Abstract
We previously demonstrated that HIV infection is associated with peripheral and central lipoatrophy in women. We now describe the association of specific antiretroviral drugs (ARV) with body fat changes over a four-year period from 1999 to 2003. 775 HIV-positive and 205 HIV-negative women in the Women’s Interagency HIV Study with anthropometric measurements, weight, bioelectric impedance analysis and ARV collected semiannually were included in analysis. Exposure to ARV was defined as report of use for 3 consecutive semiannual study visits. The average 6–month change in weight, percent total body fat, and circumference measurements (i.e., hip, waist, chest, arm, and thigh) was compared between those exposed and those unexposed to the specific ARV for any of the same three consecutive visits. Weight, percent total body fat, and hip, waist, thigh, chest, and arm circumferences decreased in HIV-positive women, but increased in HIV-negative women on average for every six-month interval over the 4-year study period. Among the HIV-positive women, didanosine was the only ARV associated with decreases in circumference measures in the hip (−0.65 cm, 95% confidence interval [CI]: −1.18, −0.12), waist (−0.71 cm, 95% CI: −1.37, −0.04), chest (−0.71 cm, 95% CI: −1.17, −0.26), and arm (−0.23 cm, 95% CI: −0.48, 0.03; p = 0.08). These prospective data suggest that fat loss continues to predominate in HIV-positive women and exposure to didanosine for at least 12 months may further worsen fat loss.
INTRODUCTION
Since the first descriptions of an HIV-protease inhibitor (PI)-associated syndrome of lipodystrophy (defined as the presence of peripheral fat loss and central fat gain),1 subsequent studies have found an association between other classes of antiretroviral (ARV) drugs, specific ARV drugs,2–9 particularly stavudine,2,3,5–9 but also lamivudine,10,11 abacavir,2 nevirapine,9 and indinavir,3,9 and fat changes. An understanding of the association between individual ARV drugs and body fat changes is important, because these fat changes can be stigmatizing, particularly when involving fat loss in the face12,13 and have been associated with decreased quality of life14 and decreased ARV adherence.15 Treatment options including antiretroviral switch strategies as well as surgical implants and injectables to correct fat loss are being explored.16 However, these treatment options have been limited by incomplete recovery of fat loss several years after discontinuation of stavudine17 or are not long lasting as in the case of injectables.18 Factors other than antiretroviral drugs including CD4 nadir, older age, and HIV infection itself have also been implicated.4,9,19,20
The varying definitions used to describe the syndrome in different studies may partly explain the differences in the factors, particularly antiretroviral drug therapies, associated with fat changes. The assessment method to define body fat changes also varies and may be subject to bias. Some studies have used only self-report4,21–23 or only examination8 to define body fat changes, but self-report confirmed by examination has generally been used in most large HIV cohort studies.3,24–26 A few studies have also used direct measurement of fat.5,6,9,27
Most of these studies have included only HIV-infected men. Some have suggested that the fat changes seen in HIV-infected women may be different from those in men28; the effect of the ARV drugs on fat change in women remains unclear. One small cross-sectional study found that exposure to lamivudine was associated with lipodystrophy.10 In that study, lipodystrophy was defined as having both peripheral lipoatrophy and central lipohypertrophy using participant self-report confirmed by examination. Another small cross-sectional study in HIV-infected women found that stavudine was associated with less leg fat but not trunk fat when fat was directly measured using dual x-ray energy absorptiometry (DEXA) scans.29 These data suggest that the factors associated with fat loss should be studied separately from the factors associated with fat gain.
Furthermore, a cross-sectional study in HIV-infected men compared to controls demonstrated that regardless of the presence or absence of clinical peripheral lipoatrophy defined as self-report confirmed by examination, HIV-infected men had less subcutaneous fat in the trunk and limb (as measured by magnetic resonance imaging) than controls.9 The findings from that study suggest that fat changes should be studied using continuous measures of regional fat.
We therefore used data from a large prospective cohort of women with, and at risk for, HIV, to investigate the association of individual antiretroviral drugs with regional body fat changes over a 4–year period using anthropometry rather than a dichotomous outcome of lipodystrophy.
MATERIALS AND METHODS
Study population
The Women’s Interagency HIV Study (WIHS) is an ongoing multicenter prospective cohort study of the history of HIV-1 infection in U.S. women with and at risk for HIV. The WIHS design and baseline characteristics have been described previously.30,31 Informed consent was obtained from the participants in accordance with procedures and consent materials reviewed and approved by the committee on human experimentation at each of the collaborating institutions.
Of the 2628 women (2059 HIV-infected and 569 HIV-uninfected) enrolled in WIHS between October 1994 and November 1995, 1710 had their first anthropometric measurements taken at or after the tenth semiannual study visit starting on April 1999 (henceforth called the index visit). All participants from the two New York sites (631) were excluded from analyses, because anthropometry measurements were not consistently collected throughout the follow-up period at these two sites, leaving a total of 1079 women. At the four participating sites, anthropometry measurements were performed on average in more than 80% of the women. Of the 1079 women, 99 were excluded from the present study because of: (1) pregnancy in the prior 2 years (n = 13), (2) report of an AIDS-defining illness at the study visit (which could also cause body fat changes) (n = 59), and (3) reported steroid or growth hormone use (n = 27), leaving a sample of 980 participants, of whom 775 were HIV-infected and 205 were HIV-uninfected.
Study measurements
At each semiannual WIHS visit, participants were examined and interviewed using a standard protocol including questions regarding each ARV medication they had taken since their prior study visit and whether they were still taking each medication. Anthropometry measurements, including circumferences of the upper arm, chest, waist, hip and thigh regions were performed by trained clinicians, who completed a standardized training and certification program conducted by a single trainer prior to anthropometry data collection. Clinicians are recertified every two years in anthropometry. Anthropometry measurements were performed using the techniques described by the Third National Health and Examination Survey procedures.32 Because breast hypertrophy has been reported in some studies of HIV-infected women after starting effective ARV therapy, a chest circumference is obtained in the WIHS to serve as a marker of breast size. Anthropometry measures of the face are not performed in the WIHS, because a standardized measure of the face was not available. Percent total body fat was determined by bioelectric impedance analysis (RJL Systems, Detroit, MI) using the formulas of Kotler.33 Height was measured annually and weight semiannually. Plasma HIV-1 RNA level and CD4 count were determined, and nadir CD4 cell count calculated as the lowest CD4 available before the index visit. Clinical AIDS was defined as a report of an AIDS defining illness at or before the index visit (and did not include those with only a history of a CD4 less than 200 cells/mm3 or CD4% less than 14%).
Statistical methods
Differences in characteristics measured at the index visit by HIV status were compared using Wilcoxon rank test statistics for continuously distributed characteristics or χ2 tests for discrete characteristics.
Eleven ARV medications were studied: 5 nucleoside reverse transcriptase inhibitors (NRTI): zidovudine (AZT), stavudine (D4T), didanosine (DDI), lamivudine (3TC), and abacavir (ABC); 2 non-nucleoside reverse transcriptase inhibitors (NNRTI): efavirenz (EFV) and nevirapine (NVP), and 4 PIs: nelfinavir (NFV), indinavir (IDV), saquinavir (SQV), and ritonavir (RTV). ARV drugs not studied due to small numbers of women exposed during the study period included: zalcitabine, tenofovir, delavirdine, amprenavir, fosamprenavir, lopinavir/ritonavir, and atazanavir.
Exposure to each of the 11 ARV medications was defined as a report of ARV use for 3 consecutive visits (or at least 12 months of exposure). The 6-month change in anthropometry was determined over an 8-visit study interval from visit 10 to visit 18. Because collection of anthropometry measurements began at visit 10, the earliest visit where an ARV could be included in the 3 consecutive visit definition of exposure was at visit 8, which occurred from April 1998 through September 1998. The earliest 6–month change in anthropometry measurements that was included in the outcome occurred from visit 10 to visit 11. Because of the nature of our definition of exposure, a participant may or may not have still been on the drug at visit 11. For participants not on the drug at visit 11, the maximum time off the drug was just less than 6 months. For each sequential visit where the 3 consecutive visit definition of exposure for a particular ARV was met, the 6-month change in anthropometric measurements was calculated and averaged over the 4–year time period from visit 10 through 18 (which concluded in September 2003). The same participant may have contributed to the average more than once, because of being either on the ARV medication for more than 3 consecutive visits or stopping and then restarting the ARV medication at some later time point. This group of participants was compared to those not having taken the specific medication for any of the same 3 consecutive visits. To enhance contrasts, women who reported using the ARV for 1 or 2 visits in the 3-visit window were excluded from comparisons.
Six-month changes in body weight, percent total body fat, and circumference measurements (over the 9 semiannual study visits from visit 10 to visit 18) were analyzed using a generalized linear model with robust variance estimates to account for repeated measurements.34 Average changes were calculated by HIV status, and among the HIV-infected women, by exposure to each of the 11 ARV drugs. Factors potentially associated with fat distribution changes were adjusted for including age, nadir CD4, current smoking status, and moderate or high alcohol use (defined as more than 3 drinks per week). Analyses were also performed adjusting for current CD4, and results did not change. Analyses were conducted using SAS version 8.2 (SAS Institute, Cary NC).
RESULTS
Baseline characteristics of the 775 HIV-infected and 205 HIV-uninfected women included in this analysis are shown in Table 1. HIV-infected women weighed less than HIV-uninfected women and had lower percent total body fat. Arm, hip, and thigh circumference measurements were also smaller for HIV-infected than for HIV-uninfected women. Among HIV-infected women, 70% reported ARV use and 55% were on HAART. The body composition characteristics between HIV-infected women on HAART and those not on HAART, as well as between HIV-infected women on any ARV and those not on ARV at the index visit were similar (data not shown). Two thirds of those who were not on an ARV at the index visit started therapy during the study period.
Table 1.
Characteristics of Nine Hundred Eighty Women at the Index Visit, Between April and October 1999
| Characteristica | HIV-infected n = 775b | HIV-uninfected n = 205 | pc |
|---|---|---|---|
| Race, n (%):d | 0.07 | ||
| Black | 457 (59%) | 118 (58%) | |
| Hispanic | 147 (19%) | 52 (25%) | |
| White | 171 (22%) | 35 (17%) | |
| Age, years | 41 (36, 46) | 39 (34, 44) | <0.01 |
| Height, cm | 163 (157, 168) | 163 (157, 168) | 0.09 |
| Weight, kg | 68 (59, 81) | 76 (61, 90) | <0.01 |
| Body fat, % | 38 (32, 45) | 42 (34, 48) | <0.01 |
| Upper arm, cm | 29 (26, 32) | 30 (27, 34) | 0.02 |
| Chest, cm | 92 (86, 99) | 93 (84, 101) | 0.54 |
| Waist, cm | 89 (80, 99) | 91 (79, 102) | 0.37 |
| Hip, cm | 97 (90, 106) | 104 (94, 113) | <0.01 |
| Thigh, cm | 50 (45, 55) | 52 (47, 59) | <0.01 |
| Nadir CD4 cell count, cells/mm3 | 243 (128, 385) | 798 (601, 978) | <0.01 |
| HIV-1 RNA, copies/mL | 1400 (80, 16000) | NA | |
| Clinical AIDS, n (%) | 360 (46%) | NA | |
| ARV use at index visit, n (%) | 514 (70%) | NA | |
| HAARTe use at index visit, n (%) | 402 (55%) | NA |
Median (interquartile range), unless noted otherwise.
Number missing body composition data for HIV-infected (height = 45, weight = 63, body fat and fat free mass = 213, arms = 263, chest = 259, waist = 256, hip = 259, thigh = 257, HIV-1 RNA = 37, any ART and HAART use = 45) and HIV-negative (height = 16, weight = 19, body fat and fat free mass = 63, arms = 69, chest = 67, waist = 69, hip = 69, thigh = 67).
p value from Wilcoxon or χ2 test, as appropriate.
Thirty-three women reporting other racial groups were combined with black.
HAART is defined as taking: (a) two or more nucleoside reverse transcriptase inhibitors (NRTIs) in combination with at least one protease inhibitor (PI) or one non-nucleoside reverse transcriptase inhibitor 9NNRTI); (b) one NRTI in combination with at least one PI and at least one NNRTI; (c) a regimen containing ritonavir and saquinavir in combination with one NRTI and no NNRTIs; or (d) an abacavir-containing regimen of three or more NRTIs in the absence of both PIs and NNRTIs.
NA, not available; ART, antiretroviral therapy; HAART, highly active antiretroviral therapy.
Average 6-month changes in weight, percent total body fat, and circumferences are shown in Table 2. HIV-infected women demonstrated decreases on average in weight and percent total body fat, while HIV-uninfected women showed increases. Circumference measures showed similar patterns of decrease in HIV-infected and gain in HIV-uninfected women. Similar results were observed when circumference measures were expressed as the percent of the baseline circumference measure for each regional site in HIV-infected and uninfected women (data not shown).
Table 2.
Average Six-Month changes in Weight, Percent Body Fat, Fat Free mass, and Circumference Measurements for the Seven Hundred Seventy-Five HIV-Infected and Two Hundred Five HIV-Uninfected Women from April 1999–April 2003 and 95% Confidence Intervals and p-Values for the Difference (Δ) Between HIV-Infected and HIV-Uninfected are Shown
| HIV-positive | HIV-negative | Δ | 95% CI | p value | |
|---|---|---|---|---|---|
| Weight (kg) | −0.07 | +0.51 | 0.59 | 0.31, 0.86 | <0.01 |
| % Total body fat | −0.06 | +0.16 | 0.22 | −0.02, 0.47 | 0.08 |
| Fat free mass (kg) | −0.01 | +0.24 | 0.26 | 0.08, 0.43 | 0.01 |
| Circumference measures | |||||
| Hip (cm) | −0.18 | +0.27 | 0.45 | 0.16, 0.74 | <0.01 |
| Waist (cm) | −0.14 | +0.06 | 0.20 | 0.02, 0.39 | 0.03 |
| Thigh (cm) | −0.08 | +0.16 | 0.24 | 0.07, 0.42 | <0.01 |
| Chest (cm) | −0.12 | +0.07 | 0.20 | −0.01, 0.40 | 0.16 |
| Arm (cm) | −0.02 | +0.08 | 0.10 | −0.01, 0.21 | 0.08 |
NRTIs were the drug class participants most reported taking with 3TC reported in 45% of the 1852 person-visits, D4T in 24% of 1087 person-visits, AZT in 19% of 892 person-visits, ABC in 7% of 378 person-visits and DDI in 6% of 314 person-visits. The percentage of women using D4T halved during the study period. The percentage of person-visits with reported use of an individual NNRTI and an individual PI on three consecutive visits ranged from 7% to 9% for NNRTI and 5% to 10% for PI.
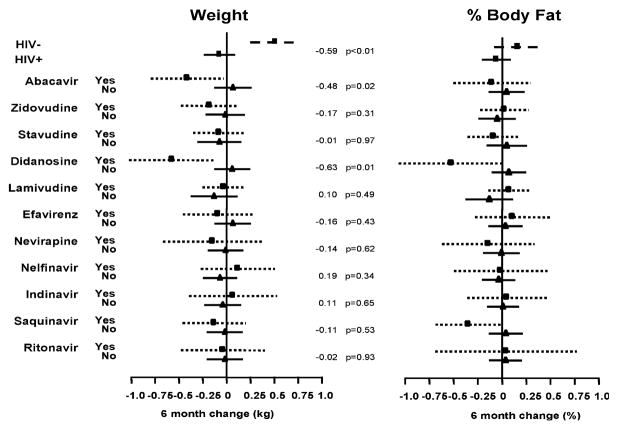
Figure 1 shows the estimate for the average 6-month change in weight and percent body fat, and the 95% confidence intervals (95% CI) for HIV-uninfected versus HIV-infected women, and for HIV-infected women reporting exposure to each of the 11 ARV medications on 3 consecutive visits versus those not taking the specific ARV in any of the same 3 visits.
FIG. 1.
Estimate for the average 6–month change in weight and percent body fat, and the 95% confidence intervals (95% CI) for HIV-infected versus HIV-uninfected women; and for HIV-infected women exposed and unexposed to each specific antiretroviral (ARV) medication, in three consecutive visits. The effect of the comparison (on kg and %, respectively), and the p values for these effects are shown to the right of each comparison group.
Participants taking DDI for 3 consecutive visits showed a significant reduction on weight (−0.63 kg; 95% CI: −1.10, −0.16; p = 0.01) and on percent body fat (−0.59%; 95% CI: −1.14, −0.04; p = 0.03). Participants taking ABC for 3 consecutive visits showed a significant reduction on weight only (−0.48 kg; 95% CI: −0.89, −0.08; p = 0.02) and participants taking SQV showed a significant reduction in percent body fat only (−0.39%, 95% CI: −0.74, −0.03; p = 0.04).
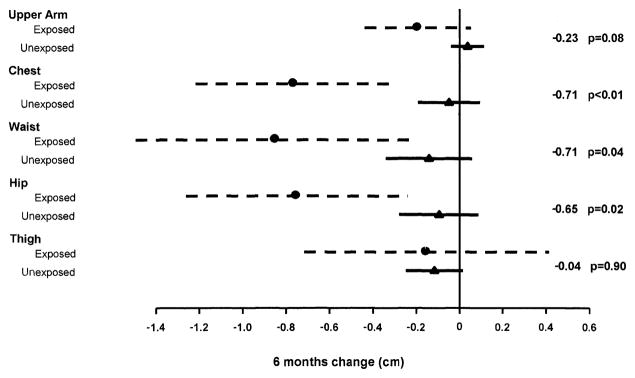
When the average 6-month change in circumference measurements were compared between those on an ARV drug and those not on the ARV drug for 3 consecutive visits, a difference was observed only for DDI. Figure 2 shows the estimate for the average 6-month change in regional circumference measures, and the 95% CI for HIV-infected women exposed to DDI on 3 consecutive visits versus those not taking DDI in any of the same 3 visits. HIV-infected women exposed to DDI demonstrated a significant average 6 month reduction in the hip, waist, and chest. A reduction was also observed in the arm although not statistically significant. There was no difference observed for the thigh. Similar findings were observed when women not taking any ARV for 3 consecutive visits were excluded from the unexposed group.
FIG. 2.
Estimate for the average 6–month change in regional body circumference, and the 95% confidence intervals (95% CI) for HIV-infected exposed and unexposed to DDI, in three consecutive visits. The effect of the comparison (cm), and the p values for these effects are shown to the right of each comparison group.
Because DDI is often used in conjunction with D4T, we analyzed the association of DDI and D4T use in body composition at baseline and during the study period. At the index visit in 1999, approximately 75% of women exposed to DDI for at least 3 consecutive visits, were also on D4T and approximately one third of women not exposed to DDI for at least 3 consecutive visits, were on D4T. During the study period from 1999 to 2003, a combination of D4T and DDI was reported in 55% of the 314 person-visits in which participants reported taking DDI on 3 consecutive visits. Among the 1087 person-visits where participants reported taking D4T on 3 consecutive visits, the combination of D4T and DDI was reported in only 16% of those person-visits.
At the index visit in 1999, there was no difference in the body composition characteristics between women exposed to DDI and those not exposed to DDI for 3 consecutive visits, including weight (67 kg versus 68 kg, respectively p = 0.56) and percent body fat (37% versus 39%, respectively, p = 0.23). However, the body composition characteristics were different between women on D4T and those not on D4T for 3 consecutive visits. D4T users had lower weight (66 kg versus 70 kg, p = 0.01), percent body fat (36% versus 40%, p < 0.01), and decreased arm (28 cm versus 29 cm, p = 0.03), hip (93 cm versus 98 cm, p < 0.01), and thigh circumferences (47 cm versus 50 cm, p < 0.01). During the 4-year study period, the average 6-month decrease in weight and percent body fat in women on a combination of D4T and DDI for 3 consecutive visits appeared greater than women on D4T without DDI, although not statistically different (data not shown).
When exploring the effect of at least 12 months of exposure to the other individual ARV medications on circumference measures, a significant 6-month average increase in the arms was observed in women exposed to D4T (0.20 cm increase, 95% CI: 0.07, 0.35; p < 0.01) and to NFV (0.18 cm increase, 95% CI: 0.03, 0.39; p = 0.04). No other significant differences were noted for circumference measures in patients on the other ARV medications.
DISCUSSION
Our findings show that over a 4-year time period, HIV-infected women in our cohort demonstrate on average a loss of weight and total percent body fat, while HIV-uninfected women on average demonstrate a gain of weight and total percent body fat. These results are consistent with previous published findings from our cohort after 2.5 years of assessment35; and suggest ongoing loss of weight and percent total body fat in HIV-infected women. However, the average 6-month decreases in weight and body fat in HIV-infected women during the study period were minimal compared to the large gains in weight and body fat in HIV-uninfected women, which might be expected with aging.
Using anthropometry, we find decreases in circumference measures at both peripheral body sites (arm, thigh, hip) and central body sites (chest, waist) in HIV-infected women, and increases in peripheral and central sites in HIV-uninfected women. Our findings are contrary to studies that suggest that lipohypertrophy predominates in HIV-infected women10,28,36,37 and highlight the importance of including an HIV-uninfected comparison group and using a continuous measure of regional body fat (instead of self-report and/or clinical exam of fat change).
We found evidence that the fat loss that appears to occur as a result of HIV infection in our women may be further worsened by the use of didanosine for at least 12 months. Didanosine was the only ARV consistently associated with fat loss when compared to those not on didanosine for the same time period. Our findings differ from other studies, which have been mainly in men. Most studies that have used direct measures of fat have found an association primarily between stavudine and fat loss5,6,9,27,29; one study also found an association between indinavir and fat loss.9 Some have suggested that the combination of didanosine and stavudine is particularly associated with fat loss.6,38 Given that stavudine and didanosine share several common adverse effects including pancreatitis, peripheral neuropathy, and the syndrome of lactic acidosis and steatosis, it is plausible that didanosine may also be associated with fat loss. In our cohort, we found that participants who took didanosine for 3 consecutive visits were also on stavudine more than half the time. However, use of stavudine and the other ARV drugs studied separately was not statistically associated with decreases in measures of body fat.
There may be two possible reasons for the lack of an effect of stavudine in our cohort. First, significant amounts of fat loss as a result of stavudine use may have occurred prior to the start of our study. We found that women, who were exposed to stavudine in 1999 when circumference measurements were first performed in our cohort, already had lower arm, hip, and thigh circumferences than those not exposed to stavudine. Second, use of stavudine in our cohort peaked in 1998 and declined to half the peak level during the study period.
A primary limitation of our study is that we did not use direct measures of fat such as DXA and magnetic resonance imaging (MRI). Anthropometry however provides a continuous measure of fat change and is less subjective than self-report and clinical exam, which has generally been used in large cohort studies to describe lipodystrophy. To minimize variability in anthropometric measurements in our cohort, all clinicians performing anthropometry in the WIHS were trained and certified with re-certification performed every 2 years. Anthropometry provides a more cost effective tool than the other imaging modalities in the measurement of fat changes over time and in relation to starting and switching antiretroviral therapies. We did not adjust for multiple comparisons in these analyses, and so some of the effects observed could be attributed to chance. However, we do not believe our observation of an association between didanosine and fat loss was caused by chance because didanosine was the only drug that showed an effect in nearly every body site and measurement.
In conclusion, our findings suggest that fat loss in HIV-infected women predominates when compared to HIV-uninfected women. Exposure to the nucleoside analog didanosine for at least 12 months compared to those not on didanosine for a similar time period appears to further worsen fat loss in our cohort of women during a 4-year time period. While our results regarding didanosine are intriguing, one should temper the present findings with the possibilities that the results are a chance finding or that there remains uncontrolled confounding. To ameliorate these concerns independent studies are needed.
Acknowledgments
Data in this manuscript were collected by the WIHS Collaborative Study Group with centers (Principal Investigators) at New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, New York (Howard Minkoff); Washington DC Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt); Los Angeles County/Southern California Consortium (Alexandra Levine); Chicago Consortium (Mardge Cohen); Data Coordinating Center (Stephen Gange). The WIHS is funded by the National Institute of Allergy and Infectious Diseases, with supplemental funding from the National Cancer Institute, the National Institute of Child Health & Human Development, The National Institute on Drug Abuse, and the National Institute of Dental and Craniofacial Research (U01-AI-35004, U01-AI-31834, U01-AI-34994, U01-AI-34989, U01-HD-32632, U01-AI-34993, U01-AI-42590). Funding is also provided by the National Center for Research Resources (M01-RR00071, M01-RR00079, and M01-RR00083).
References
- 1.Carr A, Samaras K, Burton S, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998;12:F51–58. doi: 10.1097/00002030-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Bernasconi E, Boubaker K, Junghans C, et al. Abnormalities of body fat distribution in HIV-infected persons treated with antiretroviral drugs. J Acquir Immune Defic Syndr. 2002;31:50–55. doi: 10.1097/00126334-200209010-00007. [DOI] [PubMed] [Google Scholar]
- 3.Galli M, Cozzi Lepri A, Ridolfo AL, et al. Incidence of adipose tissue alterations in first-line antiretroviral therapy—The LipoICoNa study. Arch Intern Med. 2002;162:2621–2628. doi: 10.1001/archinte.162.22.2621. [DOI] [PubMed] [Google Scholar]
- 4.Heath K, Hogg R, Singer J, Chan K, O’Shaughnessy M, Montaner J. Antiretroviral treatment patterns and incident HIV-associated morphologic and lipid abnormalities in a population-based cohort. J Acquir Immune Defic Syndr. 2002;30:440–447. doi: 10.1097/00042560-200208010-00010. [DOI] [PubMed] [Google Scholar]
- 5.Mallal SA, John M, Moore CB, James IR, McKinnon EJ. Contribution of nucleoside analogue reverse transcriptase inhibitors to subcutaneous fat wasting in patients with HIV infection. AIDS. 2000;14:1309–1316. doi: 10.1097/00002030-200007070-00002. [DOI] [PubMed] [Google Scholar]
- 6.Mallon P, Miller J, Cooper D, Carr A. Prospective evaluation of the effects of antiretroviral therapy on body composition in HIV-1 infected men starting therapy. AIDS. 2003;17:971–979. doi: 10.1097/00002030-200305020-00005. [DOI] [PubMed] [Google Scholar]
- 7.Saint-Marc T, Partisani M, Poizot-Martin I, et al. A syndrome of peripheral fat wasting (lipodystrophy) in patients receiving long-term nucleoside analogue therapy. AIDS. 1999;13:1659–1667. doi: 10.1097/00002030-199909100-00009. [DOI] [PubMed] [Google Scholar]
- 8.Saves M, Raffi F, Capeau J, et al. Factors related to lipodystrophy and metabolic alterations in patients with human immunodeficiency virus infection receiving highly active antiretroviral therapy. Clin InfectDis. 2002;34:1397–1405. doi: 10.1086/339866. [DOI] [PubMed] [Google Scholar]
- 9.FRAM Study Investigators. Fat distribution in men with HIV Infection. J Acquir Immune Defic Syndr. 2005;40:121–131. doi: 10.1097/01.qai.0000182230.47819.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gervasoni C, Ridolfo AL, Trifiro G, et al. Redistribution of body fat in HIV-infected women undergoing combined antiretroviral therapy. AIDS. 1999;13:465–471. doi: 10.1097/00002030-199903110-00004. [DOI] [PubMed] [Google Scholar]
- 11.Heath KV, Hogg RS, Chan KJ, et al. Lipodystrophy-associated morphological, cholesterol and triglyceride abnormalities in a population-based HIV/AIDS treatment database. AIDS. 2001;15:231–239. doi: 10.1097/00002030-200101260-00013. [DOI] [PubMed] [Google Scholar]
- 12.Blanch J, Rousaud A, Martinez E, et al. Factors associated with severe impact of lipodystrophy on the quality of life of patients infected with HIV-1. Clin Infect Dis. 2004;38:1464–1470. doi: 10.1086/383573. [DOI] [PubMed] [Google Scholar]
- 13.Hawkins T. Appearance-related side effects of HIV-1 treatment. AIDS Patient Care STDS. 2006;20:6–18. doi: 10.1089/apc.2006.20.6. [DOI] [PubMed] [Google Scholar]
- 14.Corless IB, Kirksey KM, Kemppainen J, et al. Lipodystrophy-associated symptoms and medication adherence in HIV/AIDS. AIDS Patient Care STDs. 2005;19:577–586. doi: 10.1089/apc.2005.19.577. [DOI] [PubMed] [Google Scholar]
- 15.Ammassari A, Murri R, Pezzotti P, et al. Self-reported symptoms and medication side effects influence adherence to highly active antiretroviral therapy in persons with HIV infection. J Acquir Immune Defic Syndr. 2001;28:445–449. doi: 10.1097/00042560-200112150-00006. [DOI] [PubMed] [Google Scholar]
- 16.Engelhard P. Correction options for lipoatrophy in HIV-infected patients. AIDS Patient Care STDs. 2006;20:151–160. doi: 10.1089/apc.2006.20.151. [DOI] [PubMed] [Google Scholar]
- 17.Martin A, Smith DE, Carr A, et al. Reversibility of lipoatrophy in HIV-infected patients 2 years after switching from a thymidine analogue to abacavir: The MITOX Extension Study. AIDS. 2004;18:1029–1036. doi: 10.1097/00002030-200404300-00011. [DOI] [PubMed] [Google Scholar]
- 18.Lafaurie M, Dolivo M, Porcher R, Rudant J, Madelaine I, Molina JM. Treatment of facial lipoatrophy with intradermal injections of polylactic acid in HIV-infected patients. J Acquir Immune Defic Syndr. 2005;38:393–398. doi: 10.1097/01.qai.0000152834.02912.98. [DOI] [PubMed] [Google Scholar]
- 19.Kotler DP, Rosenbaum K, Wang J, Pierson RN. Studies of body composition and fat distribution in HIV-infected and control subjects. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:228–237. doi: 10.1097/00042560-199903010-00003. [DOI] [PubMed] [Google Scholar]
- 20.Lichtenstein KA, Ward DJ, Moorman AC, et al. Clinical assessment of HIV-associated lipodystrophy in an ambulatory population. AIDS. 2001;15:1389–1398. doi: 10.1097/00002030-200107270-00008. [DOI] [PubMed] [Google Scholar]
- 21.Hadigan C, Miller K, Corcoran C, Anderson E, Basgoz N, Grinspoon S. Fasting hyperinsulinemia and changes in regional body composition in human immunodeficiency virus-infected women. J Clin Endocrinol Metab. 1999;84:1932–1937. doi: 10.1210/jcem.84.6.5738. [DOI] [PubMed] [Google Scholar]
- 22.Madge S, Kinloch-de-Loes S, Mercey D, Johnson MA, Weller IV. Lipodystrophy in patients naive to HIV protease inhibitors. AIDS. 1999;13:735–737. doi: 10.1097/00002030-199904160-00020. [DOI] [PubMed] [Google Scholar]
- 23.Mercie P, Viallard JF, Thiebaut R, et al. Efavirenz-associated breast hypertrophy in HIV-infection patients. AIDS. 2001;15:126–129. doi: 10.1097/00002030-200101050-00021. [DOI] [PubMed] [Google Scholar]
- 24.Carr A, Samaras K, Thorisdottir A, Kaufmann GR, Chisholm DJ, Cooper DA. Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor- associated lipodystrophy, hyperlipidaemia, and diabetes mellitus: A cohort study. Lancet. 1999;353:2093–2099. doi: 10.1016/S0140-6736(98)08468-2. [DOI] [PubMed] [Google Scholar]
- 25.Lichtenstein K, Delaney K, Armon C, et al. Incidence of and risk factors for lipoatrophy (abnormal fat loss) in ambulatory HIV-1 infected patients. J Acquir Immune Defic Syndr. 2003;32:48–56. doi: 10.1097/00126334-200301010-00007. [DOI] [PubMed] [Google Scholar]
- 26.Martinez E, Mocroft A, Garcia-Viejo MA, et al. Risk of lipodystrophy in HIV-1–infected patients treated with protease inhibitors: A prospective cohort study. Lancet. 2001;357:592–598. doi: 10.1016/S0140-6736(00)04056-3. [DOI] [PubMed] [Google Scholar]
- 27.Saint-Marc T, Partisani M, Poizot-Martin I, et al. Fat distribution evaluated by computed tomography and metabolic abnormalities in patients undergoing anti-retroviral therapy: Preliminary results of the LIPOCO study. AIDS. 2000;14:37–49. doi: 10.1097/00002030-200001070-00005. [DOI] [PubMed] [Google Scholar]
- 28.Galli M, Veglia F, Angarano G, et al. Gender Differences in antiretroviral drug-related adipose tissue alterations. J Acquir Immune Defic Syndr. 2003;34:58–56. doi: 10.1097/00126334-200309010-00008. [DOI] [PubMed] [Google Scholar]
- 29.Mulligan K, Anastos K, Justman J, et al. Fat distribution in HIV-infected women in the United States: DEXA substudy in the Women’s Interagency HIV Study. J Acquir Immune Defic Syndr. 2005;38:18–22. doi: 10.1097/00126334-200501010-00004. [DOI] [PubMed] [Google Scholar]
- 30.Bacon MC, von Wyl V, Alden C, et al. The Women’s Interagency HIV Study: An observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol. 2005;12:1013–1019. doi: 10.1128/CDLI.12.9.1013-1019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barkan SE, Melnick SL, Preston-Martin S, et al. The Women’s Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998;9:117–125. [PubMed] [Google Scholar]
- 32.National Center for Health Statistics. The Third National Health and Examination Survey Reference Manuals and Reports [CD-ROM] 1996 [Google Scholar]
- 33.Kotler DP, Burastero S, Wang J, Pierson RN., Jr Prediction of body cell mass, fat-free mass, and total body water with bioelectrical impedance analysis: Effects of race, sex, and disease. Am J Clin Nutr. 1996;64:489S–497S. doi: 10.1093/ajcn/64.3.489S. [DOI] [PubMed] [Google Scholar]
- 34.Diggle P, Heagerty P, Liang K, Zeger S. Analysis of Longitudinal Data. 2. Oxford: Oxford University Press; 2002. [Google Scholar]
- 35.Tien PC, Cole SR, Williams CM, et al. Incidence of lipoatrophy and lipohypertrophy in the Women’s Interagency HIV Study. J Acquir Immune Defic Syndr. 2003;34:461–466. doi: 10.1097/00126334-200312150-00003. [DOI] [PubMed] [Google Scholar]
- 36.Dong KL, Bausserman LL, Flynn MM, et al. Changes in body habitus and serum lipid abnormalities in HIV-positive women on highly active antiretroviral therapy (HAART) J Acquir Immune Defic Syndr. 1999;21:107–113. [PubMed] [Google Scholar]
- 37.Herry I, Bernard L, de Truchis P, Perronne C. Hypertrophy of the breasts in a patient treated with indinavir. Clin Infect Dis. 1997;25:937–938. doi: 10.1086/597649. [DOI] [PubMed] [Google Scholar]
- 38.Dube MP, Zackin R, Tebas P, et al. Prospective study of regional body composition in antiretroviral-naive subjects randomized to receive zidovudine+lamivudine or didanosine+stavudine combined with nelfinavir, efavirenz, or both: A5005s, a study of ACTG 384. Antivir Ther. 2002:L18. [Google Scholar]