Abstract
Oncolytic herpes simplex virus (HSV) is currently in phase III clinical trials for development as a novel therapeutic agent against a broad range of human tumors. Although results have been promising, clinical outcome is likely to be compromised by intrinsic and acquired resistance to HSV replication, leading us to test agents that may overcome this obstacle. We found that, despite showing no effect on HSV replication in tumor cells fully permissive to the virus growth, the mTOR inhibitor rapamycin dramatically increased the yield and dissemination of oncolytic HSVs in semipermissive tumor cells. Similar results were obtained in tumor-bearing mice. Co-administration of rapamycin with an HSV-derived oncolytic virus either blocked or reversed the growth of tumor xenografts established from semipermissive human tumor cells, while use of either agent alone produced only transient inhibitory effect. Together, our results suggest that rapamycin could be used to potentiate the activity of oncolytic HSVs against difficult-to-treat human tumors or perhaps to prevent the emergence of resistant tumor cells during virotherapy.
Keywords: oncolytic virus, herpes simplex virus, rapamycin, mTOR
Introduction
Virotherapy has shown substantial promise as a new treatment modality for a broad range of human tumors 1, 2. The antitumor activity of an oncolytic virus derives mainly from its ability to replicate after it infects a tumor cell, with subsequent spread of the progeny virus to the nearby tumor cells. The resultant extent of tumor destruction often exceeds that achieved with many other types of cancer biotherapeutic agents 3. Consequently, the ability of an oncolytic virus to replicate robustly in tumor cells is a key factor in securing a favorable outcome from virotherapy 4.
Herpes simplex virus (HSV) has a broad cell tropism, and oncolytic viruses derived from parental HSV strains can lyse tumor cells of many different tissue origins 5. Nonetheless, tumor cells that are resistant HSV oncolysis are encountered from time to time and pose significant barriers to therapeutic outcomes. Several strategies have been proposed to overcome the resistance of tumor cells to HSV. It has been reported, for example, that serial passage of an oncolytic HSV (a γ34.5-deleted mutant) in resistant glioma cells can select for viral progeny that replicate more efficiently in the tumor cells and then show an enhanced antitumor effect against these resistant gliomas in vivo 6. In our own studies, we showed that, although cyclophosphamide did not improve oncolytic HSV replication in the resistant Lewis lung carcinoma cells, its in vivo administration still enhanced the antitumor effect of the virotherapy 7.
Two groups have recently reported that rapamycin, an inhibitor of the mTOR (mammalian target of rapamycin) pathway, can increase the permissiveness of some resistant tumor cells to oncolytic myxoma virus or vesicular stomatitis virus (VSV). Stanford et al, for example, used rapamycin to pretreat human tumor cell lines that normally restrict myxoma virus replication and observed a striking increase in viral tropism and spread 8. The enhanced replication of the myxoma virus in cells with a silenced mTOR pathway appeared to be linked to an increase in Akt kinase, suggesting that rapamycin could be used to improve the efficacy of oncolytic poxviruses in cancer treatment. Alain et al reported that rapamycin could significantly increase the replicative capability of an interferon (IFN)-sensitive VSV mutant (DeltaM51) in malignant glioma cells 9. This enhancing effect apparently derived from the reduced inhibitory effect of type I IFNs on VSV (DeltaM51) replication once mTORC1 signaling was interrupted by rapamycin. More recently, rapamycin has been reported to enhance the therapeutic effect of a recombinant adenovirus carrying the gene encoding eukaryotic initiation factor 4E binding protein-1 10 and a newly developed mTOR inhibitor, Torin1, has been shown to enhance human cytomegalovirus replication 11. However, sensitizing effects of rapamycin on oncolytic viruses from other viruses have not yet been reported.
In this study, we examined the effect of rapamycin on the replication and antitumor efficacy of an HSV-based oncolytic virus, Baco-1. When tested in fully permissive tumor cells to HSV, rapamycin lacked any substantial effect on virus replication. However, in cultured tumor cells that were highly resistant to Baco-1 replication, rapamycin inhibition of mTOR signaling led to dramatic increases in virus replication and spread. Similar results were obtained in a mouse model bearing xenografted tumors established from highly resistant human tumor cells. Our results suggest a strategy to augment the activity of HSV virotherapy against otherwise resistant tumors.
Materials and Methods
Cell lines and viruses
Vero (African green monkey kidney) cells, MCF-7 and MDA-MB-231 (human breast cancer cell lines), HeLa (a human cervical adenocarcinoma line), HepG2 and HuH-7 (human hepatocellular carcinoma lines) were originally obtained from the American Type Culture Collection (Rockville, MD). EC9706, a human esophageal carcinoma cell line, was a gift of Dr. Mingrong Wang. All of the cell lines were authenticated by a microscopic morphology check according to the Cell Line Verification Test Recommendations of ATCC Technical Bulletin No. 8 (2008). This check was done within 3 months from the time when the data reported in this manuscript were generated. All cells were cultured in DMEM containing 10% fetal bovine serum (FBS). Baco-1 is an HSV-1-based oncolytic virus, constructed by deleting both copies of the ICP34.5 gene. Details of its construction have been published 12. FusOn-H2 is an HSV-2 based oncolytic virus, constructed by replacing the PK domain of the ICP10 gene with the gene encoding green fluorescent protein (GFP); the details of this construct are described elsewhere 13. ApE-Mir-3 designates an HSV-1-based oncolytic virus in which the glycoprotein H (gH) gene is controlled by tissue-specific microRNAs (miRNAs) including let-7 and mir-122 (Fu et al, unpublished data). Virus stocks were prepared by releasing the virus from infected Vero cells with heparin, followed by high-speed centrifugation as described 14.
Quantification of virus replication in vitro
For in vitro assay of virus replication, we seeded cells into 24-well plates and infected them with each virus at 0.1 pfu/cell for 1 h. They were then washed once with serum-free medium to remove unadsorbed and uninternalized viruses. The infected cells were then cultured in normal medium with or without the addition of 100 nM rapamycin (LC Laboratories, Woburn, MA). In some experiments, the cells were pretreated with the same concentration of rapamycin for 3 h before being infected with the virus. Cells were harvested at 72 to 96 h after infection and subjected to sonication to release virus. The viruses were titrated on Vero cells by a plaque assay.
In vivo animal experiments
Immune-deficient nu/nu female mice (4-6 weeks old) were purchased from The Jackson Laboratory (Bar Harbor, ME). All in vivo experimental protocols were approved by University of Houston Institutional Animal Care and Use Committee. Freshly harvested EC9706 cells (5 × 106) were injected into the right flanks of nude mice, which were then randomly divided into four groups. When tumor diameters had reached the approximate size of 5 mm, mice received a single intratumoral injection of either PBS or 1 × 106 pfu of Baco-1 in a volume of 100 μl, or daily intraperitoneal (i.p.) injection of rapamycin (50 μg/kg body weight) for 2 weeks. Another group of mice received the combined treatment. Two mice from each group were killed at day 1 after therapeutic injection for immunohistochemical staining for GFP expression. The remaining mice were kept for 3 weeks, during which time the growth of tumors was monitored after virus administration by measuring two perpendicular tumor diameters with a caliper. Tumor volume was calculated by the formula: tumor volume (mm3) = [length (mm)] × [width (mm)]2 × 0.52.
Statistical analyses
All quantitative data were normally distributed, so that Student’s t-test (two-tailed) was used to determine the statistical significance (P<0.05) of different comparisons. Results are reported as means ± SD.
Results
Rapamycin does not enhance oncolytic HSV replication in permissive tumor cells
In previous in vitro and in vivo studies, we identified a panel of tumor cell lines that are fully permissive to the replication of oncolytic HSVs 12, 13, 15. We selected three of these lines for in vitro evaluation of the effects of rapamycin on the growth of an HSV-1-based oncolytic virus, Baco-1, that is deficient for the γ34.5 gene. Baco-1 possesses several features that made it an attractive choice for these studies. First, it contains the gene encoding green fluorescent protein (GFP), so that its pattern of infectivity can be easily and conveniently monitored. Second, unlike many of the oncolytic HSVs that we have constructed, Baco-1 is nonfusogenic, enabling its replication capacity and ability to spread in tumor cell masses to be assessed without confounding interference from the fusogenic property of the virus. Finally, the oncolytic activity of Baco-1 has been shown to be less effective than the fusogenic HSVs 12, 16; hence, any enhancing effect of rapamycin on the antitumor effect of the virus would be more readily apparent.
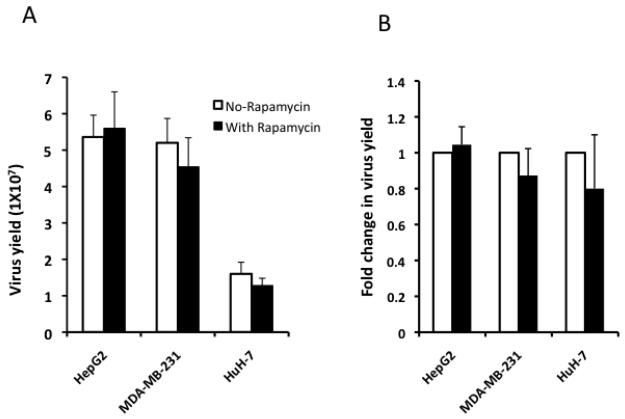
Tumor cells from each of the three permissive lines were infected with Baco-1 at a relatively low multiplicity of infection (MOI, 0.1) and then cultured in medium with or without rapamycin. The concentration of rapamycin was initially determined by incubating the cells with the drug at an increasing dose. Rapamycin showed visible toxicity to the cells at the concentration of 1 μM and above. At the concentration of 500 nM, it did not cause cytotoxicity but inhibited cell growth. It did not show any obvious effect on cell growth below the concentration of 250 nM. Thus, we chose to incubate the cells with rapamycin at a concentration of 100 nM for all the in vitro studies. The cells were harvested 72 h later for virus titration. Baco-1 replicated to a high titer in these tumor cells, reaching more than 1×107 plaque-forming-units (pfu) per milliliter (Fig.1A). The presence of rapamycin in the culture medium did not significantly enhance the virus yield in any tumor cell line (Fig. 1B), indicating that it lacks the capability to enhance the oncolytic effect of Baco-1 against tumor cells fully permissive to virus replication.
Fig. 1. Rapamycin does not enhance oncolytic HSV replication in permissive tumor cells.
Three lines of tumor cells growing in 24-well plates were infected in triplicate with Baco-1 at 0.1 pfu per cell for 1 h. The infection solution was removed and cells washed before medium without or with rapamycin at a concentration of 100 nM was added. The cells were cultured for 72 h before harvesting for virus titration. Panels A and B show the absolute and fold change in virus yield, respectively. Fold change was calculated by dividing the virus yield from the rapamycin well by that from the well lacking the drug. Values represent the mean ± SD of triplicate experiments.
Rapamycin increases oncolytic HSV replication and spread in highly resistant tumor cells
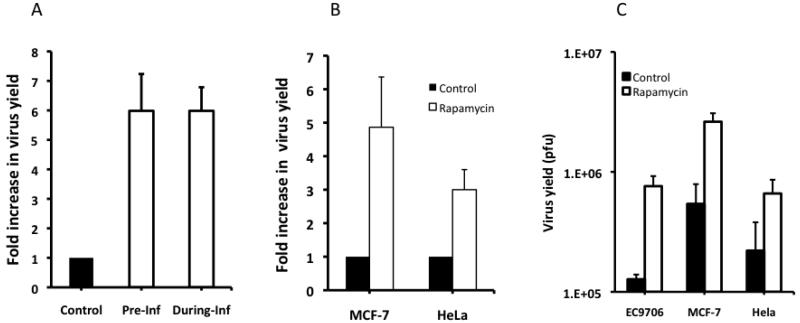
Despite the demonstrated permissiveness of many tumor cell lines to the replication of oncolytic HSVs, we often encounter tumor cells in our in vitro studies that are only partly permissive to these viruses. To test the hypothesis that inhibition of mTOR signaling might improve the replication potential of Baco-1 in otherwise resistant cells, we examined virus growth in EC9706 cells, a human esophageal carcinoma line shown in our previous studies to be highly resistant to oncolytic HSV replication 17. In contrast to the results in the permissive tumor cells, the presence of rapamycin in the medium increased the replication of Baco-1 in EC9706 cells more than 6-fold (Fig.2A). Pre-incubation of EC9706 cells with rapamycin led to the same level of virus increase as seen when the drug was present during virus infection. Similar results (Fig. 2B) were obtained with the two remaining resistant tumor cell lines; the human breast cancer line MCF-7 and HeLa adenocarcinoma cells. Thus, rapamycin-induced inhibition of mTOR signaling enhances the replication of an oncolytic HSV in tumor cells highly resistant to virus replication.
Fig. 2. Rapamycin enhances oncolytic HSV replication in highly resistant tumor cells.
A, EC9706 cells were either preincubated with rapamycin overnight (Pre-Inf) or incubated with the drug during the virus infection (During-Inf). They were then infected with Baco-1 at 0.1 pfu/cell and for 72 h. Fold increase in virus yield was calculated by dividing the yield in the control well with that in the rapamycin treated well. B, MCF-7 and HeLa cells were infected with Baco-1 at 0.01 pfu/cell for 1 h. Then the cells were cultured in medium without (control) or with rapamycin at a concentration of 100 nM for 72 h before harvesting for virus titration. C, Virus yield, as determined by plaque assay. The number in EC9706 cells was from the well in which rapamycin was present during virus infection (i.e., During-Inf in 2A). Values represent the mean ± SD of triplicate experiments.
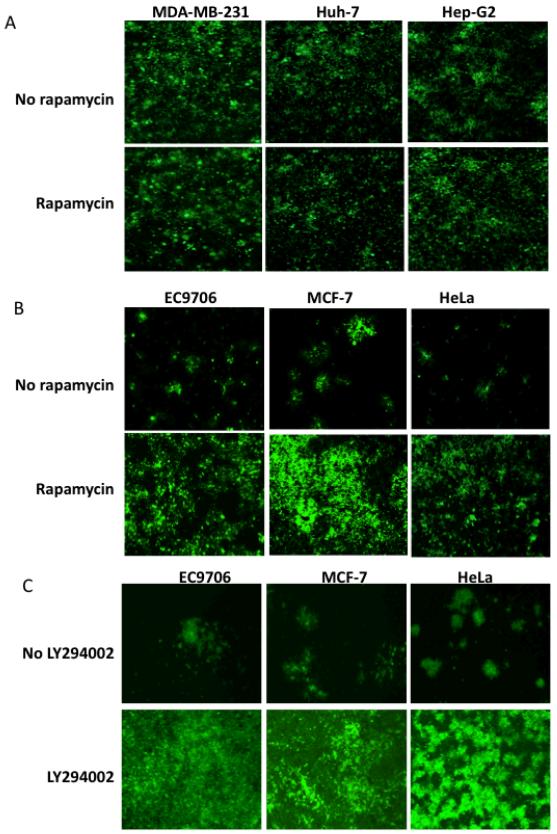
Because Baco-1 contains the GFP gene, we were able to visualize GFP expression during virus infection and thus monitor the spread of virus among tumor cells. Baco-1 had infected a majority of the permissive cells by 48 h, a result that was not substantially affected by the addition of rapamycin (Fig. 3A). By contrast, the virus did not spread extensively among these resistant tumor cells even at 96 h postinfection. The infection foci were sparsely distributed across the cell monolayer, and many of the initially infected cells remained either as single GFP-positive cells or spread to only a few surrounding cells (Fig. 3B). In the presence of rapamycin, however, the virus spread more widely, infecting almost the entire cell monolayer by 96 h postinfection. We subsequently examined the effect of rapamycin on Baco-1 replication in several additional cell lines and the results, together with those shown in figures 1 and 2, are summarized in Table 1. These results demonstrate that, despite lacking any substantial effect on virus replication in fully permissive tumor cells, rapamycin can enhance the replication and dissemination of an oncolytic HSV in highly resistant tumor cells.
Fig.3. Rapamycin promotes the spread of oncolytic HSV in highly resistant, but not fully permissive tumor cells.
A, three permissive tumor cells were infected with Baco-1 at 0.1 pfu/cell and incubated without or with rapamycin (100 nM). Micrographs were taken at 48 h postinfection. B, three highly resistant tumor cells were infected with Baco-1 at 0.01 pfu/cell and incubated without or with rapamycin at a concentration of 100 nM. C, the experiment was done essentially the same way as in B, except that LY294002 was used at 50 μM concentration. Micrographs were taken at 96 h after infection. Original magnification: X200.
Table 1.
Summary of changes in virus yield in different cells after incubation with rapamycin
| Cancer cell lines |
Tissue origin |
Relative permissiveness to Baco-11 |
Fold of change in virus yield2 |
|---|---|---|---|
| EC9706 | esophagus | + | 5.98 |
| MCF-7 | breast | + | 4.86 |
| HeLa | cervix | + | 3.0 |
| 293T | kidney | + | 8.01 |
| SW480 | colon | ++ | 5.31 |
| HepG2 | liver | ++++ | 1.04 |
| HuH-7 | liver | +++ | 0.8 |
| Hep3B | liver | +++ | 1.10 |
| MDA-MB-231 | breast | +++ | 0.87 |
| MDA-MB-435 | breast | ++++ | 0.6 |
| A549 | lung | +++ | 0.42 |
| DLD-1 | colon | +++ | 1.02 |
| HCT116 | colon | +++ | 0.5 |
| PANC-1 | pancreas | ++ | 0.99 |
| MPanc-96 | pancreas | +++ | 0.55 |
Relative permissiveness was estimated by checking for GFP positive cells 24 h after Baco-1 infection at 0.1 pfu/cell.
Fold of change in virus yield was calculated by dividing the virus yield from the well with rapamycin with that from the well without the drug.
To determine if the upstream component of the PI3K/Akt/mTOR pathway is also involved in regulating oncolytic HSV replication in the highly resistant cancer cells, we examined the effect of PI3K inhibitor, LY294002, on the replication of Baco-1 in these cells. We infected the three highly resistant tumor cell lines with Baco-1 with or without the presence of LY294002 in the medium. As shown in Fig. 3C, LY294002 significantly enhanced the replication and spread of the virus in all three cell lines. Interestingly, incubation of the infected cells with another PI3K inhibitor (wortmannin) or two Akt inhibitors (Akt Inhibitor IV and V) did not result in any significant increase in Baco-1 replication (data not shown). The reason for this differential effect is currently unclear. Nevertheless, these results indicate that more than one component of the PI3K/Akt/mTOR axis is involved in regulating oncolytic HSV replication in these highly resistant tumor cells, and that drugs as such rapamycin and LY294002 may be combined with HSV-based virotherapy to enhance the therapeutic effect.
Potentiating effect of rapamycin on virus replication in highly resistant tumor cells extends to a range of oncolytic HSVs
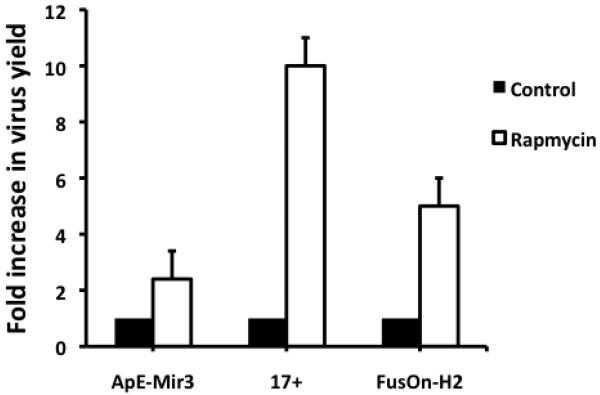
Is the ability of rapamycin to potentiate HSV replication in these highly resistant tumor cells restricted to Baco-1 or does it extend to oncolytic HSVs in general? To address this issue, we examined the effect of the drug on strain 17 (17+), a wild-type HSV-1; FusOn-H2, which was constructed from an HSV-2 by mutating the N-terminal region of the ICP 10 gene 13 and, ApE-Mir-3, an HSV-1-based oncolytic virus in which the glycoprotein H (gH) gene is controlled by tissue-specific microRNAs (miRNAs), including let-7 and mir-122 (Fu et al, unpublished data). We infected EC9706 cells with these viruses at 0.1 pfu/cell for 1 h and then cultured the cells in media with or without rapamycin at a concentration of 100 nM. The results (Fig. 4) show that rapamycin increases the replication of all three viruses. The wild type-strain, 17+, showed the greatest increase in virus yield (more than 10-fold) when the drug was present. Replication of the HSV-2-based oncolytic virus, FusOn-H2, was increased by approximately 5-fold, similar to those seen with Baco-1. The ApE-Mir-3 virus showed only a 2.5-fold increase, probably because of the intrinsic limitations imposed by the miRNA profiles in the tumor cells. These results suggest that the enhancement of HSV replication by rapamycin is a general phenomenon in the resistant tumor cells infected by these viruses.
Fig.4. Rapamycin enhances the replication of several types of HSVs in highly resistant tumor cells.
EC9706 cells were infected with each of the indicated viruses at 0.1 pfu/cell and then incubated with medium without or with rapamycin (100 nM) for 72 h before harvesting for virus titration. The fold increase in virus yield was calculated by dividing the yield in the control well by that in the rapamycin treated well. Values represent the mean ± SD of triplicate experiments.
Coadministration of rapamycin can significantly increase the antitumor effect of Baco-1 in vivo
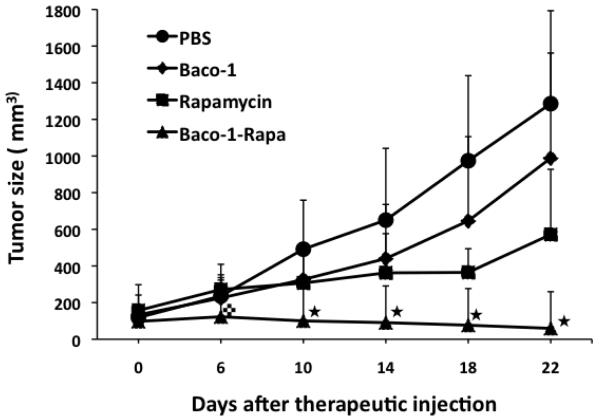
To test the beneficial effect of rapamycin on the oncolytic activity of Baco-1 in vivo, we established tumors from the highly resistant EC9706 line of human esophageal carcinoma cells by implanting tumor cells into right flank of immune-deficient mice. When tumors reached the approximate size of 5 mm in diameter, the mice were divided randomly into four groups and treated by: (i) intratumoral injection of PBS only; (ii) intratumoral injection of Baco-1; (iii) intraperitoneal administration of rapamycin; and (iv) intratumoral injection of Baco-1 and intraperitoneal administration of rapamycin. Rapamycin was given daily at the dose of 50 μg/kg body weight, a dose that had been shown to only marginally affect EC9706 tumor growth when the drug was given alone 18. Antitumor effects were assessed over 3 weeks. While Baco-1 and rapamycin given alone slowed the growth of EC9706 tumors in mice, combined administration of these agents (Baco-1-Rapa) blocked it entirely (Fig. 5). Notably, by 10-18 days postinjection, the tumors treated with Baco-1 or rapamycin alone had regained their initial growth rates, whereas those exposed to Baco-1-Rapa continued to shrink over the 22-day observation period. Thus, as in cell cultures, coadministration of rapamycin with an oncolytic HSV in vivo can potentiate the antitumor effect of virotherapy against tumor xenografts established from highly resistant human tumor cells.
Fig.5. Rapamycin potentiates the antitumor effect of Baco-1 against EC9706 tumors growing in vivo.
Mice bearing implanted EC9706 tumors on the right flank were mocked treated (PBS), treated with either Baco-1 (1×106 intratumorally) or rapamycin (50 μg/kg intraperitoneally), or treated with the combination of these agents (n=8 mice per group). Tumor size was periodically monitored after treatment.  P<0.05 vs. all other groups; □P<0.01 vs. all other groups. Two-tailed t-test.
P<0.05 vs. all other groups; □P<0.01 vs. all other groups. Two-tailed t-test.
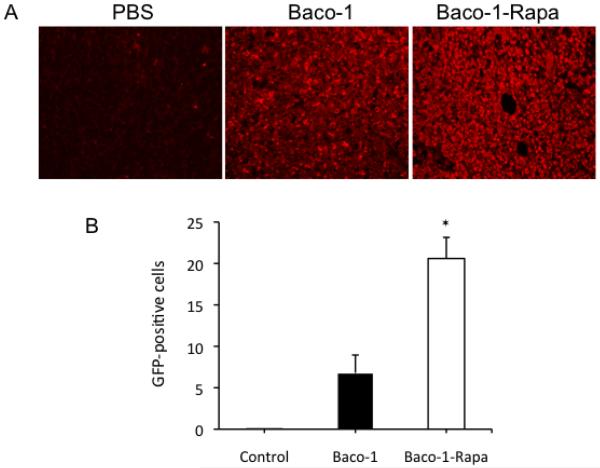
To confirm the results presented in Fig. 5, we collected tumors from mice 24 h after treatment to determine the spread of virus within the tumor tissues. Interference from the autofluorescence emitted from tissue samples was avoided by staining the tumor sections immunohistochemically for GFP expression. As compared with tumors in the Baco-1 only group, those treated by coadministration of rapamycin and Baco-1 had visually stronger staining of GFP (Fig. 6A). Quantification of GFP positive cells showed three times more GFP positive cells in tumors in the Baco-1-Rapa group compared with the Baco-1 only group (Fig. 6B).
Fig.6. Immunohistochemical staining for GFP in tumor tissues.
Tumor samples were collected 3 days after mock or therapeutic injection and sectioned. Because of possible interference of nonspecific autofluorescence from the tumor tissues, we stained the tumor sections immunohistochemically for GFP, as a means to assess the distribution of Baco-1 within tumor masses. The first antibody is rabbit anti-GFP polyclonal antibody and the second antibody is Texas red-conjugated goat anti-rabbit IgG antibody. A, Micrographs showing a typical field from tumor sections from each treatment group. Original magnification: X200. B, Quantification of GFP-positive cells by fluorescence microscopy with Micro Suite™ Five software. ⊗P<0.01 vs. Baco-1 treatment alone.
Discussion
Oncolytic viruses have the potential to improve clinical outcome for a spectrum of human tumors, but this promise is compromised by the existence or emergence of treatment-resistant tumor cells. Tumor cells become resistant to virotherapy for several reasons. They may lack receptors for a particular oncolytic virus, as reported for adenovirus-based oncolytic viruses 19, but this mechanism is unlikely to apply to oncolytic HSVs, which rely on more ubiquitous cellular receptors for entry 20. Instead, tumor cell resistance to HSV-based oncolytic viruses probably reflects failure of virus replication once the cell has been infected. Our data show that rapamycin can release the restriction placed on oncolytic HSV replication in tumor cells by increasing the virus yield as well as the spread to nearby cells. The enhanced antitumor effect of an HSV-1-based oncolytic virus against tumors established in mice from a highly resistant human esophageal carcinoma line warrants further exploration of combining rapamycin with an oncolytic HSV for clinical evaluation.
Previous studies by others have shown that rapamycin can increase the antitumor effect of some oncolytic viruses that are naturally incapable of replicating in human cells. This agent apparently acts through silencing of mTOR signaling, leading directly to an increase in Akt kinase 8. Moreover, the same study shows that rapamycin does not increase myxoma virus replication in rabbit cell lines that are intrinsically permissive or in permissive human tumor cell lines, an observation confirmed by the inability of rapamycin to enhance oncolytic HSV replication in permissive tumor cells in the present study. In the case of VSV, rapamycin inhibition of the mTOR pathway is believed to enhance virus replication by reducing type I IFN production via an inhibitory effect on phosphorylation of its effector proteins, 4E-BPs and S6Ks 9, 21. This mechanism appears relevant to the potentiation of oncolytic HSV replication by rapamycin, as the highly resistant tumor cells we studied, such as EC9706, have high levels of endogenous interferon response activity (Fu et al, submitted for publication). Studies are under way to clarify the mechanism by which rapamycin stimulates the replication of oncolytic HSV in resistant tumor cells.
Although the potentiating effect of rapamycin on oncolytic HSV replication was largely restricted to highly resistant tumor cells, we would emphasize that tumor cells in patients are more primitive than established cell lines (which has been cultured in the laboratory for generations) and therefore are likely to show resistance to initial virotherapy, suggesting that coadministration of rapamycin would be beneficial in a higher proportion of patients than predicted by our results. Confirmation of this hypothesis in tumor cells taken directly from patients would provide a strong rationale for definitive clinical testing.
Acknowledgements
We thank Dr. Haiwei Xu for technical help with immunohistochemical staining. This work was supported in part by NIH/National Cancer Institute grants R01CA106671 and R01CA132792 (to XZ).
References
- 1.Russell SJ, Peng KW. Viruses as anticancer drugs. Trends Pharmacol Sci. 2007;28:326–33. doi: 10.1016/j.tips.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu TC, Galanis E, Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat Clin Pract Oncol. 2007;4:101–17. doi: 10.1038/ncponc0736. [DOI] [PubMed] [Google Scholar]
- 3.Thorne SH, Hermiston T, Kirn D. Oncolytic virotherapy: approaches to tumor targeting and enhancing antitumor effects. Semin Oncol. 2005;32:537–48. doi: 10.1053/j.seminoncol.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Everts B, van der Poel HG. Replication-selective oncolytic viruses in the treatment of cancer. Cancer Gene Ther. 2005;12:141–61. doi: 10.1038/sj.cgt.7700771. [DOI] [PubMed] [Google Scholar]
- 5.Rabkin SD, Driever PH. Replication-Competent Herpes Simplex Virus Vectors for Cancer Therapy. In: Driever PH, Rabkin SD, editors. Replication-Competent Viruses for Cancer Therapyed. Karger; Basel: 2001. pp. 1–45. [Google Scholar]
- 6.Shah AC, Price KH, Parker JN, Samuel SL, Meleth S, Cassady KA, Gillespie GY, Whitley RJ, Markert JM. Serial passage through human glioma xenografts selects for a Deltagamma134.5 herpes simplex virus type 1 mutant that exhibits decreased neurotoxicity and prolongs survival of mice with experimental brain tumors. J Virol. 2006;80:7308–15. doi: 10.1128/JVI.00725-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li H, Zeng Z, Fu X, Zhang X. Coadministration of a herpes simplex virus-2 based oncolytic virus and cyclophosphamide produces a synergistic antitumor effect and enhances tumor-specific immune responses. Cancer Res. 2007;67:7850–5. doi: 10.1158/0008-5472.CAN-07-1087. [DOI] [PubMed] [Google Scholar]
- 8.Stanford MM, Barrett JW, Nazarian SH, Werden S, McFadden G. Oncolytic virotherapy synergism with signaling inhibitors: Rapamycin increases myxoma virus tropism for human tumor cells. J Virol. 2007;81:1251–60. doi: 10.1128/JVI.01408-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alain T, Lun X, Martineau Y, Sean P, Pulendran B, Petroulakis E, Zemp FJ, Lemay CG, Roy D, Bell JC, Thomas G, Kozma SC, et al. Vesicular stomatitis virus oncolysis is potentiated by impairing mTORC1-dependent type I IFN production. Proc Natl Acad Sci U S A. 2010;107:1576–81. doi: 10.1073/pnas.0912344107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mishra R, Miyamoto M, Yoshioka T, Ishikawa K, Matsumura Y, Shoji Y, Ichinokawa K, Itoh T, Shichinohe T, Hirano S, Kondo S. Adenovirus-mediated eukaryotic initiation factor 4E binding protein-1 in combination with rapamycin inhibits tumor growth of pancreatic ductal adenocarcinoma in vivo. Int J Oncol. 2009;34:1231–40. [PubMed] [Google Scholar]
- 11.Moorman NJ, Shenk T. Rapamycin-resistant mTORC1 kinase activity is required for herpesvirus replication. J Virol. 2010;84:5260–9. doi: 10.1128/JVI.02733-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu X, Tao L, Jin A, Vile R, Brenner M, Zhang X. Expression of a fusogenic membrane glycoprotein by an oncolytic herpes simplex virus provides potent synergistic anti-tumor effect. Mol. Ther. 2003;7:748–54. doi: 10.1016/s1525-0016(03)00092-3. [DOI] [PubMed] [Google Scholar]
- 13.Fu X, Tao L, Cai R, Prigge J, Zhang X. A Mutant Type 2 Herpes Simplex Virus Deleted for the Protein Kinase Domain of the ICP10 Gene Is a Potent Oncolytic Virus. Mol Ther. 2006;13:882–90. doi: 10.1016/j.ymthe.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Nakamori M, Fu X, Meng F, Jin A, Tao L, Bast RCJ, Zhang X. Effective Therapy of metastatic ovarian cancer with an oncolytic herpes simplex virus incorporating two membrane-fusion mechanisms. Clinical Cancer Res. 2003;9:2727–33. [PubMed] [Google Scholar]
- 15.Fu X, Zhang X. Potent systemic antitumor activity from an oncolytic herpes simplex virus of syncytial phenotype. Cancer Res. 2002;62:2306–12. [PubMed] [Google Scholar]
- 16.Nakamori M, Fu X, Pettaway CA, Zhang X. Potent antitumor activity after systemic delivery of a doubly fusogenic oncolytic herpes simplex virus against metastatic prostate cancer. Prostate. 2004;60:53–60. doi: 10.1002/pros.20056. [DOI] [PubMed] [Google Scholar]
- 17.Fu X, Tao L, Zhang X. An HSV-2-based oncolytic virus deleted in the PK domain of the ICP10 gene is a potent inducer of apoptotic death in tumor cells. Gene Ther. 2007 doi: 10.1038/sj.gt.3302971. [DOI] [PubMed] [Google Scholar]
- 18.Hou G, Zhang Q, Wang L, Liu M, Wang J, Xue L. mTOR inhibitor rapamycin alone or combined with cisplatin inhibits growth of esophageal squamous cell carcinoma in nude mice. Cancer Lett. 2010;290:248–54. doi: 10.1016/j.canlet.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 19.van Beusechem VW, Mastenbroek DC, van den Doel PB, Lamfers ML, Grill J, Wurdinger T, Haisma HJ, Pinedo HM, Gerritsen WR. Conditionally replicative adenovirus expressing a targeting adapter molecule exhibits enhanced oncolytic potency on CAR-deficient tumors. Gene Ther. 2003;10:1982–91. doi: 10.1038/sj.gt.3302103. [DOI] [PubMed] [Google Scholar]
- 20.Spear PG. Herpes simplex virus: receptors and ligands for cell entry. Cell Microbiol. 2004;6:401–10. doi: 10.1111/j.1462-5822.2004.00389.x. [DOI] [PubMed] [Google Scholar]
- 21.Costa-Mattioli M, Sonenberg N. RAPping production of type I interferon in pDCs through mTOR. Nat Immunol. 2008;9:1097–9. doi: 10.1038/ni1008-1097. [DOI] [PubMed] [Google Scholar]