Abstract
Aurora kinase B (AURKB) is critical to the process of mitosis, aiding in chromosome condensation by phosphorylating histone H3. We investigated the effects of AZD1152, an AURKB inhibitor, on radiosensitivity of androgen-insensitive prostate cancer cells. The goal of this study was to test whether AZD1152 increases the susceptibility of hormone-refractory prostate cancer cells to radiation-induced DNA damage and to determine the conditions of AZD1152 treatment that maximize radiosensitization. PC3 and DU145 cells were treated with various AZD1152 doses for various durations to elucidate the conditions that yielded maximal increases in G2/M-phase and polyploid cells. To assess DNA damage, γ-H2AX phosphorylation was quantified for cells grown under radiosensitizing conditions and subjected to either no radiation or 5 Gy radiation. Radiosensitivity was determined by clonogenic assays. Cell cycle effects in both cell lines were maximized by treatment with 60 nM AZD1152 for 48 h. AZD1152-treated cells exhibited significantly increased DNA damage 30 min postirradiation (PC3: 100% compared to 68%, P = 0.035; DU145: 100% compared to 69%, P = 0.034), with additional DNA damage 6 h postirradiation (PC3: 85% compared to 15%, P = 0.002; DU145: 67% compared to 21%, P = 0.012). Radiosensitivity was increased in both cell lines, with dose enhancement ratios of 1.53 for PC3 cells (P = 0.017) and 1.71 for DU145 cells (P = 0.02). This study identifies the optimal AZD1152 treatment conditions to maximize the radiosensitization of PC3 and DU145 cells. These results suggest a major role for DNA damage and impairment of DNA repair mechanisms in AZD1152-induced radiosensitization of prostate cancer cells.
INTRODUCTION
Prostate cancer is the most commonly diagnosed non-cutaneous malignancy in men in the U.S., with an estimated 186,320 new cases in 2008 (1). Surgical resection, radiation therapy and hormone therapy are the main treatment modalities for prostate cancer. Although there are several promising treatment strategies, prostate cancer continues to be a major cause of cancer death in males in the U.S. The most challenging cases of prostate cancer include those that are insensitive to androgen blockade (hormone treatment) and those that have become hormone-refractory after initial hormone and radiotherapy treatment.
Aurora Kinase B (AURKB) has recently emerged as a promising therapeutic target for several malignancies. Aurora kinases are a class of serine/threonine kinases necessary for cell cycle progression. AURKB is a component of the chromosomal passenger complex, functioning in chromosome orientation (2) and in regulation of spindle attachment (3). AURKB phosphorylates histone H3 (p-H3) at the serine 10 position, allowing for chromosome condensation, thus facilitating cytokinesis (4). In normal cell lines, expression of AURKB naturally peaks at the G2/M cell cycle phase transition, thus facilitating cell cycle progression at this juncture (5, 6).
AURKB overexpression is associated with increased genomic instability, and upregulation of the protein has been detected in a number of solid tumors, including prostate cancer (7–9). Additionally, its expression has been associated with poorer prognoses in ovarian, brain and hepatocellular carcinomas (10–12). Inhibition of AURKB activity has been shown to result in shrinkage of tumor xenografts via induction of apoptosis and radiosensitization (13–15). Because of the association of AURKB upregulation with tumorigenesis, inhibition of this kinase may prove to be a promising treatment strategy for a variety of cancers.
AZD1152, along with other inhibitors of AURKB, is known to induce cell cycle arrest, yielding G2/M-phase cells or polyploidy (16–18). Previous studies have linked G2/M-phase cells with increased radiosensitization in adenocarcinoma and colon carcinoma cell lines (14). Because AURKB inhibition results in increased levels of cellular polyploidy (16), inhibition of AURKB results in increased susceptibility to apoptosis (17). This provides a strong rationale that other treatments administered concurrently with AURKB inhibitors, including radiation therapy, could be quite effective in increasing treatment efficacy.
Among the various types of prostate cancer cell lines that have been established for preclinical testing, both PC3 and DU145 human-derived prostate cancer cells lines are notable for their relative insensitivity to androgen treatment, owing to their lack of the intracellular androgen receptor (19). These cell lines model an important population of patients who have prostate cancer that is resistant or refractory to hormone ablation therapy.
The effects of AZD1152 on prostate cancer have not been studied previously, and it is unknown whether the AURKB inhibitor AZD1152 (13) increases the sensitivity of androgen-resistant human prostate cancer cells to radiation treatment. Herein we examined the effects of AZD1152 on cell cycle distribution, DNA damage and radiosensitivity of PC3 and DU145 prostate cancer cells. We tested the hypothesis that AZD1152 increases the radiosensitivity of androgen-insensitive PC3 and DU145 human prostate cancer cells. If AZD1152 or other AURKB inhibitors could be demonstrated to increase the therapeutic index for androgen-resistant prostate cancer, this would have a significant clinical impact.
MATERIALS AND METHODS
Cell Culture and Reagents
PC-3 and DU145 cells (American Type Culture Collection, Rockville, MD) were cultured in RPMI 1640 medium containing 10 % fetal bovine serum and 1% penicillin/streptomycin. All cells were incubated at 37°C in 95% air/5% CO2. AZD1152 was obtained from AstraZeneca (London, England).
Western Immunoblotting
Cells (0.5 × 106) were treated with various concentrations of AZD1152. They were collected at various times and then washed with ice-cold PBS twice before the addition of lysis buffer (M-PER Mammalian Protein Extraction reagent, Pierce, Rockford, IL) including protease inhibitor cocktail (5 μl/ml) and phosphatase inhibitor cocktail I (5 μl/ml, Sigma-Aldrich, St. Louis, MO). Protein concentration was quantified by the Bio-Rad method. Equal amounts of protein were loaded into each well and separated by 12.5% or 15% SDS-PAGE gel, followed by transfer onto PVDF membranes (Bio-Rad). Membranes were blocked with 5% nonfat dry milk in PBST for 1 h at room temperature. The blots were then incubated with anti-phosphohistone H3 (Upstate, Lake Placid, NY), anti-Aurora B (Cell Signaling, Danvers, MA), and anti-Actin (Sigma-Aldrich) for 1 h at 4°C. Goat anti-rabbit IgG secondary (1:5000, Santa Cruz Biotechnologies, Santa Cruz, CA) was incubated for 45 min at room temperature. Western blots were developed using the chemiluminescence detection system (PerkinElmer, Waltham, MA) according to the manufacturer’s protocol and autoradiography.
Cell Cycle Analysis
Cells (5 ×105) were seeded in 10-cm2 dishes 24 h before AZD1152 treatment and then treated with various doses of AZD1152 for 48 h. The cells were then collected by trypsinization, fixed with 70% ethanol, and stored overnight at −20°C. Cells were then collected by centrifugation, resuspended in 1 ml of PBS with 100 μl of 200 g/ml DNase-free RNase A, and incubated at 37°C for 30 min. Propidium iodide (50 μg/ml) was then added, and the cells were incubated at room temperature for 5 min. The number of cells in each phase of the cell cycle was determined and calculated as a percentage of the total cell population.
Clonogenic Assay
Cells were treated with AZD1152 (60 nM, 48 h) or DMSO (control). Cells were irradiated with 0 to 6 Gy at a dose rate of 1.8 Gy/min using a 137Cs irradiator (J. L. Shepherd and Associates, Glendale, CA). After irradiation, the medium was changed and cells were incubated at 37°C for 8 days. Cells were then fixed for 30 min with 70% methanol and stained for 30 min with 1% methylene blue (Sigma-Aldrich) in water. After staining, colonies were counted by eye with a cutoff of 50 viable cells. The surviving fraction was calculated as (mean colony counts)/(cells plated) × plating efficiency (PE), where PE was defined as (mean colony counts)/(cells plated for irradiated controls).
Immunofluorescence for γ-H2AX
PC3 and DU145 cells were grown on sterile cover slips in six-well plates with 3 ml medium. After 24 h, the cells were incubated with DMSO or 60 nM AZD1152. After 48 h of incubation, cells were irradiated with either 0 or 5 Gy. Either 30 min or 6 h after irradiation, the cover slips were washed with cold PBS, and cells were fixed with 4% formaldehyde for 10 min at room temperature. Cells were then washed twice in PBS and placed on cover slips in ice-cold wells. Then 2 ml of ice-cold Triton X-100 solution was added. After 15 min, the cover slips were washed three times in PBS. The cover slips were then blocked in 1% BSA/PBS overnight at 4°C. The next day, mouse anti-human γ-H2AX (Abcam, Cambridge, MA) was added at a dilution of 1:100 in antibody buffer and incubated at 37°C for 30 min. Cells were washed three times in PBS and incubated with a rhodamine green-labeled goat anti-mouse IgG secondary antibody (Molecular Probes, Eugene, OR) at a dilution of 1:100 in antibody buffer at room 37°C for 30 min in the dark. The cover slips were then washed three times in PBS and placed on ice. The cells were then counterstained with 2 ml of 4, 6-diamidino-2-phenylindole (DAPI) for 5 min. The cover slips were washed three times in PBS and mounted using Vectashield on microscope slides. Three random regions of 50 cells each were examined under a microscope with 100× magnification. Nuclei containing ≥40 foci were counted as positive for γ-H2AX focus formation. The percentages of positive cells were calculated and plotted.
Statistical Analysis
All assays performed in this study, including cell culture, immunoblotting, cell cycle quantification, clonogenic analysis and immunofluorescence, were performed in triplicate (n = 3) for each culture of cells randomized to one of the following treatment conditions: (1) control, (2) drug alone, (3) radiation alone and (4) drug and radiation combined. This provided 80% power to detect a difference between two groups using a two-group t test with a 0.05 significance level, assuming an average difference of 15% between any of the two groups and a standard deviation of 5%. Standard deviations for the PC3 and DU145 cells were based on preliminary data obtained in our laboratory. The experimental observations were performed by analysts who were blinded to each of the various treatment conditions. The statistical software SPSS (SPSS, Chicago, IL) was used for all statistical analyses. All tests were two-tailed. Values are expressed as means ± SD.
RESULTS
AZD1152 Results in Decreased Phosphorylation of Histone H3 in PC3 and DU145 Prostate Cancer Cells
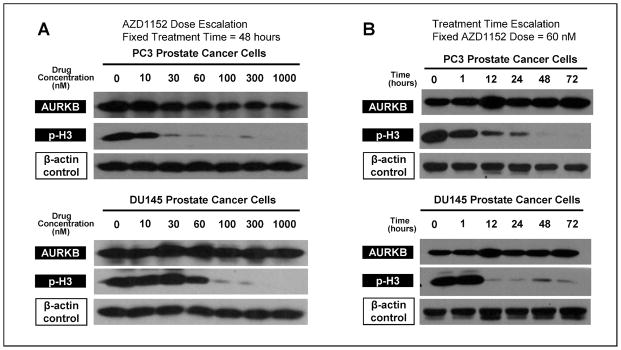
PC3 and DU145 cells were treated with varying concentrations (0–1000 nM) of the Aurora kinase B (AURKB) inhibitor AZD1152 for a total of 48 h. Western blot analysis was used to quantify resulting AURKB. The functionality of AURKB was also assessed by quantifying p-H3, the active, phosphorylated form of histone H3 required for normal chromosomal condensation. As is shown in Fig. 1A, the AURKB expression was stable at all doses for both PC3 and DU145 cells; however, AZD1152 concentrations of at least 60 nM of AZD1152 resulted in diminution of p-H3, consistent with inhibition of AURKB H3-phosphorylating activity. Thus a concentration of 60 nM AZD1152 reached the threshold required to inhibit the activity of AURKB without affecting its expression.
FIG. 1.
AZD1152 treatment of PC3 and DU145 prostate cancer cells results in stable expression of AURKB but decreased levels of phosphorylated histone H3, with increasing effect with escalation of concentration and treatment duration. Panel A: PC3 and DU145 cells were treated with various concentrations of AZD1152 for 48 h, with subsequent immunoblotting analysis. β-Actin was probed as a positive control to demonstrate equal loading. Panel B: PC3 and DU145 cells were treated with 60 nM AZD1152 for various treatment times.
PC3 and DU145 cells were exposed to 60 nM AZD1152 for various times to determine the maximal time dependence of AURKB inhibition. Figure 1B shows that AURKB inhibition was stable at all exposure times tested. However, PC3 cells demonstrated significantly diminished p-H3 levels with 48 h or more of exposure to AZD1152, and DU145 cells demonstrated significantly diminished levels of p-H3 with 12 or more hours of exposure. These data indicate that the inhibition of AURKB by AZD1152 is both dose- and time-dependent.
AZD1152 Induces G2/M Cell Cycle Arrest and Polyploidy
Because AURKB normally facilitates progression beyond the G2/M cell cycle transition point, we used flow cytometry to measure the effects of AURKB inhibition on the distribution of cell cycle phases in PC3 and DU145 cells exposed to AZD1152 for 48 h. Figure 2A (top panel) shows the resulting percentages of each of the cell cycle phases in PC3 cells. At low concentrations of AZD1152, there was a relatively high level of G0/G1-phase cells (67%) and a relatively low level of G2/M-phase cells (18%), indicative of fully functional AURKB. However, as the concentrations were increased from 3 nM to 30 nM, G2/M-phase cells reached levels above 50% and G0/G1-phase cells represented less than 5% of cells. Additionally, the fraction of polyploid cells increased at concentrations of 30 nM. At AZD1152 concentrations above 30 nM, to the maximum tested concentration of 1000 nM, these cell cycle effects were sustained. Cells in the S phase and sub-G0 phase each represented less than 10% of the total population at all dose levels.
FIG. 2.
AZD1152 induces G2/M and polyploidy cell cycle arrest in PC3 and DU145 prostate cancer cells in a dose-responsive and treatment-time-responsive manner. Panel A: PC3 and DU145 cells were treated with increasing concentrations of AZD1152 for 48 h. Panel B: PC3 cells and DU145 cells were treated 60 nM AZD1152 for increasing treatment durations. Cell numbers in each of the cell cycle phases were determined using flow cytometry.
With AZD-induced AURKB inhibition, DU145 cells (Fig. 2A, bottom panel) similarly demonstrated a dose-dependent decreased percentage of G0/G1-phase cells (73% at baseline to <5% at high concentrations) and increased percentage of polyploid cells (<5% to 70%); the transition in cell cycle composition over a concentration range from 10 nM to 100 nM AZD1152. The percentage of G2/M-phase cells increased to a maximal level of 35% at a concentration of 60 nM, with higher concentrations resulting in a somewhat lower G2/M fraction, but still higher than baseline, at concentrations of 100 nM or greater. These cell cycle analyses indicated that AZD1152-induced AURKB inhibition is maximized at concentrations of 60 nM for both PC3 and DU145 prostate cancer cell populations exposed to AZD1152 for 48 h.
Next, the cell cycle effects of AZD1152 treatment were tested in both PC3 and DU145 cells using a fixed concentration of 60 nM AZD1152 but varying the duration of treatment. As shown in Fig. 2B, top panel, PC3 cells demonstrated a time-dependent decrease in the fraction of G0/G1 cells (45% at baseline to <10% at prolonged treatment time), an increased fraction of G2/M cells (35% to 63%), and an increased fraction of polyploid cells (<10% to 35%). Maximal treatment effects were seen with a treatment time of 24 to 48 h. S-phase and sub-G0-phase cells each comprised less than 15% of the total fraction at all treatment times.
The cell cycle effects of durations of varying treatment AZD1152 on DU145 cells is shown in Fig. 2B, bottom panel. As observed in PC3 cells, increasing treatment time resulted in a gradually decreased fraction of G0/G1-phase cells (23% at baseline down to ~1% with prolonged treatments). Like PC3 cells, DU145 cells showed peak levels of G2/M-phase cells at 24 h and a maximal fraction of polyploid cells at 48 to 72 h. Optimal inhibition of AURKB was seen with 60 nM for 48 h for both PC3 and DU145 cells.
Neoadjuvant AZD1152 Followed by Radiation Results in Increased and Sustained DNA Damage
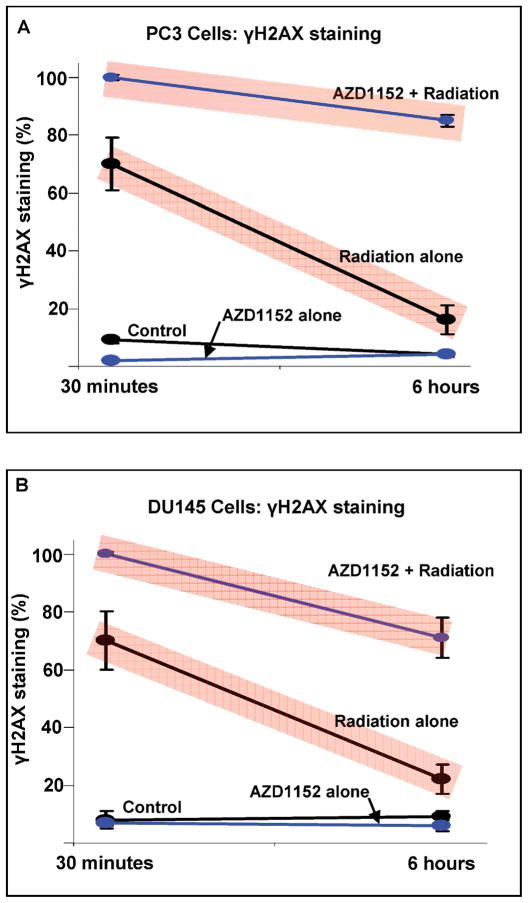
Employing the optimal regimen of 60 nM AZD1152 for 48 h, PC3 and DU145 cells were exposed to radiation and the resulting DNA damage was quantified. Figure 3 shows that PC3 cells not receiving radiation AZD1152 alone demonstrated minimal evidence of DNA double-strand breaks, as indicated by low levels of γ-H2AX foci (Fig. 3A). However, 68% of the PC3 cell population that received 5 Gy radiation alone exhibited evidence of DNA damage. Those PC3 cells that received the combination of AZD1152 and 5 Gy radiation had DNA damage in the entire population of cells, demonstrating a level of DNA damage that was significantly higher than cells exposed to radiation without AZD1152 (68% compared to 100%, P = 0.030). Furthermore, the significantly increased amounts of γ-H2AX foci in PC3 cells were sustained 6 h after radiation treatment (15% for treatment alone compared to 85% for AZD1152 and radiation; P = 0.002). Again, unirradiated cells, either with or without AZD1152, demonstrated minimal evidence of DNA damage at 6 h.
FIG. 3.
AZD1152 increases radiation-induced DNA damage and reduces DNA damage repair processes in PC3 and DU145 cells. The results for four treatment groups are shown: (1) AZD1152 t (60 nM for 48 h) followed by radiation (5 Gy); (2) radiation alone; (3) AZD1152 alone; (4) no radiation and no AZD1152. γ-H2AX was used to measure DNA damage. The means ± SD of three separate experiments are shown.
The response of DU145 cells to single-agent and combination therapy with radiation and AZD1152 (Fig. 3B) was similar to the response exhibited by PC3 cells: 69.3% of DU145 cells treated with radiation alone demonstrated γ-H2AX foci 30 min after irradiation compared to 100% of DU145 cells treated with a combination of radiation and ACS1152 (P = 0.030). These levels of DNA damage were sustained at 6 h after the completion of radiation treatment (21% for treatment alone compared to 70% for combination of AZD1152 and radiation; P = 0.010). Again, unirradiated cells, either with or without AZD1152, demonstrated minimal evidence of DNA damage.
Inhibition of Aurora Kinase B with AZD1152 Results in Radiosensitization of PC3 and DU145 Prostate Cancer Cells
To investigate whether AZD1152 radiosensitizes PC3 and DU145 cells, clonogenic assays were performed on cells treated with the optimal treatment for AZD1152 and varying doses of radiation (Fig. 4A). PC3 cells receiving AZD1152 in combination with radiation had increased sensitivity to the lethal effect of radiation at all doses tested, with a drug enhancement ratio (DER) of 1.53 (surviving fraction = 0.1; P = 0.017). DU145 cells (Fig. 4B) demonstrated significant radiosensitivity with increasing dose, with a DER of 1.71 at a surviving fraction of 0.4 (P = 0.02). The DER was calculated at a surviving fraction of 0.4 because the fraction of control treated DU145 cells never reached the level of 0.1 after 1 to 6 Gy radiation.
FIG. 4.
AZD1152 results in radiosensitization in PC3 and DU145 prostate cancer cells. Cells were treated with AZD1152 (60 nM for 48 h duration) and were then exposed to radiation (0–6 Gy). After 8 days, colonies were stained and scored. Values shown are the means ± SD of three separate experiments. Radiosensitivity was increased in both cell lines.
DISCUSSION
One of the goals of this study was to elucidate the mechanism by which AZD1152, an AURKB inhibitor, affects cell cycling in human-derived PC3 and DU145 prostate cancer cells. AURKB is an interesting therapeutic target because of its ability to facilitate and control cell cycle progression. AURKB phosphorylates histone H3, inducing chromosome condensation and facilitating cytokinesis (4). Several studies have shown that AZD1152 is capable of inhibiting phosphorylation of histone H3 (13–17). While our findings regarding the AZD1152-mediated effects on histone H3 were consistent with the published results for other cell lines, the data presented here did reveal some differences in the response of the PC3 and DU145 cells to AZD1152. We found that p-H3 levels are both dose- and time-dependent with a trend toward decreased levels of p-H3 by 60 nM for 48 h in both cell lines.
Consistent with previous reports detailing the effects of AURKB inhibition, including cell cycle arrest (17,18), our results showed that AZD1152 maximizes the proportion of cells in G2/M phase and polyploidy in PC3 and DU145 cells. The maximum in G2/M phase and polyploid cells occurred at 48 h, also in agreement with previously published data (19).
Previous studies have shown that the expression of p53 appears to predict the effects of AZD1152. Among HCT116 colon cancer cells, those that have a double p53 knockout (p53−/−) demonstrate increased polyploidy compared to wild-type (p53+/+) cells (14). Although we found that AZD1152 resulted in increased levels of both polyploid and G2/M-phase cells in PC3 cells, which are p53−/−, the G2/M phase showed overall predominance. For the DU145 cells, which are characteristically p53+/+, our results showed a prevalence of polyploid cells. This is not entirely unanticipated, however, because DU145 cells express heterozygous 233Leu and 274Phe p53 mutations, neither of which behaves as a dominant negative mutation. Some studies have suggested that mutations expressed simultaneously are able to completely inactivate p53 growth suppressive function (20). Thus it is plausible that p53 dysfunctionality is responsible for the accumulation of polyploid cells in the presence of an AURKB inhibitor.
Previous reports of the effects of AZD1152 on cells of acute myeloid leukemia cell lines showed increased fractions of both G2/M-phase and polyploid cells and a simultaneous increase in S-phase cells (16). In contrast, our data for PC3 and DU145 prostate cancer cells showed decreases in S-phase cells in response to AZD1152 treatment.
The results presented here have confirmed our hypothesis that AZD1152 treatment of human-derived PC3 and DU145 prostate cancer cells results in increased sensitivity to radiation. One of the further primary goals of these investigations was to maximize the radiosensitizing effects of AZD1152 for these androgen-insensitive prostate cancer cell lines. Because G2/M and polyploid cells predominately contain double-stranded DNA, we sought to determine the treatment conditions with AZD1152 that result in the greatest proportion of G2/M-phase and polyploid cells. Our experiments showed that AZD1152-induced inhibition of AURKB is both dose- and time-dependent and that 60 nM AZD1152 for 48 h resulted in the largest increase in polyploid and G2/M-phase cells in both PC3 and DU145 cells. These conditions were subsequently used to investigate the effects of radiation on DNA damage and cell survival.
To better characterize the temporal effects of radiation and AURKB inhibition on PC3 and DU145 cells, we quantified DNA damage at two times. The first, at 30 min postirradiation, reflects the initial susceptibility of these cells to radiation-induced DNA damage. The second, at 6 h postirradiation, when compared longitudinally to the first time, is indicative of the extent of DNA repair. DNA repair begins soon after irradiation. γ-H2AX foci peak within an hour, and focus half-lives average between 2 and 4 h (21–23). More damage was induced by radiation in both treated and control cells, though it was more sustained in AZD1152-treated populations. PC3 cells, which exhibited an increase in both G2/M-phase and polyploid cells, sustained more damage than DU145 cells, in which polyploid cells predominated. Also of note, PC3 cells lack p53 while DU145 cells express p53 mutations. These data are thus consistent with previous observations that p53-deficient cells have a longer H2AX half-life (22).
Individual cells that are incapable of repairing DNA breaks will eventually undergo cell death (24). Thus either an increase in DNA damage or a delay in DNA repair or both may result in increased radiosensitization. This was borne out in the radiation survival data (Fig. 4). Greater cytotoxicity was exhibited by PC3 cells treated with AZD1152 compared to control (DMSO), with a drug enhancement ratio of 1.53 at a surviving fraction of 0.1 (P = 0.017). In comparison, DU145 cells, which were previously shown to be composed primarily of polyploid cells after AZD1152 treatment, also showed enhanced radiosensitization, with a drug enhancement ratio of 1.71 at a surviving fraction of 0.4 (P = 0.02). Although it is possible that factors other than DNA damage may play a role in radiosensitization, these data indicate that polyploid cells may be more susceptible to radiation-induced cell death.
AURKB is highly expressed in hormone-refractory prostate cancer in patients and in DU145 and PC3 cells (8). Inhibition of AURKB using siRNA technology was associated with inhibition of growth of prostate cancer xenografts (25). Additionally, concomitant use of siRNAs against AURKB and EGFR resulted in further suppression of tumor growth. These results demonstrate the value of targeting several pathways and using multiple modalities to achieve optimal response to therapy. Radiotherapy is an essential treatment modality for prostate cancer and is frequently used with hormone therapy in managing locally advanced cases. Resistance of prostate cancer to the current available treatments, including hormone therapy, surgery and radiation therapy, is a significant clinical problem that affects the survival of patients, and the development of new treatment strategies is therefore critical for improving patient outcome. Our data indicate a potential role for AZD1152-induced AURKB inhibition in the treatment of prostate cancer with radiation therapy. AZD1152 in combination with radiation therapy results in enhanced killing of androgen-insensitive prostate cancer cells and may ultimately have the potential to improve the cure rate for patients with locally advanced prostate cancer. Further studies are warranted to assess the in vivo and clinical efficacy of AZD1152 in the treatment of hormone-refractory prostate cancer.
Acknowledgments
We would like to thank Jiaqing Li for his laboratory assistance. This work was supported in part by U.S. Department of Defense Grant DOD W23RYX-3305-N603 and Vanderbilt CTSA grant 1 UL1 RR024975 from NCRR/NIH.
References
- 1.Cancer Facts and Figures 2008. American Cancer Society; Atlanta: 2008. [Google Scholar]
- 2.Adams RR, Maiato H, Earnshaw WC, Carmena M. Essential roles of Drosophila inner centromere protein (INCENP) and aurora B in histone H3 phosphorylation, metaphase chromosome alignment, kinetochore disjunction, and chromosome segregation. J Cell Biol. 2001;153:865–880. doi: 10.1083/jcb.153.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hauf S, Cole RW, LaTerra S, Zimmer C, Schnapp G, Walter R, Heckel A, van Meel J, Rieder CL, Peters JM. The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J Cell Biol. 2003;161:281–294. doi: 10.1083/jcb.200208092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrews PD, Knatko E, Moore WJ, Swedlow JR. Mitotic mechanics: the auroras come into view. Curr Opin Cell Biol. 2003;15:672–683. doi: 10.1016/j.ceb.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Altieri DC. Molecular circuits of apoptosis regulation and cell division control: the surviving paradigm. J Cell Biochem. 2004;92:656–663. doi: 10.1002/jcb.20140. [DOI] [PubMed] [Google Scholar]
- 6.Carmena M, Earnshaw WC. The cellular geography of aurora kinases. Nat Rev Mol Cell Biol. 2003;4:842–854. doi: 10.1038/nrm1245. [DOI] [PubMed] [Google Scholar]
- 7.Lee EC, Frolov A, Li R, Ayala G, Greenberg NM. Targeting Aurora kinases for the treatment of prostate cancer. Cancer Res. 2006;66:4996–5002. doi: 10.1158/0008-5472.CAN-05-2796. [DOI] [PubMed] [Google Scholar]
- 8.Chieffi P, Cozzolino L, Kisslinger A, Libertini S, Staibano S, Mansueto G, De Rosa G, Villacci A, Vitale M, Tramontano D. Aurora B expression directly correlates with prostate cancer malignancy and influence prostate cell proliferation. Prostate. 2006;66:326–333. doi: 10.1002/pros.20345. [DOI] [PubMed] [Google Scholar]
- 9.Ota T, Suto S, Katayama H, Han ZB, Suzuki F, Maeda M, Tanino M, Terada Y, Tatsuka M. Increased mitotic phosphorylation of histone H3 attributable to AIM-1/Aurora-B overexpression contributes to chromosome number instability. Cancer Res. 2002;62:5168–5177. [PubMed] [Google Scholar]
- 10.Araki K, Nozaki K, Ueba T, Tatsuka M, Hashimoto N. High expression of Aurora-B/Aurora and Ipll-like midbody-associated protein (AIM-1) in astrocytomas. J Neurooncol. 2004;67:53–64. doi: 10.1023/b:neon.0000021784.33421.05. [DOI] [PubMed] [Google Scholar]
- 11.Kulkarni AA, Loddo M, Leo E, Rashid M, Eward KL, Fanshawe TR, Butcher J, Frost A, Ledermann JA, Stoeber K. DNA replication licensing factors and aurora kinases are linked to aneuploidy and clinical outcome in epithelial ovarian carcinoma. Clin Cancer Res. 2007;13:6153–6161. doi: 10.1158/1078-0432.CCR-07-0671. [DOI] [PubMed] [Google Scholar]
- 12.Zeng WF, Navaratne K, Prayson RA, Weil RJ. Aurora B expression correlates with aggressive behaviour in glioblastoma multiforme. J Clin Pathol. 2007;60:218–221. doi: 10.1136/jcp.2006.036806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilkinson RW, Odedra R, Heaton SP, Wedge SR, Keen NJ, Crafter C, Foster JR, Brady MC, Bigley A, Green S. AZD1152, a selective inhibitor of Aurora B kinase, inhibits human tumor xenograft growth by inducing apoptosis. Clin Cancer Res. 2007;13:3682–3688. doi: 10.1158/1078-0432.CCR-06-2979. [DOI] [PubMed] [Google Scholar]
- 14.Tao Y, Zhang P, Girdler F, Frascogna V, Castedo M, Bourhis J, Kroemer G, Deutsch E. Enhancement of radiation response in p53-deficient cancer cells by the Aurora-B kinase inhibitor AZD1152. Oncogene. 2008;27:3244–3255. doi: 10.1038/sj.onc.1210990. [DOI] [PubMed] [Google Scholar]
- 15.Kim KW, Mutter RW, Willey CD, Subhawong TK, Shinohara ET, Albert JM, Ling G, Cao C, Gi YJ, Lu B. Inhibition of surviving and aurora B kinase sensitizes mesothelioma cells by enhancing mitotic arrests. Int J Radiat Oncol Biol Phys. 2007;67:1519–1525. doi: 10.1016/j.ijrobp.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 16.Walsby E, Walsh V, Pepper C, Burnett A, Mills K. Effects of the aurora kinase inhibitors AZD1152-HQPA and ZM447439 on growth arrest and polyploidy in acute myeloid leukemia cell lines and primary blasts. Haematologica. 2008;93:662–669. doi: 10.3324/haematol.12148. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Anderson MG, Tucker LA, Shen Y, Glaser KB, Shah OJ. Inhibition of Aurora B kinase sensitizes a subset of human glioma cells to TRAIL concomitant with induction of TRAIL-R2. Cell Death Differ. 2009;16:498–511. doi: 10.1038/cdd.2008.174. [DOI] [PubMed] [Google Scholar]
- 18.Yang J, Ikezoe T, Nishioka C, Tasaka T, Taniguchi A, Kuwayama Y, Komatsu N, Bandobashi K, Togitani K, Yokoyama A. AZD1152, a novel and selective aurora B kinase inhibitor, induces growth arrest, apoptosis, and sensitization for tubulin depolymerizing agent or topoisomerase II inhibitor in human acute leukemia cells in vitro and in vivo. Blood. 2007;110:2034–2040. doi: 10.1182/blood-2007-02-073700. [DOI] [PubMed] [Google Scholar]
- 19.van Bokhoven A, Varella-Garcia M, Korch C, Johannes WU, Smith EE, Miller HL, Nordeen SK, Miller GJ, Lucia MS. Molecular characterization of human prostate carcinoma cell lines. Prostate. 2003;57:205–225. doi: 10.1002/pros.10290. [DOI] [PubMed] [Google Scholar]
- 20.Gurova KV, Rokhlin OW, Budanov AV, Burdelya LG, Chumakov PM, Cohen MB, Gudkov AV. Cooperation of two mutant p53 alleles contributes to Fas resistance of prostate carcinoma cells. Cancer Res. 2003;63:2905–2912. [PubMed] [Google Scholar]
- 21.Galleani J, Miranda C, Pierotti MA, Greco A. H2AX phosphorylation and kinetics of radiation-induced DNA double strand break repair in human primary thyrocytes. Thyroid. 2009;19:257–264. doi: 10.1089/thy.2008.0195. [DOI] [PubMed] [Google Scholar]
- 22.Banath JP, MacPhail SH, Olive PL. Radiation sensitivity, H2AX phosphorylation, and kinetics of repair of DNA strand breaks in irradiated cervical cancer cell lines. Cancer Res. 2004;64:7144–7149. doi: 10.1158/0008-5472.CAN-04-1433. [DOI] [PubMed] [Google Scholar]
- 23.Mahrhofer H, Burger S, Oppitz U, Flentje M, Djuzenova CS. Radiation induced DNA damage and damage repair in human tumor and fibroblast cell lines assessed by histone H2AX phosphorylation. Int J Radiat Oncol Biol Phys. 2006;64:573–580. doi: 10.1016/j.ijrobp.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 24.Riches LC, Lynch AM, Gooderham NJ. Early events in the mammalian response to DNA double-strand breaks. Mutagenesis. 2008;23:331–339. doi: 10.1093/mutage/gen039. [DOI] [PubMed] [Google Scholar]
- 25.Addepalli MK, Ray KB, Kumar B, Ramnath RL, Chile S, Rao H. RNAi-mediated knockdown of AURKB and EGFR shows enhanced therapeutic efficacy in prostate tumor regression. Gene Ther. 2010;17:352–359. doi: 10.1038/gt.2009.155. [DOI] [PubMed] [Google Scholar]