Abstract
Graded distributions of extracellular cues guide developing axons toward their targets. A network of second messengers, Ca2+ and cyclic nucleotides, shapes cue-derived information into either attractive or repulsive signals that steer growth cones bidirectionally. Emerging evidence suggests that such guidance signals create a localized imbalance between exocytosis and endocytosis, which in turn redirects membrane, adhesion and cytoskeletal components asymmetrically across the growth cone to bias the direction of axon extension. These recent advances allow us to propose a unifying model of how the growth cone translates shallow gradients of environmental information into polarized activity of the steering machinery for axon guidance.
The formation of functional neuronal networks depends crucially on the spatial accuracy of axon growth and navigation. Each growing axon in the developing nervous system is tipped by a growth cone, a specialized amoeboid structure that is able to interpret secreted and membrane-bound molecular “guidance cues” that direct its migration along the correct path. A series of these events deliver the axon to an approximate target region, which is followed typically by axonal arborization and contacts with appropriate postsynaptic partners at particular subcellular locations1. Such specificity of synaptic connections within the target region relies on multiple distinct mechanisms including further cue-mediated axon guidance2,3. In this way, guidance cues in the microenvironment play crucial roles in neuronal network formation.
It is widely accepted that graded distribution of guidance cues controls the direction of axon growth (FIG. 1a). Such gradients can be generated by diffusion of a secreted cue away from its source of synthesis4 or by differential expression of non-diffusible cues5. When a growth cone migrates in a guidance cue gradient, the side of the growth cone facing higher concentrations of the cue will experience higher receptor occupancy. This asymmetric receptor occupancy polarizes the growth cone for turning either toward increasing concentrations of the cue (attraction) or away from the cue (repulsion), via intracellular generation of second messengers such as Ca2+ and cyclic nucleotides6–11. An extracellular shallow gradient can be transformed into steeply graded12 or, in extreme cases, compartmentalized signals11,13 inside the growth cone. The asymmetrically produced second messengers orchestrate multiple cellular machineries including membrane trafficking, adhesion dynamics and cytoskeletal reorganization to execute bidirectional turning of the growth cone (FIG. 1b).
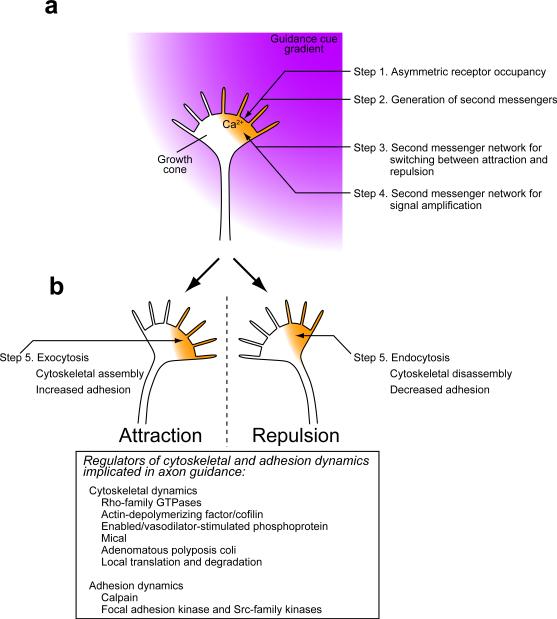
Figure 1. Overview of signaling and mechanical events during bidirectional growth cone guidance.
(a) Involvement of second messengers in signal transduction. A guidance cue gradient causes asymmetric occupancy of guidance cue receptors across the growth cone (Step 1) and initial generation of second messengers such as Ca2+ (orange) and cyclic nucleotides (Step 2). Second messenger networks determine whether the growth cone turns toward or away from the side with Ca2+ signals (Step 3) and may amplify guidance information into steeply graded or even compartmentalized signals in the growth cone (Step 4). Steps 3 and 4 may be functionally coupled and temporally overlapping processes. (b) Steering machinery for growth cone guidance. Amplified signals on one side of the growth cone break the symmetry of membrane trafficking, cytoskeletal organization and adhesiveness, which causes attractive or repulsive turning of the growth cone (Step 5). Listed in the box are examples of regulators of the cytoskeleton and adhesion dynamics that are either activated or inactivated by Ca2+, cyclic nucleotides, and other signaling components.
These basic mechanisms could be sufficient to explain short-distance guidance of axons such as those of local circuit neurons. However, additional complex mechanisms are required for long projecting axons that are guided by intermittently positioned sources of attractants en route, referred to as “intermediate targets”14. To leave an intermediate target after passing through this once attractive region, the growth cone must change its responsiveness and even reverse the polarity of guidance. Such switching can be accomplished through multiple mechanisms including an induction of different second messenger profiles that direct opposing steering machinery15,16.
Extracellular diffusible molecules showing axon guidance activities in vitro have been regarded as “guidance cues” and have been well documented (TABLE 1), even if their functional significance in vivo is less clear. This review will seek to synthesize these findings and provide an integrated picture of how axon guidance works in situ. We first suggest, based largely on in vitro data, the second messenger network models explaining how the growth cone translates shallow gradients of guidance information into either attractive or repulsive turning. Second, we examine recently identified target molecules and mechanisms that link the second messenger system with the steering apparatus. In addition to established mechanisms that act in parallel to remodel the cytoskeleton and substrate adhesions17–21, we here propose the hierarchical organization model of cellular machineries in which asymmetric membrane trafficking redirects cytoskeletal and adhesion components in the growth cone to drive its bidirectional turning. Finally, we provide our viewpoint on how the growth cone makes guidance decisions in vivo where it encounters and integrates multiple cues simultaneously to navigate through complex environmental terrain with high fidelity.
Table 1.
Ca2+ and cyclic nucleotide signaling elicited by guidance cues
| Guidance cues | Cell types and culture substrates | Turning direction | Receptors | Ca2+ channels involved in turning | Cue-induced Ca2+ increase in growth cones | Cue-induced cyclic nucleotide activation |
|---|---|---|---|---|---|---|
| Netrin-1 | Stage 22–25 Xenopus spinal neurons 12–26 h on uncoated glass (REFS. 6,9,37,98) |
Attraction | DCC | TRPC1 + L-VDCC + RyR (REFS. 6,9) |
Asymmetric Ca2+ elevation (REFS. 6,98) |
cAMP elevation in Xenopus retinal ganglion cell growth cones (REF. 15) |
| Repulsion | DCC/UNC5 | TRPC1 with or without L-VDCC (REFS. 6,9) (Involvement of these channels downstream of DCC/UNC5 is unclear) |
Not determined | cGMP elevation suggested but not observed (REF. 37) |
||
| NGF | E9–10 chicken DRG neurons on L1 or laminin (REF. 11) |
Attraction | TrkA | IP3R (REF. 11) |
Asymmetric IP3 production and IICR (REF. 11) |
cAMP elevation determined in rat DRG neurons and PC12 cells by enzyme immunoassay (REFS. 52,54) |
| BDNF | P0 rat cerebellar granule neurons on Matrigel (REF. 8) |
Attraction | TrkB | TRPC3/6 + IP3R (REF. 8) |
Ca2+ elevation, asymmetry not determined (REF. 8) |
cAMP elevation determined by enzyme immunoassay (REF. 52) |
| NT-4 | E9–10 chicken DRG neurons on laminin (REF. 38) |
Repulsion | TrkB | Not determined | Asymmetric Ca2+ elevation (REF. 38) |
Not determined |
| MAG | Stage 22–25 Xenopus spinal neurons 14–20 h on uncoated glass (REFS. 7,34) |
Repulsion | NgR complex | TRPC1 (REF. 34) RyR (REF. 7) |
Asymmetric Ca2+ elevation (REF. 7) |
MAG antagonizes neurotrophin-induced cAMP elevation in rat neurons (REF. 52) |
| E9–10 chicken DRG neurons on laminin (REF. 89) |
Attraction | Not determined | RyR (T. Tojima, R. Itofusa and H. Kamiguchi, unpublished data) | Ca2+ elevation, asymmetry not determined (REF. 89) |
Not determined | |
| Sema3A | Stage 26 Xenopus spinal neurons 16–20 h on uncoated glass (REFS. 10,56) |
Repulsion | Npn1/PlexA1 | CNGC (REF. 10) |
Asymmetric Ca2+ elevation (REF. 56) |
Asymmetric cGMP production in growth cones (REF. 10) |
| Wnt5a | P0–3 hamster cortical neurons on laminin (REF. 153) |
Repulsion | Ryk/Frizzled | SKF-96365-sensitive TRP channels (REF. 153) |
Ca2+ elevation, asymmetry not determined (REF. 153) |
Not determined |
| BMP7 | Stage 20–22 Xenopus spinal neurons 4–8 h on laminin (REF. 63) |
Attraction | BMPRII | Ca2+ signals are not required for turning (REF. 63) |
No elevation (REF. 63) |
Not determined |
| Stage 20–22 Xenopus spinal neurons 20–24 h on laminin (REF. 63) |
Repulsion | BMPRII | TRPC1 (REF. 63) |
Ca2+ elevation, asymmetry not determined (REF. 63) |
Not determined | |
| PACAP | One-day-old Xenopus spinal neurons on laminin (REF. 35) |
Attraction | PAC1 | Ca2+ signals are not required for turning (REF. 35) |
No elevation (REF. 35) |
cAMP elevation in many cell types (REF. 154) |
| ACh | One-day-old Xenopus spinal neurons 6–10 h on uncoated glass (REF. 155) |
Attraction | nAChR | Not determined | Asymmetric Ca2+ elevation (REF. 155) |
Not determined |
| GABA | E14 rat spinal neurons on serum-coated glass (REF. 156) |
Attraction | GABAAR | Not determined | Asymmetric Ca2+ elevation (REF. 156) |
Not determined |
ACh, acetylcholine; BDNF, brain-derived neurotrophic factor; BMP7, bone morphogenetic protein; BMPRII, BMP receptor II; cAMP, cyclic AMP; cGMP, cyclic GMP; CNGC, cyclic nucleotide-gated channel; DCC, deleted in colorectal cancer; DRG, dorsal root ganglion; E, embryonic day; GABA, γ-aminobutyric acid; GABAAR, GABAA receptor; IP3, inositol 1,4,5-trisphosphate; IP3R, IP3 receptor; IICR, IP3-induced Ca2+ release; L-VDCC, L-type voltage-dependent Ca2+ channel; MAG, myelin-associated glycoprotein; nAChR, nicotinic ACh receptor; NGF, nerve growth factor; NgR, Nogo-66 receptor; Npn1, neuropilin 1; NT-4, neurotrophin-4; P, postnatal day; PACAP, pituitary adenylate cyclase-activating polypeptide; PAC1, PACAP type 1 receptor; PlexA1, plexin A1; Ryk, receptor related to tyrosine kinase; RyR, ryanodine receptor; Sema3A, Semaphorin 3A; Trk, tropomyosin-related kinase; TRPC, transient receptor potential cation channel, subfamily C; UNC5, uncoordinated 5.
Involvement of second messengers in signal transduction
Second messenger networks involving Ca2+ influx and secondary Ca2+ release controlled by cyclic AMP (cAMP) and cyclic GMP (cGMP) are the primary means by which growth cones interpret and amplify signals encountered in the local environment. Here we explain how second messenger networks are shaped into growth cone guidance signals.
Generation of second messengers
In vitro studies indicate that Ca2+ signals are almost universally required for growth cone guidance with a few notable exceptions (TABLE 1). For all of the guidance cues that have been shown to elicit asymmetric Ca2+ elevations across the growth cone when applied directionally to induce axon turning, higher Ca2+ concentrations are observed on the side of the growth cone facing the source of the cues, regardless of whether the cues are attractive or repulsive (FIG. 1). Thus, localized Ca2+ elevations on one side of the growth cone can act as both attractive and repulsive signals22,23.
How do Ca2+ elevations of the same polarity elicit bidirectional turning? This most likely relies on the gating of differential sets of Ca2+ channels. The generation of cytoplasmic Ca2+ signals takes advantage of a steep gradient of Ca2+ concentrations across the plasma membrane that drives Ca2+ influx through various cation channels such as transient receptor potential type C (TRPC) channels and L-type voltage-dependent Ca2+ channels (L-VDCCs) (REF. 24). Ca2+ influx can be further amplified and extended in space and duration by secondary Ca2+ release from the endoplasmic reticulum (ER) through ryanodine receptors (RyRs) or inositol 1,4,5-trisphosphate (IP3) receptors (IP3Rs). The limited diffusion of cytoplasmic Ca2+ (REF. 25) allows spatial confinement of Ca2+ elevation to the vicinity of single open channels or discrete clusters of open channels, referred to as Ca2+ nanodomains or microdomains, respectively26.
These diverse spatiotemporal patterns of Ca2+ signals contribute to the growth cone's bidirectional responses to various guidance cues (TABLE 1 and FIG. 2). High amplitude Ca2+ produced by Ca2+-induced Ca2+ release (CICR) through RyRs or IP3-induced Ca2+ release (IICR) through IP3Rs are sufficient to initiate growth-cone turning toward the side with Ca2+ signals6,11,13 and can mediate cue-induced attraction (FIG. 2a,c,e). Conversely, repulsion is triggered by lower-amplitude Ca2+ signals that are mostly generated by Ca2+ influx through plasma membrane channels and not accompanied by CICR or IICR (REFS.7,27) (TABLE 1 and FIG. 2b,f). The amplitude and nanodomain or microdomain localization of this Ca2+ signal can vary depending on the gating of differential sets of Ca2+ channels, and mediate bidirectional growth cone turning23. The differences in amplitude can be detected by at least two Ca2+ effectors: Ca2+/calmodulin-dependent protein kinase II (CaMKII) and the Ca2+/calmodulin-dependent protein phosphatase, calcineurin (REF. 28). They are able to do so by virtue of their differing affinity for Ca2+-calmodulin: calcineurin has a higher affinity for Ca2+-calmodulin than CaMKII (REFS. 29,30). In this Ca2+ affinity model, low-amplitude Ca2+ signals activate calcineurin over CaMKII for repulsion whereas high-amplitude Ca2+ signals preferentially activate CaMKII for attraction28. However, the source of Ca2+ signals can be another determinant of the turning direction, because Ca2+ signals of equivalent amplitude with or without CICR elicit growth cone attraction or repulsion, respectively27.
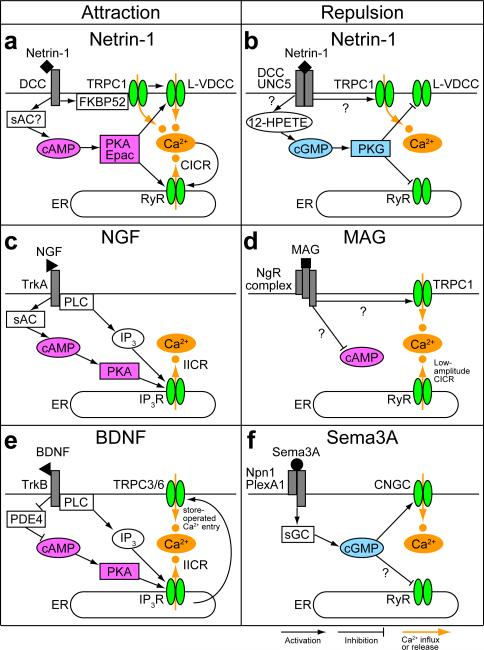
Figure 2. Second messenger systems for growth cone guidance.
(a) Netrin-1-induced attraction involves Ca2+ influx through transient receptor potential type C1 (TRPC1) and L-type voltage-dependent Ca2+ channels (L-VDCC) and Ca2+-induced Ca2+ release (CICR) from the endoplasmic reticulum (ER) through ryanodine receptors (RyR). Upon netrin-1 binding to the receptor deleted in colorectal cancer (DCC), the peptidyl-prolyl isomerase FK506-binding protein 52 (FKBP52) mediates the isomerization-elicited opening of TRPC1 (REF. 49). The activated TRPC1 triggers membrane depolarization9 leading to further Ca2+ entry through L-VDCC (REF. 37). This Ca2+ entry most likely triggers CICR that causes growth cone attraction6. Gating of L-VDCC and RyR requires the activity of the cyclic AMP (cAMP)-protein kinase A (PKA) pathway27,37. A sufficient cAMP level is maintained possibly by the action of soluble adenylate cyclase (sAC) (REF. 50), although conflicting results have been reported51. The cAMP effector Epac has also been implicated in netrin-1-induced attraction36. (b) Netrin-1-induced repulsion is probably mediated by Ca2+ influx through TRPC1 only9, because the cyclic GMP (cGMP)-protein kinase G (PKG) pathway inactivates L-VDCC (REF. 37) and RyR (REF. 38). The DCC-uncoordinated 5 (UNC5) receptor complex, which mediates netrin-1-induced repulsion, has been postulated to stimulate the production of cGMP via the lipid mediator 12-hydroperoxyeicosatetraenoic acid (12-HPETE) (REF. 37). (c) Nerve growth factor (NGF)-induced attraction involves the receptor tropomyosin-related kinase A (TrkA) and its downstream effector, phospholipase C (PLC) (REF. 53) that catalyzes the production of inositol 1,4,5-trisphosphate (IP3) in the cytosol. NGF also increases cAMP levels via sAC (REF. 54), facilitating IP3-induced Ca2+ release (IICR) upon IP3 binding to the IP3 receptor (IP3R) (REF. 11). (d) Myelin-associated glycoprotein (MAG) binds the Nogo-66 receptor complex (NgR complex) and repels growth cones via low-amplitude Ca2+ release from the ER (REF. 7) and potentially Ca2+ influx through TRPC1 (REF. 34). The TRPC1 may also participate in maintaining ER-stored Ca2+. MAG antagonizes neurotrophin-induced cAMP elevations in postnatal rat neurons under conditions in which MAG inhibits axon growth52, suggesting that MAG has a bias toward inhibiting the cAMP pathway during repulsive guidance. (e) Brain-derived neurotrophic factor (BDNF)-induced TrkB-mediated attraction requires IICR together with Ca2+ influx through TRPC3/6, in which TRPC3/6 may participate in store-operated Ca2+ entry for replenishment of the ER with Ca2+ (REF. 8). BDNF also increases cAMP levels via inhibition of phosphodiesterase 4 (PDE4) (REF. 55). (f) Semaphorin 3A (Sema3A) binds a complex of neuropilin 1 (Npn1) and plexinA1 (PlexA1) and repels growth cones by elevating cGMP levels via soluble guanylate cyclase (sGC), which in turn triggers Ca2+ influx through cyclic nucleotide-gated channel (CNGC) (REF. 10). Whether RyR is inactivated downstream of cGMP remains unclear.
To reconcile these seemingly contradictory observations, we propose to refine the Ca2+ affinity model28 by assuming that Ca2+ effectors for repulsion and attraction are localized to differential subcellular compartments: high-affinity Ca2+ effectors such as calcineurin exist diffusely in the growth cone cytosol whereas low-affinity Ca2+ effectors such as CaMKII are localized in close proximity to ER Ca2+ channels. Both calmodulin and CaMKII have been shown to associate, although not exclusively31, with RyRs and IP3Rs (REFS. 32,33), and thus are favorably situated for the detection of Ca2+ release from the ER. In this refined model, Ca2+ influx through plasma membrane channels alone would activate repulsive, but not attractive effectors, leading to growth cone repulsion. Conversely, attractive turning would require activation of attractive effectors by means of Ca2+ release from the ER, abundant Ca2+ influx across the plasma membrane that can elevate cytoplasmic Ca2+ concentrations at the ER, or combinations of the two. Consistent with this model, for example, myelin-associated glycoprotein (MAG)-induced repulsion depends on low-amplitude Ca2+ release from the ER (REF. 7) and possibly Ca2+ influx through TRPC1 channels (REF. 34) (FIG. 2d), but MAG-induced attraction requires high-amplitude Ca2+ signals accompanied by CICR (REF. 7; T. Tojima, R. Itofusa and H. Kamiguchi, unpublished data).
Asymmetric cyclic nucleotide signaling can also mediate axon guidance. For example, activation of the cAMP pathway on one side of the growth cone is sufficient to trigger attractive turning35,36. Such instructive cAMP signaling has been implicated in pituitary adenylate cyclase-activating polypeptide-induced attraction35 and netrin-1-induced attraction36, although there is no direct evidence for asymmetric cAMP distribution across the growth cone in gradients of these cues. The extracellular guidance cue Semaphorin 3A (Sema3A) stimulates the production of cGMP preferentially on the side of the growth cone facing the Sema3A source, leading to a Ca2+ influx pattern for repulsive turning10. Among several guidance cues that have been shown to influence cyclic nucleotide levels, attractive and repulsive cues tend to elevate cAMP and cGMP, respectively (TABLE 1 and FIG. 2).
Second messenger crosstalk for directional switching and signal amplification
Axon pathfinding involves not only attraction or repulsion, but often a switch from one to the other. Cyclic nucleotides can act as such a switch by modulating Ca2+ channel activities37. The Ca2+ release from the ER that determines growth cone turning polarity is regulated counteractively by cAMP and cGMP pathways11,27,38: cAMPv facilitates ligand-dependent activation of RyRs and IP3Rs for an attractive response. Conversely, cGMP inactivates RyRs and blocks CICR although the involvement of cGMP in the regulation of IICR during axon guidance remains to be determined. Biochemical and molecular structural studies have shown that the gating of ER Ca2+ channels can be modulated by phosphorylation by the cAMP- and cGMP-dependent kinases protein kinase A (PKA) and protein kinase G (PKG) (REFS. 32,33). The roles of PKA and PKG described in this review, which are based on experimental results using non-specific pharmacological agents, should be confirmed with recently developed cyclic nucleotide analogs and RNA interference technology that can specifically target these kinases36,39,40.
Evidence from other biological systems helps in the development of a hypothetical model of how growth cones can translate and amplify shallow concentration gradients of guidance cues into steeper gradients of intracellular signals. In addition to the pathway by which cAMP facilitates Ca2+ mobilization, Ca2+ in turn stimulates the production of cAMP via Ca2+-dependent adenylate cyclases (ACs) such as AC1 and AC8 (REF. 41). Indeed, AC8 has been shown to increase cAMP levels in retinal ganglion cells downstream of Ca2+-calmodulin activation42. Furthermore, recent work has demonstrated that Ca2+ depletion from the ER, an experimental equivalent of the consequence of CICR or IICR, stimulates AC-mediated cAMP production by a mechanism that is independent of the cytoplasmic Ca2+ concentration43. If this store-operated cAMP production pathway operates in growth cones, it could mediate CICR- and IICR-elicited cAMP elevations. Such positive-feedback mechanisms would potentially participate in amplifying attractive guidance signals. Both mathematical modeling of cAMP and IICR regulation and imaging of spontaneous cAMP and Ca2+ in neuronal cell bodies have shown the existence of positive-feedback regulation, further opening the possibility that this might occur in growth cones44.
This positive-feedback augmentation of guidance signals can be terminated by multiple mechanisms. The cytoplasmic Ca2+ concentration can regulate the open probability of RyRs and IP3Rs biphasically: open probability increases as Ca2+ concentrations elevate, but further Ca2+ increase above threshold levels of 0.2 to hundreds of micromolars begins to inhibit opening of these Ca2+ channels32,33,45. Also, cyclic nucleotide levels can be self-suppressed via cyclic nucleotide-dependent phosphodiesterases (PDEs) (REF. 46). It is likely that growth cones employ these mechanisms to limit the amplitude of guidance signals.
Importantly, intracellular signals may also be refined via reciprocal inhibition pathways between cAMP and cGMP. Shelly et al47 recently identified such pathways in neuronal growth cones: cAMP elevation causes cGMP reduction via cGMP-specific PDE5 whereas cGMP elevation causes cAMP reduction via cAMP-specific PDE4. These reciprocal inhibition pathways allow attractive and repulsive signals to reduce cGMP and cAMP, respectively, and possibly contribute to signal amplification by inhibiting these counteractive regulators of ER Ca2+ channels. This may force the guidance machinery toward a clear-cut decision between attractive or repulsive response. It is generally thought that gradient amplification mechanisms in chemotactic cells involve both “local augmentation” and “global inhibition” of the guidance signal, and indeed, such mechanisms appear to be employed by growth cones48. This has led to the development of a molecular model that utilizes the positive feedback loops described in this and subsequent sections.
Bidirectional growth cone turning: a molecular model
Although guidance cues signal through structurally diverse receptors, many participating second messengers are shared. This has allowed us to propose a general model for second messenger networks that facilitate attractive and repulsive turning. Signaling networks including positive-feedback loops and reciprocal inhibition help to explain how a growth cone shapes Ca2+ signals for precise navigational responses to shallow gradients of attractive cues (FIG. 3). In this hypothetical model, netrin-1, for example, induces primary Ca2+ influx through TRPC1 channels (REFS. 9,49) and elevates the cAMP concentration15,36,50,51, preferentially on the side of the growth cone facing the netrin-1 source. The cAMP elevation would cause cGMP reduction via reciprocal inhibition47. The resultant increase in the ratio of cAMP to cGMP pushes L-VDCCs and RyRs to the active state and facilitates the generation of secondary Ca2+ entry through these channels27,37,38, which in turn stimulates further production of cAMP (REFS. 41–43). Similarly, second messenger crosstalk shapes attractive guidance signals downstream of nerve growth factor (NGF) (REFS. 11,52–54) and brain-derived neurotrophic factor (BDNF) (REFS. 8,52,55) (FIG. 3a). It is tempting to speculate that these positive-feedback loops act as the machinery for “local augmentation” and produce high-amplitude CICR or IICR on one side of the growth cone for attractive guidance.
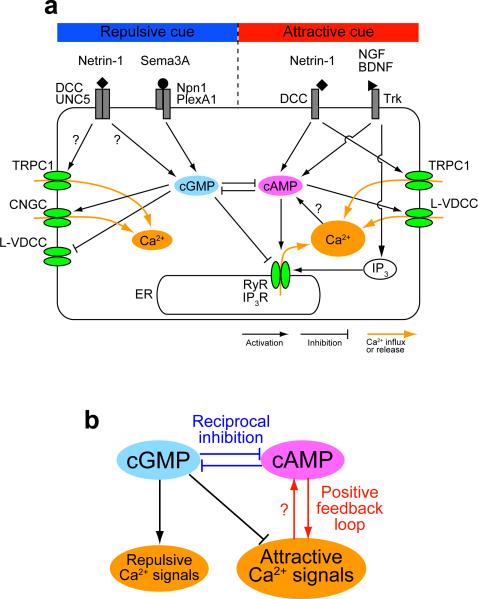
Figure 3. Second messenger network shapes attractive and repulsive Ca2+ signals.
(a) Signaling network and Ca2+ mobilization downstream of guidance cue receptors. A growth cone expresses transient receptor potential type C1 (TRPC1) channels, cyclic nucleotide-gated channels (CNGC) and L-type voltage-dependent Ca2+ channels (L-VDCC) in the plasma membrane and ryanodine receptors (RyR) and inositol 1,4,5-trisphosphate (IP3) receptors (IP3R) in the endoplasmic reticulum (ER) membrane. Cyclic AMP (cAMP) and cyclic GMP (cGMP) counteractively regulate Ca2+ mobilization: e.g., Ca2+ release through RyR is facilitated by cAMP and inhibited by cGMP. Ca2+ might in turn increase the cAMP level, forming a positive-feedback loop between Ca2+ and cAMP. Reciprocal inhibition pathways exist between cAMP and cGMP. BDNF, brain-derived neurotrophic factor; DCC, deleted in colorectal cancer; NGF, nerve growth factor; Npn1, neuropilin 1; PlexA1, plexinA1; Sema3A, Semaphorin 3A; Trk, tropomyosin-related kinase; UNC5, uncoordinated 5. (b) The core signaling network for growth cone guidance. The interactions among Ca2+ and cyclic nucleotides, including positive-feedback loops and the reciprocal inhibition, would shape either of the two types of Ca2+ signals: high-amplitude Ca2+ signals accompanied by Ca2+ release from the ER (attractive Ca2+ signals) or low-amplitude Ca2+ influx that does not trigger Ca2+ release from the ER (repulsive Ca2+ signals).
Conversely, molecular mechanisms underlying “global inhibition” remain more elusive. Recent work47 has provided insight into the global inhibition mechanisms: when the cAMP pathway is activated within a single growth cone of a hippocampal neuron, all other neurites of that neuron exhibit cAMP reduction and cGMP elevation presumably involving active transport of unidentified regulators of cyclic nucleotides. If this self-down-regulation of cAMP can operate within an even shorter range, one would imagine that attractant-induced cAMP elevation on one side of the growth cone results in cAMP reduction and cGMP elevation on the other side. This would block the generation of secondary Ca2+ signals on the side facing lower attractant concentrations, thereby contributing to the confinement of attractive Ca2+ signals to the leading side of the growth cone.
During repulsive guidance, cGMP levels are elevated by Sema3A (REF. 10) and probably by netrin-1 (REF. 37). The cGMP elevation likely causes cAMP reduction via reciprocal inhibition47. In this situation, cue-induced primary Ca2+ influx would fail to trigger further Ca2+ entry through L-VDCCs, RyRs or IP3Rs (REFS. 11,27,37,38) (FIG. 3a). Such Ca2+ influx without Ca2+ release from the ER acts as a repulsive signal. The signaling network for repulsive guidance may lack a positive-feedback loop between Ca2+ and cyclic nucleotides (FIG. 3b), which is consistent with the concept that the amplitude of repulsive Ca2+ signals is low. Thus, an intriguing model that remains to be thoroughly tested is that amplification of repellent gradients occurs downstream of Ca2+ signals.
There are exceptions, however, such as Sema3A, which attracts growth cones if cGMP levels increase to levels that are high enough to activate PKG, which induces membrane depolarization and high-amplitude Ca2+ influx through voltage-dependent Ca2+ channels (REF. 56). The proposed model (FIG. 3b) can be scrutinized by simultaneous imaging of multiple second messengers and analyses of their spatiotemporal dynamics in growth cones during attraction and repulsion.
Steering machinery for growth cone guidance
In order to accomplish growth cone turning, the external signals that have been interpreted, amplified and transduced need to result in changes in the growth cone morphology. The classic machinery that drives such morphological changes includes the cytoskeleton and adhesion complexes, where the former facilitates plasma membrane protrusion and the latter mediates membrane anchoring to extracellular matrix. Adhesion receptors like integrins link to the underlying actin cytoskeleton at adhesion complexes by means of adaptor proteins including talin (REF. 57). Together, these machineries cooperate to drive migration. Accumulating evidence highlights the importance of their asymmetric reorganization during bidirectional guidance (FIG. 1b): the growth cone turns preferentially toward the side with cytoskeletal and adhesion complex assembly or away from the side with disassembly. Thus, effector processes that control cytoskeletal and adhesion dynamics must be regulated asymmetrically across the growth cone to initiate chemotactic turning. A growing number of effector proteins have been implicated in growth cone guidance, including Rho-family GTPases (REFS. 13,58–62), actin-depolymerizing factor/cofilin (REFS. 63,64), Enabled/vasodilator-stimulated phosphoprotein (REF. 65), Mical (REFS.66,67), adenomatous polyposis coli (REF. 68), calpain (REF. 69), focal adhesion kinase (FAK) and Src-family kinases (REFS. 70–78). Local translation or degradation of cytoskeletal components and their regulators also participates in growth cone guidance79–85.
Remarkably, several recent studies86–89 have demonstrated that regulated membrane trafficking is a critical effector process for chemotactic guidance by gradients of diffusible cues. In these circumstances, membrane trafficking becomes asymmetric across the growth cone immediately after the onset of guidance signals, preceding any detectable changes in cytoskeletal dynamics. These findings are consistent with the hypothesis that regulated membrane trafficking facilitates the subsequent cytoskeletal remodeling that is necessary for axon guidance, although future studies are warranted to determine the causal relationship between these cellular processes. In the following sections, we will examine how asymmetric membrane trafficking can be an instructive step in the initiation of growth cone steering.
Exocytosis and endocytosis in the growth cone
Developing neurons undergo dramatic morphological changes that require alterations in the surface area by means of exocytosis and endocytosis90–95. Axon extension depends on the expansion of growth cone plasma membrane92, which can be mediated by vesicle-associated membrane protein 7 (VAMP7)-dependent exocytosis96. Regulated exocytosis can also occur locally in the growth cone, whereby synaptic protein-containing vesicles migrate along filopodia and fuse with the filopodial surface97. Conversely, growth cone collapse is accompanied by a reduction in the surface area via endocytosis87,93,94. These findings have led to the hypothesis that axon guidance involves asymmetric membrane trafficking across the growth cone98,99.
It is now evident that regulated exocytosis and endocytosis occur asymmetrically across the growth cone in response to guidance cue gradients86–89 (FIG. 4). A gradient of chemoattractants promotes vesicle transport and subsequent VAMP2-mediated exocytosis on the gradient side of the growth cone. Conversely, a chemorepellent gradient causes asymmetric clathrin-mediated endocytosis on the side facing the gradient. It is likely that differential regulation of these opposing forces remodels the composition of the surface membrane and associated molecules locally to initiate turning. Regulated membrane trafficking can participate in multiple steps in the axon guidance process such as receptor-mediated signal transduction and downstream mechanical processes.
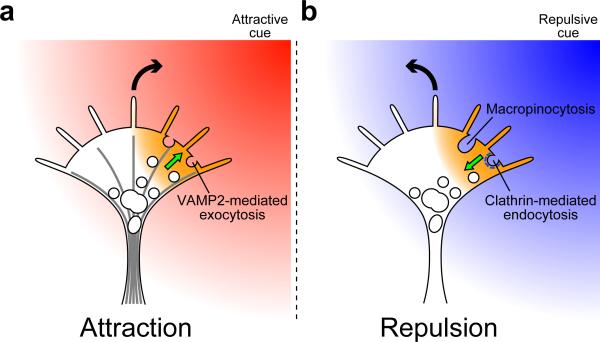
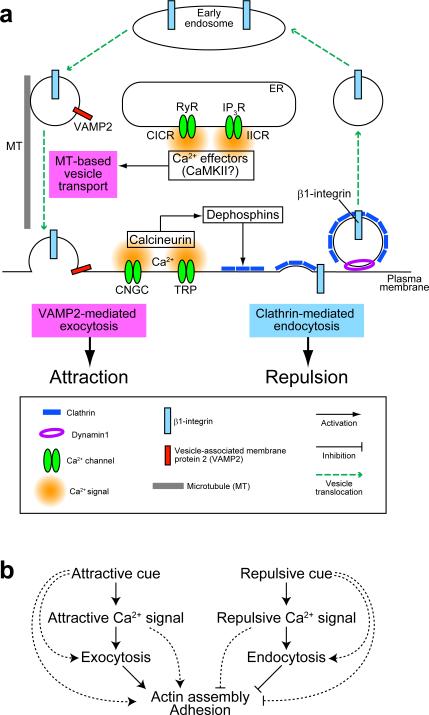
Figure 4. Asymmetric membrane trafficking drives bidirectional growth cone turning.
Extracellular gradients of guidance cues trigger the generation of Ca2+ signals on one side of growth cones (orange areas). (a) Attractive Ca2+ signals promote centrifugal transport of vesicle-associated membrane protein 2 (VAMP2)-containing vesicles (white circles) along microtubules (radial lines), with exocytosis ensuing in the growth cone periphery. (b) Repulsive Ca2+ signals facilitate the formation of clathrin (white dashes)-coated pits that migrate toward the growth cone center followed by internalization. The migration of clathrin-coated pits depends on retrograde flow of actin filaments89. Asymmetric macropinocytosis has been implicated in repulsive guidance87, although it remains unclear whether repulsive Ca2+ signals enhance this type of endocytosis in growth cones. The curved arrows indicate the direction of growth cone turning. The straight arrows indicate the transport direction of VAMP2-containing vesicles and clathrin-coated pits.
The participation of exocytic and endocytic trafficking in the control of signaling events in the growth cone has been well documented. For example, the growth cone's responses to guidance cues can be modulated by membrane trafficking that alters the surface availability of relevant receptors such as deleted in colorectal cancer, uncoordinated 5 and neuropilin 1 (REFS. 100–102). The growth cone can adjust its sensitivity to guidance cues by the process of adaptation103, which depends on endocytosis-mediated desensitization102 and protein synthesis-dependent resensitization102,103: endocytosis of the relevant receptors transiently desensitizes the growth cone to collapse-inducing activities of Sema3A and netrin-1 (REF. 102). Receptor endocytosis may be necessary for the full generation of intracellular guidance signals from early endosomes104–106. Also, Ca2+-permeable cation channels packaged into VAMP-positive vesicles are carried to the growth cone, which leads to changes in morphology107.
In addition to a role in signaling, emerging evidence suggests that membrane trafficking can be a primary and instructive driving machinery for growth cone turning86,89. First, asymmetric exocytosis and endocytosis are necessary for growth cone attraction and repulsion, respectively, induced by direct Ca2+ manipulation that can bypass receptor activation and any upstream signaling events. Second, when the turning polarity is switched between attraction and repulsion by modulating second messenger profiles, the balance of exocytosis and endocytosis is also reversed. Importantly, the reversed turning polarity also depends on the newly acquired dominant trafficking mechanism, with attractive Ca2+ signals favoring exocytosis and repulsive Ca2+ signals triggering endocytosis. Finally, asymmetric perturbation of the balance of exocytosis and endocytosis is sufficient to initiate turning toward the side with more exocytosis or less endocytosis. Taken together, these findings indicate that the balance of exocytosis and endocytosis can dictate bidirectional growth cone turning downstream of second messenger signaling.
How do second messengers control membrane trafficking?
Asymmetric Ca2+ signals attract and repel growth cones via CaMKII and calcineurin, respectively28 (FIG. 5a). Both the magnitude and subcellular localization of Ca2+ signals might dictate which effector pathways are activated, and consequently favor either exocytosis or endocytosis. During attractive turning, Ca2+ signals promote microtubule-based centrifugal transport of membrane vesicles and their subsequent VAMP2-mediated exocytosis on the side with elevated Ca2+ (REFS. 86,108). The attractive Ca2+ signals might locally activate CaMKII, which could phosphorylate motor proteins like myosin-V and KIF17 and trigger release of these motors from cargo vesicles109,110. After releasing these motors, the vesicles could be incorporated into a readily-releasable pool of vesicles docked beneath the plasma membrane, followed by Ca2+-dependent activation of synaptotagmins and other effector proteins on the vesicle membrane that regulate exocytosis111. Although the exact mechanisms remain to be elucidated, it is likely that attractive Ca2+ signals facilitate at least two distinct but successive processes: vesicle translocation into the growth cone periphery and regulated exocytosis. Repulsive Ca2+ signals elicit asymmetric clathrin-mediated endocytosis in a calcineurin-dependent manner89. The role of calcineurin in clathrin-mediated endocytosis has been extensively studied in presynaptic terminals112. Calcineurin dephosphorylates, and thereby activates, dephosphins, a group of at least eight endocytic-adaptor proteins113,114. In synaptic transmission, activated dephosphins facilitate synaptic vesicle endocytosis after exocytic neurotransmitter release. In growth cones, the requirement for calcineurin activity in repulsive turning28 and clathrin-mediated endocytosis89 suggests that dephosphins play a similar role in axon guidance. Taken collectively, repulsive Ca2+ signals facilitate clathrin-mediated endocytosis via calcineurin, whereas attractive Ca2+ signals promote exocytic trafficking possibly via CaMKII (FIG. 5a).
Figure 5. Working model of the growth cone steering machinery: membrane trafficking as a master organizer.
(a) Trafficking of membrane vesicles and their cargo proteins in growth cones. Repulsive Ca2+ signals, e.g., Ca2+ influx through cyclic nucleotide-gated channels (CNGC) or transient receptor potential (TRP) channels, facilitate the formation of clathrin-coated pits perhaps via calcineurin-mediated dephosphorylation of dephosphins. β1-integrin can be captured by coated pits for internalization and most likely sorted into early endosomes. Attractive Ca2+ signals, e.g., Ca2+ release from the endoplasmic reticulum (ER) through ryanodine receptors (RyR) or inositol 1,4,5-trisphosphate receptors (IP3R), facilitate microtubule-based centrifugal transport of vesicle-associated membrane protein 2 (VAMP2)-containing vesicles and subsequent exocytosis in the growth cone periphery. Ca2+/calmodulin-dependent protein kinase II (CaMKII) might link attractive Ca2+ signals to exocytic trafficking. In non-neuronal cells, β1-integrin is recycled to the plasma membrane via VAMP2-dependent exocytosis. Endocytic and exocytic vesicles could also carry cytoskeletal regulators (not shown). CICR, Ca2+-induced Ca2+ release; IICR, IP3-induced Ca2+ release. (b) Mechanistic model for growth cone guidance. Attractive and repulsive Ca2+ signals promote exocytosis and endocytosis, respectively, on the side with elevated Ca2+. Such asymmetric membrane trafficking is likely to control the surface addition and removal of membrane and cargo proteins including adhesion molecules and cytoskeletal regulators. The polarized targeting of driving machinery would generate asymmetric traction and protrusion forces that are essential for growth cone turning. The parallel pathways also operate that bypass Ca2+ signals or membrane trafficking (broken lines).
In addition to regulating the Ca2+ signal within growth cones, cyclic nucleotides can regulate membrane trafficking. For example, cAMP activation of PKA elicits phosphorylation of synapsin I (REF. 115), which then dissociates from the cytoplasmic surface of VAMP2-positive synaptic vesicles116. This potentiates regulated exocytosis in presynaptic terminals116 and facilitates vesicle translocation from the growth cone center toward the periphery117. Furthermore, the importance of synapsin phosphorylation in growth cone motility has been supported by the observation that expression of a synapsin mutant mimicking constitutive phosphorylation by PKA is sufficient to stimulate axon extension even in the presence of a PKA inhibitor118. These results suggest that cAMP might collaborate with Ca2+ to facilitate centrifugal vesicle transport and exocytosis during growth cone attraction. cGMP elevation can accelerate synaptic vesicle endocytosis via elevating presynaptic levels of phosphatidylinositol 4,5-biphosphate (REF. 119), but the role of cGMP in membrane trafficking in growth cones remains undefined.
Polarized remodeling of adhesion and cytoskeletal machinery
Growth cone turning depends on membrane trafficking, adhesion dynamics and cytoskeletal reorganization. While these mechanical processes can function independently, membrane trafficking might also polarize the activity of adhesion and cytoskeletal machinery by redistributing their components and regulators. For example, clathrin-mediated endocytosis retrieves integrins from the cell surface and sorts them into early endosomes for subsequent degradation or recycling back to the cell surface120. In non-neuronal cells, clathrin mediates β1-integrin endocytosis, a process that is required for focal adhesion disassembly and efficient cell migration121. Endocytosed β1-integrin is also present in VAMP2-positive vesicles in these cells, and depletion of VAMP2 reduces the amount of β1-integrin on the cell surface, which inhibits cell adhesion and chemotactic migration on a fibronectin substrate122. These findings indicate that cell adhesion to extracellular matrix can be controlled by endocytic and exocytic trafficking of integrins. An analogous mechanism might operate in axon guidance whereby growth cone repulsion would be driven by clathrin-mediated endocytosis of β1-integrin and asymmetric adhesion-complex disassembly88, whereas growth cone attraction would require exocytic trafficking of VAMP2-positive vesicles86 that could carry β1-integrin (REF. 122) (FIG. 5a). In this way, integrins may be transported to the growth cone periphery in a targeted manner and undergo exocytosis in regions where integrin-containing adhesions assemble, such as the lamellipodia and filopodia97,123,124. Importantly, asymmetric alterations in growth cone-substrate adhesion by blocking β1-integrin are sufficient to initiate growth cone turning away from the side with reduced β1-integrin function88. Thus, asymmetric trafficking of β1-integrin and potentially other adhesion molecules would be expected to cause polarized adhesion and turning of growth cones.
Downstream of repulsive guidance signals, endocytic removal of integrins must be coupled spatiotemporally with adhesion complex disassembly that often accompanies cell retraction and detachment from extracellular matrix57. As expected, a gradient of the chemorepellent Sema3A causes polarized endocytosis89 and asymmetric growth cone detachment from a laminin substrate as assessed by internal reflection microscopy125. Moreover, activation of the Ca2+-calcineurin pathway leads to disappearance of phosphorylated FAK, a component of integrin-based adhesion machinery, and growth cone detachment from a laminin substrate73. These findings imply that repulsive guidance signals can orchestrate multiple effector processes including dispersal of adhesion structures and selective retrieval of integrins. One possible mechanism underlying this orchestration is that Ca2+-dependent effectors modify components of integrin-containing adhesions for subsequent integrin internalization. For example, spontaneous Ca2+ transients in growth cone filopodia promote repulsive turning when generated asymmetrically, through local activation of the Ca2+-dependent protease calpain (REF. 69) that cleaves several components of the adhesion machinery such as FAK (REF. 126), Src (REF. 127) and talin (REF. 128). Thus, calpain may mediate the linkage between Ca2+-induced integrin endocytosis and integrin disengagement from the underlying cytoskeleton.
Cytoskeleton-associated proteins can also be a cargo of intracellular vesicles. Activation of the GTPase Rac can occur on early endosomes in non-neuronal cells129. Intracellular vesicles containing the activated Rac then move toward the cell periphery where actin-based membrane protrusions form129. Similarly, the GTPase Cdc42 can localize to intracellular vesicles and be delivered to the leading edge for directed cell migration130. Proteomic analyses have identified a large number of proteins including actin, tubulin, Rac and actin-related protein 2/3 complex that associate with VAMP2-positive synaptic vesicle and clathrin-coated vesicle preparations isolated from the brain131,132, supporting the notion of membrane trafficking as a means for the spatial restriction of cytoskeletal remodeling. Furthermore, phosphatidylinositol 3,4,5-trisphosphate (PIP3) is delivered to neuronal growth cones through microtubule-based anterograde vesicle transport133, where PIP3 facilitates axon formation via multiple signaling cascades including Cdc42 and Rac1 activation134, opening the possibility that asymmetric delivery of lipid mediators across the growth cone might participate in axon guidance. Therefore, it is reasonable to speculate that polarized trafficking of cytoskeletal regulators in the growth cone causes asymmetric cytoskeletal remodeling and growth cone turning (FIG. 5a). Another possibility is that local exocytosis and endocytosis influence the rate of actin polymerization by decreasing and increasing tension in the growth cone plasma membrane, respectively. This possibility is consistent with the Brownian-ratchet model of actin polymerization135 and has been supported by experimental results that the plasma membrane tension is inversely correlated with the rate of lamellipodial protrusion136.
We would like to propose a mechanistic model for bidirectional axon guidance, in which asymmetric membrane trafficking acts, downstream of second messengers, as a “master organizer” to spatially localize the growth cone driving machinery (FIG. 5b). In response to attractive Ca2+ signals, exocytic trafficking of VAMP2-containing vesicles would deliver positive regulators of adhesion complexes, cytoskeletal machinery and bulk membrane preferentially to the side of the growth cone with elevated Ca2+. Conversely, repulsive Ca2+ signals enhance endocytic removal of adhesion molecules, cytoskeletal components and membrane to reshape the growth cone. In this way, attractive and repulsive Ca2+ signals are transformed into exocytosis and endocytosis, respectively, which in turn potentiate asymmetric traction and protrusion forces for growth cone turning through polarized targeting of the driving machinery.
Future prospects: how does a growth cone integrate multiple guidance signals?
A growth cone must integrate signals of multiple guidance cues when navigating through complex environmental terrain in vivo. For example, commissural axon pathfinding along the dorsal-ventral axis of the spinal cord requires the release of multiple cues from either the ventral midline floor plate or the dorsal roof plate. The floor-plate-derived attractants netrin-1 (REF. 137) and Sonic hedgehog (REF. 138), and the roof-plate-derived repellents bone morphogenetic proteins (REF. 139) and possibly draxin (REF. 140) cooperate to guide commissural axons toward the ventral midline. After midline crossing, commissural axons turn rostrally, guided by counter gradients of the repellent Sonic hedgehog (REF. 141) and an attractive Wnt activity142,143 along the longitudinal axis. Another example comes from studies of the thalamocortical tract where axons are guided by counter gradients of the repellent ephrin-A5 (REF. 144) and the attractant netrin-1 (REF. 145). The growth cone's detection of multiple attractants and repellents with complementary gradient polarities likely serves to ensure correct pathway choices.
It is well known that guidance cues can regulate axonal responses to other guidance cues at the level of receptors146 or second messengers147. In addition to this hierarchical and regulatory interaction, recent work has demonstrated a “push and pull” mechanism in which both a repellent and an attractant play instructive and mutually supportive roles by confronting the same growth cone from opposite sides148. This study has also provided in vitro evidence that simultaneous presentation of two opposing cues as counter gradients improves the fidelity of growth cone turning.
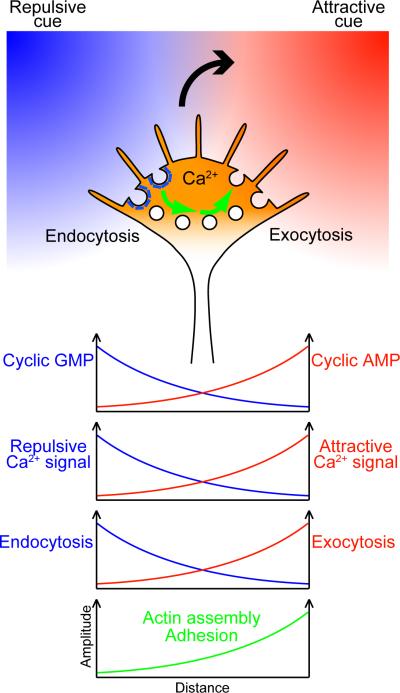
Because many guidance cues signal through cytoplasmic Ca2+, counter gradients of two opposing cues could cause Ca2+ elevations on both sides of the growth cone (FIG. 6). For the growth cone to turn in this situation, spatial asymmetry in the type of Ca2+ and its downstream signaling should form across its axis. One plausible mechanism for this asymmetry is that attractive and repulsive cues differentially regulate cyclic nucleotide signaling such that cAMP and cGMP levels have counter gradients. These counter gradients could be generated as a result of attractant/repellent-induced cAMP/cGMP increases, the reciprocal inhibition between cAMP and cGMP, and the self down-regulation of cAMP as discussed in the previous section. The high cAMP/cGMP ratio on the attractant side would facilitate CICR and IICR while the low cAMP/cGMP ratio on the repellent side would prevent Ca2+ release from the ER, which causes the reciprocal distribution of attractive and repulsive Ca2+ signals in the growth cone. These reciprocal Ca2+ signals would elicit the counter gradients of exocytic and endocytic activities that cause asymmetric alterations in actin assembly and growth cone adhesiveness. In this way, the growth cone could translate multiple guidance signals into turning behavior with high fidelity. Although future studies are necessary to elucidate the growth cone's responses to various combinations of guidance cues, one could speculate that a growth cone would bifurcate in response to attractants on both sides or would stall in response to repellents on both sides. Coexistence of an attractant and a repellent on the same side of the growth cone can be counterbalancing149 but may induce attraction or repulsion depending on whether the positive-feedback loop between Ca2+ and cAMP has been turned on.
Figure 6. Hypothetical model for growth cone guidance by multiple cues.
When a growth cone encounters both an attractant on the right side and a repellent on the left side, counter gradients of cyclic AMP and cyclic GMP could be created via several mechanisms described in the text. These counter gradients control the open probability of ryanodine receptors and inositol 1,4,5-trisphosphate receptors, leading to asymmetric release of Ca2+ from the endoplasmic reticulum across the growth cone. Even if the two guidance cues together were to cause symmetric elevations of cytoplasmic Ca2+ concentrations, the nature of Ca2+ signals would differ between both sides of the growth cone: i.e., attractive and repulsive Ca2+ signals would be reciprocally distributed. These reciprocal Ca2+ signals elicit the counter gradients of exocytic and endocytic activities that cause relatively increased filamentous actin assembly and growth cone adhesiveness on the attractant side. The larger curved arrow indicates the direction of growth cone turning. The smaller arrows indicate intracellular vesicle trafficking, although it remains unclear whether endocytosed membrane components are recycled directly to the other side of the growth cone. The x-axis of each graph corresponds to the width of the growth cone.
Conclusions
Pioneering work in the 1990s led to the discovery of many important axon guidance cues and their receptors. Subsequent studies in the 2000s elucidated signaling mechanisms by which guidance cues polarize the growth cone for turning, e.g., specific ion channels responsible for asymmetric Ca2+ signals, a second messenger network that controls switching between attraction and repulsion, and Ca2+-dependent enzymes involved in axon guidance. More recent work has identified target molecules and mechanisms that link the second messenger system with cellular machinery for growth cone motility. Whereas Ca2+ and cyclic nucleotides can influence the machinery via multiple pathways, we propose that asymmetric membrane trafficking plays a fundamental role in transforming guidance signals into polarized activity of adhesion and cytoskeletal dynamics for bidirectional turning. These revelations, along with future progress in growth cone research will contribute not only to developmental neurobiology but also to understanding mechanisms of human disorders with aberrant axon connectivity150,151 and to technological innovation for guiding regenerating axons to their appropriate targets after injury to the adult central nervous system152.
Online Summary
During neural development, graded distribution of extracellular cues in the microenvironment causes asymmetric generation of second messengers across the growth cone in order to guide the axon along its correct path. Asymmetrically generated Ca2+ signals are sufficient to initiate growth cone turning toward the side with higher Ca2+ concentrations (attraction) or with lower Ca2+ concentrations (repulsion).
Gating of differential sets of Ca2+ channels, which are regulated counteractively by cyclic AMP and cyclic GMP, can be responsible for switching between growth cone attraction and repulsion. We propose that high-amplitude Ca2+ elevation involving Ca2+ release from the endoplasmic reticulum (ER) mediates attractive guidance, whereas low-amplitude Ca2+ influx that does not trigger substantial Ca2+ release from the ER mediates repulsive guidance.
Shallow concentration gradients of guidance cues can shape growth cone Ca2+ signals for precise navigational responses. This process may depend on second messenger networks including positive-feedback augmentation between Ca2+ and cyclic AMP.
Repulsive Ca2+ signals cause asymmetric clathrin-mediated endocytosis across the growth cone with more endocytosis on the side with elevated Ca2+. Attractive Ca2+ signals promote centrifugal transport of membrane vesicles and their subsequent exocytosis mediated by vesicle-associated membrane protein 2 on the side with elevated Ca2+.
We propose that asymmetric membrane trafficking is an early and instructive step in the initiation of growth cone turning. Localized imbalance between endocytosis and exocytosis may trigger redistribution of adhesion molecules, cytoskeletal components and bulk membrane, which would potentiate asymmetric traction and protrusion forces essential for turning.
The growth cone in complex environmental terrain in vivo must integrate multiple guidance signals simultaneously to navigate with high fidelity. Spatiotemporally regulated interactions among Ca2+ and cyclic nucleotides potentially play crucial roles in this integration process, which will need to be scrutinized by quantitative imaging of multiple second messengers in navigating growth cones.
Acknowledgements
We apologize to investigators whose work could not be cited owing to space limitations. The authors' work is supported by RIKEN Brain Science Institute (H.K.), Grants-in-Aid from MEXT (H.K. and T.T.), JST PRESTO program (T.T.), funding by the National Institutes of Health (J.R.H.), and a John M. Nasseff, Sr., Career Development Award in Neurologic Surgery Research from the Mayo Clinic (J.R.H).
glossary
- Local augmentation
A theoretical reaction of chemotactic cells for gradient sensing, in which signals are augmented locally in the cellular area containing higher receptor occupancy.
- Global inhibition
Another theoretical reaction for gradient sensing. The locally augmented signals can be further isolated by spreading antagonistic signals over the whole cell.
- Adaptation
The ability of a growth cone to readjust its sensitivity over a wide range of guidance cue concentrations during long-distance chemotaxis.
- Early endosome
An intracellular membrane compartment where endocytosed molecules are sorted and directed to the plasma membrane for recycling or to lysosomes for degradation. Early endosomes can also serve as a platform where internalized receptors generate signals.
- Rac and Cdc42
Rho-family GTPases that link extracellular signals to cytoskeletal rearrangements. Rac and Cdc42 can, for example, promote actin assembly through their major effector actin-related protein 2/3 complex.
Biographies
Takuro Tojima obtained his Ph.D. (2002) from Graduate School of Science, Hokkaido University, Japan. He joined the Laboratory for Neuronal Growth Mechanisms directed by Hiroyuki Kamiguchi at RIKEN Brain Science Institute. He is also supported by JST PRESTO program. His research interests are in the molecular mechanisms of axon guidance and regeneration.
Jacob Hines earned a Ph.D. in Biomedical Sciences (2010) from the Mayo Clinic, working in the Developmental and Regenerative Neurobiology laboratory led by John Henley. His research interests are in the mechanisms of cell migration in the developing nervous system.
John Henley earned a Ph.D. in Biomedical Sciences (1997) from the Mayo Clinic and did postdoctoral training in molecular neurobiology with Mu-ming Poo at UC San Diego and UC Berkeley. He joined the faculty of the College of Medicine, Mayo Clinic (2006) and directs the Developmental and Regenerative Neurobiology laboratory (http://mayoresearch.mayo.edu/mayo/research/henley_lab/).
Hiroyuki Kamiguchi obtained his M.D. (1989) from Keio University, Japan, and was board certified in neurosurgery (1995). He completed his Ph.D. (1996) at Keio University and postdoctoral training at Case Western Reserve University, USA. He joined the faculty of RIKEN Brain Science Institute in 1999 and studies developmental mechanisms of neuronal circuits.
References
- 1.Sanes JR, Yamagata M. Many paths to synaptic specificity. Annu Rev Cell Dev Biol. 2009;25:161–195. doi: 10.1146/annurev.cellbio.24.110707.175402. [DOI] [PubMed] [Google Scholar]
- 2.Pecho-Vrieseling E, Sigrist M, Yoshida Y, Jessell TM, Arber S. Specificity of sensory-motor connections encoded by Sema3e-Plxnd1 recognition. Nature. 2009;459:842–846. doi: 10.1038/nature08000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakano H. Neural map formation in the mouse olfactory system. Neuron. 2010;67:530–542. doi: 10.1016/j.neuron.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Kennedy TE, Wang H, Marshall W, Tessier-Lavigne M. Axon guidance by diffusible chemoattractants: a gradient of netrin protein in the developing spinal cord. J Neurosci. 2006;26:8866–8874. doi: 10.1523/JNEUROSCI.5191-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flanagan JG. Neural map specification by gradients. Curr Opin Neurobiol. 2006;16:59–66. doi: 10.1016/j.conb.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Hong K, Nishiyama M, Henley J, Tessier-Lavigne M, Poo M. Calcium signalling in the guidance of nerve growth by netrin-1. Nature. 2000;403:93–98. doi: 10.1038/47507. [DOI] [PubMed] [Google Scholar]
- 7.Henley JR, Huang KH, Wang D, Poo MM. Calcium mediates bidirectional growth cone turning induced by myelin-associated glycoprotein. Neuron. 2004;44:909–916. doi: 10.1016/j.neuron.2004.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y, et al. Essential role of TRPC channels in the guidance of nerve growth cones by brain-derived neurotrophic factor. Nature. 2005;434:894–898. doi: 10.1038/nature03477. [DOI] [PubMed] [Google Scholar]
- 9.Wang GX, Poo MM. Requirement of TRPC channels in netrin-1-induced chemotropic turning of nerve growth cones. Nature. 2005;434:898–904. doi: 10.1038/nature03478. [DOI] [PubMed] [Google Scholar]
- 10.Togashi K, et al. Cyclic GMP-gated CNG channels function in Sema3A-induced growth cone repulsion. Neuron. 2008;58:694–707. doi: 10.1016/j.neuron.2008.03.017. [DOI] [PubMed] [Google Scholar]; The first report providing direct evidence of asymmetric cyclic nucleotide signaling across the growth cone during chemotactic guidance.
- 11.Akiyama H, Matsu-ura T, Mikoshiba K, Kamiguchi H. Control of neuronal growth cone navigation by asymmetric inositol 1,4,5-trisphosphate signals. Sci Signal. 2009;2:ra34. doi: 10.1126/scisignal.2000196. [DOI] [PubMed] [Google Scholar]
- 12.Song H, Poo M. The cell biology of neuronal navigation. Nat Cell Biol. 2001;3:E81–E88. doi: 10.1038/35060164. [DOI] [PubMed] [Google Scholar]
- 13.Jin M, et al. Ca2+-dependent regulation of Rho GTPases triggers turning of nerve growth cones. J Neurosci. 2005;25:2338–2347. doi: 10.1523/JNEUROSCI.4889-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chao DL, Ma L, Shen K. Transient cell-cell interactions in neural circuit formation. Nat Rev Neurosci. 2009;10:262–271. doi: 10.1038/nrn2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hopker VH, Shewan D, Tessier-Lavigne M, Poo M, Holt C. Growth-cone attraction to netrin-1 is converted to repulsion by laminin-1. Nature. 1999;401:69–73. doi: 10.1038/43441. [DOI] [PubMed] [Google Scholar]
- 16.Shewan D, Dwivedy A, Anderson R, Holt CE. Age-related changes underlie switch in netrin-1 responsiveness as growth cones advance along visual pathway. Nat Neurosci. 2002;5:955–962. doi: 10.1038/nn919. [DOI] [PubMed] [Google Scholar]
- 17.Maness PF, Schachner M. Neural recognition molecules of the immunoglobulin superfamily: signaling transducers of axon guidance and neuronal migration. Nat Neurosci. 2007;10:19–26. doi: 10.1038/nn1827. [DOI] [PubMed] [Google Scholar]
- 18.Lin AC, Holt CE. Function and regulation of local axonal translation. Curr Opin Neurobiol. 2008;18:60–68. doi: 10.1016/j.conb.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lowery LA, Van Vactor D. The trip of the tip: understanding the growth cone machinery. Nat Rev Mol Cell Biol. 2009;10:332–343. doi: 10.1038/nrm2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Donnell M, Chance RK, Bashaw GJ. Axon growth and guidance: receptor regulation and signal transduction. Annu Rev Neurosci. 2009;32:383–412. doi: 10.1146/annurev.neuro.051508.135614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dent EW, Gupton SL, Gertler FB. The growth cone cytoskeleton in axon outgrowth and guidance. Cold Spring Harb Perspect Biol. 2010 November 24; doi: 10.1101/cshperspect.a001800. doi:10.1101/cshperspect.a001800 published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng JQ. Turning of nerve growth cones induced by localized increases in intracellular calcium ions. Nature. 2000;403:89–93. doi: 10.1038/47501. [DOI] [PubMed] [Google Scholar]
- 23.Gomez TM, Zheng JQ. The molecular basis for calcium-dependent axon pathfinding. Nat Rev Neurosci. 2006;7:115–125. doi: 10.1038/nrn1844. [DOI] [PubMed] [Google Scholar]
- 24.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 25.Gabso M, Neher E, Spira ME. Low mobility of the Ca2+ buffers in axons of cultured Aplysia neurons. Neuron. 1997;18:473–481. doi: 10.1016/s0896-6273(00)81247-7. [DOI] [PubMed] [Google Scholar]
- 26.Augustine GJ, Santamaria F, Tanaka K. Local calcium signaling in neurons. Neuron. 2003;40:331–346. doi: 10.1016/s0896-6273(03)00639-1. [DOI] [PubMed] [Google Scholar]
- 27.Ooashi N, Futatsugi A, Yoshihara F, Mikoshiba K, Kamiguchi H. Cell adhesion molecules regulate Ca2+-mediated steering of growth cones via cyclic AMP and ryanodine receptor type 3. J Cell Biol. 2005;170:1159–1167. doi: 10.1083/jcb.200503157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wen Z, Guirland C, Ming GL, Zheng JQ. A CaMKII/calcineurin switch controls the direction of Ca2+-dependent growth cone guidance. Neuron. 2004;43:835–846. doi: 10.1016/j.neuron.2004.08.037. [DOI] [PubMed] [Google Scholar]; The first demonstration of molecular mechanisms that mediate bidirectional growth cone turning downstream of Ca2+ signals.
- 29.Rusnak F, Mertz P. Calcineurin: form and function. Physiol Rev. 2000;80:1483–1521. doi: 10.1152/physrev.2000.80.4.1483. [DOI] [PubMed] [Google Scholar]
- 30.Hudmon A, Schulman H. Neuronal Ca2+/calmodulin-dependent protein kinase II: the role of structure and autoregulation in cellular function. Annu Rev Biochem. 2002;71:473–510. doi: 10.1146/annurev.biochem.71.110601.135410. [DOI] [PubMed] [Google Scholar]
- 31.Hudmon A, et al. CaMKII tethers to L-type Ca2+ channels, establishing a local and dedicated integrator of Ca2+ signals for facilitation. J Cell Biol. 2005;171:537–547. doi: 10.1083/jcb.200505155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zalk R, Lehnart SE, Marks AR. Modulation of the ryanodine receptor and intracellular calcium. Annu Rev Biochem. 2007;76:367–385. doi: 10.1146/annurev.biochem.76.053105.094237. [DOI] [PubMed] [Google Scholar]
- 33.Foskett JK, White C, Cheung KH, Mak DO. Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev. 2007;87:593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shim S, et al. XTRPC1-dependent chemotropic guidance of neuronal growth cones. Nat Neurosci. 2005;8:730–735. doi: 10.1038/nn1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guirland C, Buck KB, Gibney JA, DiCicco-Bloom E, Zheng JQ. Direct cAMP signaling through G-protein-coupled receptors mediates growth cone attraction induced by pituitary adenylate cyclase-activating polypeptide. J Neurosci. 2003;23:2274–2283. doi: 10.1523/JNEUROSCI.23-06-02274.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murray AJ, Tucker SJ, Shewan DA. cAMP-dependent axon guidance is distinctly regulated by Epac and protein kinase A. J Neurosci. 2009;29:15434–15444. doi: 10.1523/JNEUROSCI.3071-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]; An influential study showing that two cAMP effectors, Epac and protein kinase A, play antagonistic roles in growth cone turning.
- 37.Nishiyama M, et al. Cyclic AMP/GMP-dependent modulation of Ca2+ channels sets the polarity of nerve growth-cone turning. Nature. 2003;423:990–995. doi: 10.1038/nature01751. [DOI] [PubMed] [Google Scholar]; An influential study showing the fundamental rule for cyclic nucleotide-mediated conversion of growth cone turning direction.
- 38.Tojima T, Itofusa R, Kamiguchi H. The nitric oxide-cGMP pathway controls the directional polarity of growth cone guidance via modulating cytosolic Ca2+ signals. J Neurosci. 2009;29:7886–7897. doi: 10.1523/JNEUROSCI.0087-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murray AJ. Pharmacological PKA inhibition: all may not be what it seems. Sci Signal. 2008;1:re4. doi: 10.1126/scisignal.122re4. [DOI] [PubMed] [Google Scholar]
- 40.Poppe H, et al. Cyclic nucleotide analogs as probes of signaling pathways. Nat Methods. 2008;5:277–278. doi: 10.1038/nmeth0408-277. [DOI] [PubMed] [Google Scholar]
- 41.Willoughby D, Cooper DM. Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol Rev. 2007;87:965–1010. doi: 10.1152/physrev.00049.2006. [DOI] [PubMed] [Google Scholar]
- 42.Xu H, Leinwand SG, Dell AL, Fried-Cassorla E, Raper JA. The calmodulin-stimulated adenylate cyclase ADCY8 sets the sensitivity of zebrafish retinal axons to midline repellents and is required for normal midline crossing. J Neurosci. 2010;30:7423–7433. doi: 10.1523/JNEUROSCI.0699-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lefkimmiatis K, et al. Store-operated cyclic AMP signalling mediated by STIM1. Nat Cell Biol. 2009;11:433–442. doi: 10.1038/ncb1850. [DOI] [PubMed] [Google Scholar]
- 44.Gorbunova YV, Spitzer NC. Dynamic interactions of cyclic AMP transients and spontaneous Ca2+ spikes. Nature. 2002;418:93–96. doi: 10.1038/nature00835. [DOI] [PubMed] [Google Scholar]
- 45.Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- 46.Zaccolo M, Movsesian MA. cAMP and cGMP signaling cross-talk: role of phosphodiesterases and implications for cardiac pathophysiology. Circ Res. 2007;100:1569–1578. doi: 10.1161/CIRCRESAHA.106.144501. [DOI] [PubMed] [Google Scholar]
- 47.Shelly M, et al. Local and long-range reciprocal regulation of cAMP and cGMP in axon/dendrite formation. Science. 2010;327:547–552. doi: 10.1126/science.1179735. [DOI] [PubMed] [Google Scholar]; An important crosstalk of cyclic nucleotides demonstrated by imaging of their spatiotemporal dynamics in neurons.
- 48.Meinhardt H. Orientation of chemotactic cells and growth cones: models and mechanisms. J Cell Sci. 1999;112:2867–2874. doi: 10.1242/jcs.112.17.2867. [DOI] [PubMed] [Google Scholar]
- 49.Shim S, et al. Peptidyl-prolyl isomerase FKBP52 controls chemotropic guidance of neuronal growth cones via regulation of TRPC1 channel opening. Neuron. 2009;64:471–483. doi: 10.1016/j.neuron.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]; An important finding implicating proline isomerization of the transient receptor potential family of cation channels as an initial signaling event in growth cone guidance.
- 50.Wu KY, et al. Soluble adenylyl cyclase is required for netrin-1 signaling in nerve growth cones. Nat Neurosci. 2006;9:1257–1264. doi: 10.1038/nn1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moore SW, et al. Soluble adenylyl cyclase is not required for axon guidance to netrin-1. J Neurosci. 2008;28:3920–3924. doi: 10.1523/JNEUROSCI.0547-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cai D, Shen Y, De Bellard M, Tang S, Filbin MT. Prior exposure to neurotrophins blocks inhibition of axonal regeneration by MAG and myelin via a cAMP-dependent mechanism. Neuron. 1999;22:89–101. doi: 10.1016/s0896-6273(00)80681-9. [DOI] [PubMed] [Google Scholar]
- 53.Ming G, et al. Phospholipase C-γ and phosphoinositide 3-kinase mediate cytoplasmic signaling in nerve growth cone guidance. Neuron. 1999;23:139–148. doi: 10.1016/s0896-6273(00)80760-6. [DOI] [PubMed] [Google Scholar]
- 54.Stessin AM, et al. Soluble adenylyl cyclase mediates nerve growth factor-induced activation of Rap1. J Biol Chem. 2006;281:17253–17258. doi: 10.1074/jbc.M603500200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gao Y, Nikulina E, Mellado W, Filbin MT. Neurotrophins elevate cAMP to reach a threshold required to overcome inhibition by MAG through extracellular signal-regulated kinase-dependent inhibition of phosphodiesterase. J Neurosci. 2003;23:11770–11777. doi: 10.1523/JNEUROSCI.23-37-11770.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nishiyama M, von Schimmelmann MJ, Togashi K, Findley WM, Hong K. Membrane potential shifts caused by diffusible guidance signals direct growth-cone turning. Nat Neurosci. 2008;11:762–771. doi: 10.1038/nn.2130. [DOI] [PubMed] [Google Scholar]
- 57.Parsons JT, Horwitz AR, Schwartz MA. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat Rev Mol Cell Biol. 2010;11:633–643. doi: 10.1038/nrm2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hu H, Marton TF, Goodman CS. Plexin B mediates axon guidance in Drosophila by simultaneously inhibiting active Rac and enhancing RhoA signaling. Neuron. 2001;32:39–51. doi: 10.1016/s0896-6273(01)00453-6. [DOI] [PubMed] [Google Scholar]
- 59.Yuan XB, et al. Signalling and crosstalk of Rho GTPases in mediating axon guidance. Nat Cell Biol. 2003;5:38–45. doi: 10.1038/ncb895. [DOI] [PubMed] [Google Scholar]
- 60.Iwasato T, et al. Rac-GAP α-chimerin regulates motor-circuit formation as a key mediator of EphrinB3/EphA4 forward signaling. Cell. 2007;130:742–753. doi: 10.1016/j.cell.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 61.Briancon-Marjollet A, et al. Trio mediates netrin-1-induced Rac1 activation in axon outgrowth and guidance. Mol Cell Biol. 2008;28:2314–2323. doi: 10.1128/MCB.00998-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li X, et al. Netrin signal transduction and the guanine nucleotide exchange factor DOCK180 in attractive signaling. Nat Neurosci. 2008;11:28–35. doi: 10.1038/nn2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wen Z, et al. BMP gradients steer nerve growth cones by a balancing act of LIM kinase and Slingshot phosphatase on ADF/cofilin. J Cell Biol. 2007;178:107–119. doi: 10.1083/jcb.200703055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marsick BM, Flynn KC, Santiago-Medina M, Bamburg JR, Letourneau PC. Activation of ADF/cofilin mediates attractive growth cone turning toward nerve growth factor and netrin-1. Dev Neurobiol. 2010;70:565–588. doi: 10.1002/dneu.20800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lebrand C, et al. Critical role of Ena/VASP proteins for filopodia formation in neurons and in function downstream of netrin-1. Neuron. 2004;42:37–49. doi: 10.1016/s0896-6273(04)00108-4. [DOI] [PubMed] [Google Scholar]
- 66.Terman JR, Mao T, Pasterkamp RJ, Yu HH, Kolodkin AL. MICALs, a family of conserved flavoprotein oxidoreductases, function in plexin-mediated axonal repulsion. Cell. 2002;109:887–900. doi: 10.1016/s0092-8674(02)00794-8. [DOI] [PubMed] [Google Scholar]
- 67.Hung RJ, et al. Mical links semaphorins to F-actin disassembly. Nature. 2010;463:823–827. doi: 10.1038/nature08724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koester MP, Muller O, Pollerberg GE. Adenomatous polyposis coli is differentially distributed in growth cones and modulates their steering. J Neurosci. 2007;27:12590–12600. doi: 10.1523/JNEUROSCI.2250-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Robles E, Huttenlocher A, Gomez TM. Filopodial calcium transients regulate growth cone motility and guidance through local activation of calpain. Neuron. 2003;38:597–609. doi: 10.1016/s0896-6273(03)00260-5. [DOI] [PubMed] [Google Scholar]
- 70.Li W, et al. Activation of FAK and Src are receptor-proximal events required for netrin signaling. Nat Neurosci. 2004;7:1213–1221. doi: 10.1038/nn1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu G, et al. Netrin requires focal adhesion kinase and Src family kinases for axon outgrowth and attraction. Nat Neurosci. 2004;7:1222–1232. doi: 10.1038/nn1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ren XR, et al. Focal adhesion kinase in netrin-1 signaling. Nat Neurosci. 2004;7:1204–1212. doi: 10.1038/nn1330. [DOI] [PubMed] [Google Scholar]
- 73.Conklin MW, Lin MS, Spitzer NC. Local calcium transients contribute to disappearance of pFAK, focal complex removal and deadhesion of neuronal growth cones and fibroblasts. Dev Biol. 2005;287:201–212. doi: 10.1016/j.ydbio.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 74.Robles E, Gomez TM. Focal adhesion kinase signaling at sites of integrin-mediated adhesion controls axon pathfinding. Nat Neurosci. 2006;9:1274–1283. doi: 10.1038/nn1762. [DOI] [PubMed] [Google Scholar]
- 75.Bechara A, et al. FAK-MAPK-dependent adhesion disassembly downstream of L1 contributes to semaphorin3A-induced collapse. EMBO J. 2008;27:1549–1562. doi: 10.1038/emboj.2008.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goh EL, et al. beta1-integrin mediates myelin-associated glycoprotein signaling in neuronal growth cones. Mol Brain. 2008;1:10. doi: 10.1186/1756-6606-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Woo S, Rowan DJ, Gomez TM. Retinotopic mapping requires focal adhesion kinase-mediated regulation of growth cone adhesion. J Neurosci. 2009;29:13981–13991. doi: 10.1523/JNEUROSCI.4028-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yam PT, Langlois SD, Morin S, Charron F. Sonic hedgehog guides axons through a noncanonical, Src-family-kinase-dependent signaling pathway. Neuron. 2009;62:349–362. doi: 10.1016/j.neuron.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 79.Wu KY, et al. Local translation of RhoA regulates growth cone collapse. Nature. 2005;436:1020–1024. doi: 10.1038/nature03885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leung KM, et al. Asymmetrical β-actin mRNA translation in growth cones mediates attractive turning to netrin-1. Nat Neurosci. 2006;9:1247–1256. doi: 10.1038/nn1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yao J, Sasaki Y, Wen Z, Bassell GJ, Zheng JQ. An essential role for β-actin mRNA localization and translation in Ca2+-dependent growth cone guidance. Nat Neurosci. 2006;9:1265–1273. doi: 10.1038/nn1773. [DOI] [PubMed] [Google Scholar]
- 82.Hengst U, Deglincerti A, Kim HJ, Jeon NL, Jaffrey SR. Axonal elongation triggered by stimulus-induced local translation of a polarity complex protein. Nat Cell Biol. 2009;11:1024–1030. doi: 10.1038/ncb1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Drinjakovic J, et al. E3 ligase Nedd4 promotes axon branching by downregulating PTEN. Neuron. 2010;65:341–357. doi: 10.1016/j.neuron.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sasaki Y, et al. Phosphorylation of zipcode binding protein 1 is required for brain-derived neurotrophic factor signaling of local β-actin synthesis and growth cone turning. J Neurosci. 2010;30:9349–9358. doi: 10.1523/JNEUROSCI.0499-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tcherkezian J, Brittis PA, Thomas F, Roux PP, Flanagan JG. Transmembrane receptor DCC associates with protein synthesis machinery and regulates translation. Cell. 2010;141:632–644. doi: 10.1016/j.cell.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tojima T, et al. Attractive axon guidance involves asymmetric membrane transport and exocytosis in the growth cone. Nat Neurosci. 2007;10:58–66. doi: 10.1038/nn1814. [DOI] [PubMed] [Google Scholar]; The first demonstration that polarized vesicle trafficking mediates chemotactic guidance of growth cones.
- 87.Kolpak AL, et al. Negative guidance factor-induced macropinocytosis in the growth cone plays a critical role in repulsive axon turning. J Neurosci. 2009;29:10488–10498. doi: 10.1523/JNEUROSCI.2355-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study suggests that asymmetric membrane retrieval via macropinocytosis, a type of fluid-phase endocytosis, mediates repulsive growth cone guidance.
- 88.Hines JH, Abu-Rub M, Henley JR. Asymmetric endocytosis and remodeling of β1-integrin adhesions during growth cone chemorepulsion by MAG. Nat Neurosci. 2010;13:829–837. doi: 10.1038/nn.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]; The first demonstration that a growth cone redistributes adhesion machinery across its axis for initiating chemotactic turning.
- 89.Tojima T, Itofusa R, Kamiguchi H. Asymmetric clathrin-mediated endocytosis drives repulsive growth cone guidance. Neuron. 2010;66:370–377. doi: 10.1016/j.neuron.2010.04.007. [DOI] [PubMed] [Google Scholar]; This paper demonstrates that asymmetric removal of growth cone plasma membrane via clathrin-coated pits is required and sufficient for repulsive turning.
- 90.Pfenninger KH. Plasma membrane expansion: a neuron's Herculean task. Nat Rev Neurosci. 2009;10:251–261. doi: 10.1038/nrn2593. [DOI] [PubMed] [Google Scholar]
- 91.Sann S, Wang Z, Brown H, Jin Y. Roles of endosomal trafficking in neurite outgrowth and guidance. Trends Cell Biol. 2009;19:317–324. doi: 10.1016/j.tcb.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 92.Craig AM, Wyborski RJ, Banker G. Preferential addition of newly synthesized membrane protein at axonal growth cones. Nature. 1995;375:592–594. doi: 10.1038/375592a0. [DOI] [PubMed] [Google Scholar]
- 93.Fournier AE, et al. Semaphorin3A enhances endocytosis at sites of receptor-F-actin colocalization during growth cone collapse. J Cell Biol. 2000;149:411–422. doi: 10.1083/jcb.149.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jurney WM, Gallo G, Letourneau PC, McLoon SC. Rac1-mediated endocytosis during ephrin-A2- and semaphorin 3A-induced growth cone collapse. J Neurosci. 2002;22:6019–6028. doi: 10.1523/JNEUROSCI.22-14-06019.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gupton SL, Gertler FB. Integrin signaling switches the cytoskeletal and exocytic machinery that drives neuritogenesis. Dev Cell. 2010;18:725–736. doi: 10.1016/j.devcel.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alberts P, et al. Cdc42 and actin control polarized expression of TI-VAMP vesicles to neuronal growth cones and their fusion with the plasma membrane. Mol Biol Cell. 2006;17:1194–1203. doi: 10.1091/mbc.E05-07-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sabo SL, McAllister AK. Mobility and cycling of synaptic protein-containing vesicles in axonal growth cone filopodia. Nat Neurosci. 2003;6:1264–1269. doi: 10.1038/nn1149. [DOI] [PubMed] [Google Scholar]
- 98.Henley J, Poo MM. Guiding neuronal growth cones using Ca2+ signals. Trends Cell Biol. 2004;14:320–330. doi: 10.1016/j.tcb.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.van Horck FP, Weinl C, Holt CE. Retinal axon guidance: novel mechanisms for steering. Curr Opin Neurobiol. 2004;14:61–66. doi: 10.1016/j.conb.2004.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bouchard JF, et al. Protein kinase A activation promotes plasma membrane insertion of DCC from an intracellular pool: A novel mechanism regulating commissural axon extension. J Neurosci. 2004;24:3040–3050. doi: 10.1523/JNEUROSCI.4934-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bartoe JL, et al. Protein interacting with C-kinase 1/protein kinase Ca-mediated endocytosis converts netrin-1-mediated repulsion to attraction. J Neurosci. 2006;26:3192–3205. doi: 10.1523/JNEUROSCI.3469-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Piper M, Salih S, Weinl C, Holt CE, Harris WA. Endocytosis-dependent desensitization and protein synthesis-dependent resensitization in retinal growth cone adaptation. Nat Neurosci. 2005;8:179–186. doi: 10.1038/nn1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ming GL, et al. Adaptation in the chemotactic guidance of nerve growth cones. Nature. 2002;417:411–418. doi: 10.1038/nature745. [DOI] [PubMed] [Google Scholar]
- 104.Zweifel LS, Kuruvilla R, Ginty DD. Functions and mechanisms of retrograde neurotrophin signalling. Nat Rev Neurosci. 2005;6:615–625. doi: 10.1038/nrn1727. [DOI] [PubMed] [Google Scholar]
- 105.Mann F, Miranda E, Weinl C, Harmer E, Holt CE. B-type Eph receptors and ephrins induce growth cone collapse through distinct intracellular pathways. J Neurobiol. 2003;57:323–336. doi: 10.1002/neu.10303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Joset A, Dodd DA, Halegoua S, Schwab ME. Pincher-generated Nogo-A endosomes mediate growth cone collapse and retrograde signaling. J Cell Biol. 2010;188:271–285. doi: 10.1083/jcb.200906089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Greka A, Navarro B, Oancea E, Duggan A, Clapham DE. TRPC5 is a regulator of hippocampal neurite length and growth cone morphology. Nat Neurosci. 2003;6:837–845. doi: 10.1038/nn1092. [DOI] [PubMed] [Google Scholar]
- 108.Akiyama H, Kamiguchi H. Phosphatidylinositol 3-kinase facilitates microtubule-dependent membrane transport for neuronal growth cone guidance. J Biol Chem. 2010;285:41740–41748. doi: 10.1074/jbc.M110.156489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Karcher RL, et al. Cell cycle regulation of myosin-V by calcium/calmodulin-dependent protein kinase II. Science. 2001;293:1317–1320. doi: 10.1126/science.1061086. [DOI] [PubMed] [Google Scholar]
- 110.Guillaud L, Wong R, Hirokawa N. Disruption of KIF17-Mint1 interaction by CaMKII-dependent phosphorylation: a molecular model of kinesin-cargo release. Nat Cell Biol. 2008;10:19–29. doi: 10.1038/ncb1665. [DOI] [PubMed] [Google Scholar]
- 111.Pang ZP, Sudhof TC. Cell biology of Ca2+-triggered exocytosis. Curr Opin Cell Biol. 2010;22:496–505. doi: 10.1016/j.ceb.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Marks B, McMahon HT. Calcium triggers calcineurin-dependent synaptic vesicle recycling in mammalian nerve terminals. Curr Biol. 1998;8:740–749. doi: 10.1016/s0960-9822(98)70297-0. [DOI] [PubMed] [Google Scholar]
- 113.Cousin MA, Robinson PJ. The dephosphins: dephosphorylation by calcineurin triggers synaptic vesicle endocytosis. Trends Neurosci. 2001;24:659–665. doi: 10.1016/s0166-2236(00)01930-5. [DOI] [PubMed] [Google Scholar]
- 114.Lee SY, et al. Regulation of the interaction between PIPKIγ and talin by proline-directed protein kinases. J Cell Biol. 2005;168:789–799. doi: 10.1083/jcb.200409028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ellis L, Katz F, Pfenninger KH. Nerve growth cones isolated from fetal rat brain. II. Cyclic adenosine 3':5'-monophosphate (cAMP)-binding proteins and cAMP-dependent protein phosphorylation. J Neurosci. 1985;5:1393–1401. doi: 10.1523/JNEUROSCI.05-06-01393.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Menegon A, et al. Protein kinase A-mediated synapsin I phosphorylation is a central modulator of Ca2+-dependent synaptic activity. J Neurosci. 2006;26:11670–11681. doi: 10.1523/JNEUROSCI.3321-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bonanomi D, et al. Phosphorylation of synapsin I by cAMP-dependent protein kinase controls synaptic vesicle dynamics in developing neurons. J Neurosci. 2005;25:7299–7308. doi: 10.1523/JNEUROSCI.1573-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kao HT, et al. A protein kinase A-dependent molecular switch in synapsins regulates neurite outgrowth. Nat Neurosci. 2002;5:431–437. doi: 10.1038/nn840. [DOI] [PubMed] [Google Scholar]
- 119.Micheva KD, Buchanan J, Holz RW, Smith SJ. Retrograde regulation of synaptic vesicle endocytosis and recycling. Nat Neurosci. 2003;6:925–932. doi: 10.1038/nn1114. [DOI] [PubMed] [Google Scholar]
- 120.Caswell PT, Vadrevu S, Norman JC. Integrins: masters and slaves of endocytic transport. Nat Rev Mol Cell Biol. 2009;10:843–853. doi: 10.1038/nrm2799. [DOI] [PubMed] [Google Scholar]
- 121.Ezratty EJ, Bertaux C, Marcantonio EE, Gundersen GG. Clathrin mediates integrin endocytosis for focal adhesion disassembly in migrating cells. J Cell Biol. 2009;187:733–747. doi: 10.1083/jcb.200904054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hasan N, Hu C. Vesicle-associated membrane protein 2 mediates trafficking of α5β1 integrin to the plasma membrane. Exp Cell Res. 2010;316:12–23. doi: 10.1016/j.yexcr.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 123.Woo S, Gomez TM. Rac1 and RhoA promote neurite outgrowth through formation and stabilization of growth cone point contacts. J Neurosci. 2006;26:1418–1428. doi: 10.1523/JNEUROSCI.4209-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Eva R, et al. Rab11 and its effector Rab coupling protein contribute to the trafficking of β1 integrins during axon growth in adult dorsal root ganglion neurons and PC12 cells. J Neurosci. 2010;30:11654–11669. doi: 10.1523/JNEUROSCI.2425-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gatlin JC, Estrada-Bernal A, Sanford SD, Pfenninger KH. Myristoylated, alanine-rich C-kinase substrate phosphorylation regulates growth cone adhesion and pathfinding. Mol Biol Cell. 2006;17:5115–5130. doi: 10.1091/mbc.E05-12-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chan KT, Bennin DA, Huttenlocher A. Regulation of adhesion dynamics by calpain-mediated proteolysis of focal adhesion kinase (FAK) J Biol Chem. 2010;285:11418–11426. doi: 10.1074/jbc.M109.090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Oda A, Druker BJ, Ariyoshi H, Smith M, Salzman EW. pp60src is an endogenous substrate for calpain in human blood platelets. J Biol Chem. 1993;268:12603–12608. [PubMed] [Google Scholar]
- 128.Franco SJ, et al. Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nat Cell Biol. 2004;6:977–983. doi: 10.1038/ncb1175. [DOI] [PubMed] [Google Scholar]
- 129.Palamidessi A, et al. Endocytic trafficking of Rac is required for the spatial restriction of signaling in cell migration. Cell. 2008;134:135–147. doi: 10.1016/j.cell.2008.05.034. [DOI] [PubMed] [Google Scholar]; This paper demonstrates that delivery of Rac-containing vesicles to specific subcellular locations controls local actin remodeling.
- 130.Osmani N, Peglion F, Chavrier P, Etienne-Manneville S. Cdc42 localization and cell polarity depend on membrane traffic. J Cell Biol. 2010;191:1261–1269. doi: 10.1083/jcb.201003091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Blondeau F, et al. Tandem MS analysis of brain clathrin-coated vesicles reveals their critical involvement in synaptic vesicle recycling. Proc Natl Acad Sci U S A. 2004;101:3833–3838. doi: 10.1073/pnas.0308186101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Takamori S, et al. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 133.Horiguchi K, Hanada T, Fukui Y, Chishti AH. Transport of PIP3 by GAKIN, a kinesin-3 family protein, regulates neuronal cell polarity. J Cell Biol. 2006;174:425–436. doi: 10.1083/jcb.200604031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Arimura N, Kaibuchi K. Neuronal polarity: from extracellular signals to intracellular mechanisms. Nat Rev Neurosci. 2007;8:194–205. doi: 10.1038/nrn2056. [DOI] [PubMed] [Google Scholar]
- 135.Peskin CS, Odell GM, Oster GF. Cellular motions and thermal fluctuations: the Brownian ratchet. Biophys J. 1993;65:316–324. doi: 10.1016/S0006-3495(93)81035-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Raucher D, Sheetz MP. Cell spreading and lamellipodial extension rate is regulated by membrane tension. J Cell Biol. 2000;148:127–136. doi: 10.1083/jcb.148.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Serafini T, et al. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–1014. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- 138.Charron F, Stein E, Jeong J, McMahon AP, Tessier-Lavigne M. The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell. 2003;113:11–23. doi: 10.1016/s0092-8674(03)00199-5. [DOI] [PubMed] [Google Scholar]
- 139.Butler SJ, Dodd J. A role for BMP heterodimers in roof plate-mediated repulsion of commissural axons. Neuron. 2003;38:389–401. doi: 10.1016/s0896-6273(03)00254-x. [DOI] [PubMed] [Google Scholar]
- 140.Islam SM, et al. Draxin, a repulsive guidance protein for spinal cord and forebrain commissures. Science. 2009;323:388–393. doi: 10.1126/science.1165187. [DOI] [PubMed] [Google Scholar]
- 141.Bourikas D, et al. Sonic hedgehog guides commissural axons along the longitudinal axis of the spinal cord. Nat Neurosci. 2005;8:297–304. doi: 10.1038/nn1396. [DOI] [PubMed] [Google Scholar]
- 142.Lyuksyutova AI, et al. Anterior-posterior guidance of commissural axons by Wnt-frizzled signaling. Science. 2003;302:1984–1988. doi: 10.1126/science.1089610. [DOI] [PubMed] [Google Scholar]
- 143.Domanitskaya E, et al. Sonic hedgehog guides post-crossing commissural axons both directly and indirectly by regulating Wnt activity. J Neurosci. 2010;30:11167–11176. doi: 10.1523/JNEUROSCI.1488-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dufour A, et al. Area specificity and topography of thalamocortical projections are controlled by ephrin/Eph genes. Neuron. 2003;39:453–465. doi: 10.1016/s0896-6273(03)00440-9. [DOI] [PubMed] [Google Scholar]
- 145.Powell AW, Sassa T, Wu Y, Tessier-Lavigne M, Polleux F. Topography of thalamic projections requires attractive and repulsive functions of Netrin-1 in the ventral telencephalon. PLoS Biol. 2008;6:e116. doi: 10.1371/journal.pbio.0060116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Stein E, Tessier-Lavigne M. Hierarchical organization of guidance receptors: silencing of netrin attraction by slit through a Robo/DCC receptor complex. Science. 2001;291:1928–1938. doi: 10.1126/science.1058445. [DOI] [PubMed] [Google Scholar]
- 147.Parra LM, Zou Y. Sonic hedgehog induces response of commissural axons to Semaphorin repulsion during midline crossing. Nat Neurosci. 2010;13:29–35. doi: 10.1038/nn.2457. [DOI] [PubMed] [Google Scholar]
- 148.Dudanova I, Gatto G, Klein R. GDNF acts as a chemoattractant to support ephrinA-induced repulsion of limb motor axons. Curr Biol. 2010;20:2150–2156. doi: 10.1016/j.cub.2010.11.021. [DOI] [PubMed] [Google Scholar]; This study suggests that two opposing cues presented as counter gradients push and pull the growth cone to ensure its correct navigation.
- 149.Schmitt AM, et al. Wnt-Ryk signalling mediates medial-lateral retinotectal topographic mapping. Nature. 2006;439:31–37. doi: 10.1038/nature04334. [DOI] [PubMed] [Google Scholar]
- 150.Engle EC. Human genetic disorders of axon guidance. Cold Spring Harb Perspect Biol. 2010;2:a001784. doi: 10.1101/cshperspect.a001784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tischfield MA, et al. Human TUBB3 mutations perturb microtubule dynamics, kinesin interactions, and axon guidance. Cell. 2010;140:74–87. doi: 10.1016/j.cell.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Alto LT, et al. Chemotropic guidance facilitates axonal regeneration and synapse formation after spinal cord injury. Nat Neurosci. 2009;12:1106–1113. doi: 10.1038/nn.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Li L, Hutchins BI, Kalil K. Wnt5a induces simultaneous cortical axon outgrowth and repulsive axon guidance through distinct signaling mechanisms. J Neurosci. 2009;29:5873–5883. doi: 10.1523/JNEUROSCI.0183-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Vaudry D, et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev. 2009;61:283–357. doi: 10.1124/pr.109.001370. [DOI] [PubMed] [Google Scholar]
- 155.Zheng JQ, Felder M, Connor JA, Poo MM. Turning of nerve growth cones induced by neurotransmitters. Nature. 1994;368:140–144. doi: 10.1038/368140a0. [DOI] [PubMed] [Google Scholar]
- 156.Bouzigues C, Morel M, Triller A, Dahan M. Asymmetric redistribution of GABA receptors during GABA gradient sensing by nerve growth cones analyzed by single quantum dot imaging. Proc Natl Acad Sci U S A. 2007;104:11251–11256. doi: 10.1073/pnas.0702536104. [DOI] [PMC free article] [PubMed] [Google Scholar]