methods. 1989;26(3):217. doi: 10.1016/0165-0270(89)90119-2. [DOI] [PubMed] [Google Scholar]
- 167.Cass WA, Gerhardt GA, Mayfield RD, Curella P, Zahniser NR. J Neurochem. 1992;59(1):259. doi: 10.1111/j.1471-4159.1992.tb08899.x. [DOI] [PubMed] [Google Scholar]
- 168.Varanda WA, Aracava Y, Sherby SM, VanMeter WG, Eldefrawi ME, Albuquerque EX. Mol Pharmacol. 1985;28(2):128. [PubMed] [Google Scholar]
- 169.Dwoskin LP, Crooks PA. J Pharmacol Exp Ther. 2001;298(2):395. [PubMed] [Google Scholar]
- 170.Drew AE, Werling LL. Schizophr Res. 2003;65(1):47. doi: 10.1016/s0920-9964(02)00500-5. [DOI] [PubMed] [Google Scholar]
- 171.Rapier C, Lunt GG, Wonnacott S. J Neurochem. 1988;50(4):1123. doi: 10.1111/j.1471-4159.1988.tb10582.x. [DOI] [PubMed] [Google Scholar]
- 172.Grady S, Marks MJ, Wonnacott S, Collins AC. J Neurochem. 1992;59(3):848. doi: 10.1111/j.1471-4159.1992.tb08322.x. [DOI] [PubMed] [Google Scholar]
- 173.Carr LA, Rowell PP, Pierce WM., Jr. Neurochem Res. 1989;14(6):511. doi: 10.1007/BF00964911. [DOI] [PubMed] [Google Scholar]
- 174.Yamashita H, Kitayama S, Zhang YX, Takahashi T, Dohi T, Nakamura S. Biochem Pharmacol. 1995;49(5):742. doi: 10.1016/0006-2952(94)00422-i. [DOI] [PubMed] [Google Scholar]
- 175.Middleton LS, Crooks PA, Wedlund PJ, Cass WA, Dwoskin LP. Synapse. 2007;61(3):157. doi: 10.1002/syn.20351. [DOI] [PubMed] [Google Scholar]
- 176.Parish CL, Nunan J, Finkelstein DI, McNamara FN, Wong JY, Waddington JL, Brown RM, Lawrence AJ, Horne MK, Drago J. Mol Pharmacol. 2005;68(5):1376. doi: 10.1124/mol.104.004820. [DOI] [PubMed] [Google Scholar]
- 177.Solanto MV. Behav Brain Res. 1998;94(1):127. doi: 10.1016/s0166-4328(97)00175-7. [DOI] [PubMed] [Google Scholar]
- 178.Volkow ND, Swanson JM. Am J Psychiatry. 2003;160(11):1909. doi: 10.1176/appi.ajp.160.11.1909. [DOI] [PubMed] [Google Scholar]
- 179.Swanson JM, Volkow ND. Neurosci Biobehav Rev. 2003;27(7):615. doi: 10.1016/j.neubiorev.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 180.Heron C, Costentin J, Bonnet JJ. Eur J Pharmacol. 1994;264(3):391. doi: 10.1016/0014-2999(94)00502-8. [DOI] [PubMed] [Google Scholar]
- 181.Volkow ND, Wang GJ, Fowler JS, Fischman M, Foltin R, Abumrad NN, Gatley SJ, Logan J, Wong C, Gifford A, Ding YS, Hitzemann R, Pappas N. Life Sci. 1999;65(1):PL7. doi: 10.1016/s0024-3205(99)00225-8. [DOI] [PubMed] [Google Scholar]
- 182.John CE, Jones SR. Neuropharmacology. 2007;52(8):1596. doi: 10.1016/j.neuropharm.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Dougherty DD, Bonab AA, Spencer TJ, Rauch SL, Madras BK, Fischman AJ. Lancet. 1999;354(9196):2132. doi: 10.1016/S0140-6736(99)04030-1. [DOI] [PubMed] [Google Scholar]
- 184.Dresel S, Krause J, Krause KH, LaFougere C, Brinkbaumer K, Kung HF, Hahn K, Tatsch K. Eur J Nucl Med. 2000;27(10):1518. doi: 10.1007/s002590000330. [DOI] [PubMed] [Google Scholar]
- 185.Szobot CM, Shih MC, Schaefer T, Junior N, Hoexter MQ, Fu YK, Pechansky F, Bressan RA, Rohde LA. Neuroimage. 2008;40(3):1195. doi: 10.1016/j.neuroimage.2007.12.050. [DOI] [PubMed] [Google Scholar]
- 186.Volkow ND, Fowler JS, Wang GJ, Ding YS, Gatley SJ. Eur Neuropsychopharmacol. 2002;12(6):557. doi: 10.1016/s0924-977x(02)00104-9. [DOI] [PubMed] [Google Scholar]
- 187.Volkow ND, Fowler JS, Wang G, Ding Y, Gatley SJ. J Atten Disord. 2002;6(Suppl 1):S31. doi: 10.1177/070674370200601s05. [DOI] [PubMed] [Google Scholar]
- 188.Kimmel HL, O’Connor JA, Carroll FI, Howell LL. Pharmacol Biochem Behav. 2007;86(1):45. doi: 10.1016/j.pbb.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wayment HK, Deutsch H, Schweri MM, Schenk JO. J Neurochem. 1999;72(3):1266. doi: 10.1046/j.1471-4159.1999.0721266.x. [DOI] [PubMed] [Google Scholar]
- 190.McElvain JS, Schenk JO. Biochem Pharmacol. 1992;43(10):2189. doi: 10.1016/0006-2952(92)90178-l. [DOI] [PubMed] [Google Scholar]
- 191.Wayment H, Meiergerd SM, Schenk JO. J Neurochem. 1998;70(5):1941. doi: 10.1046/j.1471-4159.1998.70051941.x. [DOI] [PubMed] [Google Scholar]
- 192.Wu Q, Reith ME, Kuhar MJ, Carroll FI, Garris PA. J Neurosci. 2001;21(16):6338. doi: 10.1523/JNEUROSCI.21-16-06338.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Volz TJ, Schenk JO. Synapse. 2005;58(2):72. doi: 10.1002/syn.20183. [DOI] [PubMed] [Google Scholar]
- 194.Volz TJ, Bjorklund NL, Schenk JO. Synapse. 2005;57(3):175. doi: 10.1002/syn.20161. [DOI] [PubMed] [Google Scholar]
- 195.Sandoval V, Riddle EL, Hanson GR, Fleckenstein AE. J Neurosci. 2002;22(19):8705. doi: 10.1523/JNEUROSCI.22-19-08705.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sandoval V, Riddle EL, Hanson GR, Fleckenstein AE. J Pharmacol Exp Ther. 2003;304(3):1181. doi: 10.1124/jpet.102.045005. [DOI] [PubMed] [Google Scholar]
- 197.Volz TJ, Farnsworth SJ, King JL, Riddle EL, Hanson GR, Fleckenstein AE. J Pharmacol Exp Ther. 2007;323(2):738. doi: 10.1124/jpet.107.126888. [DOI] [PubMed] [Google Scholar]
- 198.Brodie MS, Shefner SA, Dunwiddie TV. Brain Res. 1990;508(1):65. doi: 10.1016/0006-8993(90)91118-z. [DOI] [PubMed] [Google Scholar]
- 199.Mereu G, Gessa GL. Brain Res. 1985;360(1-2):325. doi: 10.1016/0006-8993(85)91249-1. [DOI] [PubMed] [Google Scholar]
- 200.Gonzales RA, Job MO, Doyon WM. Pharmacol Ther. 2004;103(2):121. doi: 10.1016/j.pharmthera.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 201.Yim HJ, Gonzales RA. Alcohol. 2000;22(2):107. doi: 10.1016/s0741-8329(00)00121-x. [DOI] [PubMed] [Google Scholar]
- 202.Di Chiara G, Imperato A. Proc Natl Acad Sci U S A. 1988;85(14):5274. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Grace AA. Addiction. 2000;95(Suppl 2):S119. doi: 10.1080/09652140050111690. [DOI] [PubMed] [Google Scholar]
- 204.Self DW, Nestler EJ. Annu Rev Neurosci. 1995;18:463. doi: 10.1146/annurev.ne.18.030195.002335. [DOI] [PubMed] [Google Scholar]
- 205.Hooks MS, Kalivas PW. Neuroscience. 1995;64(3):587. doi: 10.1016/0306-4522(94)00409-x. [DOI] [PubMed] [Google Scholar]
- 206.Tiihonen J, Kuikka J, Bergstrom K, Hakola P, Karhu J, Ryynanen OP, Fohr J. Nat Med. 1995;1(7):654. doi: 10.1038/nm0795-654. [DOI] [PubMed] [Google Scholar]
- 207.Schmidt LG, Harms H, Kuhn S, Rommelspacher H, Sander T. Am J Psychiatry. 1998;155(4):474. doi: 10.1176/ajp.155.4.474. [DOI] [PubMed] [Google Scholar]
- 208.Mash DC, Staley JK, Doepel FM, Young SN, Ervin FR, Palmour RM. Neuroreport. 1996;7(2):457. doi: 10.1097/00001756-199601310-00020. [DOI] [PubMed] [Google Scholar]
- 209.Savelieva KV, Caudle WM, Findlay GS, Caron MG, Miller GW. Alcohol Clin Exp Res. 2002;26(6):758. [PubMed] [Google Scholar]
- 210.Maiya R, Buck KJ, Harris RA, Mayfield RD. J Biol Chem. 2002;277(34):30724. doi: 10.1074/jbc.M204914200. [DOI] [PubMed] [Google Scholar]
- 211.Jiao X, Pare WP, Tejani-Butt SM. Brain Res. 2006;1073-1074:175. doi: 10.1016/j.brainres.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 212.Kiianmaa K, Nurmi M, Nykanen I, Sinclair JD. Pharmacol Biochem Behav. 1995;52(1):29. doi: 10.1016/0091-3057(95)00097-g. [DOI] [PubMed] [Google Scholar]
- 213.Yim HJ, Schallert T, Randall PK, Gonzales RA. Alcohol Clin Exp Res. 1998;22(2):367. [PubMed] [Google Scholar]
- 214.Sabeti J, Gerhardt GA, Zahniser NR. Neurosci Lett. 2003;343(1):9. doi: 10.1016/s0304-3940(03)00301-x. [DOI] [PubMed] [Google Scholar]
- 215.Wang Y, Palmer MR, Cline EJ, Gerhardt GA. Alcohol. 1997;14(6):593. doi: 10.1016/s0741-8329(97)00054-2. [DOI] [PubMed] [Google Scholar]
- 216.Lin AM, Chai CY. Brain Res. 1995;696(1-2):15. doi: 10.1016/0006-8993(95)00688-m. [DOI] [PubMed] [Google Scholar]
- 217.Robinson DL, Volz TJ, Schenk JO, Wightman RM. Alcohol Clin Exp Res. 2005;29(5):746. doi: 10.1097/01.alc.0000164362.21484.14. [DOI] [PubMed] [Google Scholar]
- 218.Budygin EA, Phillips PE, Robinson DL, Kennedy AP, Gainetdinov RR, Wightman RM. J Pharmacol Exp Ther. 2001;297(1):27. [PubMed] [Google Scholar]
- 219.Mayfield RD, Maiya R, Keller D, Zahniser NR. J Neurochem. 2001;79(5):1070. doi: 10.1046/j.1471-4159.2001.00656.x. [DOI] [PubMed] [Google Scholar]
- 220.Mathews TA, John CE, Lapa GB, Budygin EA, Jones SR. Synapse. 2006;60(4):288. doi: 10.1002/syn.20301. [DOI] [PubMed] [Google Scholar]
- 221.Jones SR, Mathews TA, Budygin EA. Synapse. 2006;60(3):251. doi: 10.1002/syn.20294. [DOI] [PubMed] [Google Scholar]
- 222.Budygin EA, John CE, Mateo Y, Daunais JB, Friedman DP, Grant KA, Jones SR. Synapse. 2003;50(3):266. doi: 10.1002/syn.10269. [DOI] [PubMed] [Google Scholar]
- 223.Vivian JA, Green HL, Young JE, Majerksy LS, Thomas BW, Shively CA, Tobin JR, Nader MA, Grant KA. Alcohol Clin Exp Res. 2001;25(8):1087. [PubMed] [Google Scholar]
- 224.Budygin EA, Oleson EB, Mathews TA, Lack AK, Diaz MR, McCool BA, Jones SR. Psychopharmacology (Berl) 2007;193(4):495. doi: 10.1007/s00213-007-0812-1. [DOI] [PubMed] [Google Scholar]
- 225.Svingos AL, Chavkin C, Colago EE, Pickel VM. Synapse. 2001;42(3):185. doi: 10.1002/syn.10005. [DOI] [PubMed] [Google Scholar]
- 226.Meshul CK, McGinty JF. Neuroscience. 2000;96(1):91. doi: 10.1016/s0306-4522(99)90481-5. [DOI] [PubMed] [Google Scholar]
- 227.Spanagel R, Herz A, Shippenberg TS. J Neurochem. 1990;55(5):1734. doi: 10.1111/j.1471-4159.1990.tb04963.x. [DOI] [PubMed] [Google Scholar]
- 228.Thompson AC, Zapata A, Justice JB, Jr., Vaughan RA, Sharpe LG, Shippenberg TS. J Neurosci. 2000;20(24):9333. doi: 10.1523/JNEUROSCI.20-24-09333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Fuentealba JA, Gysling K, Andres ME. Synapse. 2007;61(9):771. doi: 10.1002/syn.20424. [DOI] [PubMed] [Google Scholar]
- 230.Spanagel R, Herz A, Shippenberg TS. Proc Natl Acad Sci U S A. 1992;89(6):2046. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Chefer VI, Czyzyk T, Bolan EA, Moron J, Pintar JE, Shippenberg TS. J Neurosci. 2005;25(20):5029. doi: 10.1523/JNEUROSCI.0854-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Fuentealba JA, Gysling K, Magendzo K, Andres ME. J Neurosci Res. 2006;84(2):450. doi: 10.1002/jnr.20890. [DOI] [PubMed] [Google Scholar]
- 233.Heidbreder CA, Schenk S, Partridge B, Shippenberg TS. Synapse. 1998;30(3):255. doi: 10.1002/(SICI)1098-2396(199811)30:3<255::AID-SYN3>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 234.Fuentealba K. G. M. E. A. José Antonio. Synapse. 2007;61(9):771. doi: 10.1002/syn.20424. [DOI] [PubMed] [Google Scholar]
- 235.Chefer VI, Moron JA, Hope B, Rea W, Shippenberg TS. Neuroscience. 2000;101(3):619. doi: 10.1016/s0306-4522(00)00417-6. [DOI] [PubMed] [Google Scholar]
- 236.Gray AM, Rawls SM, Shippenberg TS, McGinty JF. J Neurochem. 1999;73(3):1066. doi: 10.1046/j.1471-4159.1999.0731066.x. [DOI] [PubMed] [Google Scholar]
- 237.Mello NK, Negus SS. Ann N Y Acad Sci. 2000;909:104. doi: 10.1111/j.1749-6632.2000.tb06678.x. [DOI] [PubMed] [Google Scholar]
- 238.Prisinzano TE, Tidgewell K, Harding WW. Aaps J. 2005;7(3):E592. doi: 10.1208/aapsj070361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Izenwasser S, Acri JB, Kunko PM, Shippenberg T. Synapse. 1998;30(3):275. doi: 10.1002/(SICI)1098-2396(199811)30:3<275::AID-SYN5>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 240.Shippenberg TS, Bals-Kubik R, Herz A. J Pharmacol Exp Ther. 1993;265(1):53. [PubMed] [Google Scholar]
- 241.Johnson SW, North RA. J Neurosci. 1992;12(2):483. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Piepponen TP, Honkanen A, Kivastik T, Zharkovsky A, Turtia A, Mikkola JA, Ahtee L. Pharmacol Biochem Behav. 1999;63(2):245. doi: 10.1016/s0091-3057(98)00241-x. [DOI] [PubMed] [Google Scholar]
- 243.Vihavainen T, Mijatovic J, Piepponen TP, Tuominen RK, Ahtee L. Behav Brain Res. 2006;173(1):85. doi: 10.1016/j.bbr.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 244.Shippenberg TS, Heidbreder C, Lefevour A. Eur J Pharmacol. 1996;299(1-3):33. doi: 10.1016/0014-2999(95)00852-7. [DOI] [PubMed] [Google Scholar]
- 245.Piazza PV, Le Moal ML. Annu Rev Pharmacol Toxicol. 1996;36:359. doi: 10.1146/annurev.pa.36.040196.002043. [DOI] [PubMed] [Google Scholar]
- 246.Piazza PV, Deminiere JM, Le Moal M, Simon H. Science. 1989;245(4925):1511. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- 247.Hooks MS, Jones GH, Smith AD, Neill DB, Justice JB., Jr. Pharmacol Biochem Behav. 1991;38(2):467. doi: 10.1016/0091-3057(91)90308-o. [DOI] [PubMed] [Google Scholar]
- 248.Hooks MS, Jones GH, Neill DB, Justice JB., Jr. Pharmacol Biochem Behav. 1992;41(1):203. doi: 10.1016/0091-3057(92)90083-r. [DOI] [PubMed] [Google Scholar]
- 249.Exner M, Clark D. Behav Pharmacol. 1993;4(1):47. [PubMed] [Google Scholar]
- 250.Pierce RC, Kalivas PW. Brain Res Brain Res Rev. 1997;25(2):192. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- 251.Klebaur JE, Bevins RA, Segar TM, Bardo MT. Behav Pharmacol. 2001;12(4):267. doi: 10.1097/00008877-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 252.Cain ME, Smith CM, Bardo MT. Psychopharmacology (Berl) 2004;176(2):129. doi: 10.1007/s00213-004-1870-2. [DOI] [PubMed] [Google Scholar]
- 253.Misslin R, Herzog F, Koch B, Ropartz P. Psychoneuroendocrinology. 1982;7(2-3):217. doi: 10.1016/0306-4530(82)90015-4. [DOI] [PubMed] [Google Scholar]
- 254.Cain ME, Dotson WF, Bardo MT. Exp Clin Psychopharmacol. 2006;14(3):389. doi: 10.1037/1064-1297.14.3.389. [DOI] [PubMed] [Google Scholar]
- 255.Benjamin J, Li L, Patterson C, Greenberg BD, Murphy DL, Hamer DH. Nat Genet. 1996;12(1):81. doi: 10.1038/ng0196-81. [DOI] [PubMed] [Google Scholar]
- 256.Wills TA, Vaccaro D, McNamara G. J Subst Abuse. 1994;6(1):1. doi: 10.1016/s0899-3289(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 257.Bailey JN, Breidenthal SE, Jorgensen MJ, McCracken JT, Fairbanks LA. Psychiatr Genet. 2007;17(1):23. doi: 10.1097/YPG.0b013e32801140f2. [DOI] [PubMed] [Google Scholar]
- 258.Hooks MS, Juncos JL, Justice JB, Jr., Meiergerd SM, Povlock SL, Schenk JO, Kalivas PW. J. Neurosci. 1994;14(10):6144. doi: 10.1523/JNEUROSCI.14-10-06144.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Chefer VI, Zakharova I, Shippenberg TS. J Neurosci. 2003;23(7):3076. doi: 10.1523/JNEUROSCI.23-07-03076.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Zhuang X, Oosting RS, Jones SR, Gainetdinov RR, Miller GW, Caron MG, Hen R. Proc Natl Acad Sci U S A. 2001;98(4):1982. doi: 10.1073/pnas.98.4.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Segal DS, Kuczenski R. J Pharmacol Exp Ther. 1987;242(3):917. [PubMed] [Google Scholar]
- 262.Segal DS, Kuczenski R. Psychopharmacol Bull. 1987;23(3):417. [PubMed] [Google Scholar]
- 263.Sabeti J, Gerhardt GA, Zahniser NR. J Pharmacol Exp Ther. 2002;302(3):1201. doi: 10.1124/jpet.102.035816. [DOI] [PubMed] [Google Scholar]
- 264.Sabeti J, Gerhardt GA, Zahniser NR. J Pharmacol Exp Ther. 2003;305(1):180. doi: 10.1124/jpet.102.047258. [DOI] [PubMed] [Google Scholar]
- 265.Briegleb SK, Gulley JM, Hoover BR, Zahniser NR. Neuropsychopharmacology. 2004 doi: 10.1038/sj.npp.1300536. [DOI] [PubMed] [Google Scholar]
- 266.Gulley JM, Hoover BR, Larson GA, Zahniser NR. Neuropsychopharmacology. 2003;28(12):2089. doi: 10.1038/sj.npp.1300279. [DOI] [PubMed] [Google Scholar]
- 267.Johnson LA, Furman CA, Zhang M, Guptaroy B, Gnegy ME. Neuropharmacology. 2005;49(6):750. doi: 10.1016/j.neuropharm.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 268.Robinson TE, Berridge KC. Brain Res Brain Res Rev. 1993;18(3):247. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 269.Covington HE, 3rd, Miczek KA. Psychopharmacology (Berl) 2001;158(4):388. doi: 10.1007/s002130100858. [DOI] [PubMed] [Google Scholar]
- 270.Vanderschuren LJ, De Vries TJ, Wardeh G, Hogenboom FA, Schoffelmeer AN. Eur J Neurosci. 2001;14(9):1533. doi: 10.1046/j.0953-816x.2001.01775.x. [DOI] [PubMed] [Google Scholar]
- 271.Merikangas KR, Stolar M, Stevens DE, Goulet J, Preisig MA, Fenton B, Zhang H, O’Malley SS, Rounsaville BJ. Arch Gen Psychiatry. 1998;55(11):973. doi: 10.1001/archpsyc.55.11.973. [DOI] [PubMed] [Google Scholar]
- 272.Leshner AI. Am J Med Genet. 2000;96(5):590. doi: 10.1002/1096-8628(20001009)96:5<590::aid-ajmg2>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 273.Kendler KS, Prescott CA, Myers J, Neale MC. Arch Gen Psychiatry. 2003;60(9):929. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- 274.Rhee SH, Hewitt JK, Young SE, Corley RP, Crowley TJ, Stallings MC. Arch Gen Psychiatry. 2003;60(12):1256. doi: 10.1001/archpsyc.60.12.1256. [DOI] [PubMed] [Google Scholar]
- 275.Jones GH, Marsden CA, Robbins TW. Psychopharmacologia. 1990;102(3):364. doi: 10.1007/BF02244105. [DOI] [PubMed] [Google Scholar]
- 276.Bowling SL, Rowlett JK, Bardo MT. Neuropharmacology. 1993;32(9):885. doi: 10.1016/0028-3908(93)90144-r. [DOI] [PubMed] [Google Scholar]
- 277.Smith JK, Neill JC, Costall B. Psychopharmacology. 1997;131(1):23. doi: 10.1007/s002130050261. [DOI] [PubMed] [Google Scholar]
- 278.Bardo MT, Bowling SL, Rowlett JK, Manderscheid P, Buxton ST, Dwoskin LP. Pharmacol Biochem Behav. 1995;51(2-3):397. doi: 10.1016/0091-3057(94)00413-d. [DOI] [PubMed] [Google Scholar]
- 279.Green TA, Cain ME, Thompson M, Bardo MT. Psychopharmacology (Berl) 2003;170(3):235. doi: 10.1007/s00213-003-1538-3. [DOI] [PubMed] [Google Scholar]
- 280.Schrijver NC, Bahr NI, Weiss IC, Wurbel H. Pharmacol Biochem Behav. 2002;73(1):209. doi: 10.1016/s0091-3057(02)00790-6. [DOI] [PubMed] [Google Scholar]
- 281.Larsson F, Winblad B, Mohammed AH. Pharmacol Biochem Behav. 2002;73(1):193. doi: 10.1016/s0091-3057(02)00782-7. [DOI] [PubMed] [Google Scholar]
- 282.Smith JK, Neill JC, Costall B. Psychopharmacology (Berl) 1997;131(1):23. doi: 10.1007/s002130050261. [DOI] [PubMed] [Google Scholar]
- 283.Renner MJ, Rosenzweig MR. Dev Psychobiol. 1986;19(4):303. doi: 10.1002/dev.420190403. [DOI] [PubMed] [Google Scholar]
- 284.Zimmermann A, Stauffacher M, Langhans W, Wurbel H. Behav Brain Res. 2001;121(1-2):11. doi: 10.1016/s0166-4328(00)00377-6. [DOI] [PubMed] [Google Scholar]
- 285.Bardo MT, Klebaur JE, Valone JM, Deaton C. Psychopharmacology (Berl) 2001;155(3):278. doi: 10.1007/s002130100720. [DOI] [PubMed] [Google Scholar]
- 286.Green TA, Cain ME, Thompson M, Bardo MT. Psychopharmacology (Berl) 2003 doi: 10.1007/s00213-003-1538-3. [DOI] [PubMed] [Google Scholar]
- 287.Schultz W, Tremblay L, Hollerman JR. Cereb. Cortex. 2000;10(3):272. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- 288.Gulley JM, Zahniser NR. European Journal of Pharmacology. 2003;479(1-3):139. doi: 10.1016/j.ejphar.2003.08.064. [DOI] [PubMed] [Google Scholar]
- 289.Cao YJ, Surowy CS, Puttfarcken PS. Neuropharmacology. 2005;48(1):72. doi: 10.1016/j.neuropharm.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 290.Cao YJ, Surowy CS, Puttfarcken PS. J Pharmacol Exp Ther. 2005;312(3):1298. doi: 10.1124/jpet.104.076794. [DOI] [PubMed] [Google Scholar]
- 291.Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA. Nat Neurosci. 2002;5(2):169. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- 292.Bezard E, Dovero S, Belin D, Duconger S, Jackson-Lewis V, Przedborski S, Piazza PV, Gross CE, Jaber M. J Neurosci. 2003;23(35):10999. doi: 10.1523/JNEUROSCI.23-35-10999.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Faherty CJ, Shepherd K. Raviie, Herasimtschuk A, Smeyne RJ. Brain Res Mol Brain Res. 2005;134(1):170. doi: 10.1016/j.molbrainres.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 294.Hyman C, Hofer M, Barde YA, Juhasz M, Yancopoulos GD, Squinto SP, Lindsay RM. Nature. 1991;350(6315):230. doi: 10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- 295.Nestler EJ, Carlezon WA., Jr. Biol Psychiatry. 2006;59(12):1151. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 296.Zhu J, Bruntz RC, Apparsundaram S, Dwoskin LP. Acta Pharmacol Sinica. 2006;(Supplement 1):116. [Google Scholar]