Abstract
OBJECTIVES
We sought to determine the impact of implantable cardioverter-defibrillator (ICD) therapy in patients with familial arrhythmogenic right ventricular cardiomyopathy (ARVC).
BACKGROUND
Arrhythmogenic right ventricular cardiomyopathy is a cause of sudden cardiac death, which may be prevented by ICD.
METHODS
We studied 11 families in which a 3p25 deoxyribonucleic acid (DNA) haplotype at locus ARVD5 segregated with disease and compared mortality in subjects who received an ICD with that in control subjects who were matched for age, gender, ARVC status, and family. Subjects (n = 367) at 50% a priori risk of inheriting ARVC were classified as high risk (HR) (n = 197), low risk (n = 92), or unknown (n = 78) on the basis of clinical events, DNA haplotyping, and/or pedigree position. Forty-eight HR subjects (30 males, [median age 32 years] and 18 females [median age 41 years]) were followed after ICD (secondary to ventricular tachycardia [VT] in 27%). Survival was compared with 58 HR control subjects who were alive at the same age to-the-day at which the ICD subject received the device.
RESULTS
In the HR group, 50% of males were dead by 39 years and females by 71 years: relative risk of death was 5.1 (95% confidence interval 3 to 8.5) for males. The five-year mortality rate after ICD in males was zero compared with 28% in control subjects (p = 0.009). Within five years, the ICD fired for VT in 70% and for VT >240 beats/min in 30%, with no difference in discharge rate when analyzed by ICD indication.
CONCLUSIONS
The unknown mutation at the ARVD5 locus causing ARVC results in high mortality. Risk stratification using genetic haplotyping and ICD therapy produced improved survival for males.
Treatment of ventricular tachyarrhythmias, which often cause sudden cardiac death (SCD), includes antiarrhythmic drugs and implantable cardioverter-defibrillator (ICD) therapy. Several randomized control trials have compared these treatment modalities (1–4) and showed that antiarrhythmic medications have limited effectiveness (5). Therefore, ICD therapy often is considered to be the treatment of choice for ventricular tachyarrhythmias (6). In addition, it is considered safe; rare complications include infections (7), surgical complications (8), electrode problems (9), and inappropriate shocks (10).
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is a cause of SCD, usually due to tachyarrhythmias (11), for which ICD therapy may be effective. Arrhythmogenic right ventricular cardiomyopathy may be a significant cause of SCD in young adults in the U.S. (12) and Italy (13), although its true prevalence is unknown (14). Diagnostic criteria are determined on the basis of clinical, structural, and functional features, with the gold standard for diagnosis being the presence of fat and fibrous tissue on cardiac biopsy or autopsy (15–17). However, antemortem diagnosis is difficult despite investigations, including 12-lead electrocardiogram (ECG), signal-averaged electrocardiogram (SAECG), echocardiography, magnetic resonance imaging (MRI), and Holter monitoring (HM). These tests are nonspecific, particularly as the true penetrance of the disease is not clear and variable expression is present both between and within families (15).
Arrhythmogenic right ventricular cardiomyopathy is recognized to be familial (15,18–23) in an estimated 30% of cases (17,24). However, comprehensive assessments of extended family trees rarely are documented (25), and familial cases are likely underrecognized (26). Known causes of autosomal-dominant ARVC include mutations in the cardiac ryanodine receptor (RYR2) (27,28) and desmoplakin (29) genes. Other autosomal-dominant ARVC loci include regions on chromosomes 2 (30), 3 (31), 10 (32,33), and 14 (34,35). It is clear that ARVC is a genetically heterogeneous group of diseases with phenotypic overlap (36). The number of ARVC subjects in previous studies defined by genotype is small (27,29,30,32–35,37–41).
A Newfoundland family with autosomal-dominant ARVC was reported in 1988 (42). The diagnosis of ARVC was made after postmortem documentation of fat and fiber in the myocardium and of clinical features, fulfilling published criteria (17). In this family, ARVC was linked subsequently to the short arm of chromosome 3 encompassing a critical region of 9.3cM (31) at 3p25 (ARVD5). Subsequently, 10 additional families with ARVC were studied, and the critical region was reduced to 2cM, at which locus the identical set of DNA markers (haplotype) cosegregates with ARVC. This segregation with disease across generations suggests a common founder in these 11 Newfoundland families (43) and provides by far the largest group of subjects with ARVC defined by genotype. Therefore, risk stratification of subjects by ARVC status is feasible in these families on the basis of family history, clinical history, and genetic haplotype (linkage analysis) data (a methodology used clinically in several genetic disorders before determining an underlying causative mutation) (44,45).
Previous studies of ICD therapy in patients with ARVC retrospectively assessed efficacy in individual patients in the absence of appropriate controls (9,46–49). We investigated mortality after the implantation of ICDs in 48 high-risk (HR) subjects from 11 families with an autosomal-dominant homogeneous form of ARVC linked to 3p25 and compared mortality with that in HR control subjects, who were matched for age and gender, from the same families.
METHODS
Sample population
Thirty-five families were referred to the provincial genetics program because of SCD and a family history of cardiomyopathy. Eleven were determined to have autosomal-dominant ARVC (Fig. 1) on the basis of established histopathologic, clinical, and genetic criteria (17). One of these families was the original family in which the linkage to chromosome 3 was defined (31,50), and 10 other families were found to be linked to the same region. Five hundred fifty-one individuals from extant and ancestral generations from these 11 families who were born at a priori 50% pedigree risk (ARVC-affected individuals and their first-degree relatives) were identified. To minimize the effects of ascertainment and referral bias, 367 subjects (212 male, 155 female) from “well-ascertained” sibships were studied. Well-ascertained sibships were defined as those where ≥50% of siblings were known to be at high or low risk of ARVC as determined on the basis of clinical, pedigree, and/or haplotype data. High risk was defined as documented sustained ventricular tachycardia (VT) or otherwise-unexplained SCD <50 years of age, the presence of a HR DNA haplotype, and/or obligate carrier status (affected offspring and parent) by pedigree analysis. Subjects could be defined based on any or all of these criteria for HR status. Low risk (LR) was defined as LR DNA haplotype. Otherwise, subjects were considered unknown risk status (UK). The terms HR and LR are used because the common disease haplotype is assumed to cosegregate with the as-yet unknown disease-causing mutation.
Figure 1.
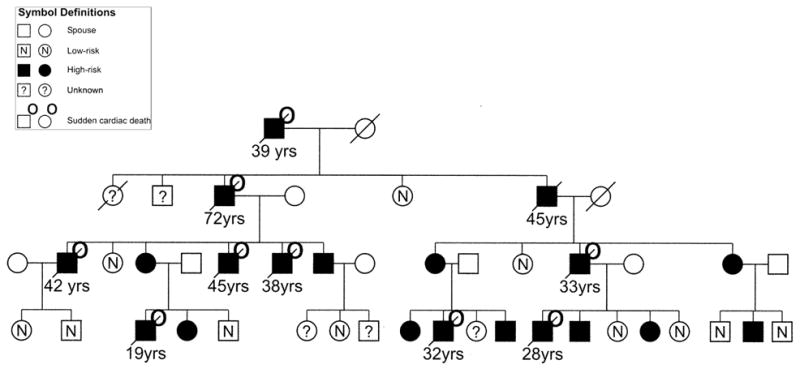
Part of one of 11 autosomal-dominant families with arrhythmogenic right ventricular cardiomyopathy linked to 3p25 showing family members at high risk (n = 197), low risk (n = 92), and unknown (n = 78) status.
Consent for analysis of past and current cardiology records and for DNA analysis was obtained from each subject (or surrogate) in compliance with the Human Investigation Committee requirements of the Health Care Corporation of St. John’s. All available clinical records and autopsy reports were obtained.
A cardiomyopathy genetics research clinic was initiated in 1998. Serial data (retrospective and prospective) on subjects at a priori 50% risk were collected (including clinical events, 12-lead ECG, SAECG, HM echocardiography, and MRI), and subjects were offered genetic haplotype analysis. Implantable cardioverter-defibrillator therapy was offered to these subjects on the basis of a HR DNA haplotype and an abnormal clinical test that was compatible with ARVC (n = 15 males and 18 females). None of these subjects had documented VT. Two more males received an ICD after a HR DNA result alone. Seven males before 1998 and six males who received implants in other North American centers had ICDs because of sustained VT that required cardioversion. No individual to date has declined an ICD, although one HR subject refused follow-up and died suddenly at 31 years of age. One subject died six weeks after first attending clinic during clinical work-up, and prior to receipt of a HR haplotype result. Thus, we report on the outcome of 30 male and 18 female subjects with ICDs, 73% of which were implanted for the primary prevention of VT.
All ICD recipients were followed biannually, at which time the ICD was examined. After confirmation of sustained ventricular tachyarrhythmias, verified by the stored electrocardiograms after ICD discharge, time to first appropriate discharge for documented VT was recorded, as was time to first VT >240 beats/min. A rate of 240 beats/min was chosen to delineate potentially fatal arrhythmias (in the absence of the ICD) from probable nonfatal arrhythmias (51,52). Implantable cardioverter-defibrillator discharge was not included if it occurred within one week of the implant date. Inappropriate ICD discharge was defined as atrial fibrillation, sinus tachycardia, or non-sustained VT.
Haplotype analysis
Of 367 subjects from well ascertained sibships, 201 (55%) had a venous blood sample collected. DNA was extracted from peripheral lymphocytes as previously described (53). For each polymorphic short tandem repeat DNA marker, fluorescent-labeled primers were used to amplify di-, tri-, or tetra nucleotide repeats by the polymerase chain reaction, and fragments were analyzed by capillary gel electrophoresis by the use of an ABI 310 genetic analyzer (Applied Biosystems, Foster City, California). The presence or absence of the 2cM HR haplotype was determined for each family member assessed.
Study design
Age to death was determined in the initial cohort of 197 HR, 92 LR, and 78 UK subjects from the 11 ARVC families, with censoring at last follow-up or at time of ICD implantation. Survival after ICD was determined and compared with that of control subjects after entry to the study. For each ICD subject, control HR subjects were selected who did not receive an ICD, were the same gender, were alive at the age to-the-day at which the ICD subject received the ICD, and were first- or second-degree relatives of the ICD subject. All available first- and second-degree relatives meeting these criteria were used as controls (n = 40; 69%). If there were no age/gender-matched first- or second-degree relatives, one third- or fourth-degree relative was used (n = 11; 19%). In the absence of any appropriate relatives, one age- and gender-matched control subject from a family in the same geographical region was used (n = 7; 12%).
The primary outcome was age at death defined to the day. Secondary outcomes were time to initial appropriate ICD device discharge for sustained VT and time to first VT >240 beats/min. Time to first appropriate discharge was compared in those who had ICD for primary versus secondary prophylaxis and in those with versus those without left ventricular enlargement (LVE) based on the Henry calculations defined as 2 SD above the mean for LVE (112%) (54,55).
Statistical analysis
We used version 11.5 of the SPSS statistics package for statistical analyses of clinical tests (SPSS, Chicago, Illinois). Categorical data were compared by the chi-square test. A p value of <0.05 was considered significant. Survival was calculated according to the Kaplan-Meier product limit method. Relative risk was calculated using Cox’s regression model. Follow-up continued until death or June 3, 2003.
RESULTS
An affected-only linkage analysis was performed in the first large Newfoundland family described (42). Of 17 affected individuals who had DNA samples available for molecular analysis, 11 manifested symptomatic sustained VT before they were 50 years of age, 1 demonstrated ARVC pathology at autopsy, and 5 were obligate ARVC disease gene carriers. Twenty-one deceased family members who transmitted ARVC through five generations also were considered obligate carriers. Linkage of disease to 3p25 was confirmed with a maximum multipoint logarithm of the odds score of 9.3 at D3S1585. Analysis of eight markers allowed the construction of a chromosome 3p25 HR haplotype with D3S3610-Fibulin2-D3S2385-D3S1585-D3S1554-708d1CA-316A10CA-D3S3613 ordered from telomere to centromere shared by all affected family members. The HR haplotype also was identified in all affected subjects from 10 other families with autosomal-dominant ARVC.
As shown in Table 1, 197 (54%) of the well-ascertained sample was designated HR. The method of determining risk status in men was approximately evenly divided between pedigree position (33%), DNA haplotype (31%), and subjects who were <50 years of age with SCD or VT (36%). Few women were diagnosed as HR because they had SCD or VT. Two hundred seven (56%) of the 367 subjects were born after 1950. Most HR male subjects had died, in comparison with approximately one quarter of HR female subjects, and 76% of male and 39% of female deaths were due to SCD. Only four LR subjects died, which is compatible with survival being necessary to obtain a blood sample for DNA tests (Table 1). It is likely that most of those with UK status are in fact LR.
Table 1.
Demographic Data, Method of Risk Determination, and Cause of Death in 367 Subjects at an a Priori 50% Risk of Inheriting ARVC, From 11 Families Linked to 3p25: Defined by ARVC Risk Status
| High Risk (n = 197)
|
Low Risk (n = 92)
|
Unknown (n = 78)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male (n = 123)
|
Female (n = 74)
|
Male (n = 46)
|
Female (n = 46)
|
Male (n = 43)
|
Female (n = 35)
|
|||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |
| Born <1950 (n = 160) | 61 | 50 | 32 | 43 | 10 | 22 | 13 | 28 | 27 | 63 | 17 | 49 |
| Born ≥1950 (n = 207) | 62 | 50 | 42 | 57 | 36 | 78 | 33 | 72 | 16 | 37 | 18 | 51 |
| Methods of diagnosis | ||||||||||||
| Obligate carrier (OC) |
41 | 33 | 30 | 41 | — | — | — | — | — | — | — | — |
| DNA haplotype |
38 | 31 | 41 | 55 | 46 | 100 | 46 | 100 | — | — | — | — |
| Clinical event (SCD or VT) | 44 | 36 | 3 | 4 | — | — | — | — | — | — | — | — |
| Dead | 82 | 67 | 18 | 24 | 1 | 2 | 3 | 6 | 19 | 44 | 11 | 31 |
| SCD | 62 | 50 | 7 | 9 | — | — | — | — | 1 | 2 | — | — |
ARVC = arrhythmogenic right ventricular cardiomyopathy; SCD = sudden cardiac death; VT = ventricular tachycardia.
Overall mortality in the ARVC population
Median survival in HR males (n = 123) was 39 years (95% confidence interval [CI] 36 to 42), in LR males (n = 46) was 76 years (95% CI 76), and in unknown males (n = 43) was 67 years (95% CI 63 to 71). Median survival for HR females (n = 74) was 71 years (95% CI 64 to 78), for LR females (n = 46), the median age could not be computed because of the lack of events, and UK females (n = 35) was 83 years (95% CI 51 to 115 years). The mortality rate was significantly greater in HR than LR males (p = 0.00001) and HR than LR females (p = 0.01). The youngest age at death in HR males was 19 years, and the oldest was 72 years. In HR females, the respective ages were 37 and 88 years. Female HR subjects survived significantly longer than male HR subjects (p = 0.00001) (Fig. 2). The relative risk of death for males compared with females in the HR group was 5.1 (95% CI 3 to 8.5). We also assessed mortality in subjects born before and after 1950 to take into account possible effects of differences in risk status determination: results remained the same (data not shown).
Figure 2.
Time to death or last follow-up (censored) in high-risk males and females with arrhythmogenic right ventricular cardiomyopathy linked to 3p25.
Subjects studied
There were 30 males who received ICDs at median age of 33 years (range, 15 to 51 years) and 18 females at median age of 42 years (range, 22 to 56 years). Implantable cardioverter-defibrillators were implanted in 17 males and 18 females in the absence of documented VT (primary prophylaxis) and in the remaining 13 males after VT (secondary prophylaxis). Fifty-eight control subjects consisted of 36 males whose median age at study entry was 33 years (range, 15 to 51 years) and 22 female subjects whose median age at study entry was 39 years (range, 22 to 56 years) (Table 2).
Table 2.
Demographic Data and Method of Risk Determination of ARVC in 48 Subjects at High Risk Who Received an ICD and 58 Matched Control Subjects From 11 Families With ARVC Linked to 3p25
| Demographic Parameters |
ICD Cohort
|
Control Cohort
|
ICD Cohort M+F 48 | Control Cohort M+F 58 | p Value* | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Males (n = 30)
|
Females (n = 18)
|
Males (n = 36)
|
Females (n = 22)
|
||||||||
| n | % | n | % | n | % | n | % | ||||
| Born <1950 | 2 | 6 | 1 | 5 | 25 | 69 | 19 | 86 | 3 | 44 | <0.0001 |
| Born ≥1950 | 28 | 93 | 17 | 95 | 11 | 31 | 3 | 14 | 45 | 14 | |
| Methods of diagnosis | |||||||||||
| Obligate carrier (OC) | 4 | 13 | 4 | 50 | 18 | 51 | 17 | 49 | 8 | 35 | <0.0001 |
| DNA haplotype | 22 | 74 | 14 | 39 | 5 | 50 | 5 | 50 | 36 | 10 | <0.0001 |
| Clinical event (SCD or VT) | 4 | 13 | 0 | 0 | 13 | 10 | 0 | 0 | 4 | 13 | <0.05 |
| Mean age at entry (yrs) | 33.3 | 40.3 | 32.2 | 40 | ns | ||||||
| Median age at entry (yrs) | 32.9 | 42 | 32.7 | 39.2 | ns | ||||||
| Standard deviation (yrs) | 9.8 | 9.0 | 9.6 | 9.5 | ns | ||||||
Chi-square with 1 df for ICD M+F vs. control M+F.
ARVC = arrhythmogenic right ventricular cardiomyopathy; ICD = implantable cardioverter-defibrillator; SCD = sudden cardiac death; VT = ventricular tachycardia.
Most of the ICD subjects had their risk status defined by DNA haplotype and were born after 1950. In contrast, 36 of 58 of the control subjects were born before 1950 and had risk status defined by pedigree position. The age at entering the study was the same in the control group as the age of receipt of ICD, reflecting the matching strategy. There were no statistically significant differences between ICD cases and control subjects in medication profile, the presence of syncope or chest pain, or abnormalities on SAECG, ECG, HM, or MRI. However, a significantly greater proportion of cases had documented palpitation and presyncope than did the controls (Table 3). The abnormalities noted on clinical testing are listed in Table 3 (17,54,56–64).
Table 3.
Clinical Manifestations of ARVC in 48 Subjects at High Risk Who Received an ICD and 58 Matched Control Subjects From 11 Families With ARVC Linked to 3p25
| ICD Cohort (n = 48)
|
Control Cohort (n = 58)
|
Chi-Square 1 df, p Value | |||||
|---|---|---|---|---|---|---|---|
| No. With Manifestation |
No. With Documentation |
% | No. With Manifestation |
No. With Documentation |
% | ||
| Antiarrhythmic medications* | |||||||
| Class 1 | 10 | 44 | 23 | 11 | 42 | 26 | ns |
| Sotalol | 13 | 44 | 33 | 6 | 31 | 13 | ns |
| Amiodarone | 11 | 44 | 27 | 5 | 30 | 17 | ns |
|
| |||||||
| Symptoms | |||||||
| Palpitations | 35 | 44 | 79 | 15 | 32 | 47 | 0.003 |
| Presyncope | 29 | 44 | 66 | 12 | 32 | 37 | 0.01 |
| Syncope | 15 | 44 | 34 | 5 | 33 | 15 | ns |
| Chest pain | 14 | 44 | 32 | 18 | 35 | 51 | ns |
| Heart failure | 6 | 47 | 13 | 10 | 31 | 32 | 0.04 |
|
| |||||||
| Abnormal clinical tests† | |||||||
| 12-lead ECG‡ | 33 | 48 | 69 | 32 | 33 | 97 | 0.002 |
| SAECG§ | 17 | 31 | 55 | 5 | 12 | 42 | ns |
| Echocardiogram¶ | 24 | 42 | 57 | 20 | 25 | 80 | ns |
| Holter monitor|| | 33 | 42 | 79 | 20 | 24 | 83 | ns |
| MRI** | 4 | 23 | 23 | 0 | 4 | 0 | ns |
| Catheterization†† | 0 | 18 | 0 | 3 | 15 | 20 | 0.05 |
| Autopsy‡‡ | 0 | 0 | 0 | 16 | 17 | 94 | |
Class 1 medications: mexilitine, propafenone, procainamide, quinidine, and phenytoin. The larger denominator for the class 1 medication takes into account those with no records because they never visited a doctor.
Abnormalities on clinical tests.
Electrocardiogram (ECG) T-wave inversion, septal Q waves, poor R-wave progression in V1, V2, and V3, or presence of PVCs (17, 56).
Signal-averaged ECG (SAECG): >115 ms QRS duration (17).
Echocardiogram: >112% Left ventricular dilatation (54), any right ventricular dilatation, focal or global hypokinesis, or akinesis of any cardiac wall (17, 57–59).
Magnetic resonance imaging (MRI): evidence of fatty/fibrous infiltrate or ventricular thinning (61–63).
Cardiac catheterization: any critical coronary artery disease.
ARVC = arrhythmogenic right ventricular cardiomyopathy; ICD = implantable cardioverter-defibrillator.
Mortality after ICD
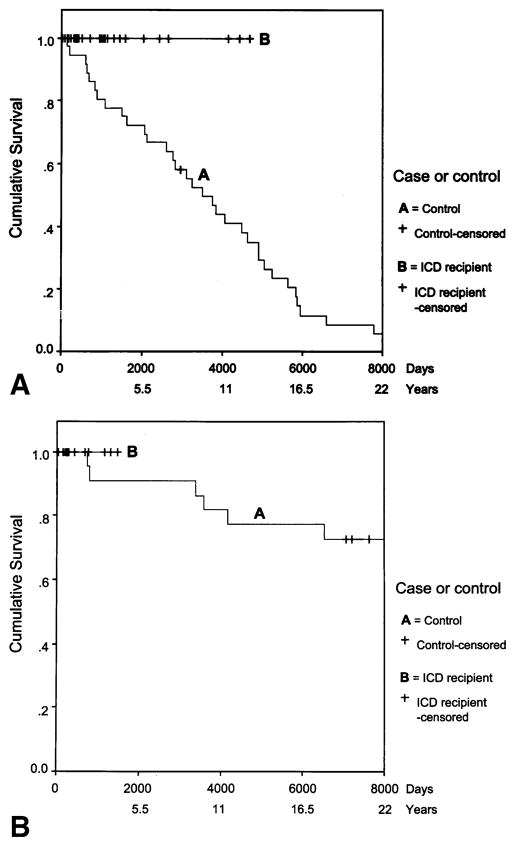
The male ICD group was followed for a median of 2.6 years (from 3 weeks to 12.8 years) and the control group for a median of 9.5 years (from 4.5 months to 31 years). There were no deaths in the ICD group. Three subjects went on to successfully receive a heart transplant after several years (range, 2 to 10 years) of ICD therapy. Indications for transplant included increasing heart failure as a secondary consequence of cardiomyopathy and intractable VT. One subject in the control group received a heart transplant; the remaining 35 subjects died. The five-year mortality rate for males after ICD therapy was 0 compared to 28% in controls (p = 0.009) (Fig 3A).
Figure 3.
(A) Time to death or last follow-up (censored) in 30 male subjects at high-risk for arrhythmogenic right ventricular cardiomyopathy linked to 3p25 who received implantable cardioverter-defibrillators (ICDs) and 36 matched control subjects. (B) Time to death or last follow-up (censored) in 18 female subjects at high risk for arrhythmogenic right ventricular cardiomyopathy linked to 3p25 who received ICDs and 22 matched control subjects.
The female ICD group (n = 18) was followed for a median of 0.7 years, from 2 weeks to 3.9 years, and the control group (n = 22) for a median of 28.8 years, from 1.9 years to 37.8 years. There were no deaths in the ICD group, whereas in the control group, 10 of 22 (45%) subjects had died. The five-year mortality rate in the control group was 9%, which was not significantly different from that in the females who received an ICD (Fig. 3B).
Time to appropriate first discharge of the ICD
In the male ICD group (n = 30), 14 subjects (47%) had at least one appropriate discharge for VT. The median time to first discharge was 2.9 years (95% CI 1.17 to 4.6 years; range, 2 weeks to 5.1 years). The five-year cumulative frequency for first appropriate ICD discharge was 70%, which was significantly greater than the five-year mortality rate for the control group (p = 0.0005). However, the five-year mortality in the control group was similar to the five-year cumulative rate of 28% for VT >240 beats/min in the ICD group (Fig. 4). Two (11%) of 18 female ICD subjects had an appropriate first discharge, both for VT >240 beats/min.
Figure 4.
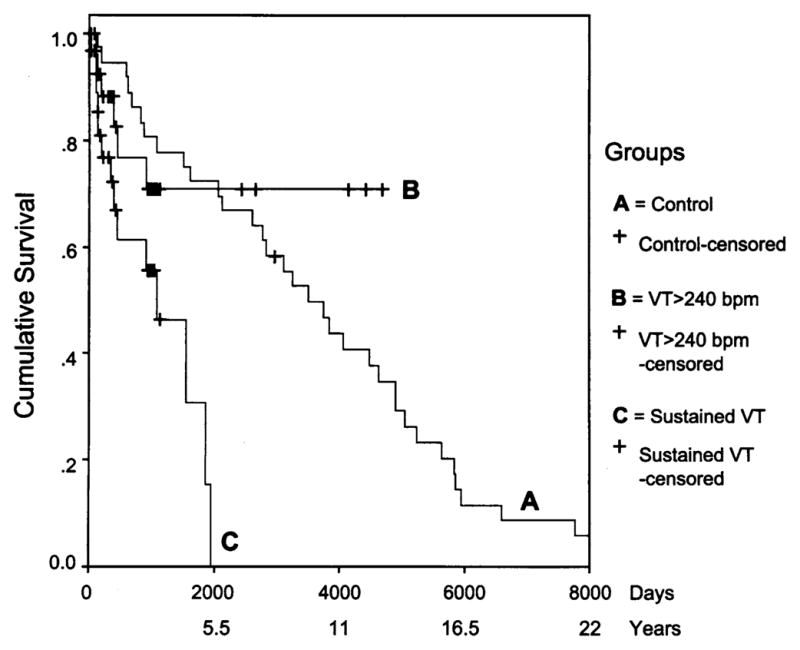
Time to first appropriate discharge or last follow-up (censored) for sustained ventricular tachycardia (VT) and for VT >240 beats/min in males who received an implantable cardioverter-defibrillator and time to death or last follow-up (censored) in matched controls with arrhythmogenic right ventricular cardiomyopathy linked to 3p25.
We compared discharge for VT in males based on whether the ICD was for primary or secondary indications. No significant difference existed between the groups (median time 2.5 years, 95% CI 0 to 5.2 years vs. 2.9 years, 95% CI 0 to 6 years, respectively). A similar observation was made when time to first discharge for VT >240 beats/min was assessed (results not shown). No significant difference in discharge in those with or without LVE was observed. In 16 subjects with LVE, 45% had fired within five years, and in 10 subjects without LVE, all had fired. With respect to side effects observed, 5 (10%) of the 48 ICD subjects have had single inappropriate shocks, and 3 had lead fracture, requiring replacement.
DISCUSSION
Mortality
The results of this study, from a large genetically homogenous population, demonstrate that ARVC, which is caused by an unknown mutation at the ARVD5 locus on chromosome 3, is a life-shortening disorder. There is a significantly increased relative risk of earlier death in men compared with women, in agreement with previous studies (25,65), although the mortality rates in the current study are higher than those for other familial forms of ARVC (41).
Guidelines for the prophylactic use of ICD in ARVC are not established (66). Most subjects in this study were essentially asymptomatic, presenting a dilemma with respect to therapy application. The use of ICD was thus influenced by the fact that the presenting symptom often was SCD and the extensive family histories defined a malignant natural history. Clinical testing in subjects from ARVC families in this study who were at an a priori 50% pedigree risk, and the availability of genetic haplotyping, allowed accurate determination of those at greatest risk, permitting prophylactic implantation of an ICD.
Efficacy of ICD
Few studies have assessed the efficacy of ICD therapy in ARVC (9,46–49,67). The largest series to date (48) studied a multicenter group of 132 patients, a smaller study assessed mainly primary prophylaxis (49), and a single-center study (9) reported on 60 subjects with advanced disease. These studies of heterogenous ARVC subjects estimated ICD mortality benefit based on discharge-free survival. In contrast, the 11 large ARVC families linked by a founder haplotype in this current study provided the etiologic homogeneity that facilitated the identification of a robust control group matched for HR status, age, gender, and family. As a result, a closer estimate of the true magnitude of ICD therapy could be derived. The results clearly demonstrated that ICD therapy significantly reduced mortality in HR men from the ARVC families studied. Interestingly, these ICD mortality benefit results (28% five-year total mortality reduction in males compared with familial controls) appear similar to those in previous studies where discharge-free survival was estimated: 36% five-year total reduction (9) and 28% three-year reduction (48). Few side effects of ICD therapy occurred, and all were resolved. It is notable that decreased mortality was observed despite the fact that most men studied received an ICD before the documented onset of ventricular arrhythmias. The results for women were not statistically significant, which was likely attributable to the reduced statistical power due to the less-malignant course of ARVC in women, the smaller sample size, and shorter duration of follow-up.
Theoretically, first ICD discharge would be analogous to death given certain criteria: that the ICD is programmed to fire at only those times when a potentially lethal arrhythmia occurs and that the insertion of the ICD is not proarrhythmic (68), the latter being very unlikely given previous primary prevention studies (69). By the end of this study, approximately half of the male group and 10% of the female group had had an appropriate ICD discharge. However, it was demonstrated that time to first discharge in the ICD group occurred much earlier than death occurred in the control group. There are several potential explanations for this finding. First, subjects with ICDs may have progressed further in the disease process than the controls and thus were at greater risk of having an arrhythmic event. If this explanation were correct, then the benefit of ICD may be greater than it would appear in this study. Against this possibility, however, is that the degree of ectopy observed on HM was similar in the ICD and control groups, and there were fewer ICD subjects with an abnormal echocardiogram. However, the conclusion that ICD and control groups were similar should be tempered by the fact that the course of disease in the control group was determined retrospectively, and data on clinical manifestations of disease were missing in a substantial minority of controls. Finally, individuals in the control group may have survived runs of VT, which triggered discharge in the ICD group. This explanation seems the most likely, given that we found that time to first ICD discharge for sustained VT >240 beats/min was similar to time to death in the control group. Unlike one recent study of ICD efficacy in ARVC (48), we did not find a relationship between LVE and rate of discharge of the ICD.
Study limitations
There are several limitations to this study. First, it is a cohort study with historic matched controls. Second, there was selection bias in the choice of cases for the ICD group and to a lesser extent the control group. Third, clinical data from the control group and from a minority of the ICD group were collected retrospectively. Finally, only families linked to 3p25 were studied, and the results may not be generalizable to all families with ARVC. However, a randomized controlled trial is not possible in ARVC, and all previous studies have been descriptive, without an appropriate control group. The control group for this study was matched for multiple factors that may influence outcome: ARVC risk status, family, sex, gender, and survival. Selection bias was limited by including all available controls and by studying only well ascertained sibships in which ≥50% of siblings at 50% risk had a known ARVC status. Furthermore, selection bias in the cases was likely to limit the effect size of the ICD as some cases had ICD because they had already developed VT and may therefore have been more severely affected. However, these individuals did not seem to have ICD discharge more often than other subjects. The retrospective nature of the study did not influence the determination of the primary outcome (mortality) but did limit the conclusions that could be drawn on the comparability of the clinical manifestations of disease in the ICD and control groups. Although these results may not be generalizable to other causes of ARVC, this ARVD5-linked population is by far the largest genetically homogeneous group of ARVC subjects studied, and ARVC caused by this mutation may be prevalent in other populations. Careful family history will detect an autosomal-dominant mode of inheritance consistent with this form of ARVC, but positive family histories can be missed if the mutation is transmitted through several generations of females. Further studies will be necessary to determine the long-term clinical course of ARVC after ICD therapy and to assess the utility of ICD in females with ARVC.
Conclusions
We conclude that this form of ARVC is a malignant disease, particularly in males, who frequently die suddenly in early adulthood. The study is unique in that DNA haplotyping and extensive family history data are used for risk stratification and subsequent prophylactic ICD therapy in a large genetically homogeneous sample. Our results demonstrate that this genetic risk stratification model for ICD therapy has a beneficial impact on survival in males in this population regardless of indication (primary or secondary therapy), implying that SCD may be the first symptom in those at high risk. These results support the use of ICDs as a primary prevention therapy in familial ARVC linked to 3p25 for individuals at high genetic risk.
Acknowledgments
This research was supported by grants from the Canadian Institute for Health Research/ACOA (Regional Partnerships Program) Newfoundland and Labrador Centre for Applied Health Research, Janeway Children’s Hospital Foundation, Memorial University Opportunities Fund, General Hospital Foundation, St. John’s, Newfoundland, the Ernst and Berta Grimmke Stiftung Foundation (Berlin), and St. Judes Medical Research Grant, Canada. Dr. Parfrey holds a CIHR (RPP) Distinguished Scientist Award.
We wish to sincerely thank the families with ARVC who have supported this research and who live with the effects of this genetic disease. We also thank pathologists Dr. Simon Avis, Dr. Lynn Morris-Larkin, and Dr. Barry Gallagher; Drs. Elizabeth Ives and William Marshall for their original interest and work with this population; Mr. Philip Abdelmalik and Dr. Nick Barrowman for statistical advice; and Ms. Anne Marie Whalen for technical assistance.
Abbreviations and Acronyms
- ARVC
arrhythmogenic right ventricular cardiomyopathy
- ARVD
arrhythmogenic right ventricular dysplasia
- ARVD5
the locus for the mutation causing ARVC on chromosome 3p25
- ECG
electrocardiogram
- HM
Holter monitoring
- HR
high risk
- ICD
implantable cardioverter-defibrillator
- LR
low risk
- LVE
left ventricular enlargement
- MRI
magnetic resonance imaging
- SAECG
signal-averaged electrocardiogram
- SCD
sudden cardiac death
- UK
unknown
- VT
ventricular tachycardia
References
- 1.Buxton AE, Lee KL, Fisher JD, et al. A randomized study of the prevention of sudden death in patients with coronary artery disease. N Engl J Med. 1999;341:1882–90. doi: 10.1056/NEJM199912163412503. [DOI] [PubMed] [Google Scholar]
- 2.The Antiarrhythmics Versus Implantable Defibrillators (AVID) investigators. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N Engl J Med. 1997;337:1576–84. doi: 10.1056/NEJM199711273372202. [DOI] [PubMed] [Google Scholar]
- 3.Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med. 1996;335:1933–40. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 4.Connolly SJ, Gent M, Roberts RS, et al. Canadian Implantable Defibrillator Study (CIDS): a randomized trial of the implantable cardioverter defibrillator against amiodarone. Circulation. 2000;101:1297–302. doi: 10.1161/01.cir.101.11.1297. [DOI] [PubMed] [Google Scholar]
- 5.Echt D, Liebson P, Mitchell L, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324:781–8. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 6.Gregoratos G, Abrams J, Epstein AE, et al. ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmic Devices: Summary Article. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2002;106:2145–61. doi: 10.1161/01.cir.0000035996.46455.09. [DOI] [PubMed] [Google Scholar]
- 7.Karchmer AW, Longworth DL. Infections of intracardiac devices. Infect Dis Clin North Am. 2002;16:477–502. doi: 10.1016/s0891-5520(01)00005-8. [DOI] [PubMed] [Google Scholar]
- 8.Pavia S, Wilkoff B. The management of surgical complications in pacemaker and implantable cardioverter defibrillators. Curr Opin Cardiol. 2001;16:66–71. doi: 10.1097/00001573-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Wichter T, Paul M, Wollmann C, et al. Implantable cardioverter/defibrillator therapy in arrhythmogenic right ventricular cardiomyopathy. Single-centre experience of long term follow-up and complications in 60 patients. Circulation. 2004;109:1503–8. doi: 10.1161/01.CIR.0000121738.88273.43. [DOI] [PubMed] [Google Scholar]
- 10.Schser B, Osswald S. Methods of minimising inappropriate cardioverter-defibrillator shocks. Curr Opin Cardiol. 2000;2:346–52. doi: 10.1007/s11886-000-0092-4. [DOI] [PubMed] [Google Scholar]
- 11.Peters S, Reil G. Risk factors of cardiac arrest in arrhythmogenic right ventricular dysplasia. Eur Heart J. 1995;16:77–80. doi: 10.1093/eurheartj/16.1.77. [DOI] [PubMed] [Google Scholar]
- 12.Shen W, Edwards W, Hammill S, Bailey K, Ballard D, Gersh B. Sudden unexpected non-traumatic death in 54 young adults: a 30 year population study. Am J Cardiol. 1995;76:148–52. doi: 10.1016/s0002-9149(99)80047-2. [DOI] [PubMed] [Google Scholar]
- 13.Corrado D, Thiene G, Nava A, Rossi L, Pennelli N. Sudden death in young competitive athletes: clinicopathologic correlations in 22 cases (see comments) Am J Med. 1990;89:588–96. doi: 10.1016/0002-9343(90)90176-e. [DOI] [PubMed] [Google Scholar]
- 14.Corrado D, Fontaine G, Marcus F, et al. Arrhythmogenic right ventricular dysplasia/cardiomyopathy: need for an international registry. Circulation. 2000;101:e101–6. doi: 10.1161/01.cir.101.11.e101. [DOI] [PubMed] [Google Scholar]
- 15.Nava A, Scognamiglio R, Thiene G, et al. A polymorphic form of familial arrhythmogenic right ventricular dysplasia. Am J Cardiol. 1987;59:1405–9. doi: 10.1016/0002-9149(87)90929-5. [DOI] [PubMed] [Google Scholar]
- 16.Fontaine G, Fontaliran F, Herbert J, et al. Arrhythmogenic right ventricular dysplasia. Annu Rev Med. 1999;50:17–35. doi: 10.1146/annurev.med.50.1.17. [DOI] [PubMed] [Google Scholar]
- 17.McKenna WJ, Thiene G, Nava A, et al. Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Task Force of the Working Group Myocardial and Pericardial disease of the European Society of Cardiology and the Scientific Council on Cardiomyopathies of the International Society and Federation of Cardiology. Br Heart J. 1994;71:215–8. doi: 10.1136/hrt.71.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruder M, Winston S, Davis J, Abbott J, Eldar M, Scheinman M. Arrhythmogenic right ventricular dysplasia in a family. Am J Cardiol. 1985;56:799–800. doi: 10.1016/0002-9149(85)91144-0. [DOI] [PubMed] [Google Scholar]
- 19.Buja G, Nava A, Martini B, Canciani B, Thiene G. Right ventricular dysplasia: a familial cardiomyopathy? Eur Heart J. 1989;10:13–15. doi: 10.1093/eurheartj/10.suppl_d.13. [DOI] [PubMed] [Google Scholar]
- 20.Nava A, Thiene G, Canciani B, et al. Familial occurrence of right ventricular dysplasia: a study involving nine families. J Am Coll Cardiol. 1988;12:1222–8. doi: 10.1016/0735-1097(88)92603-4. [DOI] [PubMed] [Google Scholar]
- 21.Graber H, Unverferth D, Baker P, Ryan J, Baba N, Wooley C. Evolution of a hereditary cardiac conduction and muscle disorder: a study involving a family with six generations affected. Circulation. 1986;74:21–35. doi: 10.1161/01.cir.74.1.21. [DOI] [PubMed] [Google Scholar]
- 22.Ibsen H, Baandrup U, Simonsen E. Familial right ventricular cardiomyopathy. Br Heart J. 1985;54:156–9. doi: 10.1136/hrt.54.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Racovec P, Rossi L, Fontaine G, Sasel B, Markez J, Voncina D. Familial arrhythmogenic right ventricular disease. Am J Cardiol. 1986;58:377–8. doi: 10.1016/0002-9149(86)90089-5. [DOI] [PubMed] [Google Scholar]
- 24.Thiene G, Brasso D, Danieli G, Rampazzo A, Corrado D, Nava A. Arrhythmogenic right ventricular cardiomyopathy. Trends Cardiovasc Med. 1997;7:84–90. doi: 10.1016/S1050-1738(97)00011-X. [DOI] [PubMed] [Google Scholar]
- 25.Marcus F, Fontaine G, Guiraudon G. Right ventricular dysplasia: a report of 24 adult cases. Circulation. 1982;65:384–98. doi: 10.1161/01.cir.65.2.384. [DOI] [PubMed] [Google Scholar]
- 26.Hamid MS, Norman M, Quraishi A, et al. Prospective evaluation of relatives for familial arrhythmogenic right ventricular cardiomyopathy/dysplasia reveals a need to broaden diagnostic criteria. J Am Coll Cardiol. 2002;40:1445–50. doi: 10.1016/s0735-1097(02)02307-0. [DOI] [PubMed] [Google Scholar]
- 27.Tiso N, Stephan DA, Nava A, et al. Identification of mutations in the cardiac ryanodine receptor gene in families affected with arrhythmogenic right ventricular cardiomyopathy type 2 (ARVD2) Hum Mol Genet. 2001;10:189–94. doi: 10.1093/hmg/10.3.189. [DOI] [PubMed] [Google Scholar]
- 28.Priori SG, Napolitano C, Tiso N, et al. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 2001;103:196–200. doi: 10.1161/01.cir.103.2.196. [DOI] [PubMed] [Google Scholar]
- 29.Rampazzo A, Nava A, Malacrida S, et al. Mutation in human desmoplakin domain binding to plakoglobin causes a dominant form of arrhythmogenic right ventricular cardiomyopathy. Am J Hum Genet. 2002;71:1200–6. doi: 10.1086/344208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rampazzo A, Nava A, Miorin M, et al. ARVD4, a new locus for arrhythmogenic right ventricular cardiomyopathy maps to chromosome 2 long arm. Genomics. 1997;45:259–63. doi: 10.1006/geno.1997.4927. [DOI] [PubMed] [Google Scholar]
- 31.Ahmad F, Li D, Karibe A, et al. Localisation of a gene responsible for arrhythmogenic right ventricular dysplasia to chromosome 3p23. Circulation. 1998;98:2791–5. doi: 10.1161/01.cir.98.25.2791. [DOI] [PubMed] [Google Scholar]
- 32.Li D, Ahmad F, Gardner M, et al. The locus of a novel gene responsible for arrhythmogenic right ventricular dysplasia characterised by early onset and high penetrance maps to chromosome 10p12-p14. Am J Hum Genet. 2000;66:148–56. doi: 10.1086/302713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melburg A, Oldors A, Blomstrom-Lundquist C, et al. Autosomal dominant myofibrillar myopathy with arrhythmogenic right ventricular cardiomyopathy linked to chromosome 10q. Ann Neurol. 1999;46:684–92. doi: 10.1002/1531-8249(199911)46:5<684::aid-ana2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 34.Rampazzo A, Nava A, Danieli GA, et al. The gene for arrhythmogenic right ventricular cardiomyopathy maps to chromosome 14q23-q24. Hum Mol Genet. 1994;3:959–62. doi: 10.1093/hmg/3.6.959. [DOI] [PubMed] [Google Scholar]
- 35.Severini GM, Krajinovic M, Pinamonti B, et al. A new locus for arrhythmogenic right ventricular dysplasia on the long arm of chromosome 14. Genomics. 1996;31:193–200. doi: 10.1006/geno.1996.0031. [DOI] [PubMed] [Google Scholar]
- 36.Fontaine G, Fontaliran F, Frank R. Arrhythmogenic right ventricular cardiomyopathies. Clinical forms and main differential diagnoses. Circulation. 1998;97:1532–5. doi: 10.1161/01.cir.97.16.1532. [DOI] [PubMed] [Google Scholar]
- 37.Rampazzo A, Beffagna G, Nava A, et al. Arrhythmogenic right ventricular cardiomyopathy type 1 (ARVD1): confirmation of locus assignment and mutation screening of four candidate genes. Eur J Hum Genet. 2003;11:69–76. doi: 10.1038/sj.ejhg.5200914. [DOI] [PubMed] [Google Scholar]
- 38.Bauce B, Nava A, Rampazzo A, et al. Familial effort polymorphic ventricular arrhythmias in arrhythmogenic right ventricular cardiomyopathy map to chromosome 1q42–43. Am J Cardiol. 2000;85:573–9. doi: 10.1016/s0002-9149(99)00814-0. [DOI] [PubMed] [Google Scholar]
- 39.Rampazzo A, Nava A, Erne P, et al. A new locus for arrhythmogenic right ventricular cardiomyopathy (ARVD2) maps to chromosome 1q42-q43. Hum Mol Genet. 1995;4:2151–4. doi: 10.1093/hmg/4.11.2151. [DOI] [PubMed] [Google Scholar]
- 40.Nava A, Canciani B, Daliento L, et al. Juvenile sudden death and effort ventricular tachycardias in a family with right ventricular cardiomyopathy. Int J Cardiol. 1988;21:111–23. doi: 10.1016/0167-5273(88)90212-4. [DOI] [PubMed] [Google Scholar]
- 41.Nava A, Bauce B, Basso C, et al. Clinical profile and long-term follow-up of 37 families with arrhythmogenic right ventricular cardiomyopathy. J Am Coll Cardiol. 2000;36:2226–33. doi: 10.1016/s0735-1097(00)00997-9. [DOI] [PubMed] [Google Scholar]
- 42.Marshall W, Furey M, Larsen B, et al. Right ventricular cardiomyopathy and sudden death in young people. N Engl J Med. 1988;319:174–5. doi: 10.1056/NEJM198807213190312. [DOI] [PubMed] [Google Scholar]
- 43.Rahman P, Jones A, Curtis J, et al. The Newfoundland population: a unique resource for genetic investigation of complex diseases. Hum Mol Genet. 2003;12:R167–72. doi: 10.1093/hmg/ddg257. [DOI] [PubMed] [Google Scholar]
- 44.Creighton S, Almqvist E, Macgregor D, et al. Predictive, pre-natal and diagnostic genetic testing for Huntington’s disease: the experience in Canada from 1987 to 2000. Clin Genet. 2003;63:462–75. doi: 10.1034/j.1399-0004.2003.00093.x. [DOI] [PubMed] [Google Scholar]
- 45.Elles RG, Hodgkinson K, Mallick NP, et al. Diagnosis of adult polycystic kidney disease by genetic markers and ultrasonographic imaging in a voluntary family register. J Med Genet. 1994;31:115–20. doi: 10.1136/jmg.31.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Link M, Wang P, Haugh C, et al. Arrhythmogenic right ventricular dysplasia: clinical results with implantable cardioverter defibrillators. J Int Cardiol Electrophysiol. 1997;1:41–8. doi: 10.1023/a:1009714718034. [DOI] [PubMed] [Google Scholar]
- 47.Tavernier R, Gevaert S, DeSutter J, et al. Long term results of cardioverter-defibrillator implantation in patients with right ventricular dysplasia and malignant ventricular tachyarrhythmias. Heart. 2001;85:53–6. doi: 10.1136/heart.85.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Corrado D, Leoni L, Link MS, et al. Implantable cardioverter-defibrillator therapy for prevention of sudden death in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation. 2003;108:3084–91. doi: 10.1161/01.CIR.0000103130.33451.D2. [DOI] [PubMed] [Google Scholar]
- 49.Roguin A, Bomma C, Nasir K, et al. Implantable cardioverter-defibrillators in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Am Coll Cardiol. 2004;43:1843–52. doi: 10.1016/j.jacc.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 50.OMIM. Online Mendelian Inheritance in Man (OMIM) 2004. [Accessed November 16, 2004]. Available at: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=OMIM.
- 51.Bocker D, Bansch D, Heinecke A, et al. Potential benefit from implantable cardioverter-defibrillator therapy in patients with and without heart failure. Circulation. 1998;98:1636–43. doi: 10.1161/01.cir.98.16.1636. [DOI] [PubMed] [Google Scholar]
- 52.Nisam S, Breithardt G. Mortality trials with implantable defibrillators. Am J Cardiol. 1997;79:468–71. doi: 10.1016/s0002-9149(96)00853-3. [DOI] [PubMed] [Google Scholar]
- 53.Gerull B, Osterziel KJ, Witt C, Dietz R, Thierfelder L. A rapid protocol for cardiac troponin T mutation detection in familial hypertrophic cardiomyopathy. Hum Mutat. 1998;11:179–82. doi: 10.1002/(SICI)1098-1004(1998)11:2<179::AID-HUMU12>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 54.Henry WL, Gardin JM, Ware JH. Echocardiographic measurements in normal subjects from infancy to old age. Circulation. 1980;62:1054–61. doi: 10.1161/01.cir.62.5.1054. [DOI] [PubMed] [Google Scholar]
- 55.Henry WL, Ware J, Gardin JM, Hepner SI, McKay J, Weiner M. Echocardiographic measurements in normal subjects. Growth-related changes that occur between infancy and early adulthood. Circulation. 1978;57:278–85. doi: 10.1161/01.cir.57.2.278. [DOI] [PubMed] [Google Scholar]
- 56.Stuckless S, Hodgkinson K, Norman M, et al. The phenotypic expression of poor-R wave progression on ECG in arrhythmogenic right ventricular cardiomyopathy linked to 3p25 (abstr) Am J Hum Genet. 2003;73 (Suppl):269. [Google Scholar]
- 57.Hodgkinson K, Stuckless S, Dicks E, et al. Left ventricular dilatation in a large arrhythmogenic right ventricular cardiomyopathy (OMIM#60440) population: prevalence, incidence and diagnostic utility (abstr) Am J Hum Genet. 2002;71:350. [Google Scholar]
- 58.Shoji T, Kaneko M, Onodera K, et al. Arrhythmogenic right ventricular dysplasia with massive involvement of the left ventricle. Can J Cardiol. 1991;7:303–7. [PubMed] [Google Scholar]
- 59.De-Pasquale C, Heddle W. Left sided arrhythmogenic ventricular dysplasia in siblings. Heart. 2001;86:128–30. doi: 10.1136/heart.86.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stuckless S, Hodgkinson K, Dicks E, et al. Diagnostic utility of premature ventricular complexes in a large arrhythmogenic right ventricular cardiomyopathy (OMIM 60440) population (abstr) Am J Hum Genet. 2002;71 (Suppl):351. [Google Scholar]
- 61.Molinari G, Sardanelli F, Zandrino F, et al. Adipose replacement and wall motion abnormalities in right ventricle arrhythmias: evaluation by MR imaging. Retrospective evaluation on 124 patients. Int J Card Imaging. 2000;2:105–15. doi: 10.1023/a:1006304626233. [DOI] [PubMed] [Google Scholar]
- 62.Bluemke D, Krupinski E, Ovitt T, et al. MR Imaging of arrhythmogenic right ventricular cardiomyopathy: morphologic findings and interobserver reliability. Cardiology. 2003;99:153–62. doi: 10.1159/000070672. [DOI] [PubMed] [Google Scholar]
- 63.Tandri H, Calkins H, Nasir K, et al. Magnetic resonance imaging findings in patients meeting task force criteria for arrhythmogenic right ventricular dysplasia. J Cardiovasc Electrophysiol. 2003;14:476–82. doi: 10.1046/j.1540-8167.2003.02560.x. [DOI] [PubMed] [Google Scholar]
- 64.Burke A, Farb A, Tashko G, Virmani R. Arrhythmogenic right ventricular cardiomyopathy and fatty replacement of the right ventricular myocardium. Are they different diseases? Circulation. 1998;97:1571–80. doi: 10.1161/01.cir.97.16.1571. [DOI] [PubMed] [Google Scholar]
- 65.Blomstrom-Lundqvist C, Sabel K, Olsson S. A long term follow-up of 15 patients with arrhythmogenic right ventricular dysplasia. Br Heart J. 1987;58:477–88. doi: 10.1136/hrt.58.5.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fontaine G, Prost-Squarcioni C. Implantable cardioverter defibrillator in arrhythmogenic right ventricular cardiomyopathies. Circulation. 2004;109:1445–7. doi: 10.1161/01.CIR.0000121322.91189.2E. [DOI] [PubMed] [Google Scholar]
- 67.Breithardt G, Wichter T, Haverkamp W, et al. Implantable cardioverter defibrillator therapy in patients with arrhythmogenic right ventricular cardiomyopathy, long QT syndrome, or no structural heart disease. Am Heart J. 1994;127:1151–8. doi: 10.1016/0002-8703(94)90103-1. [DOI] [PubMed] [Google Scholar]
- 68.Pinski SL, Fahy GJ. Implantable cardioverter-defibrillators. Am J Med. 1999;106:446–58. doi: 10.1016/s0002-9343(99)00047-9. [DOI] [PubMed] [Google Scholar]
- 69.Moss A, Zareba W, Hall W, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–83. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]