Abstract
Chromosomal deletions at 6p25-p24 are rare findings in patients with developmental delay. There is limited information about the adult phenotype. We present a 36-year-old patient with schizophrenia, mild mental retardation, progressive hearing deficits, and characteristic facial features. Ocular (Axenfeld–Rieger anomaly) abnormalities were diagnosed in infancy; vision, however, has remained unimpaired. There were no other major congenital anomalies. Brain imaging showed only minor changes. There was no family history of intellectual deficits or psychosis. Karyotyping revealed a 6p25 deletion, and detailed fluorescence in situ hybridization (FISH) analyses using 23 probes confirmed a 6.7 Mb 6p25-pter deletion. The breakpoint is near a possible 6p25-p24 locus for schizophrenia. Psychotic illness may be part of the neurodevelopmental abnormalities and long-term outcome of patients with 6p terminal deletions. Other similarly affected patients likely remain to be diagnosed in adult populations of schizophrenia and/or mental retardation.
Keywords: 6p, subtelomeric deletion, chromosomal abnormality, adult phenotype, schizophrenia
INTRODUCTION
6p25 deletion syndrome involves a group of rare chromosomal aberrations [Davies et al., 1996]. The syndrome is characterized by ocular anomalies (of the anterior chamber of the eye) typically Axenfeld–Rieger malformation, progressive hearing loss, and craniofacial features including brachycephaly, hypertelorism, down-slanting palpebral fissures, tented mouth, up-turned nose, smooth philtrum, and low set ears. Patients with interstitial 6p24-p22 deletions tend to have a different phenotype [Davies et al., 1999a]. Besides the variable degrees of developmental delay and mental retardation found in most patients, there is limited information on the adult or neurobehavioral phenotype. We present a case that suggests schizophrenia may be a feature in patients with 6p25 deletion syndrome.
METHODS
The 36-year-old Caucasian patient studied was initially assessed as part of a study screening for genetic syndromes in adults with schizophrenia. After providing informed consent to participate in the study, patients had a clinical screening assessment involving brief medical history and targeted physical examination. Patients considered to have features consistent with a possible genetic syndrome had further assessments and investigations. These included a standardized physical examination for dysmorphic features in adults as described elsewhere [Scutt et al., 2001], family and developmental history and medical records review. Intellectual level was assessed using WAIS-III, and functioning using Vineland Adaptive Behavioral Scale scores.
Initial clinical cytogenetic investigation involved standard G-banding of blood lymphocytes and fluorescence in situ hybridization (FISH) for 22q11.2 deletions using the TUPLE1 (Vysis/Abbott Laboratories, des Plaines, IL) probe as previously described [Bassett et al., 2000, 2005]. Detailed FISH analyses on a research basis using probes from 6p23-p25 followed initial karyotype results.
RESULTS
Clinical Report
The patient is the youngest daughter of nonconsanguineous parents. She was born at term by spontaneous vaginal delivery to a 30-year-old mother and a 32-year-old father. The pregnancy was complicated by severe morning sickness and several episodes of bleeding leading to induction of labor at 37 weeks’ gestation. Birth weight was 3,250 g (75th centile).
A hospital assessment at age 10 months found developmental delay, hypertelorism, Axenfeld anomaly (bilateral posterior embryotoxon and iridocorneal bands; bilateral iris atrophy), and pectus excavatum. There was no hypotonia, and cardiac exam, hemoglobin, calcium level, and protein electrophoresis were normal. Bilateral calcaneal valgus deformity was conservatively managed with corrective boots. Congenital strabismus was surgically corrected at age 5 years, at which time asymptomatic mitral valve incompetence and minimal left ventricular hypertrophy were clinically suspected from physical examination and electrocardiogram findings. In early adolescence, the patient underwent surgery to reduce prognathism. Recurrent serous otitis media required multiple bilateral myringotomies and tube insertions from ages 6 to 27 years. Mild bilateral conductive hearing loss at age 6 years had progressed by age 35 years to moderate left sensorineural and right severe mixed hearing loss treated with hearing aids. At age 35 years she had no functional visual impairment and had normal intraocular pressures on repeated assessment since age 10 months. A skull X-ray showed unusual skull base with angle approaching 180° and a very shallow sella.
Mild articulation difficulties and mild mental retardation were noted in early childhood, and the patient received special education throughout her schooling. At age 26 years she began to have gradually decreasing social functioning, and increasing irritability and depression. Auditory hallucinations and grandiose delusions were recognized at about age 31 years, accompanied by grossly disorganized behavior. She met DSM-IV criteria for schizophrenia, but antipsychotic treatment did not begin until age 33 years. Symptoms responded to clozapine after trials of other antipsychotics were discontinued due to lack of response and side effects. Fluoxetine, which had been used to treat associated depression, was discontinued after a single generalized seizure at age 36 years. An EEG found no lateralizing, focal, or paroxysmal features. A CT scan at age 35 years showed generalized subcortical and central brain atrophy and bilateral periventricular white matter attenuation. MRI at age 36 years confirmed diffuse moderate supratentorial atrophy and confluent and punctate white matter changes with small foci of encephalomalacia.
Physical examination at age 35 years revealed height of 154 cm (10th centile), weight 95.3 kg (BMI =40.2), and occipito-frontal circumference 55.5 cm (75–90th centile). Craniofacial features included brachycephaly, short neck, low posterior hairline; long, asymmetric face with maxillary flattening and prognathism; hypertelorism and small, slightly up-slanted eyes; large, low set and posteriorly rotated ears with simplified helices; short nose with up-turned tip, broad nasal bridge and anteverted nares; flat philtrum; mouth with thin upper lip and slightly down-turning corners (Fig. 1). Palate was narrow and high-arched. Pectus excavatum was evident. Hands showed slight hyperflexibility with tapered fingers and bilaterally triphalangeal thumbs. Lower limbs showed bilateral flat feet, hallux valgus, and 4–5th camptodactyly. There were no other congenital anomalies noted. She had a wide-based gait and difficulties with coordination. Echocardiogram and abdominal ultrasound found no cardiac, renal, or other congenital anomalies; however, the ultrasound showed gallstones.
Fig. 1.
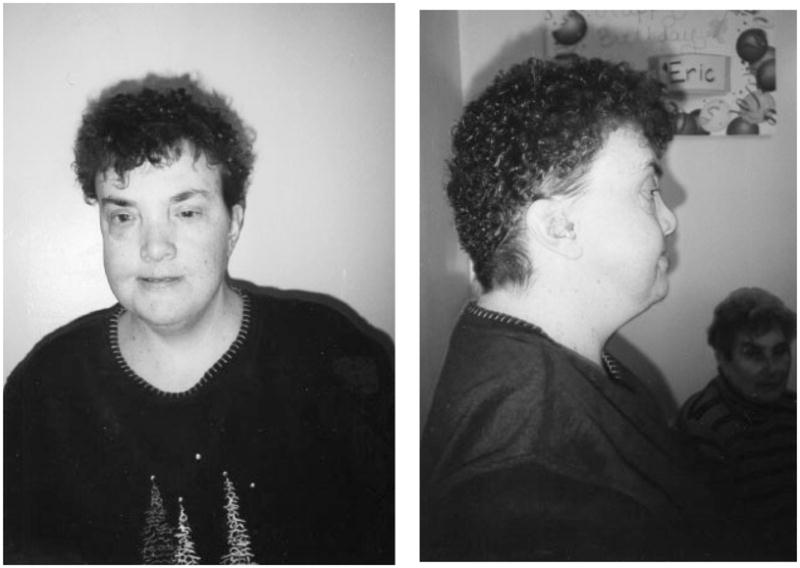
Frontal and profile views of patient at age 36 years showing characteristic facial features of 6p25 deletion syndrome.
Cognitive assessments performed at age 36 years showed a WAIS-III full scale IQ of 53 (Verbal IQ =59, Performance IQ =56), corresponding to mild to moderate mental retardation, with moderate to severe impairments in tests of attention, memory, and executive functioning. Vineland Adaptive Behavior Scale scores in age equivalents showed overall functioning at 8 years 11 months, daily living skills at 9 years 6 months, communication skills at 8 years 3 months, and socialization skills at 8 years 11 months. Currently, at age 37 years she lives in a group home in frequent contact with her family and participates in and enjoys day programs in the community (e.g., hairdressing, cooking).
There was no family history of psychotic illness or intellectual deficits. The proband’s father had died suddenly at age 57 years of cirrhosis. There was a history of glaucoma with onset in middle age in the patient’s mother and maternal grandmother.
Cytogenetic and FISH Studies
A karyotype performed in 1967, likely involving solid Giemsa staining, showed normal female chromosomes. At age 36 years, screening and follow-up assessments confirmed a history of Axenfeld anomaly and showed suggestive features indicating the likelihood of a genetic syndrome consistent with 6p25 deletion. Clinical chromosomal assessment using standard G-banding at a resolution of 500 bands showed 46,XX,del(6)(p25), and FISH for 22q11 deletion syndrome using TUPLE1 probe was negative. Testing of available family members (mother and sister) showed normal chromosomes. Molecular cytogenetic studies using FISH and 6p probes described elsewhere [Davies et al., 1999b; Mirza et al., 2004], with the addition of clones DJ470L22 and DJ69L16 selected from the Ensembl database (http://www.ensembl.org/Homo_sapiens/contingview?mapfrag=RP3-470L22; http://www.ensembl.orgHomo_sapiens/contingview?mapfrag=RP11-69L16), were used to refine the breakpoint. The research FISH analysis (Fig. 2) confirmed the clinical diagnosis of a 6.7 Mb 6p25-pter terminal deletion; results are summarized in Table I.
Fig. 2.
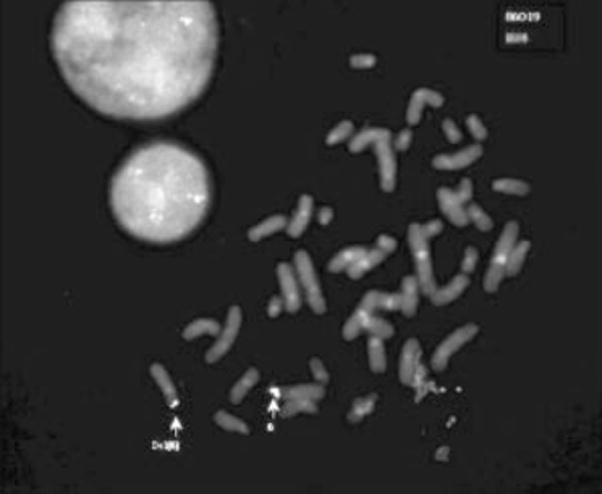
Example of a result of in situ hybridization on metaphase chromosomes of the case. Probes were labeled with biotin and digoxigenin and detected using fluorescein isothiocyanate or tetramethylrhodamine isothiocyanate systems, respectively. Chromosomes were counterstained with 4′,6-diamidino-2-phenylindole. Probe DJ 86O19 (green) hybridized on chromosomes 6 and del(6) whereas the more distal probe B24 (red) hybridized only to the normal chromosome (see Table I).
TABLE I.
FISH Characterization of the 6p25 Deletion in the Patient
| Location on 6p | Distance from 6p telomere (Mb) | Clones 6p Tel (FISH probes) | Markers and genes | Current case report |
|---|---|---|---|---|
| 25.3 | 0.5 | DJ20B11 | FLJ11026 | − |
| 25.3 | 1.5 | DJ118b18 | FOXC1 | − |
| 25.3 | 1.7 | DJ 279I9 | GMDS | − |
| 25.3 | 2.1 | DJ33B19 | GMDS | − |
| 25 | B24 | P1 REGION | − | |
| 25 | DJ36I2 | PX TO CDYL | − | |
| 25 | 5.1 | DJ67E13 | ALO35653 | − |
| 25 | 5.75 | DJ182O16 | D6S1685 | − |
| 25 | 6.1 | DJ103H18 | D6S1677 | − |
| 25 | 6.4 | DJ287K15 | D6S477(F13A) | − |
| 25 | 6.4 | DJ23021 | D6S1677 | − |
| 25 | 6.5 | DJ80N2 | PRP1 | − |
| 25.1 | 6.7 | DJ470L22 | AL136361 | − |
| 25 | 7.3 | DJ69L16 | D6S1159 | − |
| 25 | 7.5 | DJ29L9 | DSP | − |
| 25 | 7.3 | DJ69L16 | D6S1159 | + |
| 25/24.3 | 7.3 | DJ155I9 | D6S1640 | + |
| 25/24.3 | 8 | DJ303A1 | BMP6 | + |
| 24.3 | 8.9 | DJ133H11 | D6S410 | + |
| 24.3 | DJ136F24 | WI-6424 | + | |
| 24.3 | DJ860I9 | + | ||
| 24.3 | 11.2 | DJ110P13 | SGC33794 | + |
| 24.3 | DJ151C13 | STSG26237 | + |
–, absence of signals on deletion chromosome 6 for that probe; +, signals detected on both intact and deletion chromosomes 6 for that probe.
DISCUSSION
To our knowledge, we report the oldest adult with 6p25 deletion syndrome and the first with schizophrenia. Table II presents a summary of this patient in the context of previous reports of adults and adolescents with distal 6p deletions [Jalal et al., 1989; Palmer et al., 1991; Law et al., 1998; Davies et al., 1999b; Le Caignec et al., 2005]. The patient presented in this report has many typical features of 6p25 deletion syndrome, and appears to have greatest similarity to patient SG in Law et al., 1998 and patient 3 in Mirza et al., 2004, with respect to the phenotype and extent of deletion [Law et al., 1998; Mirza et al., 2004]. A normal karyotype in infancy, normal vision, few other health problems and lack of genetics follow-up likely contributed to lack of recognition of a diagnosable syndrome until she was specifically assessed as an adult. The case supports recent evidence that 6p25 deletion syndrome has a recognizable clinical phenotype and supports recommendations for thorough investigations including FISH for patients presenting with characteristic features [Davies et al., 1999b; Le Caignec et al., 2005; Maclean et al., 2005].
TABLE II.
Adolescents and Adults With 6p25-pter Deletions
| Case reports | [Le Caignec et al., 2005] | [Jalal et al., 1989] | [Palmer et al., 1991] | [Law et al., 1998; Davies et al., 1999b] | [Mirza et al., 2004] | Current case |
|---|---|---|---|---|---|---|
| Age (y) | 15 | 21 | 18 | 32 | 201 | 36 |
| Sex | Female | Female | Female | Male | Female | Female |
| Extent of deletion | 6p25 8 Mb | 6p23-pter | 6p23-pter | 6p24.3-pter 6.7 Mb | 6p25.1-pter 6.57 Mb | 6p25-pter 6.7 Mb |
| Mental retardation | Severe | Severe | (Mild) learning difficulties | Mild to moderate | ||
| Neurobehavioral phenotype | Schizophrenia | |||||
| Functioning | Reduced | Reduced | Full time factory job | Day program activities | ||
| Speech | Delayed | Delayed | Delayed | Severely delayed | Slow | No delay; mild articulation problems |
| Motor | Delayed; hypotonia | Delayed | No delay or hypotonia | |||
| Structural brain anomalies | Moderate atrophy; white matter changes | |||||
| OFC | +1 SD | 2nd centile | 90th centile | Macrocephaly | 75th centile | |
| Height | −1 SD | <5th centile | 9–25th centile | 10th centile | ||
| Characteristic facies | Yes | Yes | Yes | Yes | ||
| Cardiac defect | No | Bicuspid aortic valve | No | |||
| Anterior eye chamber anomaly | Yes | Yes; bilateral | Yes | Yes; bilateral | ||
| Vision | Divergent strabismus | Poor; divergent squint | Divergent squint | Normal; strabismus | ||
| Hearing deficit | Bilateral conductive | Profound; progressive sensorineural | Conductive | Progressive sensorineural, conductive | ||
| Myringotomy tubes | Yes; bilateral | Yes | Yes; multiple | |||
| Genitalia | Hypogonadism | |||||
| Other | Left mammary hypoplasia; hypodontia; hypoplastic malleus on CT scan | Dental abnormalities; telangiectasia | Prognathism; supranumerary nipple; clumsy gait | Vasculitis; abdominal hernias; cleft uvula | Perthes disease; dental abnormalities; pigmentary retinopathy | Prognathism; gallstones; bilateral valgus deformity |
Blank, not reported/not mentioned.
Phenotypic findings from age 13 years; phenotypes with later onset would not be documented.
Neurobehavioral phenotypes, however, including psychiatric diagnoses and IQ levels, are relatively rarely mentioned in case reports of 6p deletions or other genetic syndromes, thus it is unclear whether or not they may be associated features. This is partly due to the young age of most cases, and like deafness which may be progressive, neurobehavioral features may be under-reported. This patient had onset of schizophrenia at about age 31 years. The illness is often diagnosed somewhat later in women than in men and treatment may be delayed for many reasons [Lieberman and Fenton, 2000]. Cognitive decline is common in schizophrenia and the illness is more common in patients with lower IQ; prevalence in mental retardation is about three times that of the general population rate of ~1% [Turner, 1989]. The schizophrenia in this case may be coincidental, however, there was no family history of psychiatric disorder and there is a possible schizophrenia susceptibility locus in a nearby region. In addition, recent mouse mutational models indicate that many loci in the region syntenic to distal 6p may have neurobehavioral phenotypes in the heterozygous state [Bogani et al., 2005].
There is some evidence that the 6p region may contain two susceptibility loci for schizophrenia. The most studied is a 6p22 locus containing the DTNBP1 (dysbindin) candidate gene, but there is also a locus at 6p25-p24 [Straub et al., 2002]. This more distal locus has recently been reported to be linked to a subtype of schizophrenia with greater general intellectual deficits [Hallmayer et al., 2005]. There are many genes in the 6p25-pter region, and it is likely that phenotypes associated with contiguous gene deletions may arise due to the cumulative consequence of losing several genes [Bogani et al., 2005]. One gene of potential interest is NRN-1 (neuritin), located at 6p25 about 6 Mb from the telomere. NRN-1 is involved in synapse remodeling, and thus fits a recent proposal for synaptic abnormalities [Owen et al., 2005] and the neurodevelopmental pathogenesis model of schizophrenia [Bassett et al., 2001]. The identification of patients with schizophrenia and genetic syndromes such as 6p25 deletion syndrome may thus be valuable for investigations of the molecular basis of schizophrenia [Bassett et al., 2000] and neurodevelopmental phenotypes of these syndromes using mouse models [Bogani et al., 2005].
More adults with 6p25 deletions likely remain to be found. They would help clarify the adult phenotype which remains to be characterized for 6p25 deletion syndrome as for most genetic syndromes.
Acknowledgments
The authors thank the patient and her family for their participation. This research was supported by grants from the W. Garfield Weston Foundation and by a Distinguished Investigator Award from the National Alliance for Research on Schizophrenia and Depression (A.S.B) and Canada Research Chair in Schizophrenia Genetics (A.S.B).
References
- Bassett AS, Chow EWC, Weksberg R. Chromosomal abnormalities and schizophrenia. Am J Med Genet Part C Semin Med Genet. 2000;97C:45–51. doi: 10.1002/(sici)1096-8628(200021)97:1<45::aid-ajmg6>3.0.co;2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett AS, Chow EWC, O’Neill S, Brzustowicz LM. Genetic insights into the neurodevelopmental hypothesis of schizophrenia. Schizophr Bull. 2001;27:417–430. doi: 10.1093/oxfordjournals.schbul.a006884. [DOI] [PubMed] [Google Scholar]
- Bassett AS, Chow EW, Husted J, Weksberg R, Caluseriu O, Webb GD, Gatzoulis MA. Clinical features of 78 adults with 22q11 deletion syndrome. Am J Med Genet Part A. 2005;138A:307–313. doi: 10.1002/ajmg.a.30984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogani D, Willoughby C, Davies J, Kaur K, Mirza G, Paudyal A, Haines H, McKeone R, Cadman M, Pieles G, Schneider JE, Bhattacharya S, Hardy A, Nolan PM, Tripodis N, Depew MJ, Chandrasekara R, Duncan G, Sharpe PT, Greenfield A, Denny P, Brown SD, Ragoussis J, Arkell RM. Dissecting the genetic complexity of human 6p deletion syndromes by using a region-specific, phenotype-driven mouse screen. Proc Natl Acad Sci USA. 2005;102:12477–12482. doi: 10.1073/pnas.0500584102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies AF, Mirza G, Flinter F, Ragoussis J. An interstitial deletion of 6p24-p25 proximal to the FKHL7 locus and including AP-2alpha that affects anterior eye chamber development. J Med Genet. 1999a;36:708–710. [PMC free article] [PubMed] [Google Scholar]
- Davies AF, Mirza G, Sekhon G, Turnpenny P, Leroy F, Speleman F, Law C, van Regemorter N, Vamos E, Flinter F, Ragoussis J. Delineation of two distinct 6p deletion syndromes. Hum Genet. 1999b;104:64–72. doi: 10.1007/s004390050911. [DOI] [PubMed] [Google Scholar]
- Hallmayer JF, Kalaydjieva L, Badcock J, Dragovic M, Howell S, Michie PT, Rock D, Vile D, Williams R, Corder EH, Hollingsworth K, Jablensky A. Genetic evidence for a distinct subtype of schizophrenia characterized by pervasive cognitive deficit. Am J Hum Genet. 2005;77:468–476. doi: 10.1086/432816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalal SM, Macias VR, Roop H, Morgan F, King P. Two rare cases of 6p partial deletion. Clin Genet. 1989;36:196–199. doi: 10.1111/j.1399-0004.1989.tb03188.x. [DOI] [PubMed] [Google Scholar]
- Law CJ, Fisher AM, Temple IK. Distal 6p deletion syndrome: A report of a case with anterior chamber eye anomaly and review of published reports. J Med Genet. 1998;35:685–689. doi: 10.1136/jmg.35.8.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Caignec C, De Mas P, Vincent MC, Boceno M, Bourrouillou G, Rival JM, David A. Subtelomeric 6p deletion: Clinical, FISH, and array CGH characterization of two cases. Am J Med Genet Part A. 2005;132A:175–180. doi: 10.1002/ajmg.a.30409. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Fenton WS. Delayed detection of psychosis: Causes, consequences, and effect on public health. Am J Psychiatry. 2000;157:1727–1730. doi: 10.1176/appi.ajp.157.11.1727. [DOI] [PubMed] [Google Scholar]
- Maclean K, Smith J, St Heaps L, Chia N, Williams R, Peters GB, Onikul E, McCrossin T, Lehmann OJ, Ades LC. Axenfeld-Rieger malformation and distinctive facial features: Clues to a recognizable 6p25 microdeletion syndrome. Am J Med Genet Part A. 2005;132A:381–385. doi: 10.1002/ajmg.a.30274. [DOI] [PubMed] [Google Scholar]
- Mirza G, Williams RR, Mohammed S, Clark R, Newbury-Ecob R, Baldinger S, Flinter F, Ragoussis J. Refined genotype-phenotype correlations in cases of chromosome 6p deletion syndromes. Eur J Hum Genet. 2004;12:718–728. doi: 10.1038/sj.ejhg.5201194. [DOI] [PubMed] [Google Scholar]
- Owen MJ, O’Donovan MC, Harrison PJ. Schizophrenia: A genetic disorder of the synapse? BMJ. 2005;330:158–159. doi: 10.1136/bmj.330.7484.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer CG, Bader P, Slovak ML, Comings DE, Pettenati MJ. Partial deletion of chromosome 6p: Delineation of the syndrome. Am J Med Genet. 1991;39:155–160. doi: 10.1002/ajmg.1320390208. [DOI] [PubMed] [Google Scholar]
- Scutt L, Chow EWC, Weksberg R, Honer WG, Bassett AS. Patterns of dysmorphic features in schizophrenia. Am J Med Genet Part B Neuropsychiatr Genet. 2001;105B:713–723. doi: 10.1002/ajmg.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RE, MacLean CJ, Ma Y, Webb BT, Myakishev MV, Harris-Kerr C, Wormley B, Sadek H, Kadambi B, O’Neill FA, Walsh D, Kendler KS. Genome-wide scans of three independent sets of 90 Irish multiplex schizophrenia families and follow-up of selected regions in all families provides evidence for multiple susceptibility genes. Mol Psychiatry. 2002;7:542–559. doi: 10.1038/sj.mp.4001051. [DOI] [PubMed] [Google Scholar]
- Turner TH. Schizophrenia and mental handicap: A historical review, with implications for further research. Psychol Med. 1989;19:301–314. doi: 10.1017/s0033291700012344. [DOI] [PubMed] [Google Scholar]


