Abstract
To characterize predictors of isolated hepatitis B core antibody (anti-HBc) among human immunodeficiency virus (HIV)–infected and HIV-uninfected women, we compared 702 women with anti-HBc and hepatitis B surface antibody (anti-HBs) with 490 women with isolated anti-HBc (1.8% of whom had detectable hepatitis B virus [HBV] DNA). Factors independently associated with isolated anti-HBc without viremia were detectable hepatitis C virus (HCV) RNA, HIV positivity, history of injection drug use, >10 lifetime sex partners, and HIV RNA level >100,000 copies/mL. Anti-HBs levels were lower among anti-HCV–positive women. Isolated anti-HBc was rarely explained by occult HBV in this cohort but may be explained by the influence of viral coinfections on anti-HBs level or durability.
Antibody to hepatitis B core antigen (anti-HBc) in the absence of hepatitis B surface antigen (HBsAg) or antibody to HBsAg (anti-HBs) is a common finding in HIV-infected persons [1, 2]. However, the clinical significance of isolated anti-HBc without hepatitis B (HBV) viremia is uncertain. An association between isolated anti-HBc and hepatitis C virus (HCV) infection has been noted in HIV-infected and -uninfected persons [1–6]. Although this association may be an epidemiologic artifact of shared routes of transmission, it has been theorized that HCV infection may impair the antibody response to HBsAg [7], may cause false-positive anti-HBc results [2], or may cause false-negative HBsAg results [8]. We sought to elucidate this association by investigating the relationship between isolated anti-HBc, HCV viremia, and HIV in women participating in the Women’s Interagency HIV Study (WIHS).
METHODS
The WIHS is a prospective observational study in which the prevalence of isolated anti-HBc at study entry was 238 (15%) of 1606 among HIV-infected and 33 (6%) of 526 among HIV-uninfected women tested [9]. The WIHS enrolled 2056 HIV-infected and 569 HIV-uninfected at-risk US women at 6 sites—Chicago; the San Francisco Bay area; Brooklyn and Bronx/Manhattan, New York; Washington, DC; and Los Angeles—between October 1994 and November 1995. From October 2001 through September 2002, an additional 1143 women (737 HIV infected and 406 HIV uninfected) were enrolled. Informed consent was obtained from all participants, in accordance with the US Department of Health and Human Services guidelines and the institutional review boards of participating institutions. The cohort was designed to reflect the demographics of the HIV epidemic among women in the United States. Details of recruitment and cohort demographics have been published elsewhere [10]. We compared the demographic and disease characteristics of women with isolated anti-HBc with those of women with anti-HBc/anti-HBs (considered to represent resolved natural infection).
Laboratory methods
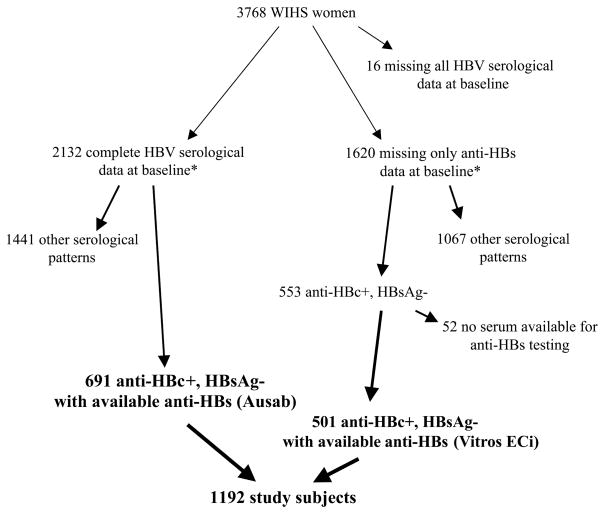
Hepatitis B serological analysis, including for anti-HBs, total anti-HBc, and HBsAg, was performed at baseline (i.e., study entry) for 2132 of the 3768 WIHS women by use of Abbott Ausab EIA, Abbott Corzyme EIA, and Abbott Auszyme Microparticle EIA, respectively (Abbott Laboratories). In addition to these 2132 women with complete baseline serological data, 1620 WIHS women had anti-HBc and HBsAg tested at baseline but not anti-HBs (figure 1). Among these 1620 women, 553 were positive for anti-HBc and negative for HBsAg; for 501 of these women, stored serum from the baseline visit or from a visit within 18 months of baseline was available for testing for anti-HBs by use of Vitros ECi (Ortho Diagnostics). We compared demographic, behavioral, and HIV and HCV disease status between study women and women who had missing HBV serological data and found no significant differences.
Figure 1.
Study subject identification flow sheet. Anti-HBc, hepatitis B core antibody; anti-HBs, hepatitis B surface antibody; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; WIHS, Women’s Interagency HIV Study. *Assays used for hepatitis serological analysis at baseline were the Abbott Ausab EIA for anti-HBs, the Abbott Corzyme EIA for anti-HBc, and the Abbott Auszyme Microparticle EIA for HBsAg (Abbott Laboratories); anti-HBs was measured on reposited serum by use of Vitros ECi (Ortho Diagnostics).
HCV antibody testing was performed at baseline using EIA 2.0 and 3.0 (Abbott Laboratories and Ortho Diagnostics, respectively). Qualitative HCV RNA testing was performed on HCV antibody–positive women by use of COBAS Amplicor HCV (version 2.0; Roche Diagnostics), which has a lower limit of detection of 100 IU/mL. The COBAS Amplicor HCV Monitor assay kit (version 2.0; Roche Diagnostics) was used for quantitative testing (lower and upper limits of detection, 600 and 500,000 IU/mL). All samples were initially tested quantitatively by a 1:10 dilution with negative human plasma per the manufacturer’s recommendations. Diluted samples negative for HCV RNA were retested undiluted by use of a quantitative Amplicor HCV assay that has a lower limit of detection of 50–100 IU/mL. All samples that were nonreactive in both HCV quantitative and qualitative polymerase chain reaction assays were considered to be HCV RNA negative.
HBV DNA testing was performed on specimens from the baseline, second, or third visit for women with isolated anti-HBc by use of the COBAS Amplicor Monitor test (Roche Diagnostics). The lower limit of detection for this assay is 200 copies/mL.
Statistical methods
Women were included in the study if they were anti-HBc positive and HBsAg negative. Because a portion of cases of isolated anti-HBc are explained by occult HBV viremia [11], we eliminated women with known HBV viremia from the study group.
Logistic regression models were used to evaluate the association between isolated anti-HBc and demographic and disease characteristics (SAS; version 9; SAS Institute). The association between each potential predictor variable, as noted in table 1, and the presence of isolated anti-HBc was first analyzed univariately. Women were not queried about lamivudine use before its FDA approval in 1995; we have coded missing lamivudine values before FDA approval as “not receiving drug.” The subsequent multivariate model included all variables with P < .10 on univariate analysis (table 1). Quantitative variables were compared by means of t tests, and correlation between quantitative variables was measured by Pearson’s correlation coefficient.
Table 1.
Baseline characteristics and univariate analysis of factors associated with isolated hepatitis B core antibody (anti-HBc) among 988 HIV-infected and 196 HIV-uninfected women seropositive for anti-HBc.
| Characteristic, parameter | Anti-HBc/anti-HBs and isolated anti-HBc, total no. | Isolated anti-HBc, no. (%) | OR (95% CI) | P |
|---|---|---|---|---|
| HIV status | ||||
| Negative | 196 | 53 (27) | 1.0 | |
| Positive | 988 | 429 (43) | 2.1 (1.5–2.9) | <.0001 |
|
| ||||
| Age | ||||
| <35 years | 394 | 143 (36) | 1.0 | |
| ≥35 years | 790 | 339 (43) | 1.3 (1.0–1.7) | .03 |
|
| ||||
| Race | ||||
| White | 157 | 71 (45) | 1.0 | |
| Black | 763 | 296 (39) | 0.8 (0.5–1.1) | .13 |
| Hispanic | 229 | 104 (45) | 1.0 (0.7–1.5) | .97 |
| Other | 35 | 11 (31) | 0.6 (0.3–1.2) | .14 |
|
| ||||
| History of injection drug use | ||||
| Never | 470 | 127 (27) | 1.0 | |
| Ever | 713 | 354 (50) | 2.7 (2.1–3.4) | <.0001 |
| Missing | 1 | |||
|
| ||||
| History of transfusion | ||||
| Never | 778 | 334 (43) | 1.0 | |
| Ever | 168 | 78 (46) | 1.2 (0.8–1.6) | .41 |
| Missing | 238 | |||
|
| ||||
| HCV status | ||||
| Ab negative | 451 | 125 (28) | 1.0 | |
| Ab positive, RNA negative | 143 | 54 (38) | 1.6 (1.1–2.4) | .02 |
| Ab positive, RNA positive | 540 | 282 (52) | 2.9 (2.2–3.7) | <.0001 |
| Missing | 50 | |||
|
| ||||
| HCV RNA status, HCV Ab positive only | ||||
| Negative | 143 | 54 (38) | 1.0 | |
| Positive | 540 | 282 (52) | 1.8 (1.2–2.6) | .002 |
|
| ||||
| Plasma log10 HCV RNA level, HCV RNA positive only | ||||
| <6.0 copies/mL | 193 | 93 (48) | 1.0 | |
| 6.0–6.75 copies/mL | 251 | 136 (54) | 1.3 (0.9–1.9) | .21 |
| >6.75 copies/mL | 96 | 53 (55) | 1.3 (0.8–2.2) | .26 |
| Missing | 37 | |||
|
| ||||
| Alanine aminotransferase level | ||||
| 0–44 IU/L | 889 | 335 (38) | 1.0 | |
| 45–90 IU/L | 208 | 106 (51) | 1.7 (1.3–2.3) | .0005 |
| >90 IU/L | 75 | 37 (49) | 1.6 (1.0–2.6) | .05 |
| Missing | 12 | |||
|
| ||||
| Lifetime no. of sex partners | ||||
| <5 | 165 | 50 (30) | 1.0 | |
| 5–10 | 313 | 121 (39) | 1.4 (1.0–2.2) | .07 |
| >10 | 670 | 292 (44) | 1.8 (1.2–2.6) | .002 |
| Missing | 36 | |||
|
| ||||
| Plasma HIV RNA level, HIV positive only | ||||
| ≤4000 copies/mL | 364 | 133 (37) | 1.0 | |
| 4001–20,000 copies/mL | 214 | 101 (47) | 1.6 (1.1–2.2) | .01 |
| 20,001–100,000 copies/mL | 213 | 95 (45) | 1.4 (1.0–2.0) | .06 |
| >100,000 copies/mL | 177 | 95 (54) | 2.0 (1.4–2.9) | .0002 |
| Missing | 20 | |||
|
| ||||
| Lamivudine therapy, HIV positive only | ||||
| No | 923 | 414 (45) | 1.0 | |
| Yes | 65 | 15 (23) | 0.4 (0.2–0.8) | .009 |
| Missing | 1 | |||
|
| ||||
| CD4 cell counts, HIV positive only | ||||
| >500 cells/mm3 | 322 | 128 (40) | 1.0 | |
| 351–500 cells/mm3 | 208 | 78 (38) | 0.9 (0.6–1.3) | .60 |
| 201–350 cells/mm3 | 218 | 107 (49) | 1.5 (1.0–2.1) | .03 |
| ≤200 cells/mm3 | 217 | 109 (50) | 1.5 (1.1–2.2) | .02 |
| Missing | 23 | |||
NOTE. Statistically significant values are in boldface. Ab, antibody; anti-HBs, hepatitis B surface antibody; CI, confidence interval; OR, odds ratio.
RESULTS
Of the 1192 women identified as being anti-HBc positive and HBsAg negative, 490 (41%) had isolated anti-HBc, and 702 (59%) were seropositive for both anti-HBc and anti-HBs (consistent with resolved natural infection). Of the 490 women with isolated anti-HBc, 452 had serum available for HBV DNA quantification. Eight women (1.8%) were found to have detectable HBV DNA and were therefore excluded from the analysis. Because the prevalence of occult HBV infection was low, the remaining 38 women who did not have samples available for HBV DNA testing were included in the analysis (comparison of these 38 women with the women who had serum available revealed no significant differences in terms of demographic, behavioral, and HIV or HCV disease characteristics).
Factors associated with isolated anti-HBc
Table 1 shows the demographic and disease characteristics of the study patients, comparing women with isolated anti-HBc to those seropositive for both anti-HBc and anti-HBs. HIV status, HCV status, a history of injection drug use (IDU), lifetime number of sex partners, elevated alanine aminotransferase level, and elevated HIV RNA level were strongly associated with the presence of isolated anti-HBc. In multivariate analysis, HIV status, a history of IDU, and >10 lifetime sex partners were found to be independently associated with isolated anti-HBc (table 2). Also, HCV viremia, but not HCV antibody positivity, was independently associated with isolated anti-HBc. When a separate analysis was done with HCV antibody status and HCV RNA status dichotomized, the results were similar; HCV antibody status was not significantly associated with isolated anti-HBc (adjusted odds ratio [aOR], 1.1; P = .75), but HCV viremia was (aOR, 1.6; P = .03). There was no difference in the prevalence of isolated anti-HBc whether women were tested for anti-HBs by Ausab EIA (271/696) or Vitros ECi (222/501) (OR, 0.8 [95% confidence interval, 0.64–1.01]).
Table 2.
Multivariate analysis of factors associated with isolated hepatitis B core antibody (anti-HBc) among 988 HIV-infected and 196 HIV-uninfected women seropositive for anti-HBc.
| Characteristic, parameter | aOR (95% CI) | P |
|---|---|---|
| HCV status | ||
| Ab negative | 1.0 | |
| Ab positive, RNA negative | 1.1 (0.6–1.8) | .77 |
| Ab positive, RNA positive | 1.7 (1.1–2.8) | .02 |
|
| ||
| HIV status | ||
| Negative | 1.0 | |
| Positive | 1.8 (1.1–2.8) | .01 |
|
| ||
| History of injection drug use | ||
| Never | 1.0 | |
| Ever | 1.7 (1.1–2.6) | .02 |
|
| ||
| Lifetime no. of sex partners | ||
| <5 | 1.0 | |
| 5–10 | 1.4 (0.9–2.2) | .10 |
| >10 | 1.7 (1.1–2.5) | .01 |
|
| ||
| Plasma HIV RNA level, HIV positive only | ||
| ≤4000 copies/mL | 1.0 | |
| 4001–20,000 copies/mL | 1.4 (0.9–2.0) | .10 |
| 20,001–100,000 copies/mL | 1.2 (0.8–1.8) | .36 |
| >100,000 copies/mL | 1.7 (1.0–2.7) | .03 |
NOTE. The model included all variables with P < .10 on univariate analysis (table 1), and only variables with P < .05 (in boldface) are displayed. Age, lamivudine use, CD4 cell count, and alanine aminotransferase level were also included in the model. Ab, antibody; aOR, adjusted odds ratio; CI, confidence interval.
Influence of HCV status on quantitative anti-HBs
To determine whether HCV status influenced the titer of anti-HBs, we studied anti-HBs results from the subset of 501 anti-HBc-positive and HBsAg-negative women whose specimens were tested with the quantitative Vitros ECi assay. We found that, overall, women with HCV antibody positivity had lower anti-HBs levels—88.4 mIU/mL, versus 351.1 mIU/mL for women with HCV antibody negativity (P < .001). We did not find a difference in mean anti-HBs levels when we compared the 27 of 296 HCV antibody–positive women with undetectable HCV RNA to the 269 of 296 women with detectable HCV RNA (mean, 60.9 vs. 91.1 mIU/mL; P = .50). Also, among the 296 HCV antibody–positive women, there was no correlation between quantitative HCV RNA and anti-HBs level (Pearson correlation, 0.0046).
DISCUSSION
In this large cohort of HIV-infected and HIV-uninfected US women, we found that isolated anti-HBc without viremia was common and was associated with ongoing HCV replication but not with resolved HCV infection. That the prevalence of isolated anti-HBc is greater in the presence of ongoing HCV viremia than of HCV antibody positivity alone suggests that the association is not an artifact of shared routes of transmission but rather represents a biological interaction between HCV infection and HBV infection or between HCV and the immunologic response to HBV infection.
There are a number of theories about why HCV infection may be associated with isolated anti-HBc. There is evidence that active HCV infection impairs antibody production. Hepatitis C appears to impair the ability to mount an appropriate immune response to hepatitis B vaccination [7], and some have found that nonresponse to HBV vaccination is associated with quantitative HCV RNA level [12]. HCV has been shown, in vitro, to decrease dendritic cell (i.e., antigen presentation) function [13]. In our cohort, we found that, overall, HCV antibody–positive women had lower anti-HBs levels, but we were unable to demonstrate a relationship between quantitative HCV RNA and anti-HBs levels, perhaps because of sample size.
Alternatively, HCV infection may cause more false-positive anti-HBc results [2], or HCV infection may inhibit HBV replication and, thus, impair the ability to detect HBsAg [1].
We identified an association between isolated anti-HBc and HIV status, although no association between isolated anti-HBc and the level of immune function as measured by CD4 cell count was observed. Several studies have reported a high prevalence of isolated anti-HBc in HIV-infected persons [1, 2] but none, to our knowledge, has compared the prevalence with an HIV-uninfected cohort or shown that the association was independent of HCV coinfection. Why HIV infection should increase the prevalence of isolated anti-HBc is unclear. It is known that HIV causes B cell dysfunction, and it is possible that those with HIV infection have a higher incidence of false-positive anti-HBc results or that the levels or durability of the anti-HBs response to natural HBV infection are perturbed in HIV infection [14].
The finding that isolated anti-HBc is associated with a history of IDU independent of HCV status was surprising. Similarly, the association between isolated anti-HBc and lifetime number of sex partners was unexpected. These associations imply an epidemiologic relationship between isolated anti-HBc and high-risk behaviors that is not explained simply by HIV or HCV status.
We found that the univariate association between isolated anti-HBc and elevated alanine aminotransferase level was not independent of hepatitis C status in our cohort. This is consistent with the results of several small studies of liver biopsy samples showing that persons with isolated anti-HBc did not have higher rates of liver inflammation than was explained by HCV coinfection [15, 16].
Limitations
Our findings should be evaluated in light of the study’s limitations. Approximately two-fifths of the anti-HBs determinations were performed on reposited serum by use of a different assay than that used for the original measurements, because the Ausab EIA was no longer available; however, the proportion of women with isolated anti-HBc among the anti-HBc–positive women was not different for the 2 assays, leading us to believe that the assays are comparable. Also, because of specimen availability, the later anti-HBs determinations were performed on serum up to 18 months after the baseline visit, although most often within 6 months of the baseline visit. Anti-HBs status was unlikely to change in a significant number of women with anti-HBc during that period of time.
Conclusion
Isolated anti-HBc was common in this cohort and was rarely explained by occult HBV viremia. We found that active HCV replication was associated with isolated anti-HBc and that HCV status may influence quantitative anti-HBs. We also found that HIV infection, particularly in the setting of high HIV RNA levels, was associated with isolated anti-HBc. Whether these coinfections interfere with the production or durability of anti-HBs in resolved HBV infection or cause false-positive anti-HBc results in the absence of HBV infection remains unclear. Further investigation into the interactions between these viruses is needed to clarify the cause of isolated anti-HBc and its clinical significance.
Acknowledgments
Financial support: Funding for the present study was provided by a National Institute on Drug Abuse supplement to the Women’s Interagency HIV Study (WIHS; grant UO1-AI-034993). Funding for hepatitis B and C virological analysis was provided by the National Institute of Allergy and Infectious Diseases (grant RO1-AI-052065 to A.K. and grant RO1-AI-057006 to H.D.S.) and the University of California, San Francisco, Liver Center (grant P30-DK-26743 to M.P.). The WIHS is funded by the National Institute of Allergy and Infectious Diseases, with supplemental funding from the National Cancer Institute and the National Institute on Drug Abuse (grants UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590); funding is also provided by the National Institute of Child Health and Human Development (grant UO1-CH-32632) and the National Center for Research Resources (grants MO1-RR-00071, MO1-RR-00079, and MO1-RR-00083).
Data in this article were collected by the Women’s Interagency HIV Study (WIHS) Collaborative Study Group, which has centers (principal investigators) at the New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, New York (Howard Minkoff); the Washington, DC, Metropolitan Consortium (Mary Young); the Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt); the Los Angeles County/Southern California Consortium (Alexandra Levine); the Chicago Consortium (Mardge Cohen); and the Data Coordinating Center (Stephen Gange).
Footnotes
Potential conflicts of interest: none reported.
Presented in part: Interscience Conference on Antimicrobial Agents and Chemotherapy, December 2005, Washington, DC (poster 1647).
References
- 1.Neau D, Winnock M, Galperinep T, et al. Isolated antibodies against the core antigen of hepatitis B virus in HIV-infected patients. HIV Med. 2004;5:171–3. doi: 10.1111/j.1468-1293.2004.00206.x. [DOI] [PubMed] [Google Scholar]
- 2.Gandhi RT, Wurcel A, Lee H. Isolated antibody to hepatitis B core antigen in HIV-infected individuals. Clin Infect Dis. 2003;36:1602–5. doi: 10.1086/375084. [DOI] [PubMed] [Google Scholar]
- 3.Jilg W, Sieger E, Zachoval R, Schatzl H. Individual with antibodies against hepatitis B core antigen: high percentage of carriers of hepatitis B and C virus. J Hepatol. 1995;23:14–20. doi: 10.1016/0168-8278(95)80305-x. [DOI] [PubMed] [Google Scholar]
- 4.Greub G, Frei PC. Isolated antibody to hepatitis B core is associated with hepatitis C virus infection. Clin Microbiol Infect. 2000;6:629. doi: 10.1046/j.1469-0691.2000.00160.x. [DOI] [PubMed] [Google Scholar]
- 5.Berger A, Doerr HW, Rabenau HF, Weber B. High frequency of HCV infection in individuals with isolated antibody to hepatitis B core antigen. Intervirology. 2000;43:71–6. doi: 10.1159/000025026. [DOI] [PubMed] [Google Scholar]
- 6.Wedemeyer H, Cornberg M, Tegtmeyer B, Frank H, Tillman HL, Manns MP. Isolated anti-HBV core phenotype in anti-HCV positive patients is associated with hepatitis C virus replication. Clin Microbiol Infect. 2004;10:70–2. doi: 10.1111/j.1469-0691.2004.00771.x. [DOI] [PubMed] [Google Scholar]
- 7.Wiedman M, Liebert UG, Oeson U, et al. Decreased immunogenicity of recombitant hepatitis B vaccine in chronic hepatitis C. Hepatology. 2000;31:230–4. doi: 10.1002/hep.510310134. [DOI] [PubMed] [Google Scholar]
- 8.Brechot C, Thiers V, Kremsdorf D, Nalpas B, Pol S, Paterlini-Brechot P. Persistent B virus infection in subjects without hepatitis B surface antigen: clinically significant or purely “occult”? Hepatology. 2001;34:194–203. doi: 10.1053/jhep.2001.25172. [DOI] [PubMed] [Google Scholar]
- 9.Tien PC, Kovacs A, Bacchetti P, et al. Association between syphilis, antibodies to herpes simplex virus type 2 and recreational drug use and hepatitis B infection in the Women’s Interagency HIV Study. Clin Infect Dis. 2004;39:1363–70. doi: 10.1086/424879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barkan SE, Melnick SL, Preston-Martin S, et al. The Women’s Inter-agency HIV Study. Epidemiology. 1998;9:117–25. [PubMed] [Google Scholar]
- 11.Gandhi RT, Wurcel A, McGovern B, et al. Low prevalence of ongoing hepatitis B viremia in HIV-positive individuals with isolated antibody to hepatitis B core antigen. J Acquir Immune Defic Syndr. 2003;34:439–41. doi: 10.1097/00126334-200312010-00013. [DOI] [PubMed] [Google Scholar]
- 12.Leroy V, Bourliere M, Durand M, et al. The antibody response to hepatitis B virus vaccination is negatively influence by the HCV viral load in patients with chronic hepatitis C: a case-control study. Eur J Gastroenterol Hepatol. 2002;14:485. doi: 10.1097/00042737-200205000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Auffermann-Gretzinger S, Keeffe E, Levy S. Impaired dendritic cell maturation in patients with chronic but not resolved hepatitis C virus infection. Blood. 2001;97:3171. doi: 10.1182/blood.v97.10.3171. [DOI] [PubMed] [Google Scholar]
- 14.Moir L, Ogwaro KM, Malaspina A, et al. Perturbations in B cell responsiveness to CD4+ T cell help in HIV-infected individuals. Proc Natl Acad Sci USA. 2003;100:6057–62. doi: 10.1073/pnas.0730819100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper C, Kilby D. Clinical significance of hepatitis B core antibody positivity in HCV-infected and HCV/HIV coinfected individuals. Clin Infect Dis. 2004;38:1335–7. doi: 10.1086/383155. [DOI] [PubMed] [Google Scholar]
- 16.Knoll A, Hartmann A, Hamoshi H, Weislmaier K, Jilg W. Serological pattern “anti-HBc alone”: characterization of 552 individuals and clinical significance. World J Gastroenterol. 2006;12:1255–60. doi: 10.3748/wjg.v12.i8.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]