Abstract
The synthesis of the potent molluscicide, cyanolide A, has been achieved in 10 steps without the use of protecting groups. The synthesis features a key Sakurai macrocyclization/dimerization reaction that simultaneously forms both tetrahydropyran rings and the macrocycle of the natural product.
The C2-symmetric macrodiolide, cyanolide A, was isolated by Gerwick and co-workers from the cyanobacteria L. bouillonii collected near Pigeon Island, Papua New Guinea.1 The dimer exhibited significant molluscicidal activity against Biomphlalaria glabrara (LC50 = 1.2 μM). This unique biological activity and its interesting structure have inspired four total syntheses. All of the completed syntheses have relied on either Yamaguchi's or Shiina's lactonization protocol to form the macrocylic dimer from complex monomers (Figure 1).2 We report an alternative synthesis of the cyanolide A aglycon in a concise process that avoids the use of protecting groups.
Figure 1.
Retrosynthesis of cyanolide A
Cyanolide A is of particular interest in human health because of its molluscicidal activity; an effective and selective molluscicide agent has the potential to eradicate schistosomiasis, an endemic parasitic infection. Over 200 million people in developing countries have been infected and greater than 700 million people are at risk of this disease.3 It is caused by a trematode flatworm (Schistomosa) that penetrates the skin, laying eggs in the bladder or bowels of the human host. An immune response to the eggs can cause a variety of symptoms including hepatomegaly, splenomegaly, kidney disease, and bladder cancer.4 The worm is transmitted from a variety of water snails that play host to the parasite, and it is transmitted to the human host while bathing in infected water sources.5 Current therapy is heavily dependent upon treatment of infected individuals with the anthelmintic praziquantel.6 Unfortunately, eliminating the parasite from the human host does not protect against future illness, and reinfection is common in patients who are repeatedly exposed to contaminated water. An alternative strategy to prevent schistosomiasis is elimination of the snail host through the use of molluscicides.7 Regrettably, the mostly widely used molluscicide, niclosamide, has low water solubility and is detrimental to the environment.8 The discovery and synthesis of an environmentally benign and cost-efficient molluscicide, such as cyanolide A, would therefore be useful in the eradication of this disease.
Recently, we disclosed a Prins dimerization/macrocyclization strategy to form similar tetrahydropyran-containing macrodiolides.9,10 Unfortunately, the geminal dimethyl group on the 3-position of the THP ring of cyanolide A precludes the use of this strategy. Prins cyclizations that might form 3,3-disubstituted THP rings are known to divert to tetrahydrofurans through either an oxonia-Cope rearrangement followed by a 5-exo cyclization or a Wagner–Meerwein ring contraction of the tetrahydropyranyl cation (eq 1).11 As a result, these ring systems are usually formed through either the intramolecular cyclization of elaborate linear molecules2,12 or addition and reduction of lactone derivatives.13
 |
(Eq 1) |
Since the previously developed Prins reaction would be ineffective in the synthesis cyanolide A, a Sakurai reaction was proposed to form the elusive 3,3-disubstituted THP rings while maintaining the simultaneous dimerization/macrocyclization strategy.14 The activated allyl silane should stabilize the tetrahydropyranyl cation formed, preventing problematic rearrangements. Based on previous results concerning the synthesis of the related dimeric macrolides, monomer 1 was identified as the ideal dimerization/macrocyclization precursor (Figure 1). It has been found that β-acyl aldehydes decompose rapidly under Lewis acidic conditions, so a dimethyl acetal was employed. The monomer would be formed from the esterification of alcohol 3 with β-hydroxy acid 2.
Synthesis of β-hydroxy acid 2 began with ethyl 3,3-diethoxy-2,2-dimethylpropanoate (4) which is obtained in one step from commercially available materials (Scheme 1).15 Cerium(III)-mediated addition to the ethyl ester would produce the allyl silane in one step, but unfortunately addition to the hindered ester 4 was not possible.16 Alternatively, formation of the methyl ketone followed by enol triflate formation afforded acetal 5 in good yield.17 Kumada coupling followed by deprotection of the acetal under mildly acidic conditions produced aldehyde 6. Standard aldol conditions using Nagao's auxiliary with either titanium tetrachloride or tin(II) triflate led exclusively to the proto-desilylated product. Fortunately, Sammakia's conditions utilizing dichlorophenylborane proved mild enough to provide the aldol adduct as a single diastereomer.18 Finally, hydrolysis of the auxiliary provided β-hydroxy acid 2.
Scheme 1.
Synthesis of β-hydroxy acid 2
Alcohol 3 is synthesized in two steps by addition of dimethylthioacetal to (R)-1,2-epoxybutane (8)19 followed by oxidation with iodine in the presence of methanol (Scheme 2). Esterification of alcohol 3 with β-hydroxy acid 2 was challenging, presumably due to the presence of the alcohol on 2. Direct DMAP mediated acylation of alcohol 3 with the thiazolidinethione aldol adduct was unsuccessful.20 Ultimately, Yamaguchi's esterification conditions provided the monomeric product in 61% yield.21
Scheme 2.
Synthesis of monomer 1
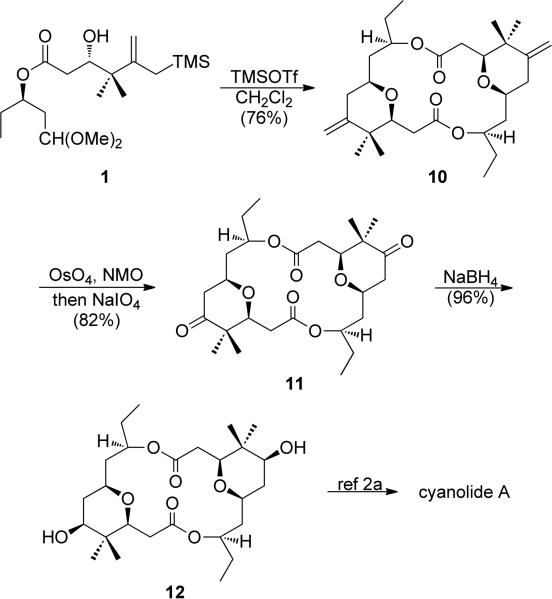
With the monomeric fragment in hand, further elaboration toward cyanolide A through the Sakurai dimerization/macrocyclization was explored (Scheme 3).22 When conditions developed for a Prins reaction (TESOTf in acetic acid) were employed,9 a single product was isolated that was determined to be the trisubstituted endo olefin isomer of the desired product 10. Similar isomerizations have been avoided by running Sakurai reactions at reduced temperatures in diethyl ether.11a Unfortunately, these conditions led to extensive decomposition of the starting material. After screening a variety of solvents, additives, and Lewis acids, it was found that TMSOTf in dichloromethane at –78 °C provided a 76% yield of exo olefin dimer 10 with only 7% of the inseparable endo product.23 Upjohn dihydroxylation24 of dimer 10 and subsequent oxidative cleavage yielded diketone 11. Finally, reduction of the ketone afforded 12, the known aglycon of cyanolide A. The spectroscopic data of this molecule closely matched the data of the previously reported syntheses and has been glycosylated to yield cyanolide A.2abd
Scheme 3.
Synthesis of cyanolide A by a Sakurai dimerization/macrocyclization reaction
The total synthesis of the aglycon of cyanolide A has been completed with a longest linear sequence of 10 steps and 18% overall yield without the use of protecting groups. This marks the shortest synthesis to date. A key Sakurai dimerization/macrocyclization reaction was exploited to develop a significant amount of the molecular complexity in a single step. This strategy allowed facile formation of 3,3-disubstituted tetrahydropyrans, which has proved challenging with other approaches. Further applications of the dimerization/macrocyclization strategy are under development.
Supplementary Material
ACKNOWLEDGMENT
Support was provided by the National Institute of General Medicine (GM-43854). M. R. G. was supported by a Lilly Graduate Research Fellowship.
Footnotes
Supporting Information. Detailed experimental procedures and compound characterization data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Pereira AR, McCue CF, Gerwick WH. J. Nat. Prod. 2010;73:217–220. doi: 10.1021/np9008128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.a Kim H, Hong J. Org. Lett. 2010;12:2880–2883. doi: 10.1021/ol101022z. [DOI] [PubMed] [Google Scholar]; b Hajare AK, Ravikumar V, Khaleel S, Bhuniya D, Reddy DS. J. Org. Chem. 2011;76:963–966. doi: 10.1021/jo101782q. [DOI] [PubMed] [Google Scholar]; c Yang Z, Xie X, Jing P, Zhao G, Zheng J, Zhao C, She X. Org. Biomol. Chem. 2011;9:984–986. doi: 10.1039/c0ob00971g. [DOI] [PubMed] [Google Scholar]; d Pabbaraja S, Satyanarayana K, Ganganna B, Yadav JS. J. Org. Chem. 2011;76:1922–1925. doi: 10.1021/jo102401v. [DOI] [PubMed] [Google Scholar]
- 3.a Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Lancet Infect. Dis. 2006;6:411–425. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]; b Chitsulo L, Engels D, Montresor A, Savioli L. Acta Trop. 2000;77:41–51. doi: 10.1016/s0001-706x(00)00122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.a World Health Organization Report of the Scientific Working Group Meeting on Schistosomiasis; Geneva, Switzerland. Nov 14–16, 2005. [Google Scholar]; b Gryseels B, Polman K, Clerinx J, Kestens L. Lancet. 2006;368:1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 5.Ross AG, Bartley PB, Sleigh AC, Olds GR, Li Y, Williams GM, McManuss DP. N. Engl. J. Med. 2002;346:1212–1220. doi: 10.1056/NEJMra012396. [DOI] [PubMed] [Google Scholar]
- 6.a Cioli D, Pica-Mattoccia L. Parasitol. Res. 2003;90:S3–9. doi: 10.1007/s00436-002-0751-z. [DOI] [PubMed] [Google Scholar]; b Bergquist R. Acta Trop. 2008;108:65–68. doi: 10.1016/j.actatropica.2008.11.002. [DOI] [PubMed] [Google Scholar]; c Melman SD, Steinauer ML, Cunningham C, Kubatko LS, Mwangi IN, Wynn NB, Mutuku MW, Karanja DMS, Colley DG, Black CL, Secor WE, Mjoki GM, Loker ES. Plos Neglected Trop. Dis. 2009;3:e504. doi: 10.1371/journal.pntd.0000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perrett S, Whitfield PJ. Parasitol. Today. 1996;12:156–159. doi: 10.1016/0169-4758(96)10001-6. [DOI] [PubMed] [Google Scholar]
- 8.Andrews P, Thyssen J, Lorke D. Pharmacol. Ther. 1982;19:245–295. doi: 10.1016/0163-7258(82)90064-x. [DOI] [PubMed] [Google Scholar]
- 9.Gesinski MR, Tadpetch K, Rychnovsky SD. Org. Lett. 2009;11:5342–5345. doi: 10.1021/ol9022062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.For a recent review of the Prins macrocyclization strategy, see: Crane EA, Scheidt KA. Angew. Chem., Int. Ed. 2010;49:8316–8326. doi: 10.1002/anie.201002809.
- 11.Lolkema LDM, Semeyn C, Ashek L, Hiemstra H, Speckamp WN. Tetrahedron. 1994;50:7129–7140. [Google Scholar]
- 12.a Smith AB, III, Jurica JA, Walsh SP. Org. Lett. 2008;10:5625–5628. doi: 10.1021/ol802466t. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Huang X, Shao N, Palani A, Aslanian R, Buevich A. Org. Lett. 2007;9:2597–2600. doi: 10.1021/ol071068n. [DOI] [PubMed] [Google Scholar]; c Ghosh AK, Gong G. Org. Lett. 2007;9:1437–1440. doi: 10.1021/ol0701013. [DOI] [PubMed] [Google Scholar]; d Shangguan N, Kiren S, Williams LJ. Org. Lett. 2007;9:1093–1096. doi: 10.1021/ol063143k. [DOI] [PubMed] [Google Scholar]; e Rölle T, Hoffmann RW. Helv. Chim. Acta. 2004;87:1214–1227. [Google Scholar]; f Willson TM, Kocienski P, Jarowicki K, Isaac K, Hitchcock PM, Faller A, Campbell SF. Tetrahedron. 1990;46:1767–1782. [Google Scholar]
- 13.a Crimmins MT, Stevens JM, Schaaf GM. Org. Lett. 2009;11:3990–3993. doi: 10.1021/ol901655e. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Brown LE, Landaverry YR, Davies JR, Milinkevish KA, Ast S, Carlson JS, Oliver AG, Konopelski JP. J. Org. Chem. 2009;74:5405–5410. doi: 10.1021/jo9009003. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Sawant KB, Ding F, Jennings MP. Tetrahedron Lett. 2007;48:5177–5180. [Google Scholar]; d Jiang X, García-Fortanet J, De Brabrander JK. J. Am. Chem. Soc. 2005;127:11254–11255. doi: 10.1021/ja0537068. [DOI] [PubMed] [Google Scholar]; e Yoshimura T, Yakushiji F, Kondo S, Wu X, Shindo M, Shishido K. Org. Lett. 2005;8:475–478. doi: 10.1021/ol0527678. [DOI] [PubMed] [Google Scholar]
- 14.a Keck GE, Covel JA, Schiff T, Yu T. Org. Lett. 2002;4:1189–1192. doi: 10.1021/ol025645d. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Markó IE, Leroy B. Tetrahedron Lett. 2000;41:7225–7230. [Google Scholar]; c Leroy B, Markó IE. Tetrahedron Lett. 2001;42:8685–8688. [Google Scholar]
- 15.Deno NC. J. Am. Chem. Soc. 1947;69:2233–2234. [Google Scholar]
- 16.a Bunnelle WH, Narayanan BA. Org. Synth. 1990;69:89–92. [Google Scholar]; b Lee TV, Channon JA, Cregg C, Porter JR, Roden FS, Yeoh HT-L. Tetrahedron. 1989;45:5877–5886. [Google Scholar]
- 17.Nicolaou KC, Li A, Edmonds DJ, Tria S, Ellery SP. J. Am. Chem. Soc. 2009;131:16905–16918. doi: 10.1021/ja9068003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.a Zhang Y, Phillips AJ, Sammakia T. Org. Lett. 2004;6:23–25. doi: 10.1021/ol036020y. [DOI] [PubMed] [Google Scholar]; b Zhang Y, Sammakia T. J. Org. Chem. 2006;71:6262–6265. doi: 10.1021/jo0605694. [DOI] [PubMed] [Google Scholar]
- 19.Schaus SE, Brandes BD, Larrow JF, Tokunaga M, Hansen KB, Gould AE, Furrow ME, Jacobsen EN. J. Am. Chem. Soc. 2002;124:1307–1315. doi: 10.1021/ja016737l. [DOI] [PubMed] [Google Scholar]
- 20.Wu Y, Sun Y-P, Yang Y-Q, Hu Q, Zhang Q. J. Org. Chem. 2004;69:6141–6144. doi: 10.1021/jo049204e. [DOI] [PubMed] [Google Scholar]
- 21.Inanaga J, Hirata K, Saeki H, Katsuki T, Yamaguchi M. Bull. Chem. Soc. Jpn. 1979;52:1989–1993. [Google Scholar]
- 22.Examples of Sakurai macrocyclization: Wender PA, Schrier AJ. J. Am. Chem. Soc. 2011 doi: 10.1021/ja203034k. ASAP. DOI: 10.1021/ja203034k.Wender PA, DeChristopher BA, Schrier AJ. J. Am. Chem. Soc. 2008;130:6658–6659. doi: 10.1021/ja8015632.
- 23.Employment of catalytic Bi(OTf)3 gave similar results, consistent with Marko's results (ref 13c).
- 24.VanRheenen V, Kelly RC, Cha DY. Tetrahedron Lett. 1976;17:1973–1976. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.