Abstract
Estradiol (E2) treatment in young adult, ovariectomized mice increases physical activity and reverses deleterious effects on skeletal muscle. Here we test the hypothesis that E2 treatment improves muscle function and physical activity in aged, ovarian-senescent mice. Plasma E2 levels and vaginal cytology confirmed ovarian senescence in 20-month-old C57BL/6 mice. Mice were then randomly divided into activity groups, having access to a running wheel or not, and further into those receiving E2 or placebo. Placebo-treated mice wheel ran more than E2-treated mice (P=0.03), with no difference between treatment groups in cage activities such as time spent being active and ambulation distance (P≥0.55). Soleus muscles from aged mice that wheel ran adapted by getting larger and stronger, irrespective of E2 status (P≤0.02). Soleus muscle fatigue resistance was greater in mice treated with E2 (P=0.02), but maximal isometric tetanic force was not affected (P≥0.79). Because E2 treatment did not improve physical activity or overall muscle function in the aged, ovarian-senescent mice as predicted, a second study was initiated to examine E2 treatment of young adult mice prematurely ovarian senescent from exposure to the chemical, 4-vinylcyclohexene diepoxide (VCD). 4-month-old C57BL/6 female mice were dosed with oil (control) or VCD. Vaginal cytology confirmed ovarian senescence in all mice treated with VCD 63 days after the onset of dosing, and then a subset of the VCD mice received E2 (VCD+E2). Wheel running distance did not differ among control, VCD, and VCD+E2 mice (P≥0.34). Soleus muscle concentric, isometric, and eccentric in vitro forces were greater in VCD+E2 than VCD mice (P<0.04), indicating beneficial estrogenic effects on muscle function. In general, aged and young mice with senescent ovaries were less responsive to E2 treatment, in terms of physical activities and muscle function, than what has previously been shown for young, ovariectomized mice. These results bring forth the possibility that some component of the residual, follicle-depleted ovarian tissue influences physical activity in mice or that aging diminishes the responsiveness of skeletal muscle and related tissues to E2 treatment.
Keywords: 4-vinylcyclohexene diepoxide, aging, exercise, skeletal muscle
1. Introduction
Aging results in many structural, functional, and biochemical changes that can impact the ability or the willingness to do physical activity [1]. This scenario is complicated in females because changes in ovarian hormones occur simultaneously with aging and some of the tissues important for regulating and doing physical activity are affected by both age and ovarian hormones. For example, in rodents the hypothalamic areas that regulate physical activity are affected by both aging and alterations in estrogen status [2,3,4]. Similarly, skeletal muscle weakness occurs with age and there is evidence for a greater decline in strength at the time of menopause in women [5] and ovarian failure in female mice [6]. One approach for dissecting out the influences of aging versus ovarian hormones is to ovariectomize young female rodents. Although this is closer to a model of surgical menopause in young women, it is advantageous because the effects of ovarian hormones can be assessed independent of age. Using this approach, physical activity in terms of voluntary wheel running decreases by as much as 90% and cage activities are reduced by about 50% in young female rodents post-surgery [7,8,9]. Similarly, skeletal muscle force generation decreases by 10 to 20% post-surgery [10,11,12]. Furthermore, it appears that estrogens are the primary hormones responsible for the decreases, as 17β-estradiol (E2) treatment reverses these ovariectomy-induced physical inactivities within a few days [7,8,9,13] and skeletal muscle function can be restored in as early as 14 days [7,11,12]. Similarly, female rodents become less active with age [1], but whether or not low circulating estrogen levels contribute to this age-reported physical inactivity is not known. Thus, the first purpose of this study was to determine the effects of E2 treatment on physical activity of older female mice that had experienced natural, age-induced ovarian senescence. Physical activity was monitored by both voluntary wheel running and several parameters of daily cage activities. We hypothesized that physical activities would be greater in aged, ovarian-senescent mice treated with E2 compared with those not treated.
It is unclear if exercise-induced skeletal muscle adaptations differ for post-menopausal women on estrogen-based hormone therapy compared to those not. Some studies have shown no differences between interventions of exercise alone or exercise in combination with estrogen-based hormone therapy [14,15,16], while others have shown additive effects of exercise and hormone therapy [17,18]. Beneficial skeletal muscle adaptations do occur in response to both physical activity and estradiol treatment in young, ovariectomized mice [7,11,12]. As such, a second purpose of this study was to determine the extent to which muscle of aged, ovarian-senescent mice adapt to physical activity and to E2 treatment. We hypothesized that mice engaged in physical activity would have better soleus muscle contractility compared with those that were relatively sedentary, and soleus muscle contractile function would be greater in aged, ovarian-senescent mice treated with E2 versus those that remained untreated. Specifically, we hypothesized that maximal isometric force and active stiffness would be greater in soleus muscles of aged, ovarian-senescent mice treated with E2 compared with those not treated. Furthermore, because there have been sex implications in resistance to muscle fatigue [19], we hypothesized that there would be a protective effect of E2 on soleus muscle fatigability.
Our initial hypotheses were not supported, as the aged, ovarian-senescent mice treated with E2 did not have improved physical activities or overall muscle function compared to the placebo-treated, aged mice. Thus, the E2 benefits on physical activity and skeletal muscle observed in young ovariectomized mice might be influenced by the absence of the ovaries. Alternatively, those tissues important for doing or regulating physical activity and muscle contractility are responsive to E2 treatment in young but not aged mice. We addressed these two possibilities using a mouse model of premature ovarian senescence allowing us to investigate senescent ovaries in otherwise young mice [20,21]. Chemical dosing of 4-vinylcyclohexene diepoxide (VCD) accelerates the normal process of apoptosis and atresia of ovarian follicles resulting in state of persistent diestrus and low circulating E2 levels, similar to what occurs with age in rodents [22,23]. The advantages of this approach is that young adult mice can be utilized to study the influences of ovarian hormones without the confounding effects of advanced age, similar to ovariectomy, but while having the ovarian tissue remain intact. Furthermore, the hormone profile of the follicle-depleted, ovary-intact young mouse after VCD treatment is similar to the aged, ovarian-senescent mouse as opposed to the abrupt changes in hormones that occur with ovariectomy [24,25]. Specifically, with VCD exposure, follicle stimulating and luteinizing hormones gradually increase, while progesterone, androstenedione, and E2 progressively decrease to levels found in age-induced, ovarian senescence mice [23,25]. VCD treatment has been shown to specifically target the ovary with no effects on other tissues [26,27,28,29,30,31,32].
Using young adult, VCD-treated mice with and without subsequent E2 treatment, we tested the hypothesis that physical activity and soleus muscle function would be reduced when circulating E2 was diminished and preserved when E2 was restored. Support of this hypothesis would lead us to believe that indeed E2 is a key ovarian hormone for physical activity and skeletal muscle function and that perhaps in our previous study using aged mice the dosing regimen was not optimal or the aged mouse was not able to respond to the E2 treatment.
2. Methods
2.1 Animals
Female C57BL/6 mice (n = 60) were purchased through the National Institute of Aging at 17 months of age and then given 1 week to acclimate to our facility upon arrival for Study I. Twelve of the initial 60 mice were not used in the study. Six of the mice were excluded before being assigned to a group; three had skin irritations in the neck area that would have compromised pellet implantation, one had an abdominal tumor, and two died of unknown causes. The other six mice were eliminated during the study due to bacterial infection (n=1), inner ear infection (n=1), distended urinary bladder (n=1), and death of undetermined cause (n=3).
A second set of female C57BL/6 mice (n=24) were purchased from Jackson Laboratories (Bar Harbor, ME) at 3 months of age for Study II. These mice were housed at the Jackson In Vivo Laboratory Facility and were dosed daily for 15 days with either sesame oil (n=8) or VCD (n=16) via intraperitoneal injections at a dose of 160 mg/kg body mass/day [23]. Five of the VCD mice died during treatment at Jackson Laboratories; technical reports indicated the mice appeared fine except for abnormal gait patterns following their last injection prior to death. Thus, a total of nineteen, 4-month-old mice arrived in our facilities for Study II.
Aged mice in Study I were housed five per cage until persistent diestrus was confirmed and at that time were placed in individual cages. Mice in Study II were group housed for the duration of the study. All mice were maintained on a 12-hr light cycle and had free access to water and a phytoestrogen-free chow (2019 Teklad Global 19% Protein Rodent Diet, Harland Teklad, Madison, WI). This chow was used to eliminate potential complications from exogenous forms of estrogens.
At the end of each study, when mice were ~22- and 8-months of age for Study I and II respectively, mice were weighed and anesthetized by intraperitoneal injection of sodium pentobarbital (100 mg/kg body weight), with supplemental doses given as needed prior to in vitro muscle contractile function testing. After muscles and uteri were harvested, mice were euthanized by an overdoes of sodium pentobarbital (200 mg/kg body weight). All protocols were approved by the Institutional Animal Care and Use Committee at the University of Minnesota.
2.2 Experimental design of Study I
Ovarian senescence was determined by vaginal cytology. Cytology was assessed 2–3 times per week and a mouse was deemed to be in persistent diestrus when two consecutive samples showed predominantly leukocytes [33]. Of the 60 mice initially received, 7 were in persistent diestrus upon arrival. By 18- and 19-months of age, 10 and 19 mice entered persistent diestrus, respectively. In all, mice had failed ovaries by an average of 19.7 ± 0.2 (mean ± SE) months of age. This was confirmed by the low plasma levels of E2 one week prior to the onset of the study; 38 of the 40 mice that were tested had values below the assay detection level (10 pg/ml) and the remaining 2 mice averaged 15.2 ± 0.4 pg/ml. Because the timing of E2 treatment following hormone deficiency is critical [34,35], within 2–4 weeks of when each mouse entered persistent diestrus, the following study design was initiated (Figure 1).
Figure 1.

Design for Study I; Upon confirmed ovarian-senescence, mice were randomly assigned to sedentary or running groups. Sedentary mice were then randomly assigned to placebo or E2 treatment, while runners were divided on a rank-order scheme to placebo or E2 treatment. Design for Study II; Approximately, half of the mice dosed with VCD were randomized to receive E2 treatment. Physical activity testing (wheel running and cage activities) occurred once prior to and twice following hormone manipulation. In both studies, following ~8 weeks of hormone manipulation, soleus muscle function was tested in vitro.
Approximately half of the mice were given running wheels for 1 week in their home cages for acclimation purposes. Distance run during the last 3 days was used to rank mice according to running performance. These mice were divided into two groups based on a rank-order scheme designed to minimize baseline differences between the two groups in wheel running performance. The mice then received pellet implants, either E2 (run-E2; n=12) or placebo (run-placebo; n=9). Wheels were taken out of cages for 3 days to allow recovery from the minor pellet-implantation surgery. Wheels were then reintroduced and voluntary running continued for approximately 8 weeks. Mice that did not wheel run, i.e., remained sedentary, were also subdivided into two groups and received pellets; either E2 (sed-E2; n=10) or placebo (sed- placebo; n=9) and remained in their individual cages for ~8 week. 24-hr cage activity measurements were conducted on all mice during week 7 of the hormone manipulation period. Body mass of each mouse was measured weekly and food intake was monitored daily throughout the study. At ~22 months of age each mouse was anesthetized and soleus muscles underwent in vitro contractile function testing.
Eight additional 17 month-old mice not included in the previous study design were used to investigate the influence of the ovarian tissue on wheel running. Once persistent diestrus was confirmed, all mice were given running wheels for 1 week. The mice were then randomized and underwent either a sham (n=3) or ovariectomy (n=5) procedure. Following ~1 week of recovery mice were re-introduced to running wheels for 2 weeks.
2.3 Experimental design of Study II
VCD-treated mice were assessed daily for estrous cycle stage until persistent diestrus and thus ovarian senescence was confirmed. VCD mice were considered acyclic after 10 days of diestrus [36], which occurred 63 ± 3 days following the onset of VCD dosing. Once persistent diestrus was confirmed, each mouse underwent physical activity testing including wheel running and cage activities. Then, ~4 weeks later (when mice were ~6 months of age), hormone manipulation began and lasted for 8 weeks; experimental groups were VCD (n=5), VCD+E2 (n=6), and control (n=8) (see Figure 1). The control group consisted of the mice that had received sesame oil injections at Jackson Labs. During the 8-week intervention, mice underwent assessments of wheel running motivation (during weeks 2 and 5), cage activities and food intake (during weeks 1 and 7), and weekly body mass. At the end of 8-week intervention, mice were ~8 months of age, and were anesthetized and soleus muscles underwent in vitro contractile function testing.
2.4 Hormone manipulation and related procedures
Mice were anesthetized in an induction chamber with isoflurane and then maintained via inhalation of 1.75% isoflurane mixed with oxygen at a flow rate of 200 ml/min delivered via a mask. Once anesthetized, mice were implanted with a 60-day slow-release E2 or placebo pellet (Innovative Research of America, Sarasota, FL) on the dorsal aspect of the neck using a trochar. In Study I, E2 pellets contained 0.09 mg of 17β-estradiol, delivering ~1.5 µg daily. This dosage was used for two reasons. First, a dosage was desired that would mimic the average circulating level of E2 in young, adult female mice over the estrous cycle, i.e., ~15 pg/ml serum [37]. In a preliminary study using these pellets we measured plasma E2 from four mice at 16.01 ± 5.3 pg/ml. Second, we also found in preliminary studies that 0.18 mg 17β-estradiol pellets caused urine retention in aged mice, which has been reported by others as well [38,39,40]. Bladder problems were minimized by using the lower dose pellets as only one mouse was excluded due to a distended urinary bladder. In Study II, a subset of the VCD-treated mice were implanted with 0.18 mg, 60-day slow-release E2 pellets, which delivers ~3 µg per day. We used the higher dose pellet for these younger mice because we desired to mimic our previous experiments on young ovariectomized mice [7,9,11]. Placebo pellets used in both studies consisted of the same matrix as the E2 pellets.
Ovariectomy of mice was conduced as previously described [7,10,11]. While under isoflurane anesthesia the ovaries were removed bilaterally. Mice undergoing the ovariectomy procedure were administered 0.15 µg of buprenorphine subcutaneously ~5 minutes post procedure. Blood was collected by facial vein bleed, plasma was separated from whole blood, and E2 was measured by ELISA (KA0234, Abnova Corporation, Taiwan). The uteri were dissected and immediately weighed as additional marker of ovarian-hormone depletion and hormone treatment.
2.5 Physical activity assessments
Voluntary wheel running was measured using an exercise wheel, 11 cm in diameter with a 2 inch wide running surface, that was mounted to the top of a standard mouse cage [9]. A digital magnetic counter was fastened to the wheel and connected to a microprocessor which stored the number of revolutions per 24 hr. In Study II, mice were allowed access to the wheels on three separate 3-day periods to assess physical activity without any anticipated training effects [41].
Cage activities were evaluated by first placing individual mice in a mock activity chamber to acclimate to the new environment for 24 hr immediately prior to the test. Mice were then placed individually into an activity chamber (Med Associates Inc., St. Albans, Vermont) for 24 hr [7,42]. Data presented are total active time, jumping counts, rearing counts, and ambulatory distance and were collected using Activity Monitor version 6.01 software (Med Associates) with a box size set to “3” (4.8 cm2). While in the activity chamber, food intake was also measured for each mouse in Study II.
2.6 In vitro assessment of soleus muscle contractility
The dissection and testing protocol for the isolated soleus muscle preparation has been described previously [6,43]. First, passive muscle stiffness was determined by stretching inactive muscle sinusoidally from 97.5% Lo to 102.5% Lo at 0.5 Hz and measuring the force response (300B-LR; Aurora Scientific Inc., Aurora, ON, Canada). Maximal isometric twitch force (Pt) was then elicited by stimulating the muscle with a 0.5-ms pulse at 150 V (Grass S48 stimulator delivered through a SIU5D stimulus isolation unit; Grass Telefactor, Warwick, RI) and maximal isometric tetanic force (Po) determined by stimulating muscles for 400 ms at 150 V and 120 Hz. Active stiffness was determined during a second maximal tetanic contraction by eliciting a sinusoidal length oscillation of 0.01% Lo at 500 Hz. Additional parameters measured from twitch and tetanic contractions include; time to peak twitch (TPT), twitch one-half relaxation time (RT½), maximal rate of tetanic force development (+dP/dt), and maximal rate of relaxation (−dP/dt).
Soleus muscles from mice in Study I then underwent a protocol employing a bout of fatiguing contractions that began 2 min following the final force and stiffness measurements [7]. For soleus muscles from Study II, they then were measured for maximal concentric force that was elicited by passively lengthening the muscle to 105% of Lo over 3 s, and then stimulating tetanically for 133 ms as the muscle was shortened to 95% of Lo at 1.5 Lo/s. Finally, eccentric force was elicited by passively shortening the muscle to 95% of Lo and then stimulating tetanically as it lengthened to 105% of Lo at 1.5 Lo/s.
Following contractility testing, each soleus muscle was removed from the bath, trimmed at the myotendinous junctions, blotted, weighed, and stored at −80° until further analysis. Fiber length was estimated as 71% of the soleus muscle length [44]. Specific Po was calculated by correcting for physiological cross-sectional area of the muscle [45].
2.7 Statistical analysis
In Study I, a two-way repeated measure ANOVA was used to determine if differences existed for wheel-running distances between mice treated and not treated with E2 over time. Student t-tests were used to examine possible differences in cage activities between mice treated and not treated with E2. A three-way repeated measure ANOVA (treatment × activity level × time) was used to determine differences in food intake. Two-way ANOVAs (treatment (E2 or placebo) × activity level (wheel runners or sedentary)) were used to determine if there were differences among the four groups for body mass, organ masses, muscle composition, and soleus muscle contractility measures. Holm-Sidak post-hoc tests were applied whenever a significant interaction or main effect was detected by ANOVA.
For Study II, two-way repeated measures ANOVAs (group × time) were conducted to assess body mass, activity and running. One-way ANOVAs were used to determine differences among groups for food intake, muscle contractile parameters, uterine masses, and muscle masses. Holm-Sidak post-hoc tests were applied whenever a significant interaction or main effect was detected by ANOVA.
Statistical analyses were done using SigmaStat version 3.5 (Systat Software Inc; Point Richmond, CA), with the exception for the three-way repeated measures ANOVA, which was conducted using JMP version 7.0 (SAS Institute, Inc; Cary NC). Significance was accepted with an α level of 0.05. Values are reported as means ± SE.
3. Results
3.1 Study I: Ovarian senescence in aged mice
Body mass was not different among groups at the beginning or end of the 8-week study (i.e., when mice were 20 and 22 months old, respectively), although there were trends for E2-treated mice to have lower body masses than those treated with placebo (Table 1). On average, mice ate 4.1 ± 0.1 g of food per 24 hr with no effect of hormone status or physical activity (P>0.457). Mice treated with E2 had uterine masses nearly twice that of placebo mice indicating effective E2 treatment (Table 1).
Table 1.
Body and uterine masses from aged, ovarian-senescent mice that were treated with placebo or E2 for 8 weeks in Study I.
| Sedentary | Runner | 2-Way ANOVA P-value |
|||||
|---|---|---|---|---|---|---|---|
|
Placebo (n=9) |
E2 (n=10) |
Placebo (n=9) |
E2 (n=12) |
Running effect |
Estradiol effect |
Interaction | |
| Body mass at 20-mo (g) |
27.8 ± 1.2 | 25.6 ± 0.6 | 27.7 ± 1.6 | 26.7 ± 0.6 | 0.602 | 0.137 | 0.543 |
| Body mass at 22-mo (g) |
28.8 ± 1.3 | 26.7 ± 0.8 | 28.7 ± 1.9 | 26.5 ± 0.5 | 0.909 | 0.067 | 0.917 |
| Uterus mass (mg) | 94.4 ± 7.4 | 199.0 ± 10.3 |
98.9 ± 10.8 |
182.5 ± 25.7 |
0.069 | <0.001 | 0.593 |
Mean ± SE. Body masses were measured when all mice were ovarian-failed but had not yet been randomized to receive E2- or placebo-treatment (~20-mo of age) and at the termination of the study (~22-mo of age) after 8 weeks of hormone intervention.
3.1.1 Physical activities
Of the non-wheel running mice, there was no difference between those treated with placebo and E2 for any parameter of daily cage activity. Mice were active for about 4 hr per day, 234 ± 31 vs. 248 ± 12 min for placebo and E2 mice, respectively (P=0.555). Distance ambulated per 24 hr was not different between groups, 415 ± 50 vs. 365 ± 72 m for placebo and E2 mice, respectively (P=0.608). During the active periods, placebo- and E2-treated mice had equivalent counts for jumping and rearing activities as well (P≥0.551).
Mice that had access to running wheels were affected by E2 as far as distances run. Prior to receiving placebo or E2 treatments, mice ran an average 2.8 ± 0.3 km per 24 hr (Figure 2). There was an effect of both time and treatment on running distances with the E2–treated mice running significantly less than placebo-treated mice during the 8-week study (P≤0.027; Figure 2).
Figure 2.
Distances run on wheels per 24 hr depicted as weekly averages for aged, ovarian-senescent mice treated with placebo or E2 (means ± SE). Pre time point was prior to treatment. Data analyzed by 2-way repeated measures ANOVA (treatment by time). Main effect of both treatment and time. N=9–12 per group. *Significantly different than the pre time point; †Significantly different than week 2.
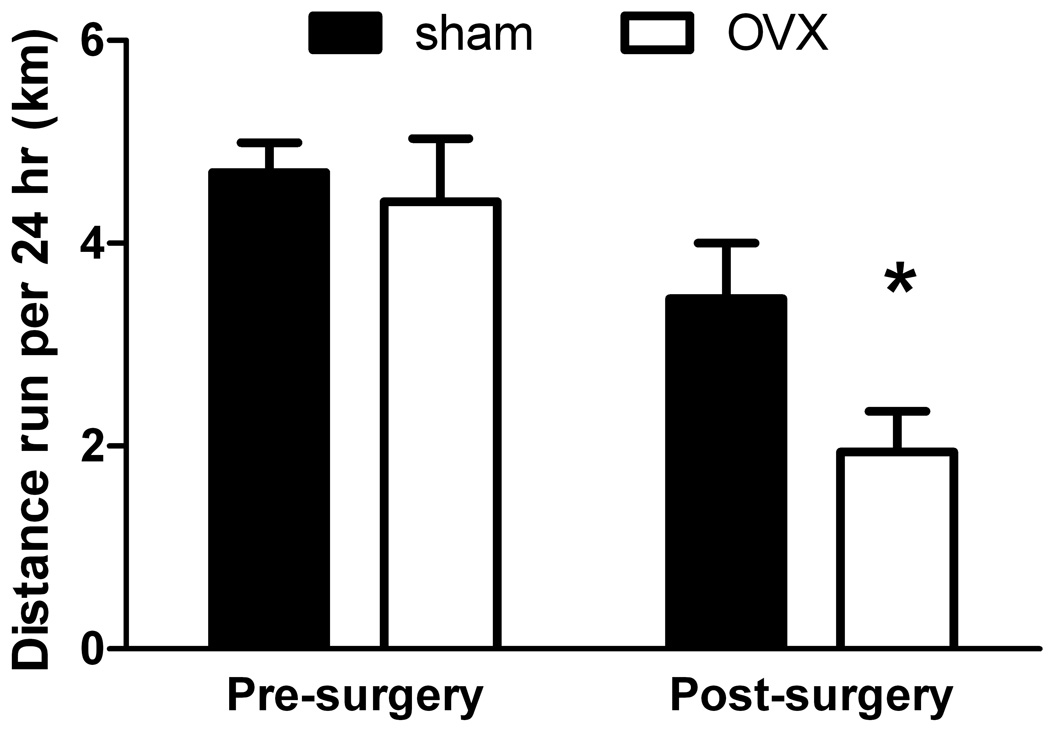
To begin to investigate discrepancies of E2 effects on physical activities between previously-reported young, ovariectomized mice and aged, ovarian-senescent mice in these studies, an additional set of 8 aged, ovarian-senescent female mice were studied. Once persistent diestrus was confirmed, mice were randomized to a surgical group, either a sham surgery or ovariectomy. However, before any surgery took place mice wheel ran for 1 week and averaged 4.5 ± 0.4 km per 24 hr (Figure 3). Mice were then subjected to surgery. During the next 2 weeks, the sham-operated, ovary-intact mice ran 3.5 ± 0.6 km per 24 hr while the ovariectomized mice ran only 1.9 ± 0.4 km (P=0.012; Figure 3). These data suggest that the presence of residual, ovarian tissue influences wheel running in aged mice.
Figure 3.
Wheel running distances for aged, ovarian-senescent mice before any surgical procedure and then following a sham operation or ovariectomy (OVX) (means ± SE). Data analyzed by 2-way repeated measures ANOVA (surgical group by time). *Significantly less than sham at given time. N=3–5 per group.
3.1.2 Muscle contractility
Soleus muscles from aged, ovarian-senescent mice that ran on wheels for ~8 week had greater capacity to generate force than those without running wheels, irrespective of E2 treatment. Specifically, soleus muscles of running mice had 21 to 23% greater mass, Po, and active stiffness (Table 2). Examination of force-time tracings revealed no effect of either wheel running or E2 on time to peak twitch (TPT), twitch one-half relaxation time (RT½), or tetanic rates of contraction (+dP/dt) or relaxation (−dP/dt) (Table 2). E2 treatment of the aged, ovarian-senescent mice affected the fatigability of the soleus muscle, but no other contractile parameter (Table 2). With the 60-contraction fatiguing bout, there was a main effect of E2 on fatigability from the 10th contraction onward (P≤0.032; Figure 4). Specifically, E2 was protective against soleus muscle fatigue with ~10% greater fatigue resistance in E2-treated mice, irrespective of wheel running. The force decrement was confirmed to be fatigue as opposed to muscle injury as Po 5 min following the fatigue protocol (post fatigue) was not different than Po pre-fatigue in any of the four groups of mice (P>≥0.229; Figure 4 insert).
Table 2.
In vitro contractile properties of soleus muscles from aged, ovarian-senescent mice treated with placebo or E2 for 8 weeks in Study I.
| Sedentary | Runner | 2-Way ANOVA P-value |
|||||
|---|---|---|---|---|---|---|---|
|
Placebo (n=9) |
E2 (n=10) |
Placebo (n=9) |
E2 (n=12) |
Running effect |
Estradiol effect |
Interaction | |
| Soleus mass (mg) | 9.4 ± 0.7 | 8.7 ± 0.5 | 10.8 ± 0.4 | 9.6 ± 0.5 | 0.024 | 0.076 | 0.612 |
| Muscle length (mm) |
11.3 ± 0.1 | 11.1 ± 0.2 | 11.2 ± 0.1 | 11.2 ± 0.1 | 0.937 | 0.292 | 0.552 |
| Pt (mN) | 23.6 ± 2.2 | 24.3 ± 1.7 | 25.9 ± 1.2 | 27.9 ± 1.3 | 0.380 | 0.899 | 0.186 |
| Twitch TPT (ms) | 40.4 ± 1.5 | 42.3 ± 1.6 | 41.9 ± 1.0 | 44.2 ± 1.5 | 0.249 | 0.155 | 0.890 |
| Twitch RT½ (ms) | 57.6 ± 7.2 | 52.4 ± 7.7 | 52.3 ± 4.0 | 58.1 ± 2.9 | 0.973 | 0.949 | 0.324 |
| Po (mN) | 139.5 ± 14.9 |
133.3 ± 10.5 | 161.5 ± 9.0 |
173.3 ± 6.8 |
0.005 | 0.788 | 0.389 |
| Specific Po (N/cm2) |
12.9 ± 1.4 | 13.0 ± 1.1 | 12.7 ± 0.7 | 15.3 ± 0.7 | 0.314 | 0.186 | 0.195 |
| Tetanic +dP/dt (N/s) |
1.5 ± 0.1 | 1.3 ± 0.1 | 1.3 ± 0.1 | 1.6 ± 0.1 | 0.060 | 0.695 | 0.547 |
| Tetanic −dP/dt (N/s) |
1.9 ± 0.2 | 1.7 ± 0.3 | 2.2 ± 0.2 | 2.2 ± 0.1 | 0.635 | 0.519 | 0.073 |
| Active stiffness (N/m) |
272.4 ± 15.2 |
278.6 ± 23.4 | 323.9 ± 10.0 |
341.1 ± 8.0 |
<0.001 | 0.716 | 0.437 |
| Passive stiffness (N/m) |
20.9 ± 3.3 | 17.3 ± 2.8 | 15.8 ± 1.9 | 16.3 ± 2.4 | 0.254 | 0.567 | 0.439 |
| Fatigue (% of initial contraction) |
49.1 ± 5.7 | 55.1 ± 6.4 | 43.3 ± 5.3 | 63.1 ± 4.0 | 0.837 | 0.022 | 0.206 |
Mean ± SE. Pt, peak twitch force; TPT, time to peak twitch force; RT1/2, one-half relaxation time; +dP/dt, maximal rate of tetanic force development; −dP/dt, maximal rate of relaxation; Po, maximal isometric tetanic force; Specific Po is normalized by physiological cross sectional area; Fatigue is the percentage of maximal isometric tetanic force on the 60th contraction relative to that of the 1st contraction of the protocol.
Figure 4.
Force decrements and recovery from a fatiguing bout of contractions performed by isolated soleus muscles from aged, ovarian-senescent mice following 60 days of ovarian hormone manipulation and being sedentary or wheel running (means ± SE). Fatigue is calculated as the percentage of force relative to that of the first contraction of the protocol. Plotted are the relative forces of every fifth contraction during the 60-contraction fatiguing protocol. There were main effects of E2 from 10th – 60th contractions. Insert is pre and post Po values for each of the four groups of mice, showing that the fatiguing bout did not induce injury to the soleus muscle because post Po values recovered to equivalent levels of pre Po values. N=7–12 per group.
3.2 Study II: Premature ovarian senescence in adult mice
Body mass did not differ between the control, VCD, and VCD+E2 groups of mice (main effect of group, P=0.251), but was affected by time. Initially mice weighed 23.0 ± 0.3 g and at the end of the 8-week study mice weighed 25.1 ± 0.4 g (main effect of time, P<0.001). Mice ate an average of 3.4 ± 0.1 g of food per 24 hr with no effect of group (P=0.485). Periodic vaginal cytology confirmed that control mice were cycling normally and that VCD and E2 interventions were effective throughout the 8-week study. At the end of the study, control mice had uterine weights different than VCD and VCD+E2-groups, indicating that both the VCD and E2-treatments were effective in atrophy and hypertrophy the uterus, respectively (118.2 ± 13.0, 50.4 ± 7.6, and 131.9 ± 27.5 g for control, VCD, and VCD+E2, respectively; P=0.021).
3.2.1 Physical activities
Acute wheel running and 24-hr cage activity were measured three times during the study as indicators of physical activity. There was no difference in running among groups and only moderate differences in cage activities (Table 3). Total active time increased by 1–2 hr per day over the 8-week study for the VCD+E2 mice and these mice were more active for ~1.5 hr per day than were control mice at week 7 (Table 3). There were no significant differences in ambulatory distance among groups and rearing counts increased over time irrespective of group (Table 3).
Table 3.
Physical activities of control mice, mice prematurely ovarian-senescent due to VCD, and VCD mice treated with E2 in Study II.
| Wheel running (km/24 hr) | ||||||
|---|---|---|---|---|---|---|
| Control | VCD | VCD+E2 | 2-Way ANOVA | |||
| (n=8) | (n=5) | (n=6) | P-value | |||
| Time effect | Group effect | Interaction | ||||
| Pre | 7.2 ± 0.5 | 8.0 ± 0.5 | 8.5 ± 0.3 | 0.338 | 0.710 | 0.434 |
| Week 2 | 8.0 ± 0.5 | 8.3 ± 0.6 | 8.0 ± 0.9 | |||
| Week 5 | 8.5 ± 0.5 | 8.1 ± 0.5 | 8.8 ± 0.5 | |||
| Total time active in cage (min/24 hr) | ||||||
| Pre | 267 ± 23 | 288 ± 22 | 291 ± 21 | - | - | 0.018 |
| Week 1 | 270 ± 21 | 292 ± 33 | 242 ± 29 | |||
| Week 7 | 270 ± 20 | 309 ± 5 | 366 ± 16 *†‡ | |||
| Distance ambulated in cage (m/24 hr) | ||||||
| Pre | 756 ± 114 | 905 ± 170 | 890 ± 150 | 0.958 | 0.064 | 0.748 |
| Week 1 | 1389 ± 125 | 833 ± 169 | 553 ± 109 | |||
| Week 7 | 1084 ± 379 | 966 ± 49 | 1210 ± 115 | |||
| Rearing counts (n/24 hr) | ||||||
| Pre | 7203 ± 1245 | 7144 ± 570 | 8466 ± 1003 | 0.044 | 0.458 | 0.637 |
| Week 1 | 8494 ± 1216 | 9181 ± 1574 | 8814 ± 1447 | |||
| Week 7 § | 8003 ± 1548 | 8868 ± 372 | 11872 ± 740 | |||
Mean ± SE. Pre is the time point prior to hormone treatment. Date were analyzed by 2-way repeated measures ANOVA with time as the repeated factor.
Significantly different than Pre within group;
Significantly different than Week 1 within group;
Significantly different than Control within Week 7;
Significantly different than Pre.
3.2.2 Muscle contractility
There was no difference among groups in soleus muscle size (Table 4). However, mice that were prematurely ovarian-senescent due to VCD and then replaced with E2 for 8 weeks had greater soleus muscle strength than VCD mice not treated. Soleus muscles from VCD+E2 mice generated 16–19% more concentric, isometric, and eccentric force than VCD mice (Figure 5; P≤0.039). This was also evident in concentric and isometric specific forces which were 10–12% greater in VCD+E2 relative to VCD mice (P≤0.009), but eccentric specific force did not differ among groups (P=0.218). There were no differences among groups in passive stiffness or peak twitch force (Table 4). Active stiffness, indicative of strong-binding myosin to actin, showed a trend for the soleus muscles from VCD+E2 mice to be greater (Table 4). Force-time tracings of twitch and tetanic contractions revealed minimal effects of VCD or E2 treatment on rates of muscle contraction and relaxation with only tetanic +dP/dt being faster in the VCD+E2 than the VCD group of mice (Table 4).
Table 4.
In vitro contractile properties of soleus muscles from young adult, prematurely ovarian-senescent mice in Study II.
| Control (n=8) |
VCD (n=5) |
VCD+E2 (n=6) |
1-way ANOVA P-value |
|
|---|---|---|---|---|
| Soleus mass (mg) | 9.9 ± 0.4 | 9.4 ± 0.5 | 9.9 ± 0.2 | 0.616 |
| Muscle length (mm) | 11.6 ± 0.1 | 11.7 ± 0.1 | 11.6 ± 0.2 | 0.828 |
| Pt (mN) | 36.8 ± 1.9 | 37.5 ± 2.7 | 38.0 ± 1.4 | 0.905 |
| Twitch TPT (ms) | 38.5 ± 1.0 | 39.4 ± 1.6 | 40.6 ± 1.4 | 0.794 |
| Twitch RT½ (ms) | 43.8 ± 1.3 | 43.8 ± 1.2 | 45.3 ± 2.3 | 0.771 |
| Tetanic +dP/dt (N/s) | 2.9 ± 0.1 | 2.6 ± 0.2 | 3.4 ± 0.2 * | 0.026 |
| Tetanic −dP/dt (N/s) | 2.3 ± 0.1 | 2.3 ± 0.1 | 2.7 ± 0.1 | 0.115 |
| Active stiffness (N/m) | 291.9 ± 7.4 | 311.9 ± 9.4 | 328.6 ± 14.6 | 0.056 |
| Passive stiffness (N/m) | 12.0 ± 0.6 | 11.4 ± 0.6 | 11.0 ± 0.2 | 0.381 |
Mean ± SE. Pt, peak twitch force; TPT, time to peak twitch force; RT1/2, one-half relaxation time; +dP/dt, maximal rate of tetanic force development; −dP/dt, maximal rate of relaxation.
Significantly different than VCD.
Figure 5.
Contractility of soleus muscles from mice treated with sesame oil (control), VCD, or VCD+E2 (means ± SE). Specific forces are corrected for physiological cross-sectional area of soleus muscle. Data analyzed by one-way ANOVA. *Significantly different than VCD; †Significantly different than control. N=5–8 per group.
4. Discussion
The overall results of these studies indicate that physical activity and skeletal muscle function in mice that are ovarian-senescent, either due to age or chemical treatment, respond to E2 treatment somewhat differently than what has previously been shown in young, ovariectomized mice. When young mice are ovariectomized physical activities plummet by as much as 90% [7,8] and muscle force generation diminishes ~10% [46]. When these mice are replaced with E2 there are increases in voluntary wheel running and physical activites [7,9,47], and reversal of ovarian hormone-induced strength losses [7,11,12,48] back to pre-surgery levels. In the first study reported here, we investigated physical activity and soleus muscle contractile function in 22-month-old, ovarian-senescent mice and found that 8 weeks of E2 treatment in those mice did not affect cage activites, decreased wheel running, and had no affect on soleus muscle force generation. These conflicting results of E2 treatment in estrogen-deficient young and aged mice prompted us to design a second study to examine the influence of the presence of failed, follicle-depleted ovaries in a young, 8-month-old mouse model of premature ovarian senescence. In these young mice, our primary findings were that physical activities were minimally altered by inducing ovarian senescence or by E2 treatment. Our hypotheses regarding soleus muscle function were partially supported as premature ovarian senescence did not result in reduced soleus muscle force production but greater forces were generated in response to E2 treatment. Collectively, the results of these two studies indicate that ovarian-senescent mice are less responsive to E2 treatment than what has previously been shown for ovariectomized mice.
Physical activity decreases with aging in many species [1]. Similarly in the studies reported here, aged female mice showed relatively low activities. For example, young mice in Study II were active for ~5 hr per day and ambulated about their cages ~1000 m per day while the aged mice in Study I were active for ~4 hr and ambulated only ~400 m per day. Like aging, young female mice that are rendered estrogen-deficient via ovariectomy have 20–55% lower cage activities relative to ovariectomized mice that are replaced with E2 [7]. Similar to cage activities, wheel running is detrimentally affected by the loss of ovarian hormones via ovariectomy in young mice, and can be preserved with treatment of E2 [8,9,12,47,49]. These observations led us to test hypothesize that cage activities and wheel running would be enhanced in aged, ovarian-senescence mice in response to E2 treatment. However, treatment with E2 evoked a decrease in running, with mice running about half the distances of those that were treated with placebo. It is possible that the dosing regimen used in these mice was not optimal for eliciting wheel running. While there is large range of E2 doses that induces rodent wheel running and the dose used in this study was likely close to that which yielded the greatest wheel running in young mice [50]. Another possibility is that perhaps the timing of treatment onset post-ovarian failure was not soon enough to recognize the protect effects of hormone treatment. Clearly, closer analyses of hormone dosing regimens in aged mice are needed.
To explore the possibility that the senescent ovarian tissue influenced wheel running, we compared running between aged mice with intact ovaries to those that had their non-functioning ovaries surgically removed. Interestingly, following the removal of the senescent ovaries, running decreased more than that in age-matched mice that underwent sham surgeries. Although there were low numbers of mice for this study, the result suggests that in the aged mouse, some component of the senescent, follicle-depleted ovary influences wheel running. This would not explain, however, why the outcome of both E2 treatment and ovariectomy of the aged mice was lowered wheel running (see Figures 2 and 3); these findings also warrant further studies.
We previously reported that in young mice, wheel running as little as 1.45 km per day can induce positive skeletal muscle adaptations [12]. Increases in force due to wheel running have been shown in aged male mice [51]. In Study I reported here, the aged female mice only ran 2.3 km per day and this was enough to produce favorable adaptations in soleus muscle. Irrespective of ovarian hormone status, soleus muscles from mice that wheel ran for 8 weeks had more mass and generated more force compared to mice that did not wheel run. Absolute force and active stiffness were both ~22% greater in mice that ran on wheels. This is an important finding in that muscle adaptations in aged female mice can occur in response to exercise independent of ovarian hormone status.
E2 treatment in aged mice had a protective effect on soleus muscle fatigue. There has been a noted difference in fatigue resistance between sexes with females exhibiting more fatigue resistance than males [19]. Recently this sex difference was hypothesized to be independent of androgen because androgen receptor knockout mice had equivalent fatigue resistance of the soleus muscle to that of wild type female mice, both of which were more resistant than muscles from wild type males [52]. It is possible that the direct effects of E2 on soleus muscle fatigue can explain the commonly found sex difference. We have previously determined that E2-mediated effects on skeletal muscle strength are independent of physical activity, thus we propose that the fatigue results presented in this study are due to direct effects of E2 on skeletal muscle [7].
Aside from the E2-mediated improvement in fatigue-resistance, hormone treatment did not affect soleus muscle force generation in aged female mice. A possible explanation is that estrogen receptor content or function could be altered with aging rendering the tissue non-responsive to E2. Evidence for this idea includes reduced binding of nuclear estrogen receptors in the hypothalamus, pituitary, and uterus of aged, 23-month-old female mice following E2 treatment [53]. Also, the proportion of nuclei positively stained for estrogen receptor alpha and beta in quadriceps muscle of postmenopausal women were reduced compared to that in muscle of young women [54]. While young ovariectomized mice responded to the loss and treatment of E2 by changing muscle levels of estrogen receptor alpha [41], it is possible that skeletal muscle of aged mice do not respond as well [55]. Brain areas have been shown to be both E2-responsive and responsible for physical activity [49,56], thus it is possible that the lack of differences in physical activity of the aged E2-treated mice could be due to changes in neurologic estrogen receptors. Further detailed studies are needed to determine both content and function of skeletal muscle and nervous system estrogen receptors in aged compared to young females.
The original hypotheses that were put forth and tested in Study II were partially supported. In young mice prematurely ovarian-senescent due to VCD, we found minor effects of E2 replacement. VCD+E2 mice spent relatively more time being active in the cage and ambulatory distance tended to be greatest in those mice, but wheel running was not improved. Notably, VCD-induced ovarian senescence did not result in reduced cage activities or wheel running compared to control mice, indicating that the presence of the ovary, even when senescent, may influence physical activity. How the senescent ovary may affect physical activity in mice is speculative. It has been shown that the ovaries of VCD mice produce non-detectable levels of E2 but still produce low levels of androgen, such that the ratio of androstenedione (the androgen precursor to testosterone) to E2 in VCD mice is quite high relative to that ratio in control mice with normal estrous cycles [23]. Therefore, it is possible that androgen or the ratio of androgen to E2 influences wheel running and cage activities. Androgen has been implicated as a behavioral modifier as exemplified by the low running distance and cage activities in androgen receptor knock out mice [57,58]. Thus, interactions of the various sex hormones and their underlying mechanisms by which running motivation in mice is affected requires more research.
Also partially supporting the hypotheses within Study II are the results of the soleus contractile function testing. In young mice with undetectable levels of E2 due to VCD-induced ovarian senescence, there was no decrement in soleus muscle function as originally hypothesized. However, in support of our hypotheses, VCD+E2 mice had better soleus muscle strength. Concentric, isometric, and eccentric forces were up to 20% higher in VCD+E2 mice compared to VCD mice. These results substantiate that in young mice, skeletal muscle is responsive to E2 treatment.
In summary, these studies report a number of novel findings on physical activity and skeletal muscle in ovarian-senescent mouse models. First, E2 treatment to aged, ovarian-senescent mice was not affective in increasing physical activities. Ovariectomy of these aged female mice impaired wheel running further, suggesting that some component of the senescent ovary in addition to estrogen production might influence physical activity. Second, E2 treatment to aged, ovarian-senescent female mice improved muscle fatigue resistance. However, force production was not affected as has been reported previously in young, ovariectomized mice suggesting a diminished responsiveness of skeletal muscle in aged mice. Third, wheel running improved soleus muscle function in aged female mice irrespective of hormone status. Fourth, in young mice exposed to the chemical VCD, producing a senescence specifically of the ovarian tissue, greater soleus muscle force generation occurred following E2 replacement. These results substantiate the beneficial effect of this hormone on muscle function. Future studies should examine the possibility that the senescent, follicle-depleted ovarian tissue can influence physical activity in female mice and determine whether or not skeletal muscle of aged, ovarian-senescent mice can respond to E2 treatment.
Highlights.
Wheel running provoked positive adaptations to soleus muscles of aged mice.
E2 protected soleus muscle fatigue but not force in aged, ovarian-senescent mice.
Young, ovarian-senescent mice had greater soleus muscle force with E2 treatment.
Acknowledgments
The authors would like to thank Greg D. Cochrane, Rachel M. Landisch, Tara L. Mader, Steve A. Nelson, Susan A. Novotny, and Gordon L. Warren for their contributions to this project. This research was supported by National Institutes of Health grants R01-AG031743 and K02-AG036827.
Abbreviations
- E2
17 β-estradiol
- VCD
4-vinylcyclohexene diepoxide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ingram DK. Age-related decline in physical activity: generalization to nonhumans. Med Sci Sports Exerc. 2000;32:1623–1629. doi: 10.1097/00005768-200009000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Fahrbach SE, Meisel RL, Pfaff DW. Preoptic implants of estradiol increase wheel running but not the open field activity of female rats. Physiol Behav. 1985;35:985–992. doi: 10.1016/0031-9384(85)90270-7. [DOI] [PubMed] [Google Scholar]
- 3.Kotz CM, Teske JA, Levine JA, Wang C. Feeding and activity induced by orexin A in the lateral hypothalamus in rats. Regul Pept. 2002;104:27–32. doi: 10.1016/s0167-0115(01)00346-9. [DOI] [PubMed] [Google Scholar]
- 4.Stranahan AM, Lee K, Mattson MP. Contributions of impaired hippocampal plasticity and neurodegeneration to age-related deficits in hormonal pulsatility. Ageing Res Rev. 2008;7:164–176. doi: 10.1016/j.arr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillips SK, Rook KM, Siddle NC, Bruce SA, Woledge RC. Muscle weakness in women occurs at an earlier age than in men, but strength is preserved by hormone replacement therapy. Clin Sci (Lond) 1993;84:95–98. doi: 10.1042/cs0840095. [DOI] [PubMed] [Google Scholar]
- 6.Moran AL, Warren GL, Lowe DA. Soleus and EDL muscle contractility across the lifespan of female C57BL/6 mice. Exp Gerontol. 2005;40:966–975. doi: 10.1016/j.exger.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Greising SM, Baltgalvis KA, Kosir AM, Moran AL, Warren GL, Lowe DA. Estradiol's beneficial effect on murine muscle function is independent of muscle activity. J Appl Physiol. 2011;110:109–115. doi: 10.1152/japplphysiol.00852.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morgan MA, Pfaff DW. Effects of estrogen on activity and fear-related behaviors in mice. Horm Behav. 2001;40:472–482. doi: 10.1006/hbeh.2001.1716. [DOI] [PubMed] [Google Scholar]
- 9.Gorzek JF, Hendrickson KC, Forstner JP, Rixen JL, Moran AL, Lowe DA. Estradiol and tamoxifen reverse ovariectomy-induced physical inactivity in mice. Med Sci Sports Exerc. 2007;39:248–256. doi: 10.1249/01.mss.0000241649.15006.b8. [DOI] [PubMed] [Google Scholar]
- 10.Moran AL, Warren GL, Lowe DA. Removal of ovarian hormones from mature mice detrimentally affects muscle contractile function and myosin structural distribution. J Appl Physiol. 2006;100:548–559. doi: 10.1152/japplphysiol.01029.2005. [DOI] [PubMed] [Google Scholar]
- 11.Moran AL, Nelson SA, Landisch RM, Warren GL, Lowe DA. Estradiol replacement reverses ovariectomy-induced muscle contractile and myosin dysfunction in mature female mice. J Appl Physiol. 2007;102:1387–1393. doi: 10.1152/japplphysiol.01305.2006. [DOI] [PubMed] [Google Scholar]
- 12.Warren GL, Moran AL, Hogan HA, Lin AS, Guldberg RE, Lowe DA. Voluntary run training but not estradiol deficiency alters the tibial bone-soleus muscle functional relationship in mice. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2015–R2026. doi: 10.1152/ajpregu.00569.2007. [DOI] [PubMed] [Google Scholar]
- 13.Lightfoot JT. Sex hormones' regulation of rodent physical activity: a review. Int J Biol Sci. 2008;4:126–132. doi: 10.7150/ijbs.4.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maddalozzo GF, Widrick JJ, Cardinal BJ, Winters-Stone KM, Hoffman MA, Snow CM. The effects of hormone replacement therapy and resistance training on spine bone mineral density in early postmenopausal women. Bone. 2007;40:1244–1251. doi: 10.1016/j.bone.2006.12.059. [DOI] [PubMed] [Google Scholar]
- 15.Teixeira PJ, Going SB, Houtkooper LB, Metcalfe LL, Blew RM, Flint-Wagner HG, Cussler EC, Sardinha LB, Lohman TG. Resistance training in postmenopausal women with and without hormone therapy. Med Sci Sports Exerc. 2003;35:555–562. doi: 10.1249/01.MSS.0000058437.17262.11. [DOI] [PubMed] [Google Scholar]
- 16.Going S, Lohman T, Houtkooper L, Metcalfe L, Flint-Wagner H, Blew R, Stanford V, Cussler E, Martin J, Teixeira P, Harris M, Milliken L, Figueroa-Galvez A, Weber J. Effects of exercise on bone mineral density in calcium-replete postmenopausal women with and without hormone replacement therapy. Osteoporos Int. 2003;14:637–643. doi: 10.1007/s00198-003-1436-x. [DOI] [PubMed] [Google Scholar]
- 17.Sipila S, Taaffe DR, Cheng S, Puolakka J, Toivanen J, Suominen H. Effects of hormone replacement therapy and high-impact physical exercise on skeletal muscle in post-menopausal women: a randomized placebo-controlled study. Clin Sci (Lond) 2001;101:147–157. [PubMed] [Google Scholar]
- 18.Taaffe DR, Sipila S, Cheng S, Puolakka J, Toivanen J, Suominen H. The effect of hormone replacement therapy and/or exercise on skeletal muscle attenuation in postmenopausal women: a yearlong intervention. Clin Physiol Funct Imaging. 2005;25:297–304. doi: 10.1111/j.1475-097X.2005.00628.x. [DOI] [PubMed] [Google Scholar]
- 19.Hicks AL, Kent-Braun J, Ditor DS. Sex differences in human skeletal muscle fatigue. Exerc Sport Sci Rev. 2001;29:109–112. doi: 10.1097/00003677-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Danilovich N, Ram Sairam M. Recent female mouse models displaying advanced reproductive aging. Exp Gerontol. 2006;41:117–122. doi: 10.1016/j.exger.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Wu JM, Zelinski MB, Ingram DK, Ottinger MA. Ovarian aging and menopause: current theories, hypotheses, and research models. Exp Biol Med (Maywood) 2005;230:818–828. doi: 10.1177/153537020523001106. [DOI] [PubMed] [Google Scholar]
- 22.Hoyer PB, Devine PJ, Hu X, Thompson KE, Sipes IG. Ovarian toxicity of 4-vinylcyclohexene diepoxide: a mechanistic model. Toxicol Pathol. 2001;29:91–99. doi: 10.1080/019262301301418892. [DOI] [PubMed] [Google Scholar]
- 23.Mayer LP, Devine PJ, Dyer CA, Hoyer PB. The follicle-deplete mouse ovary produces androgen. Biol Reprod. 2004;71:130–138. doi: 10.1095/biolreprod.103.016113. [DOI] [PubMed] [Google Scholar]
- 24.Acosta JI, Mayer L, Talboom JS, Tsang CW, Smith CJ, Enders CK, Bimonte-Nelson HA. Transitional versus surgical menopause in a rodent model: etiology of ovarian hormone loss impacts memory and the acetylcholine system. Endocrinology. 2009;150:4248–4259. doi: 10.1210/en.2008-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rivera Z, Christian PJ, Marion SL, Brooks HL, Hoyer PB. Steroidogenic capacity of residual ovarian tissue in 4-vinylcyclohexene diepoxide-treated mice. Biol Reprod. 2009;80:328–336. doi: 10.1095/biolreprod.108.070359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Springer LN, McAsey ME, Flaws JA, Tilly JL, Sipes IG, Hoyer PB. Involvement of apoptosis in 4-vinylcyclohexene diepoxide-induced ovotoxicity in rats. Toxicol Appl Pharmacol. 1996;139:394–401. doi: 10.1006/taap.1996.0180. [DOI] [PubMed] [Google Scholar]
- 27.Mayer LP, Pearsall NA, Christian PJ, Devine PJ, Payne CM, McCuskey MK, Marion SL, Sipes IG, Hoyer PB. Long-term effects of ovarian follicular depletion in rats by 4-vinylcyclohexene diepoxide. Reprod Toxicol. 2002;16:775–781. doi: 10.1016/s0890-6238(02)00048-5. [DOI] [PubMed] [Google Scholar]
- 28.Hu X, Christian PJ, Thompson KE, Sipes IG, Hoyer PB. Apoptosis induced in rats by 4-vinylcyclohexene diepoxide is associated with activation of the caspase cascades. Biol Reprod. 2001;65:87–93. doi: 10.1095/biolreprod65.1.87. [DOI] [PubMed] [Google Scholar]
- 29.Hu X, Christian P, Sipes IG, Hoyer PB. Expression and redistribution of cellular Bad, Bax, and Bcl-X(L) protein is associated with VCD-induced ovotoxicity in rats. Biol Reprod. 2001;65:1489–1495. doi: 10.1095/biolreprod65.5.1489. [DOI] [PubMed] [Google Scholar]
- 30.Hu X, Flaws JA, Sipes IG, Hoyer PB. Activation of mitogen-activated protein kinases and AP-1 transcription factor in ovotoxicity induced by 4-vinylcyclohexene diepoxide in rats. Biol Reprod. 2002;67:718–724. doi: 10.1095/biolreprod.102.004259. [DOI] [PubMed] [Google Scholar]
- 31.Toxicology and Carcinogenesis Studies of 4-Vinyl-1-cyclohexene Diepoxide (CAS No. 106-87-6) in F344/N Rats and B6C3F1 Mice (Dermal Studies) Natl Toxicol Program Tech Rep Ser. 1989;362:1–249. [PubMed] [Google Scholar]
- 32.NTP Toxicology and Carcinogenesis Studies of 4-Vinylcyclohexene (CAS No. 100-40-3) in F344/N Rats and B6C3F1 Mice (Gavage Studies) Natl Toxicol Program Tech Rep Ser. 1986;303:1–190. [PubMed] [Google Scholar]
- 33.Nelson JF, Felicio LS, Randall PK, Sims C, Finch CE. A longitudinal study of estrous cyclicity in aging C57BL/6J mice: I. Cycle frequency, length and vaginal cytology. Biol Reprod. 1982;27:327–339. doi: 10.1095/biolreprod27.2.327. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki S, Brown CM, Dela Cruz CD, Yang E, Bridwell DA, Wise PM. Timing of estrogen therapy after ovariectomy dictates the efficacy of its neuroprotective and antiinflammatory actions. Proc Natl Acad Sci U S A. 2007;104:6013–6018. doi: 10.1073/pnas.0610394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foster TC. Interaction of rapid signal transduction cascades and gene expression in mediating estrogen effects on memory over the life span. Front Neuroendocrinol. 2005;26:51–64. doi: 10.1016/j.yfrne.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Lohff JC, Christian PJ, Marion SL, Hoyer PB. Effect of duration of dosing on onset of ovarian failure in a chemical-induced mouse model of perimenopause. Menopause. 2006;13:482–488. doi: 10.1097/01.gme.0000191883.59799.2e. [DOI] [PubMed] [Google Scholar]
- 37.Nelson JF, Felicio LS, Osterburg HH, Finch CE. Altered profiles of estradiol and progesterone associated with prolonged estrous cycles and persistent vaginal cornification in aging C57BL/6J mice. Biol Reprod. 1981;24:784–794. doi: 10.1095/biolreprod24.4.784. [DOI] [PubMed] [Google Scholar]
- 38.Golub MS, Germann SL, Mercer M, Gordon MN, Morgan DG, Mayer LP, Hoyer PB. Behavioral consequences of ovarian atrophy and estrogen replacement in the APPswe mouse. Neurobiol Aging. 2008;29:1512–1523. doi: 10.1016/j.neurobiolaging.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levin-Allerhand JA, Sokol K, Smith JD. Safe and effective method for chronic 17beta-estradiol administration to mice. Contemp Top Lab Anim Sci. 2003;42:33–35. [PubMed] [Google Scholar]
- 40.Heikkinen T, Kalesnykas G, Rissanen A, Tapiola T, Iivonen S, Wang J, Chaudhuri J, Tanila H, Miettinen R, Puolivali J. Estrogen treatment improves spatial learning in APP + PS1 mice but does not affect beta amyloid accumulation and plaque formation. Exp Neurol. 2004;187:105–117. doi: 10.1016/j.expneurol.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 41.Baltgalvis KA, Greising SM, Warren GL, Lowe DA. Estrogen regulates estrogen receptors and antioxidant gene expression in mouse skeletal muscle. PLoS One. 2010;5:e10164. doi: 10.1371/journal.pone.0010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Landisch RM, Kosir AM, Nelson SA, Baltgalvis KA, Lowe DA. Adaptive and nonadaptive responses to voluntary wheel running by mdx mice. Muscle Nerve. 2008;38:1290–1303. doi: 10.1002/mus.21141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Warren GL, Hayes DA, Lowe DA, Williams JH, Armstrong RB. Eccentric contraction-induced injury in normal and hindlimb-suspended mouse soleus and EDL muscles. J Appl Physiol. 1994;77:1421–1430. doi: 10.1152/jappl.1994.77.3.1421. [DOI] [PubMed] [Google Scholar]
- 44.McCully KK, Faulkner JA. Characteristics of lengthening contractions associated with injury to skeletal muscle fibers. J Appl Physiol. 1986;61:293–299. doi: 10.1152/jappl.1986.61.1.293. [DOI] [PubMed] [Google Scholar]
- 45.Brooks SV, Faulkner JA. Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol. 1988;404:71–82. doi: 10.1113/jphysiol.1988.sp017279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greising SM, Baltgalvis KA, Lowe DA, Warren GL. Hormone therapy and skeletal muscle strength: a meta-analysis. J Gerontol A Biol Sci Med Sci. 2009;64:1071–1081. doi: 10.1093/gerona/glp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kadi F, Karlsson C, Larsson B, Eriksson J, Larval M, Billig H, Jonsdottir IH. The effects of physical activity and estrogen treatment on rat fast and slow skeletal muscles following ovariectomy. J Muscle Res Cell Motil. 2002;23:335–339. doi: 10.1023/a:1022071114344. [DOI] [PubMed] [Google Scholar]
- 48.Lowe DA, Baltgalvis KA, Greising SM. Mechanisms behind estrogen's beneficial effect on muscle strength in females. Exerc Sport Sci Rev. 2010;38:61–67. doi: 10.1097/JES.0b013e3181d496bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ogawa S, Chan J, Gustafsson JA, Korach KS, Pfaff DW. Estrogen increases locomotor activity in mice through estrogen receptor alpha: specificity for the type of activity. Endocrinology. 2003;144:230–239. doi: 10.1210/en.2002-220519. [DOI] [PubMed] [Google Scholar]
- 50.Ribeiro AC, Pfaff DW, Devidze N. Estradiol modulates behavioral arousal and induces changes in gene expression profiles in brain regions involved in the control of vigilance. Eur J Neurosci. 2009;29:795–801. doi: 10.1111/j.1460-9568.2009.06620.x. [DOI] [PubMed] [Google Scholar]
- 51.Wineinger MA, Abresch RT, Walsh SA, Carter GT. Effects of aging and voluntary exercise on the function of dystrophic muscle from mdx mice. Am J Phys Med Rehabil. 1998;77:20–27. doi: 10.1097/00002060-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 52.MacLean HE, Chiu WS, Notini AJ, Axell AM, Davey RA, McManus JF, Ma C, Plant DR, Lynch GS, Zajac JD. Impaired skeletal muscle development and function in male, but not female, genomic androgen receptor knockout mice. Faseb J. 2008;22:2676–2689. doi: 10.1096/fj.08-105726. [DOI] [PubMed] [Google Scholar]
- 53.Nelson JF, Bergman MD, Karelus K, Felicio LS. Aging of the hypothalamo-pituitary-ovarian axis: hormonal influences and cellular mechanisms. J Steroid Biochem. 1987;27:699–705. doi: 10.1016/0022-4731(87)90139-7. [DOI] [PubMed] [Google Scholar]
- 54.Wiik A, Ekman M, Johansson O, Jansson E, Esbjornsson M. Expression of both oestrogen receptor alpha and beta in human skeletal muscle tissue. Histochem Cell Biol. 2009;131:181–189. doi: 10.1007/s00418-008-0512-x. [DOI] [PubMed] [Google Scholar]
- 55.Greising SM, Landisch RM, Song ES, Lowe DA. Effects of Estrogen Replacement on Skeletal Muscle of Aged Mice that Experienced Natural Ovarian-Failure. Medicine & Science in Sports & Exercise. 2008;40:S350. [Google Scholar]
- 56.Hertrampf T, Seibel J, Laudenbach U, Fritzemeier KH, Diel P. Analysis of the effects of oestrogen receptor alpha (ERalpha)- and ERbeta-selective ligands given in combination to ovariectomized rats. Br J Pharmacol. 2008;153:1432–1437. doi: 10.1038/sj.bjp.0707664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ophoff J, Callewaert F, Venken K, De Gendt K, Ohlsson C, Gayan-Ramirez G, Decramer M, Boonen S, Bouillon R, Verhoeven G, Vanderschueren D. Physical activity in the androgen receptor knockout mouse: evidence for reversal of androgen deficiency on cancellous bone. Biochem Biophys Res Commun. 2009;378:139–144. doi: 10.1016/j.bbrc.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 58.Fan W, Yanase T, Nomura M, Okabe T, Goto K, Sato T, Kawano H, Kato S, Nawata H. Androgen receptor null male mice develop late-onset obesity caused by decreased energy expenditure and lipolytic activity but show normal insulin sensitivity with high adiponectin secretion. Diabetes. 2005;54:1000–1008. doi: 10.2337/diabetes.54.4.1000. [DOI] [PubMed] [Google Scholar]