SUMMARY
Ubiquitin (Ub) is an essential protein found in all eukaryotic cells and plays important roles in a variety of cellular functions including germ cell development. We have previously reported that targeted disruption of the polyubiquitin gene Ubb results in male and female infertility in Ubb−/− mice, with germ cells arrested at meiotic prophase I. Although reduced Ub levels in germ cells are believed to be responsible for the fertility defect in Ubb−/− mice, it is still unclear how reduced Ub levels result in sterility. Here we describe the results of a microarray analysis of the murine testicular transcriptome, which demonstrates dramatically altered gene expression patterns in Ubb−/− mice, possibly related to reduced levels of histone 2A (H2A) ubiquitylation. We find that large numbers of genes related to fertility, metabolism, transcription, and the ubiquitin–proteasome system (UPS) are misregulated in Ubb−/− mice. Such wide-ranging alterations in gene expression suggest that loss of the Ubb gene does not mimic a single-gene defect phenotype, but instead may affect gene expression more globally. These dramatic changes in gene expression could, at least in part, contribute to the complex fertility and metabolic phenotypes seen in these mice.
INTRODUCTION
The stress-regulated polyubiquitin genes Ubb and Ubc, along with the constitutive monomeric ubiquitin (Ub) ribosomal fusion genes, encode Ub, one of the most conserved eukaryotic proteins known (Wiborg et al., 1985; Finley et al., 1989). Ub is required not only for ATP-dependent proteasomal degradation of abnormal proteins or normal proteins with a rapid turnover, but also for mediation of numerous signaling functions in cells (Hochstrasser, 1996; Hershko and Ciechanover, 1998). The attachment of multiple Ub moieties to proteins, or polyubiquitylation, has diverse functions including proteasomal degradation, kinase activation, and DNA repair (Pickart and Fushman, 2004). Monoubiquitylation, on the other hand, is involved primarily in endocytosis and histone modification (Hicke, 2001).
The polyubiquitin gene Ubb consists of three or four tandem repeats of Ub-coding sequences. Proper regulation of Ubb is important for normal brain function, as a frameshift mutation of this gene has been shown to generate an aberrant form of Ub found in the cerebral cortices of Alzheimer’s and Down syndrome patients (van Leeuwen et al., 1998). Ubb is also required for fertility in both male and female mice (Ryu et al., 2008b). We have previously generated mice with a targeted deletion of Ubb, and have shown that male Ubb−/− mice have an arrest of spermatogenesis during the pachytene stage and do not produce spermatozoa, leading to infertility (Ryu et al., 2008b). The infertility phenotype of Ubb−/− mice likely arises from insufficient Ub production since Ubb−/− testes have dramatically reduced Ub levels (50% of wild-type levels at 1 month) (Ryu et al., 2008b). In addition to the fertility defect, loss of Ubb leads to obesity and sleep abnormalities in mice (Ryu et al., 2008a, 2010). These metabolic and sleep phenotypes are correlated with degeneration or dysfunction of neurons in the hypothalamus, and total Ub levels are also reduced in this brain region (Ryu et al., 2008a).
Histones are the most abundant ubiquitylated proteins in mammals (West and Bonner, 1980a,b), therefore, histone ubiquitylation could be affected as a result of reduced Ub levels in Ubb−/− mice. Although H2B is the main ubiquitylated histone in yeast (Hicke, 2001), H2A is the main ubiquitylated histone in mammals, with 5–15% of all H2A existing as monoubiquitylated H2A (Jason et al., 2002). This modification has been linked to gene repression in both Drosophila (Wang et al., 2004; Cao et al., 2005) and mammals (Minsky and Oren, 2004). It has also been shown that histone ubiquitylation is essential for eukaryotic gene regulation (Emre and Berger, 2004; Cao et al., 2005) and is critical for meiosis in yeast (Robzyk et al., 2000).
Meiosis in mammals may also rely on histone ubiquitylation; the pachytene stage of spermatogenesis in mice is associated with a dramatic upregulation of gene transcription (Shima et al., 2004), a process that may require extensive chromatin remodeling and chromatin modifications, including histone ubiquitylation. Therefore, insufficient Ub in Ubb−/− testes may prevent normal histone ubiquitylation and thus alter gene expression during development and spermatogenesis. Abnormal gene regulation in the testes may very well underlie the fertility defect in Ubb−/− mice. In this study, we have conducted a microarray analysis of testicular RNA from wild-type and Ubb−/− mice to assess the effects of reduced Ub availability on gene expression patterns. We find that several genes related to fertility, metabolism, transcription, and the ubiquitin–proteasome system (UPS) are misregulated in Ubb−/− testes at 7 and/or 14 days. In addition, ubiquitylated levels of histone H2A are significantly reduced in Ubb−/− testes. These findings potentially suggest an important role for histone ubiquitylation in mammalian fertility.
RESULTS
Overall Patterns
To test if gene expression patterns are altered in Ubb−/− mice, microarray analysis was performed on testicular lysates from male wild-type and Ubb−/− mice at 7 and 14 days after birth. We limited our analysis to these early time points because pachytene spermatocytes (which appear at 14 days postpartum) in Ubb−/− testes eventually undergo apoptosis (histologically apparent at 19 days) and round spermatids never form (Ryu et al., 2008b). Thus, later time points in development may display abnormal expression patterns not wholly related to the spermatogenesis defect.
Two striking observations emerge from a heat map representation of the data (Fig. 1A). First, more genes are upregulated in the absence of Ubb than are downregulated (yellow represents upregulation, whereas blue represents downregulation). This difference is mainly due to differential expression at 14 days; at 7 days, equal numbers of unique transcripts are up- and downregulated in wild-type and Ubb−/− testes (25 each), but at 14 days, the number of upregulated transcripts in Ubb−/− testes is more than three times the number of downregulated transcripts (272 versus 83) (Fig. 1B).
Figure 1.
Up- or downregulation of genes in Ubb−/− testes. A: Heat map representation of the gene expression profile in Ubb−/− testes at 7 and 14 days. Yellow represents upregulated genes in Ubb−/− versus wild type testes (more than two-fold increase), blue represents downregulated genes in Ubb−/− versus wild type testes (more than two-fold decrease, i.e., less than 0.5-fold increase), and black represents no significant change in gene expression in Ubb−/− versus wild type testes (less than two-fold increase, but more than 0.5-fold increase). The majority of genes are differentially regulated either at 7 or 14 days; that is, only five unique genes, which include Ubc and three obesity-related genes, are upregulated at both 7 and 14 days, and two unique genes, which include Ubb, are downregulated at both 7 and 14 days. Regulatory patterns of unique transcripts in Ubb−/− testes are shown in the box. B: Number of unique up- or downregulated transcripts in Ubb−/− testes at 7 and 14 days. Equal numbers of transcripts are up- and downregulated at 7 days, whereas the number of upregulated transcripts is three times that of the number of downregulated transcripts at 14 days.
Second, the overwhelming majority of genes (385 genes) are differentially (up- or down-) regulated at either 7 days or at 14 days, but not at both days (Fig. 1A). The reason for this pattern may have to do with the unique pattern of gene expression during spermatogenesis (Shima et al., 2004). It has been reported that certain groups of genes in the testes are highly expressed during the first week of development but are repressed by the end of the second week, whereas another, extremely large, group of genes is dramatically upregulated only after the second week of life (Shima et al., 2004). Strikingly, there is not much overlap between these groups of genes (Shima et al., 2004). Thus, it is possible that many of the genes expressed at 7 days are simply not expressed at 14 days, accounting for the fact that these genes are not misregulated at both 7 and 14 days.
Only ten genes (Ubc, Cfd, Fabp4, Car3, Adipoq, Adi1, Ubb, Fastkd2, Ggtla1, and E2f5) are differentially regulated between wild-type and Ubb−/− mice at both 7 and 14 days. Specifically, five genes including Ubc and certain obesity-related genes are upregulated at both 7 and 14 days (Fig. 2A–D), two genes including Ubb are downregulated at both 7 and 14 days (Fig. 2A–D), and, to our surprise, three genes are oppositely regulated at these different time points.
Figure 2.
Fold change of gene expression in Ubb−/− testes. A,B: Fold change of polyubiquitin gene (Ubb and Ubc) expression in Ubb−/− testes at 7 days (A) and at 14 days (B). Negative fold-change indicates decreased gene expression (i.e., a −2-fold change is the same as 50% reduced gene expression in Ubb−/− versus wild type testes). The fold change for Ubb* is based on the 1453723_x_at probe set and for Ubb** is based on the 1435643_x_at probe set. The difference between these probe sets is described in Supplementary Results and Supplementary Table 1. C: Fold change of selected gene expression in Ubb−/− testes at 7 days. Fertility or obesity-related genes are underlined. Although equal numbers of genes are up- or downregulated at 7 days, only the selected genes described in the text are shown. Details of each gene are described in the text and Supplementary Table 2. D: Fold change of selected gene expression in Ubb−/− testes at 14 days. Fertility, transcription (general or testis-specific), UPS, or obesity-related genes are underlined. Only the selected genes described in the text are shown. Details of each gene are described in the text and Supplementary Tables 3–5.
Difficulty of Accurate Polyubiquitin Gene Expression Measurements Due to the Nonunique Nature of Probe Sets
Not surprisingly, Ubb is downregulated in Ubb−/− testes at both 7 and 14 days (Fig. 2A,B). Unexpectedly, the microarray data show that Ubb−/− testes have some Ubb expression at both time points, although mRNA expression data clearly demonstrate a lack of Ubb transcripts in Ubb−/− mice (Ryu et al., 2008b). This paradoxical result may be due to the fact that the three Ubb probe sets on the Affymetrix chip share sequence similarity to other ubiquitin genes (see Supplementary Results and Supplementary Table 1). Indeed, when we quantify Ubb transcripts from Ubb−/− testes by quantitative real-time RT-PCR, we fail to detect transcripts (Supplementary Fig. 1).
Ubc is upregulated roughly three-fold at 7 days (Fig. 2A), consistent with quantitative RT- PCR results demonstrating a significant upregulation of Ubc in testes from adult Ubb−/− mice (Ryu et al., 2008b). At 14 days, Ubc is upregulated more than three-fold (Fig. 2B and Supplementary Table 5). Unlike Ubc, Uba52 is not upregulated at either 7 or 14 days, also consistent with previous quantitative RT-PCR results (Ryu et al., 2008b). It should be noted that the Affymetrix 430 2.0 mouse genome array used for this study does not contain a transcript for Uba80, the second mammalian mono-ubiquitin gene.
Specific Differentially Regulated Genes and Expression Patterns
Fertility-related genes
One of the downregulated genes of interest at 7 days is Dmc1, which is a meiosis-specific homolog of the bacterial RecA gene required for DNA recombination and repair (Fig. 2C). At 14 days, a number of testis-specific or meiosis-specific genes including Mtl5, Fabp9, Msh4, Spdya, Rec8, and Zfp318 are downregulated in Ubb−/− testes, raising the question of whether or not this altered gene expression profile of meiosis-specific genes could contribute to the pachytene arrest and subsequent germ cell apoptosis in Ubb−/− mice (Fig. 2D). These genes are detailed in Supplementary Table 3.
Metabolism-related genes
Intriguingly, a large group of genes involved with steroid/lipid metabolism and inflammation seem to be upregulated in Ubb−/− mice at 7 days (Fig. 2C and Supplementary Table 2). These genes constitute 36% (9/25) of all unique upregulated genes at this time point, or 50% (9/18) of named (known) genes (it is possible that some of the unnamed genes may also be lipid metabolism genes, so that simply considering all transcripts, including those with unknown gene function, may underestimate the true percentage). Interestingly, three of these lipid metabolism genes, Fabp4, Cfd, and Adipoq, continue to be abnormally upregulated at 14 days (2.89, 3.16, and 3.36-fold increase in gene expression, respectively; Fig. 2D). Further investigation is required to elucidate if upregulation of these obesity-related genes is connected to the adult-onset obesity phenotype in Ubb−/− mice (Ryu et al., 2008a).
Transcription-related genes
Strikingly, the largest group of upregulated genes at 14 days in Ubb−/− testes is that related to transcription (e.g., confirmed or predicted transcription factors, coactivators or corepressors, RNA binding and splicing proteins, chromatin remodeling proteins, and E3 ligases specifically linked to gene transcription) (Fig. 2D and Supplementary Table 4). Those genes with testis-specific functions or demonstrating high expression in the testis (i.e., Mysm1, E2f5, Smad5, Osr1, Itch, etc.) are summarized in Supplementary Table 4A. One possibility is that some of these genes may be upregulated in an attempt to compensate for the downregulated meiosis-specific genes.
Genes related to general transcriptional activation (i.e., Trove2 (predicted), Qk, Smarca5, Ncor1, Hdac2, Rnf12, etc.) are summarized in Supplementary Table 4B. All together, these genes constitute almost 18% (49/272 unique transcripts) of all upregulated genes, and almost 28% (49/177 unique transcripts) of named genes. In contrast, transcription-related genes are extremely underrepresented in the other groups of differentially regulated genes analyzed (2/25 of downregulated genes at 7 days, 0/25 of upregulated genes at 7 days, and 1/83 of downregulated genes at 14 days).
UPS-related genes
Another important group of upregulated genes is that related to the UPS (Fig. 2D and Supplementary Table 5). These genes include three Ub-specific proteases, or USP (Usp4, Usp47 (predicted), and Usp19), four E3 ligases (Trip12 (predicted), Fbxo32, Itch, and Rnf12), one E2 conjugase (Ube2i), and one uH2A deubiquitinase (Mysm1). Some of these genes (such as the proteases or deubiquitinase) may play important roles in increasing free Ub levels in Ubb−/− testes since these testes contain dramatically reduced levels of Ub (Ryu et al., 2008b).
Effect of Reduced Germ Cell Number on Expression Patterns
Though the testes from Ubb−/− mice appear histologically normal at 7 days (Ryu et al., 2008b), we find that they contain many fewer GCNA (germ cell nuclear antigen)-labeled germ cells at this stage (Supplementary Fig. 2). Therefore, it is possible that some of the observed downregulation may result from reduced germ cell numbers.
To investigate this possibility, we needed to know whether the genes with reduced mRNA transcripts were expressed mainly in germ cells or in somatic cells. Our microarray analysis was performed on testicular lysates containing a mixture of germ cells and somatic cells (Sertoli cells and Leydig cells). Therefore, we turned to published work (GEO accession number, GSE4193; http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE4193) (Namekawa et al., 2006) to obtain expression data for specific germ cell types.
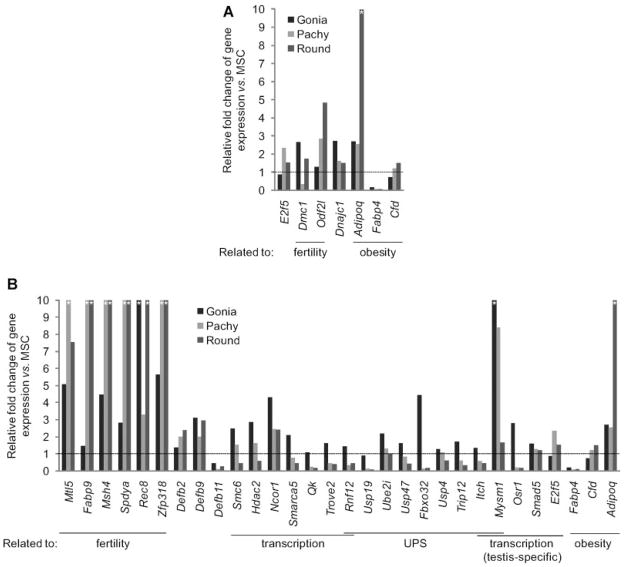
In Figure 3, we present data gathered from this analysis, which investigates gene expression by germ cell type (spermatogonia, spermatocytes, and round spermatids) over the course of spermatogenesis. For the sake of comparison to our current data, we focused on those genes that are significantly (>2-fold) up- or downregulated in Ubb−/− testes.
Figure 3.
Relative fold-change of gene expression in different germ cell types from wild-type testes by microarray analysis. A,B: Relative fold-change of gene expression in type A and B spermatogonia (Gonia), pachytene spermatocytes (Pachy), and round spermatids (Round) compared to mouse Sertoli cells (MSC). Asterisk (*) indicates more than 10-fold upregulation versus MSC. The genes corresponding to Figure 2C and D are shown; top graph (A) indicates expression profiles of genes misregulated in Ubb−/− testes at 7 days, whereas bottom graph (B) shows profiles of genes misregulated at 14 days. Complete data set is also available as Supplementary Data.
Together, these analyses show that many fertility genes with reduced transcripts in 14-day Ubb−/− testes are expressed at high levels in wild-type pachytene spermatocytes, suggesting that reduced germ cell numbers in Ubb−/− mice could contribute to the observed downregulation of these genes.
However, several genes linked to transcription, the UPS, and metabolism are expressed more highly in somatic cells at 14 days (see Fig. 3B), indicating that the observed misregulation of these genes cannot be explained by simple reductions in germ cell number. Moreover, since we do not observe an increase in germ cell or somatic cell number at either 7 or 14 days (Ryu et al., 2008b), upregulated genes at either of these time points cannot be ascribed to an increase in cell number, and almost certainly result from bona fide upregulation of gene expression. Lastly, if a reduction in germ cell number were the sole contributing factor to reduced transcript levels in Ubb−/− mice, one might expect that levels of all transcripts normally expressed in germ cells would be reduced, which is not the case.
Reduced Levels of Histone Ubiquitylation May Account for the Altered Gene Regulation in Ubb−/− Testes
To investigate whether or not the altered gene regulation in Ubb−/− testes could be a consequence of deficient histone ubiquitylation, we decided to quantify monoubiquitylated H2A (uH2A) levels in testicular lysates from 15-day-old wild-type, Ubb+/−, and Ubb−/− mice. Given that only 1.5% of histone H2B is ubiquitylated in mammals, whereas 5–15% of H2A is ubiquitylated (Jason et al., 2002), we focused on uH2A.
We found that uH2A levels in Ubb−/− testicular lysates are significantly reduced compared to wild-type or Ubb+/− lysates (Fig. 4A,B). These reduced uH2A levels may be responsible for the large number of upregulated genes at 14 days, given that uH2A has been linked to gene repression in mammals (Minsky and Oren, 2004). We have previously found that the XY body, a uH2A-rich structure that normally forms in pachytene spermatocytes, is still present in Ubb−/− testes (although its formation may be delayed) (Ryu et al., 2008b). Our previous immunofluorescence data also shows that the amount and staining pattern of uH2A present in the XY body is similar between wild-type and Ubb−/− pachytene spermatocytes (Ryu et al., 2008b).
Figure 4.

Reduced levels of H2A ubiquitylation in Ubb−/− testes. A: Western blot analysis of ubiquitylated H2A (uH2A) in testicular lysates from 15-day-old wild-type (+/+, n = 3), Ubb+/− (+/−, n = 2), and Ubb−/− (−/−, n = 3) mice. Tissue lysates were subjected to SDS–PAGE followed by western blot detection with polyclonal anti-H2A antibody. Band intensity of uH2A was normalized by the total H2A (uH2A +H2A) band intensity. Data are expressed as the mean ± SEM from the indicated number of mice. *P <0.05 versus wild-type (+/+) mice. B: Representative western blot data is shown. C: Immunofluorescence analysis of uH2A in touch prep slides from 15-day-old wild-type (Ubb+/+) and Ubb−/− mice. DNA was visualized using bisbenzamide. Representative images from three different touch prep slides are shown for each genotype. uH2A immunoreactivity was generally high in spermatocytes with a distinct intense staining pattern marked by XY body in pachytene spermatocytes. SG, spermatogonia; SC, spermatocytes; RT, round spermatids. Scale bar, 10 μm.
To determine if other testicular cells might have reduced uH2A levels, we performed immunofluorescence analysis of uH2A in individual cells isolated by touch preparation (Fig. 4C). Since the testicular cell types present at 15 days are mostly spermatogonia and spermatocytes, we compared uH2A immunoreactivity between these two cell types in wild-type and Ubb−/− testes (consistent with our earlier data, we were able to detect a few round spermatids that had already completed meiosis in touch preparation from wild-type, but not Ubb−/−, testes).
In agreement with a previous report (Baarends et al., 1999), we find that uH2A immunoreactivity is generally higher in spermatocytes than in spermatogonia, as shown in Figure 4C. We also find that pachytene spermatocytes labeled by strong uH2A immunoreactivity in the XY body are present in both wild-type and Ubb−/− spermatocytes. We do not observe a significant difference in uH2A signal intensity between wild-type and Ubb−/− testes in either cell type examined. Our immunofluorescence data therefore suggest that the reduced uH2A levels we observe in Ubb−/− testicular lysates do not arise from reduced uH2A levels in individual pachytene spermatocytes or spermatogonia (see “Discussion” Section).
DISCUSSION
Microarray analysis of the Ubb−/− testicular transcriptome shows that several groups of genes, including those related to fertility, metabolism, transcription, and the UPS demonstrate greatly altered patterns of expression. In general, fertility-related genes are downregulated and some obesity-related genes are upregulated; these patterns may show some correlation with the infertility and obesity phenotypes in Ubb−/− mice. In addition, a large group of transcription-related genes (either general or testis-specific) and several UPS-related genes are upregulated, suggesting that gene expression in Ubb−/− testes is generally misregulated, possibly due to the reduced availability of cellular ubiquitin (Ub).
Levels of ubiquitylated histone H2A (uH2A) are reduced in Ubb−/− testes, and this may contribute to the disruption of gene expression in these mice. Ubb−/− mice demonstrate more upregulation than downregulation of genes, suggesting that H2A ubiquitylation may normally contribute more to gene repression than gene activation in mammals.
Can Downregulation of Fertility-Related Genes Account for the Infertility in Ubb−/− Mice?
Downregulation of Dmc1 in Ubb−/− mice is of interest because Dmc1−/− mice exhibit spermatogenesis arrest during at the zygotene stage resulting from abnormal or absent synapsis of homologous chromosomes (Pittman et al., 1998; Yoshida et al., 1998). Downregulation of this gene is unlikely, however, to be the main cause of infertility in Ubb−/− mice for two reasons. First, Dmc1−/− mice experience zygotene arrest, whereas Ubb−/− mice make it to the pachytene stage and display normal chromosomal synapsis (Ryu et al., 2008b). Second, Dmc1 transcript levels are reduced by only approximately 50% in Ubb−/− mice, and there is no effect of Dmc1 heterozygosity on fertility (Pittman et al., 1998). However, Dmc1 downregulation in conjunction with several other transcriptional abnormalities could very well contribute to a complex infertility phenotype in Ubb−/− mice.
There are at least two possible, nonmutually exclusive explanations for the downregulation of Dmc1 at 7 days. First, microarray analysis of wild-type testes indicates that Dmc1 is expressed much more highly in spermatogonia than in Ubb−/− pachytene spermatocytes (see Fig. 3A). The reduced number of spermatogonia in Ubb−/− testes (see Supplementary Fig. 2), along with possibly reduced Dmc1 transcripts per germ cell, could account for reduced Dmc1 levels from Ubb−/− testicular lysates at 7 days. A second explanation may be related to the time course of spermatogenesis. Since Ubb−/− mice have decreased body weight at birth (Ryu et al., 2008a), the initiation of spermatogenesis may be delayed in these mice, and so fewer spermatogonia (specifically, Type B spermatogonia, which have higher Dmc1 levels) may be present at 7 days.
It is interesting that Dmc1 is not misregulated at 14 days, even though it is clearly expressed in wild-type testes at this time point. At 14 days, there are relatively more pachytene spermatocytes than spermatogonia, so Dmc1 levels may not appear significantly different between wild-type and Ubb−/− testes. In other words, the differences in Dmc1 levels are much more dramatic when comparing spermatogonia than when comparing spermatocytes.
Though the majority of the meiosis-specific genes (Msh4, Rec8, and possibly Spdya) are reduced by only 40–50% in Ubb−/− testes, the combinatorial effect of a reduction in multiple meiosis-specific genes could very well lead to a complex infertility phenotype. In addition, some genes whose transcripts are reduced more significantly (Mtl5 and Fabp9) have incompletely understood testicular functions, and may prove extremely critical for proper spermatogenesis; further investigation into the functions of these genes may shed light on the pachytene arrest in Ubb−/− mice. It is also possible that altered levels of the uncharacterized potential transcription factor Zfp318 may be responsible for some of the transcriptional changes seen at 14 days in Ubb−/− mice.
Is Upregulation of Obesity, Transcription, and UPS -Related Genes Connected to the Phenotype of Ubb−/− Mice?
Abnormal regulation of steroid-producing genes may contribute to the Ubb−/− infertility phenotype, even though gross hormone levels do not seem to be abnormal in knockout mice (Ryu et al., 2008b). An intriguing possibility is that abnormal regulation of lipid metabolism genes in the testes may reflect abnormal regulation elsewhere, such as in adipose tissue. Simultaneous dysregulation of several lipid metabolism genes may underlie, or at least contribute to, the obesity phenotype in Ubb−/− mice (Ryu et al., 2008a). Even though these transcripts are upregulated at 7 days, long before the onset of the increased fat-to-muscle ratio phenotype in Ubb−/− mice (Ryu et al., 2008a), such disrupted gene regulation in the testes may none-the-less point to abnormal regulation in other tissues. Alternatively, abnormal regulation of these genes at 7 days may contribute to the reduced birth weight of Ubb−/− mice compared to wild-type littermates (Ryu et al., 2008a).
Increased transcript levels of transcription-related genes with testis-specific functions may not necessarily reflect increased protein levels of these gene products; in fact, if protein levels of these essential genes are decreased by other transcriptional abnormalities in Ubb−/− mice, transcript levels of these genes may increase to compensate. If the compensation is not adequate, spermatogenesis defects may ensue. Regardless, the large number of upregulated transcription-related genes suggests that transcription and gene expression may be globally abnormal in Ubb−/− testes, perhaps as a result of insufficient Ub. The combinatorial effect of so many dysregulated genes may lead to a complex infertility phenotype and pachytene arrest.
Some of the UPS-related genes, especially the Ub-specific proteases (USP), may be upregulated to compensate for the absence of Ubb; increased USP activity may elevate cellular levels of free Ub (as mentioned previously, Ub levels may be limiting in Ubb−/− testes and may result in proteotoxic stress). In addition, a six-fold increase in the transcription of Mysm1, a uH2A-specific deubiquitinase, may serve to remove Ub from histone H2A molecules to increase free Ub levels. Such a decrease in histone ubiquitylation during proteotoxic stress has been described previously by Dantuma et al. (2006) and Groothuis et al. (2006).
Increased Mysm1 activity may also contribute to the large number of transcriptional changes discovered by microarray analysis in Ubb−/− testes. It is interesting that no proteasome components are up- or downregulated at either time point (the Affymetrix annotation file shows that probe sequences for several proteasome components are present on the 430 2.0 GeneChip). If the absence of sufficient Ub in Ubb−/− mice leads to impaired protein degradation in proteotoxic stress, one might initially expect that proteasomal components would be upregulated to compensate (this may not be an effective compensation, as the problem lies with protein polyubiquitylation, but it may occur nonetheless). The lack of proteasome transcript upregulation could indicate either that protein degradation is not greatly abnormal in Ubb−/− testes, or that transcriptional compensation mechanisms cannot occur without sufficient Ub (perhaps because Ub itself is needed for histone ubiquitylation and gene regulation).
Does Reduced Histone Ubiquitylation in Ubb−/− Testes Contribute to Altered Gene Expression Patterns?
The number of germ cells is reduced inUbb−/− testes, and this can certainly contribute to the observed downregulation of transcripts. In otherwords, reduced transcript levels may in part simply reflect fewer cells making those transcripts. However, we believe that germ cell number cannot wholly account for the observed gene expression patterns.
First, if misexpression of transcripts were simply a result of reduced germ cell number, one might expect levels of ALL transcripts normally expressed in germ cells to be reduced, which is clearly not the case. Second, a striking number of transcripts are upregulated, and we do not detect an increase in either somatic or germ cell number at either 7 or 14 days (Ryu et al., 2008b). Third, histone ubiquitylation has such an important role in gene regulation (Robzyk et al., 2000; Jason et al., 2002; Emre and Berger 2004; Minsky and Oren 2004; Wang et al., 2004; Cao et al., 2005), it is hard to believe that significantly reduced levels of uH2A in Ubb−/− testes (see Fig. 4A) would not have a vital impact on gene regulation.
Our inability to detect reduced levels of uH2A in Ubb−/− spermatogonia or spermatocytes by immunofluorescence (see Fig. 4C) is puzzling, but possibly points to a reduction of uH2A in nongerm cells. In other words, reduced uH2A levels in somatic cells may influence germ cell development in a noncell-autonomous manner. It is also possible that our detection method (immunofluorescence) is not ideal for measuring uH2A levels in individual cells.
The effects of histone ubiquitylation in metazoans are likely complex, and may lead to either activation or repression depending on the context. For example, H2A ubiqui-tylation is required for Hox gene silencing in Drosophila (Cao et al., 2005; Wei et al., 2006), but certain Hox genes are downregulated in the absence of H2A ubiquitylation (Cao et al., 2005). This result suggests that H2A ubiquitylation may lead to gene activation as well as repression. Indeed, our microarray analysis shows that while 349 genes are upgregulated in Ubb−/− mice, many genes (105) are also downregulated; if some of these changes result directly from decreased H2A ubiquitylation, it could suggest that histone ubiquitylation may normally activate certain genes while repressing others.
Other gene expression changes could indirectly result from reduced uH2A levels, if for example uH2A affects expression of activators or repressors. In addition, some genes may be either down- or upregulated genes in response to the gene expression changes that arise as a result of decreased uH2A levels. Though H2A is the main ubiquitylated histone in mammals (5–15% of mammalian H2A is ubiquitylated) (Jason et al., 2002), a small percentage of H2B is also ubiquitylated, so changes in uH2B levels may also contribute to the phenotypes of Ubb−/− mice.
It should be noted that an absence of Ubb and a decrease in histone ubiquitylation may only be one mechanism contributing to differential gene regulation in Ubb−/− mice. It is equally possible that insufficient Ub may lead to abnormal protein degradation and thus affect signal transduction and transcription pathways that rely on protein degradation, such as Wnt/β-catenin, Hedgehog, and NFκB/IκB. These two mechanisms are by no means mutually exclusive, and in fact would likely operate simultaneously if Ub levels were limiting. If insufficient Ub in Ubb−/− testes leads to profound changes in gene regulation, either as a result of insufficient histone ubiquitylation or insufficient protein degradation for degradation-dependent transcription pathways, the resulting infertility phenotype is likely to be complex and not a result of single gene(s) defects.
To explore whether or not insufficient histone ubiquitylation leads to altered gene regulation in Ubb−/− mice, one could use chromatin immunoprecipitation to assess histone ubiquitylation patterns along promoters and open reading frames of differentially regulated genes in wild-type and Ubb−/− mice. If histone ubiquitylation patterns are found to be correlated with gene expression, the Ubb−/−mouse (and specifically, the Ubb−/− testes) may serve as a tractable system for studying Ub-dependent gene regulation in mammals. It may also be interesting to measure transcripts of the lipid metabolism genes highlighted in Supplementary Table 2 in the adipose tissue of Ubb−/− mice. Altered regulation of many such genes may lead to the complex metabolic phenotypes seen in these mice (Ryu et al., 2008a, 2010).
MATERIALS AND METHODS
RNA Preparation and Microarray Analysis
Whole testes from wild-type (WT) and Ubb−/− (KO) mice were harvested at 7- and 14-days postpartum; two mice of each genotype were analyzed at each time point. Total RNA was extracted using Trizol reagent and the RNA samples were confirmed to be intact (nondegraded) and pure (minimal DNA contamination) by formaldehyde gel electrophoresis and spectrophotometry, respectively, before performing microarray analysis. A minimal optical density (OD260/280) ratio of 1.8 in Tris buffer was required for microarray hybridization.
Ten micrograms of total RNA was used to create biotinylated cRNA, which was then hybridized to the Affymetrix 430 2.0 mouse whole genome array. Arrays were stained and washed using the Affymetrix GeneChip Fluidics Station 400 and scanned using a GeneArray Scanner 2500A. The entire experiment was performed twice using duplicate samples. Microarray Suite 5.0 software was used to view the data and statistical analysis was performed using GeneSpring 7.0 software.
Expression levels for genes in Ubb−/− testes at each time point were considered upregulated if there was a statistically significant, two-fold increase over the wild-type expression level, and considered downregulated if there was a more than two-fold decrease (or less than 0.5-fold increase). Significance was determined using a one-way ANOVA parametric test with variances not assumed equal, a P-value cut off of 0.5 and using a Benjamini and Hochberg False discovery rate multiple test correction. These data were provided as Supplementary Data and also deposited in Gene Expression Omnibus (GEO) with accession number GSE27568 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE27568).
The data was further analyzed as follows. Using the software programs Cluster and TreeView (both written by Mike Eisen, UC Berkeley), heat maps of the data were generated to assess overall expression patterns. Moreover, the four groups of gene expression data (genes whose levels were greater in WT vs. KO at 7 days, those with levels greater in KO vs. WT at 7 days, those with levels greater in WT vs. KO at 14 days, and those with levels greater in KO vs. WT at 14 days) were analyzed manually using the Gene ontology program AmiGO (http://amigo.-geneontology.org/cgi-bin/amigo/go.cgi) to detect functional patterns of up- and downregulated genes.
To verify the microarray analysis data, we have confirmed the mRNA levels of a few selected genes by quantitative real-time RT-PCR (Supplementary Fig. 1).
Western Blot Analysis
Testes from 15-day old males of each genotype (wild-type, Ubb+/−, and Ubb−/−) were homogenized with a hand-held homogenizer in hypotonic buffer containing 10 mM HEPES (pH 7.7), 3 mM MgCl2, 0.1 mM EDTA (pH 8.0), 10% glycerol, and protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN). The suspension was centrifuged for 10 min at 4°C and the pellet was resuspended in lysis buffer containing 50 mM Tris–HCl (pH 7.4), 150 mM NaCl, 2 mM EDTA, 1% NP-40, and 0.1% SDS. The resuspended pellet was sonicated and centrifuged again for 10 min at 4°C. The supernatant was then subjected to SDS–PAGE and transferred to nitrocellulose membrane using standard protocols. The membrane was probed with a polyclonal anti-H2A antibody (1:1,000, Abcam, Cambridge, MA) and visualized using a phosphorimager (Typhoon, GE Healthcare Life Sciences, Piscataway, NJ). Band intensity of uH2A was normalized by the total H2A (uH2A +H2A) band intensity.
Touch Preparation and Immunofluorescence
Freshly isolated testes from 15-day old males were cut through the center with a razor blade. One half of the testis was picked up with a forceps and the cut surface was gently dabbed onto positively charged microscope slides (Superfrost Plus, VWR, Radnor, PA). These “touch prep” slides were air dried for 10 min and fixed with 4% paraformaldehyde for 10 min at room temperature. Touch prep slides were permeabilized with 0.3% Triton X-100/PBS for 30 min on ice, blocked with 3% BSA/PBS for 1 hr at room temperature, and then incubated with a monoclonal anti-uH2A antibody (1:500, clone E6C5, Millipore, Billerica, MA) overnight at 4°C. After washing with PBS, touch prep slides were incubated with Alexa Fluor 488-conjugated goat anti-mouse IgM (1:400, Invitrogen, Carlsbad, CA) and bisbenzamide (1:10,000, to visualize DNA) for 1 hr at room temperature. The slides were then washed and mounted with ProLong Gold antifade reagent (Invitrogen).
Supplementary Material
Acknowledgments
We thank our colleagues in the UOS cell biology lab, especially Han-Wook Ryu, for maintenance and genotyping of mice. This work was funded by a grant from the National Institute of Neurological Disorders and Stroke (NS04842) to R.R.K., and a grant from the Korea Healthcare technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A084520) to K.-Y.R.
Grant sponsor: National Institute of Neurological Disorders and Stroke, Grant number: NS04842; Grant sponsor: Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea, Grant number: A084520.
Abbreviations
- H2A
histone 2A (also uH2A, monoubiquitylated histone H2A)
- Ub
ubiquitin
- UPS
ubiquitin-proteasome system
Footnotes
Additional supporting information may be found in the online version of this article.
References
- Baarends WM, Hoogerbrugge JW, Roest HP, Ooms M, Vreeburg J, Hoeijmakers JH, Grootegoed JA. Histone ubiquitination and chromatin remodeling in mouse spermatogenesis. Dev Biol. 1999;207:322–333. doi: 10.1006/dbio.1998.9155. [DOI] [PubMed] [Google Scholar]
- Cao R, Tsukada Y, Zhang Y. Role of Bmi-1 and Ring1A in H2A ubiquitylation and Hox gene silencing. Mol Cell. 2005;20:845–854. doi: 10.1016/j.molcel.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Dantuma NP, Groothuis TA, Salomons FA, Neefjes J. A dynamic ubiquitin equilibrium couples proteasomal activity to chromatin remodeling. J Cell Biol. 2006;173:19–26. doi: 10.1083/jcb.200510071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emre NC, Berger SL. Histone H2B ubiquitylation and deubiquitylation in genomic regulation. Cold Spring Harb Symp Quant Biol. 2004;69:289–299. doi: 10.1101/sqb.2004.69.289. [DOI] [PubMed] [Google Scholar]
- Finley D, Bartel B, Varshavsky A. The tails of ubiquitin precursors are ribosomal proteins whose fusion to ubiquitin facilitates ribosome biogenesis. Nature. 1989;338:394–401. doi: 10.1038/338394a0. [DOI] [PubMed] [Google Scholar]
- Groothuis TA, Dantuma NP, Neefjes J, Salomons FA. Ubiquitin crosstalk connecting cellular processes. Cell Div. 2006;1:21. doi: 10.1186/1747-1028-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- Hicke L. Protein regulation by monoubiquitin. Nat Rev Mol Cell Biol. 2001;2:195–201. doi: 10.1038/35056583. [DOI] [PubMed] [Google Scholar]
- Hochstrasser M. Ubiquitin-dependent protein degradation. Annu Rev Genet. 1996;30:405–439. doi: 10.1146/annurev.genet.30.1.405. [DOI] [PubMed] [Google Scholar]
- Jason LJ, Moore SC, Lewis JD, Lindsey G, Ausio J. Histone ubiquitination: A tagging tail unfolds? BioEssays. 2002;24:166–174. doi: 10.1002/bies.10038. [DOI] [PubMed] [Google Scholar]
- Minsky N, Oren M. The RING domain of Mdm2 mediates histone ubiquitylation and transcriptional repression. Mol Cell. 2004;16:631–639. doi: 10.1016/j.molcel.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Namekawa SH, Park PJ, Zhang LF, Shima JE, McCarrey JR, Griswold MD, Lee JT. Postmeiotic sex chromatin in the male germline of mice. Curr Biol. 2006;16:660–667. doi: 10.1016/j.cub.2006.01.066. [DOI] [PubMed] [Google Scholar]
- Pickart CM, Fushman D. Polyubiquitin chains: Polymeric protein signals. Curr Opin Chem Biol. 2004;8:610–616. doi: 10.1016/j.cbpa.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Pittman DL, Cobb J, Schimenti KJ, Wilson LA, Cooper DM, Brignull E, Handel MA, Schimenti JC. Meiotic prophase arrest with failure of chromosome synapsis in mice deficient for Dmc1, a germline-specific RecA homolog. Mol Cell. 1998;1:697–705. doi: 10.1016/s1097-2765(00)80069-6. [DOI] [PubMed] [Google Scholar]
- Robzyk K, Recht J, Osley MA. Rad6-dependent ubiquitination of histone H2B in yeast. Science. 2000;287:501–504. doi: 10.1126/science.287.5452.501. [DOI] [PubMed] [Google Scholar]
- Ryu KY, Garza JC, Lu XY, Barsh GS, Kopito RR. Hypothalamic neurodegeneration and adult-onset obesity in mice lacking the Ubb polyubiquitin gene. Proc Natl Acad Sci USA. 2008a;105:4016–4021. doi: 10.1073/pnas.0800096105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu KY, Sinnar SA, Reinholdt LG, Vaccari S, Hall S, Garcia MA, Zaitseva TS, Bouley DM, Boekelheide K, Handel MA, Conti M, Kopito RR. The mouse polyubiquitin gene Ubb is essential for meiotic progression. Mol Cell Biol. 2008b;28:1136–1146. doi: 10.1128/MCB.01566-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu KY, Fujiki N, Kazantzis M, Garza JC, Bouley DM, Stahl A, Lu XY, Nishino S, Kopito RR. Loss of polyubiquitin gene Ubb leads to metabolic and sleep abnormalities in mice. Neuropathol Appl Neurobiol. 2010;36:285–299. doi: 10.1111/j.1365-2990.2009.01057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima JE, McLean DJ, McCarrey JR, Griswold MD. The murine testicular transcriptome: Characterizing gene expression in the testis during the progression of spermatogenesis. Biol Reprod. 2004;71:319–330. doi: 10.1095/biolreprod.103.026880. [DOI] [PubMed] [Google Scholar]
- van Leeuwen FW, de Kleijn DP, van den Hurk HH, Neubauer A, Sonnemans MA, Sluijs JA, Koycu S, Ramdjielal RD, Salehi A, Martens GJ, Grosveld FG, Peter J, Burbach H, Hol EM. Frameshift mutants of beta amyloid precursor protein and ubiquitin-B in Alzheimer’s and Down patients. Science. 1998;279:242–247. doi: 10.1126/science.279.5348.242. [DOI] [PubMed] [Google Scholar]
- Wang H, Wang L, Erdjument-Bromage H, Vidal M, Tempst P, Jones RS, Zhang Y. Role of histone H2A ubiquitination in Polycomb silencing. Nature. 2004;431:873–878. doi: 10.1038/nature02985. [DOI] [PubMed] [Google Scholar]
- Wei J, Zhai L, Xu J, Wang H. Role of Bmi1 in H2A ubiquitylation and Hox gene silencing. J Biol Chem. 2006;281:22537–22544. doi: 10.1074/jbc.M600826200. [DOI] [PubMed] [Google Scholar]
- West MH, Bonner WM. Histone 2A, a heteromorphous family of eight protein species. Biochemistry. 1980a;19:3238–3245. doi: 10.1021/bi00555a022. [DOI] [PubMed] [Google Scholar]
- West MH, Bonner WM. Histone 2B can be modified by the attachment of ubiquitin. Nucleic Acids Res. 1980b;8:4671–4680. doi: 10.1093/nar/8.20.4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiborg O, Pedersen MS, Wind A, Berglund LE, Marcker KA, Vuust J. The human ubiquitin multigene family: Some genes contain multiple directly repeated ubiquitin coding sequences. EMBO J. 1985;4:755–759. doi: 10.1002/j.1460-2075.1985.tb03693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Kondoh G, Matsuda Y, Habu T, Nishimune Y, Morita T. The mouse RecA-like gene Dmc1 is required for homologous chromosome synapsis during meiosis. Mol Cell. 1998;1:707–718. doi: 10.1016/s1097-2765(00)80070-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.