Abstract
Perfluorocarbon nanoemulsions can deliver lipophilic therapeutic agents to solid tumors and simultaneously provide for monitoring nanocarrier biodistribution via ultrasonography and/or 19F MRI. In the first generation of block copolymer stabilized perfluorocarbon nanoemulsions, perfluoropentane (PFP) was used as the droplet forming compound. Although manifesting excellent therapeutic and ultrasound imaging properties, PFP nanoemulsions were unstable at storage, difficult to handle, and underwent hard to control phenomenon of irreversible droplet-to-bubble transition upon injection. To solve the above problems, perfluoro-15-crown-5-ether (PFCE) was used as a core forming compound in the second generation of block copolymer stabilized perfluorocarbon nanoemulsions. PFCE nanodroplets manifest both ultrasound and fluorine (19F) MR contrast properties, which allows using multimodal imaging and 19F MR spectroscopy for monitoring nanodroplet pharmacokinetics and biodistribution. In the present paper, acoustic, imaging, and therapeutic properties of unloaded and paclitaxel (PTX) loaded PFCE nanoemulsions are reported. As manifested by the 19F MR spectroscopy, PFCE nanodroplets are long circulating, with about 50% of the injected dose remaining in circulation two hours after the systemic injection. Sonication with 1-MHz therapeutic ultrasound triggered reversible droplet-to-bubble transition in PFCE nanoemulsions. Microbubbles formed by acoustic vaporization of nanodroplets underwent stable cavitation. The nanodroplet size (200 nm to 350 nm depending on a type of the shell and conditions of emulsification) as well as long residence in circulation favored their passive accumulation in tumor tissue that was confirmed by ultrasonography. In the breast and pancreatic cancer animal models, ultrasound-mediated therapy with paclitaxel-loaded PFCE nanoemulsions showed excellent therapeutic properties characterized by tumor regression and suppression of metastasis. Anticipated mechanisms of the observed effects are discussed.
Keywords: Perfluorocarbon nanoemulsion, ultrasonography, fluorine MRS, fluorine MRI, ultrasound-mediated chemotherapy, pancreatic cancer
1. Introduction
Tumor-targeted chemotherapy is an area of active research with the promise to overcome serious problems of current cancer therapy, potentially realizing the “magic bullet” concept formulated by Paul Ehrlich a century ago. The promising approach towards achieving this goal is in the development of stimuli-responsive drug carriers that release their payload locally in tumor tissue under the action of internal (e.g. pH) or external stimuli, such as heat, light, or ultrasound [1]. Local application of external stimuli requires tumor imaging prior to, and during treatment. Imaging can also be used to monitor drug carrier biodistribution. Such information allows optimal timing of external stimulus application.
In the last decade, advances in nanomedicine have allowed the combination of various functionalities (e.g. chemotherapeutic agent, imaging agent, and targeting moiety) in one molecular or supramolecular construct. These constructs may be macromolecules or nanoparticles. The family of nanoparticles of biomedical importance includes polymeric micelles, liposomes, hollow particles, nano-emulsion droplets etc. Properly designed nanoparticles avoid extravasation to normal tissues and recognition by cells of the reticulo-endothelial system (RES); these properties prolong nanoparticles circulation time after systemic injection. This in turn allows passive targeting of cancerous or inflamed tissues. Passive targeting is based on the so called enhanced permeability and retention (EPR) effect [2] that allows extravasation of drug-loaded nanoparticles through defective tumor microvasculature characterized by large inter-endothelial gaps. Characteristic pore cutoff size range between 380 and 780 nm have been shown in a variety of tumors, though in some tumors the size may increase up to 2 μm [3, 4]. In addition to enhanced vascular permeability, tumors demonstrate poor lymphatic drainage ensuring prolonged retention of the extravasated particles in tumor tissue. In contrast to tumors, blood vessels in normal tissues have tight inter-endothelial junctions that do not allow extravasation of nanoparticles.
Efficient tumor accumulation of nanoparticles via the EPR effect requires sufficient particle residence time in circulation. To provide for this, nanoparticles are commonly coated with poly(ethylene oxide) chains that suppress blood protein adsorption and particle recognition by RES cells.
Among feasible external stimuli, ultrasound is especially attractive by virtue of its accessibility, cost effectiveness, and the possibility to combine imaging and therapeutic capabilities. Sonication may be performed non-invasively or with minimum invasiveness through intraluminal, laparoscopic or percutaneous means. For extracorporeal sonication, the transducer is placed in contact with a water-based gel or a water layer on the skin, and no insertion or surgery is required. As a therapeutic modality, ultrasound may be delivered with millimeter precision and may be directed toward deeply located body sites. As an imaging modality, ultrasound delivers real time information.
The information produced by ultrasound imaging may be enhanced by application of ultrasound contrast agents, i.e. microbubbles. For many decades, microbubbles have been used in clinical practice only as ultrasound contrast agents. During the last decade, microbubbles have attracted attention as drug carriers and enhancers of drug and gene delivery and are now being widely investigated for this application [5–26]. However, currently used ultrasound contrast agents present a number of inherent problems as drug carriers to solid tumors. Their short circulation time (minutes) and relatively large size (two to ten microns) do not allow effective extravasation into tumor tissue, preventing efficient tumor targeting.
One way to solve this problem is to develop nano-sized microbubble precursors that would effectively accumulate in tumor tissue (via the EPR effect or through active targeting) and then convert into microbubbles in situ under the action of tumor-directed ultrasound. With this in mind, we have recently developed novel drug-loaded perfluorocarbon nanoemulsions stabilized by biodegradable amphiphilic block copolymers [1, 5, 27–30]. Bubbles are produced from these nanodroplets under the action of ultrasound. Block copolymer shells of nanodroplets provide for high in vivo stability and allow accumulation in the tumor volume via the EPR effect; active targeting is also possible. We have shown that without ultrasound, chemotherapeutic drug (paclitaxel, PTX) was tightly retained by nanodroplets stabilized with poly(ethylene oxide)-co-polycaprolactone (PEG-PCL) block copolymer; however, drug was effectively released into tumor volume under the action of tumor-directed ultrasound, which resulted in effective tumor regression [5, 6, 27–31]. In addition, nanodroplets produced long-lasting ultrasound contrast that was substantially enhanced upon droplet-to-bubble transition [5, 6, 29–31].
Our first generation of perfluorocarbon nanoemulsion drug carrier comprised perfluoropentane (PFP) as a droplet core [1, 5, 27–31]. The PFP has a boiling temperature of 29 °C; however, thermally induced droplet-to-bubble conversion is hampered by high Laplace pressure inside nanodroplets; in contrast, nanodroplets easily convert into microbubbles under the action of therapeutic ultrasound [29, 30]. Ultrasound-induced droplet-to-bubble transition is called acoustic droplet vaporization (ADV) [32–34]. Under the action of therapeutic ultrasound, microbubbles formed via ADV undergo acoustic cavitation. It has been shown that droplet-to-bubble transition and bubble oscillation result in release of encapsulated drug and enhanced intracellular uptake [5, 30]. Stable cavitation of microbubbles has been implicated as the main mechanism of enhanced gene or drug delivery [5, 13, 35–37].
Systemically injected PTX-loaded PFP nanoemulsions combined with 1-MHz ultrasound resulted in effective tumor regression in mouse models of breast, ovarian, and pancreatic cancer [5, 28, 30]. However, PFP easily forms foam if improperly handled; in addition, its irreversible droplet-to-bubble transition is hard to control. In order to replace the PFP as a droplet core, we have screened various perfluorocarbon compounds with a higher stability. Among those, perfluoro-15-crown-5-ether (PFCE, boiling temperature of 146 °C) has attracted special attention due to a plethora of useful properties. PFCE contains 20 equivalent 19F nuclei that generate a single resonance peak in 19F magnetic resonance imaging [38–40]. It is possible to use existing proton nuclear magnetic resonance (NMR) instrumentation with minor adjustments to detect fluorinated species with high sensitivity (~83% relative to 1H). It is also highly beneficial that in vivo, the endogenous fluorine is found primarily in bones and teeth as solid fluorides with undetectable NMR signals. This allows 19F MRI to be used to track the biodistribution of exogenously administered fluorinated tracers in vivo. In the present study, we tested the feasibility of using 19F MRI to determine the localisation of fluorine nanodroplets after systemic injection. The combination of 19F and proton MRI depicted the anatomic location of the injected nanodroplets.
PFCE nanoemulsions have been investigated earlier along with perfluorooctylbromide nanoemulsions as 19F MRI contrast agents and antiangiogenic drug delivery agents in a series of works by the Lanza and Wickline’s team [41–52]. The perfluorocarbon nanoemulsions used in these works were stabilized by phospholipid membranes functionalized for active delivery to fibrin or neo-angiogenic sites of tumor bearing mice. For these systems, a unique mechanism of intracellular drug delivery was postulated, based on a so-called contact facilitated delivery where phospholipid membranes of nanodroplets merged with cell membranes of target cells thus delivering their drug payload directly into the cytoplasm.
The nanodroplets used in our studies have a different structure. They are stabilized with amphiphilic poly(ethylene oxide) (PEG)-containing block copolymers and therefore are coated with PEG chains, which are expected to show lower nanodroplet uptake by the RES cells; this can also decrease or prevent contact facilitated drug delivery. The mechanism of drug delivery by the perfluorocarbon nanodroplets described in the present work is anticipated to be different from that described for phospholipid-stabilized nanodroplets. However the properties that allow combining imaging and ultrasound-mediated therapy are inherent to both types of nanoemulsions.
This paper reports the first results on the acoustic properties, ultrasound imaging, 19F MRS and MRI, and therapeutic properties of block copolymer stabilized PFCE nanoemulsions.
2. Materials and methods
2.1. Bock copolymers
Block copolymers poly(ethylene oxide)-co-poly(D,L-lactide) (PEG-PDLA), poly(ethylene oxide)-co-poly(L-lactide) (PEG-PLLA), and poly(ethylene oxide)-co-polycaprolactone (PEG-PCL) were from Polymer Source Inc. (Montreal, Quebec, Canada). The PEG-PDLA copolymer had a total molecular weight of 9,500 Da; the molecular weights of a hydrophilic PEG block and a hydrophobic PDLA block were 5,000 D and 4,500 Da respectively. The PEG-PLLA copolymer had a molecular weight of 9,700 Da, with corresponding block weights of 5,000 Da and 4,700 Da; the PEG-PCL copolymer had a total molecular weight of 4,600 Da, with corresponding block weights of 2,000 Da and 2,600 Da.
In some experiments, PEG-phospholipid compound, 1.2-distearoyl-sn-glycero-3-phosphoethanolamone-N-[metoxy(polyethylene glycol)-2000 (PEG-PE) (Avanti Polar Lipids Inc., Alabaster, Alabama) and oligomeric PEG-containing fluorinated surfactant Zonyl FSO (Dupont product obtained through Sigma-Aldrich, St. Louis, MO) were admixed with PEG-PDLA in droplet shells. Introduction of PEG-PE allows simple conjugation of various ligands needed for active targeting of nanodroplets; we found that in mixed copolymer/PEG-PE micelles or nanodroplets, a size signal of pure PEG-PE micelles disappeared from size distribution plots indicating that PEG-PE was incorporated into mixed copolymer/PEG-PE micelles or nanodroplet shells.
Zonyl FSO is a fluorine containing surfactant that promotes solubilization of highly hydrophobic perfluorocarbon compounds. Though being not essential for the formation of PFCE nanodroplets, Zonyl FSO was used in some PFCE formulations for consistency with other perfluorocarbon formulations used in our studies.
2.2. Preparation of paclitaxel loaded PFCE nanodroplets
Paclitaxel containing PEG-PDLA micellar solution was prepared by a solid dispersion technique [53]. Typically, 50 mg of PEG-PDLA and 5 mg of paclitaxel were dissolved in 1 ml of tetrahydrofuran (THF). If desired, 10 mg PEG-PE was added. The THF was then evaporated under gentle nitrogen stream at 60 °C or evacuated in vacuum at room temperature. To produce paclitaxel containing micellar solution, the residual gel matrix was dissolved in 1 ml of phosphate buffered saline (PBS). Zonyl FSO solution in PBS was added in a desired concentration (usually 1.2% (vol.)) to this solution. Ten to fifty microliters of PFCE were introduced into 1 ml micellar solution and a mixture was emulsified by sonication in ice cold water (VCX500, Sonics and Materials, Inc., CT, USA) to obtain paclitaxel-loaded 1% to 5% (vol.) PFCE nanodroplets. PTX-loaded PFCE nanodroplets stabilized by PEG-PLLA or PEG-PCL shells were prepared as described earlier for PFP nanodroplets [27, 29, 30].
2.3. Nanodroplet introduction into gels
The nanodroplets were introduced into warm agarose solution in phosphate buffered saline (PBS) before gel formation. The liquid mixture was placed in a Samco transfer pipette (5-mm inner diameter, 0.3-mm wall thickness) (Fisher Scientific, Pittsburg, PA, USA) and cooled down to room temperature for gel formation.
2.4. Particle size distribution
Size distribution of nanoparticles was measured by dynamic light scattering at a scattering angle of 165° using Delsa Nano S instrument (Beckman Coulter, Osaka, Japan) equipped with a 658-nm laser and a temperature controller. Particle size distribution was analyzed using the non-negative least squares (NNLS) method. The instrument allows measurement of particle sizes from 0.6 nm to 7 μm; microparticles larger than 7 μm cannot be measured accurately. Optical monitoring of the samples using an inverted microscope and hemacytometer (model 3200, Hauser Scientific, Horsham, PA, USA) showed no microdroplets larger than 4 μm.
2.5. Sonication
Unfocused 1-MHz ultrasound was generated by an Omnisound 3000 instrument (Accelerated Care Plus Inc, Sparks, NV, USA) equipped with a 1-cm piezoceramic crystal and 5-cm2 probe head. Focused 1-MHz ultrasound was generated by a high intensity focused ultrasound (HIFU) transducer (H-101, Sonic Concepts, Bothell, WA, USA) with an active diameter of 64 mm and focal length of 63 mm. The −3 dB lateral and axial pressure profiles were 1.2 and 10 mm respectively. For the details of sonication experiments, see ref. [27].
2.6. Monitoring acoustic droplet vaporization
The ultrasound-induced formation of microbubbles from nanodroplets was monitored at room temperature by ultrasound imaging, based on higher echogenicity of bubbles compared to droplets [33, 34]; a 7.5-MHz linear array scanner (Scanner 250, Pie Medical, Maastricht, The Netherlands) was used for ultrasound imaging, with 14 frames per second scan rate. The details of measurements have been described in ref. [27].
2.7. Cavitation activity
These measurements were performed with the samples placed in the Samco transfer pipettes. Cavitation activity was assessed by measuring harmonic, subharmonic, and broadband noise amplitudes in a portion of the scattered beam as described in detail in ref. [27].
2.8. MRgHIFU experiments
All MRgHIFU experiments were performed in a Siemens TIM Trio 3T MRI scanner (Siemens AG, Erlangen, Germany). The MRgHIFU system (Image Guided Therapy, Bordeaux, France) uses a 256-element phased array transducer (1MHz, 13 cm radius of curvature, 2 × 10 mm focal spot) with electronic steering capabilities in all three directions (+/− 15 mm in x and y, +/− 25 mm in z), as well as mechanical movement in the x-y plane. The temperatures were measured with the proton resonance frequency method [54] using a 2D GRE sequence (TE=8 ms, TR=65 ms, flip angle=20°, 128×128 matrix, 5 slices). The maximum power deposition rate was found to be 0.0031 W/mm3 in the phantom impregnated with nanodroplets. This value is consistent with a maximum power intensity of 80 W/cm2 at the focal zone assuming an acoustic efficiency of 45%, an absorption value of 0.04 Np/cm/MHz and a beam size of 2.2 × 2.2 × 10 mm. In vivo, the maximum power deposition rate was found to be 0.001 W/mm3. This value is consistent with a maximum power intensity of 54 W/cm at the focal zone assuming an acoustic efficiency of 45%, an absorption value of 0.03 Np/cm/MHz and a beam size of 2.2 × 2.2 × 10 mm.
2.9. 19F MR imaging
To perform 19F MR spectroscopy (19F MRS) or imaging (19F MRI) on the 3T human MRI system, a transmit/receive 19F/1H dual-tune volume RF coil with hardware interface consisting of a quadrature transmit/receive (Tx/Rx) switch, coil-selector, and preamplifier was constructed in house by EKJ. The coil had a 3.8 cm inner diameter and a 7.6 cm length. The pre-amplifier had an operational bandwidth of 3–200 MHz. Tx/Rx switching was accomplished using a quarter-wave coaxial cable and PIN diode, to which the DC bias voltage was supplied from the imaging system. The isolation between Tx and Rx ports was −40 dB during the RF transmission. The Tx/Rx switching interface is equipped with a coil selection switch for 19F/1H MRI, because the X/4 length of two nuclei is similar to within 5%. In the 19F MRS measurements of excised organs, surface coil designed for the rabbit eye experiments was used.
2.10. Cells
Human breast cancer MDA MB231 cells and pancreatic cancer MiaPaCa-2 cells were obtained from American Type Culture Collection (Manassas, VA). Cells were cultured in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Gibco, Grand Island, NY) at 37 °C in humidified air containing 5% CO2. Pancreatic cancer MiaPaCa-2 cells were transfected with red fluorescence protein (RFP) according to the procedure described in refs. [55, 56]. Pancreatic cancer cells were maintained in DMEM supplemented with 10% FBS.
2.11. Animal Procedures
Four to six weeks old nu/nu mice were obtained from Charles River Laboratories (Wilmington, MA). Animals were housed in accordance with the Guide for the Care and Use of Laboratory Animals as adopted by the National Institutes of Health. All experiments were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of the University of Utah (Protocol 08–01001). For breast cancer inoculation, cells were suspended in serum-free RPMI-1640 medium and inoculated subcutaneously to the flanks of unanaesthetized mice (1 × 106 cells/100 μL/mouse). For the implantation of orthotopic pancreatic cancer tumors, 1.5×106 RFP expressing MiaPaCa-2 cells suspended in DMEM media were surgically injected into the tail of the pancreas, as previously described [28, 30, 55, 56].
2.12. Measuring nanodroplet pharmacokinetics
These experiments were performed using 3 months old Swiss Webster white mice obtained from Charles River Laboratories (Wilmington, MA). Animals were housed in accordance with the Guide for the Care and Use of Laboratory Animals as adopted by the National Institutes of Health. Each animal was systemically injected with 300 μl of 5% PFCE nanodroplet formulation. At chosen time points (30 min; 2 h; 6 h; 24 h) animals were anesthetized, blood was collected by cardiac puncture, then animals were sacrificed, organs (the liver, spleen, lung, and heart) were excised, weighted and kept refrigerated until 19F MRS measurements that were performed the same day. Experiments were run in triplicates. Two types of nanodroplet shells formed by either PEG-PDLA or PEG-PCL were tested; copolymer concentration was 1% (wt.); shell-forming copolymers were mixed with 0.25% (wt.) PEG-PE; Zonyl FSO concentration was 0.6% (vol.).
2.13. Ultrasound imaging
Ultrasound imaging was performed using Acuson Sequoia 512 linear transducer (Siemens, Mountain View, CA).
3. Results
3.1. Physical and Acoustic Properties of PFCE Nanoemulsions
3.1.1. Size distribution
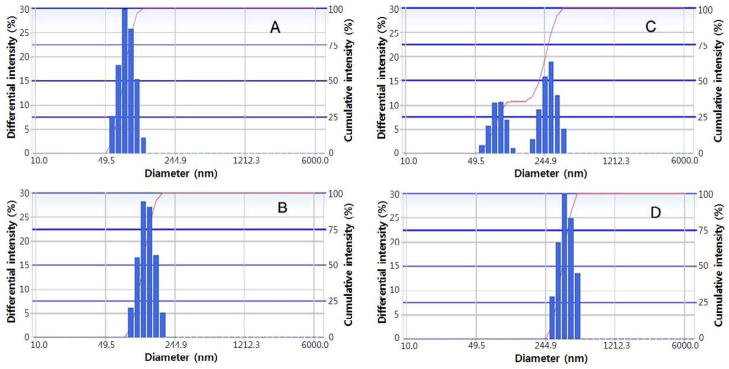
The data for empty and PTX-loaded PEG-PDLA micelles and PEG-PDLA stabilized nanodroplets are presented in Fig 1A–D. The size of both, micelles and nanodroplets increased after PTX loading. Size distributions for empty and PTX-loaded micelles are presented in Figures 1A and 1B respectively.
Fig. 1.
Particle size distribution for (A) – empty 5% PEG-PDLA micelles; size distribution parameters: 81.6 ± 14.7; (B) – PTX-loaded PEG-PDLA micelles (0.5% PTX/5% PEG-PDLA); size distribution parameters: 129 ± 23.7 nm; (C) – nanodroplet formulation (1% PFCE/5% PEG-PDLA); size distribution parameters: peak 1 – 83.7 ± 14.3 nm (33%); peak 2 – 275.1 ± 50.8 nm (67%); (D) – 5% PFCE/5% PLA nanodroplets; size distribution parameters: 392 ± 65 nm.
Typically, at a 1% (vol.) PFCE concentration in a 5% PEG-PDLA micellar solution, a bimodal distribution of nanodroplet sizes was observed corresponding to a mixture of micelles (81.6 ± 14.7 nm) and nanodroplets (275 ± 50 nm), as illustrated in Fig. 1C. The micelle/droplet ratio decreased with increasing PFCE concentration; micelles disappeared at PFCE concentration of 5% (Fig. 1D) indicating that all block copolymer was transferred from micelles onto droplet surfaces. Typically, after PFCE emulsification in the PTX-loaded micellar solutions, the size of droplets increased from 275 ± 50 nm for empty droplets to ca. 300 – 500 nm for PTX-loaded droplets, while the size of micelles dropped to values corresponding to empty micelles indicating that PTX was transferred from micelles to nanodroplets. This trend was observed for any droplet-stabilizing copolymer. Similar effects were reported earlier for PFP nanodroplets [30]. The size of PTX-loaded PFCE nanodroplets presented in this work was consistently smaller than that of the PTX-loaded PFP nanodroplets of our earlier publications [30]. This reduction in size is particularly beneficial for drug delivery applications.
Optical microscopy of PFCE formulations indicated presence of a small population (fraction of percent) of droplets of micron-range sizes (up to 3 μm) that avoided measurement by dynamic light scattering due to precipitation to the bottom of the cuvette during the measurement process.
3.1.2. Echogenic properties
Due to large differences in acoustic impedances between perfluorocarbons and water, PFCE nanodroplets generate relatively strong ultrasound contrast (Fig. 2).
Fig. 2.
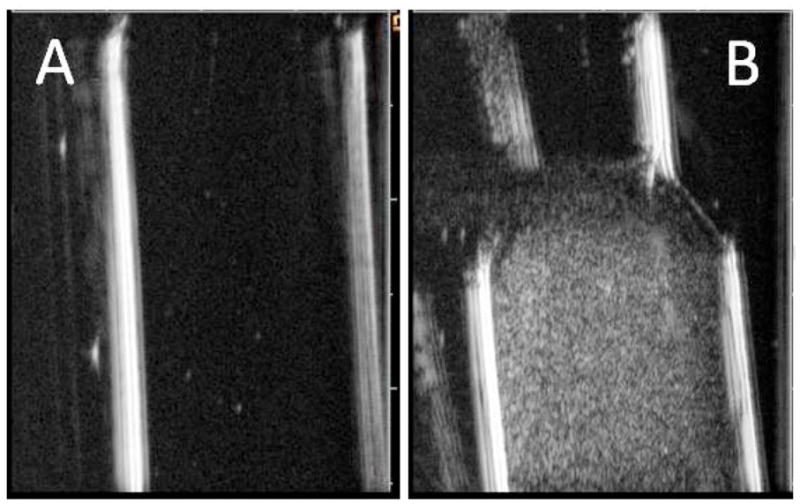
Ultrasound images of (A) – pure agarose gel in a test tube and (B) – PFCE nanodroplet loaded agarose gel; a 1.2% agarose solution in PBS was mixed with an equal volume of 2% PFCE nanodroplet emulsion at 50 °C to produce 1% (vol.) nanodroplet concentration in the gel. The mixture was cooled to room temperature to solidify. Note higher echogenicity of the nanodroplet loaded gel. This effect did not depend on the type of the copolymer used to form droplet shell.
3.1.3. Acoustic Droplet Vaporization
Despite high boiling temperature of 146 °C, nanodroplets converted into microbubbles under the action of therapeutic ultrasound, as manifested in Fig. 3 by the formation of bright specks in the ultrasound images of PFCE nanoemulsions sonicated with continuous wave (CW) (Fig. 3A) or pulsed (Fig. 3B) ultrasound. The bubbles formed under ultrasound were moving upward; however upon turning ultrasound off, they reversed direction of motion and were returning back to the bottom of the test tube thus indicating that droplet-to-bubble transition was reversible. The observation that droplet-to-bubble transition proceeded under pulsed ultrasound (Fig. 3B) suggested that the mechanical component of ultrasound played predominant role in inducing droplet vaporization because sample heating under pulsed ultrasound did not exceed two – three degrees Celsius in our experimental setting, which could be considered negligible for purely thermal vaporization of PFCE. Ultrasound-induced droplet-to-bubble transition is called acoustic droplet vaporization (ADV). ADV thresholds were slightly higher for PFCE nanodroplets compared to PFP nanodroplets stabilized by the same copolymer (Table 1). Similar effects were reported earlier by others for different types of perfluorocarbon emulsions formed by perfluorocarbon compounds with high boiling temperatures [57].
Fig. 3.
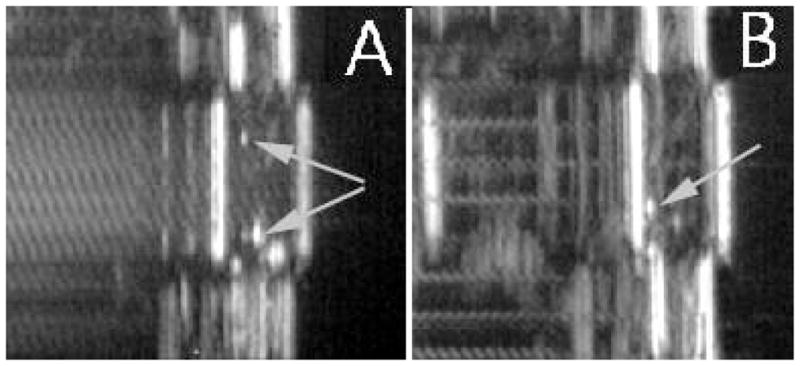
Droplet-to-bubble transition in 1% PFCE/0.25% PEG-PLLA nanoemulsions is manifested by generation of bright specks in ultrasound images (indicated by arrows). (A) – continuous wave 1-MHz ultrasound; (B) – pulsed 1-MHz ultrasound, pulse length 3 ms, 20% duty cycle. Similar results were obtained for PEG-PDLA, PEG-PCL, and copolymer mixture stabilized nanoemulsions.
Table 1.
Comparisons of ADV thresholds for the 1% PFP and 1% PFCE nanodroplets stabilized by PEG-PLLA copolymer. Peak rarefactional pressures are presented.
| Sample | 1% PFP / 0.25% PEG-PLLA | 1% PFCE / 0.25% PEG-PLLA | ||
|---|---|---|---|---|
| Duty cycle | 20% | 100% | 20% | 100% |
| Threshold (Mpa) | 0.20~0.23 | 0.14 ~0.16 | 0.34 ~0.41 | 0.20~0.23 |
3.1.4. Bubble Cavitation
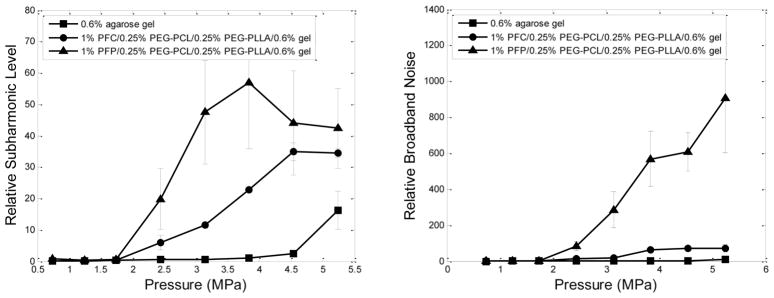
Cavitation effects of the bubbles formed via ADV were explored for unfocused ultrasound and HIFU at a frequency of 1 MHz. The appearance and amplitudes of harmonic frequencies and broadband noise were monitored in the Fast Fourier Transform emission spectra. The results shown in Fig. 4 indicate that bubbles formed by the ADV of PFCE nanodroplets oscillated under the action of ultrasound, similar to the effects observed for PFP nanoemulsions [27]. Though oscillation amplitudes were somewhat smaller for PFCE than for PFP, PFCE bubbles clearly underwent stable cavitation both in liquid emulsions and gel matrices. Larger differences between PFP and PFCE bubbles were observed for broadband noise intensities indicating that PFCE bubbles were less susceptible to inertial cavitation.
Fig. 4.
Relative subharmonic (left) and broadband noise (right) levels generated under the action of 1-MHz focused ultrasound (HIFU) at room temperature by PFP and PFCE nanodroplets inserted into the 0.6% agarose gel. Nanodroplets were stabilized by a mixture of 0.25% PEG-PLLA/0.25% PEG-PCL copolymer. Triangles – PFP droplets; circles – PFCE droplets; squares – pure agarose gel.
PFCE bubbles stabilized with PEG-PDLA shells generated higher subharmonic and broadband noise amplitudes than those stabilized with PEG-PLLA or PEG-PCL (data not shown), presumably due to amorphous PDLA blocks forming softer nanodroplet shells in comparison with those formed by crystalline PLLA or PCL blocks.
Under lower ultrasound pressures generated by an unfocused Omnisound 3000 transducer, cavitation amplitudes of PFCE bubbles inserted in a gel matrix were relatively small while inertial cavitation was not manifested (data not shown). This result directed the choice of HIFU transducer for pilot tumor therapy experiments using a breast cancer model. Strong therapeutic effect observed in this pilot study (see below) encouraged testing lower energy unfocused ultrasound in subsequent larger scale experiments with a pancreatic cancer model.
3.1.5. MRI thermometry of a PFCE droplet loaded gel phantom under focused ultrasound (FUS)
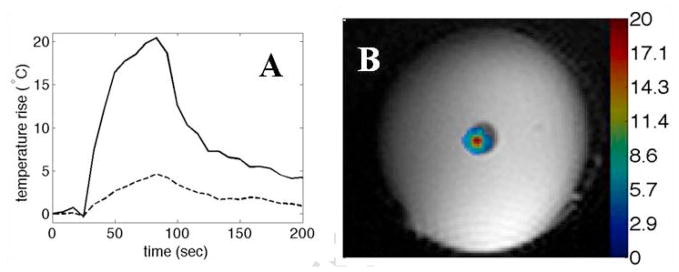
These experiments were performed in agarose gel phantoms that were either “empty” or loaded with PFCE nanodroplets. The results of temperature measurements by the MRI thermometry are shown in Fig. 5A, B. Temperature rise was much faster and the temperature achieved under FUS irradiation was much higher for the nanodroplet-containing gel in comparison to the empty gel. The data indicate that the acceleration of FUS-induced heating was associated with ultrasound absorption by PFCE nanodroplets.
Fig. 5.
(A) - Kinetics of temperature rise for the PFCE droplet-loaded (solid line) or empty (dashed line) agarose phantoms. The voxels with the maximum temperature rise are displayed in both cases. Power was applied for 60 s at 40 W (electrical). The maximum power deposition rate was found to be 0.0031 W/mm3 in the phantom impregnated with nanodroplets. This value is consistent with a maximum power intensity of 80 W/cm2 at the focal zone assuming an acoustic efficiency of 45%, an absorption value of 0.04 Np/cm/MHz and a beam size of 2.2 × 2.2 × 10 mm. A 2D-GRE sequence was used for thermometry (5 slices, 2×2×3mm, tacq=8.3s, TR/TE=65/8ms, FA=20°, 128×128 matrix). (B) - The temperature map at the time when the maximum temperature occurred (theat=60s) for the droplet-loaded gel.
3.1.6. In vivo MRI thermometry and therapeutic properties of systemically injected PFCE nanodroplets in pancreatic tumor bearing mice subjected to FUS irradiation
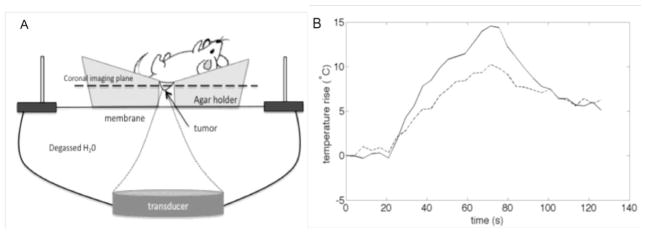
Experimental setup for the in vivo FUS application is schematically presented in Figure 6A. Kinetics of temperature rise for the subcutaneous pancreatic tumors systemically injected with the empty (solid line) or paclitaxel loaded nanodroplets (dashed line) are presented in Figure 6B. Ultrasound beam was steered for 50 s in a circle of 4-mm diameter (8 “points”, 200 ms/point, 30 circles per treatment resulting in a 6-s sonication of each “point”). Maximum temperature measured by the MRI thermometry in coronal slices was slightly higher for a mouse injected with empty nanodroplets, which could be related to different concentration of tumor-accumulated nanodroplets; however it could be also an experimental problem associated with the 1-mm separation of measured coronal slices (maximal attained temperature could be located between the two slice)s. Despite lower measured maximal temperature, the therapeutic effect was much stronger for drug-loaded nanodroplets (Figure 7). Initial tumor sizes were close for both mice (the tumor treated with PTX-loaded nanodroplets being initially slightly larger than that treated with empty nanodroplets, compare fluorescence images of Figures 7A and 7C). Note that only viable tumor cells generate Red Fluorescence Protein and therefore produce fluorescence.
Fig. 6.
A – a scheme of FUS experiments; B - kinetics of FUS-induced temperature rise for the PFCE droplet injected mice; solid line – empty droplets; dashed line – PTX-loaded droplets. Ultrasound beam was steered for 50 s in a circle of 4-mm diameter (8 “points”, 200 ms/point, 30 circles per treatment resulting in a 6-s sonication of each “point”). The maximum power deposition rate was 0.001 W/mm3. This value is consistent with a maximum power intensity of 54 W/cm2 at the focal zone assuming an acoustic efficiency of 45%, an absorption value of 0.03 Np/cm/MHz and a beam size of 2.2 × 2.2 × 10 mm.
Fig. 7.
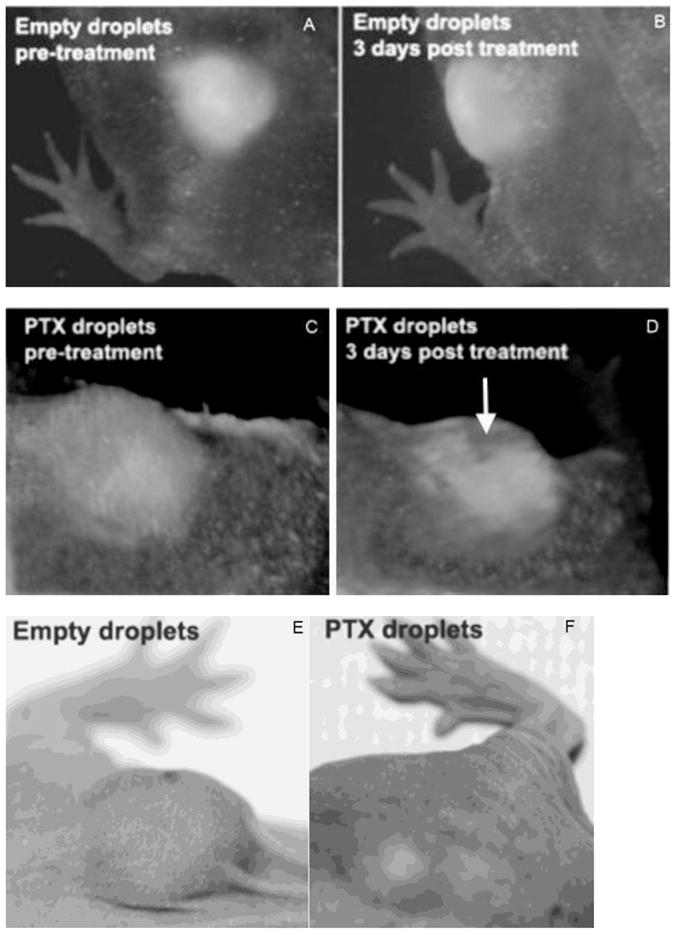
Intravital fluorescence images of subcutaneous pancreatic tumors before and after FUS treatment (A – D). Mice were injected with empty droplets (A, B) or PTX-loaded droplets (C, D). Fluorescence images of initial tumors are shown in panels A and C; images recorded three days after treatment are shown in panels B and D. Photographs of the tumors were taken 12 days after the treatment for empty droplets (E) and PTX-loaded droplets (F). Conditions of FUS treatment are presented in caption to Figure 6B. Mice were systemically injected with empty or PTX-loaded 1% PFCE/5%PEG-PDLA nanodroplets six hours before FUS treatment; DOX dose was 40 mg/kg.
No trace of cell death was observed in fluorescence images of a FUS-treated mouse injected with empty nanodroplets (Figure 7A, B). In contrast, cell death was clearly manifested in fluorescence images of a FUS-treated mouse injected with PTX-loaded nanodroplets (Figure 7C, D). The area of killed cells (approximately 4 mm diameter) corresponded to the FUS-treated area. Figure 7D shows that the PTX action on the tumor cell killing was substantially enhanced by ultrasound. “Cavernous” appearance of the tumor in Figure 7D is an optical effect associated with dead cells losing fluorescence; real tumor did not have any depression (Figure 7F). Tumor photographs taken twelve days after treatment (Figure 7E, F) indicate that tumor growth was effectively delayed after only one tumor treatment with PTX-loaded nanoemulsions and ultrasound; note that only a fraction of the total tumor volume was treated by ultrasound. Larger scale experiments on pancreatic cancer chemotherapy with PTX-loaded nanoemulsions and ultrasound using orthotopic pancreatic cancer model are presented in Section 3.2.4.
3.2. Therapeutic and Imaging Properties of PFCE nanoemulsions
3.2.1. PFCE nanodroplets are long circulating
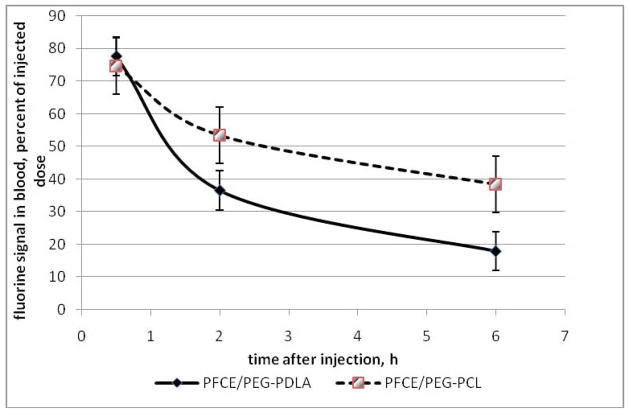
Fluorine MR spectroscopy (19F MRS) was used for quantitatively monitoring nanodroplet pharmacokinetics and biodistribution in healthy white Swiss Webster mice as described in Materials and Methods section. The pharmacokinetic curves are shown in Figure 8 for the two types of nanodroplet stabilizing copolymers. The curves indicate that both types of nanodroplets were long circulating. About seventy percent of the injected dose remained in circulation thirty minutes after the systemic injection; forty to fifty percent were still circulating two hours after the injection; for PEG-PCL stabilized nanodroplets, forty percent of the injected dose remained in circulation six hours after injection; even twenty four hours after injection, about ten percent of the injection dose was still found in the blood while about eighty percent was fond in the liver (these data are not presented in the graphs because only one mouse for each formulation was used at the 24-hour time point). Nanodroplets were taken up mainly by the liver and spleen; the uptake by other organs was below the 19F MRS sensitivity. On the per organ basis, the major nanodroplet uptake was observed for the liver, while on the per gram basis, for up to 6 hours after the injection, the uptake was significantly higher for the spleen; the uptakes by the liver and spleen were gradually equalized at later stages.
Fig. 8.
Nanodroplet pharmacokinetics measured with 19F MRS (N = 3; mean value plus/minus standard deviation is presented).
3.2.2. Nanodroplet biodistribution in tumor bearing mice
3.2.2.1.Ultrasonography
Due to the echogenic properties of PFCE nanodroplets, their tumor accumulation and biodistribution can be monitored by ultrasonography. Ultrasound imaging showed that after systemic injection, PFCE intratumoral distribution in the orthotopic human pancreatic MiaPaCa-2 tumors surgically inoculated in nude mice [55, 56] was highly nonuniform, as was reported earlier for PFP nanodroplets [28]. Note that pancreatic tumors are very poorly vascularized, with functional blood vessels localized predominantly in the tumor rim.
Similar to the tumor, nanodroplet uptake by the liver causes an increase in liver echogenicity. The degree of liver enhancement depended on the composition of the nanodroplet shell; significant differences in the liver uptake were observed between 0.25% PEG-PCL stabilized nanodroplets (Figure 9A) and those stabilized with a surfactant mixture comprising 5% PEG-PDLA, 1% PEG-PE, and 1.2% Zonyl FSO (Figure 9B). These data indicate that the liver uptake of nanodroplets can be modulated by optimizing composition of nanodroplet shells.
Fig. 9.
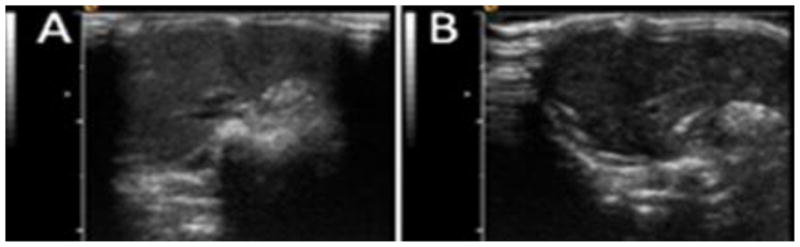
Ultrasound images of the liver. (A) – Mouse was injected with 0.25% PEG-PCL stabilized nanodroplets; (B) – mouse was injected with nanodroplets stabilized with a surfactant mixture comprising 5% PEG-PDLA, 1% PEG-PE, and 1.2% Zonyl FSO (tumor image for this mouse is shown in Figure 9B). Note significant differences in the liver echogenicity between panels 9A and 9B (50 arb.u. vs 25 arb.u. as measured by the NIH ImageJ software), which indicates lower liver uptake of nanodroplets stabilized with a surfactant mixture.
3.2.2.2.Fluorine MRI of PFCE nanodroplets after systemic injections
PFCE has twenty equivalent fluorine nuclei, which provides for a sharp fluorine peak in the MR spectra and allows 19F MR imaging of PFCE nandroplets in vitro and in vivo. In the pilot experiments described below, 19F MR images of the orthotopic pancreatic cancer bearing mouse are presented.
In the experiment presented in Fig. 10, mouse was injected every two hours with 200 μl of 2% PFCE/0.5% PEG-PCL nanodroplets; a total of four injections were given, for an injected PFCE dose of 16 μl or 2 mmol/kg. The 19F MR images superimposed on the low resolution proton images are shown in Fig. 10A,B for two coronal slices. Fluorine atom locations were clearly observed, with signal-to-noise ratio of 24. The fluorine was located in the liver and its distribution was clearly speckled. Fluorine signal was also seen in the upper locus of the tumor (indicated by arrows); however the interpretation of this signal is ambiguous because it could come from the lower end of the liver lobe fused with the tumor. Necropsy images presented in Fig. 10C show a large tumor with multiple liver metastases, which is typical for 40% of orthotopically inoculated pancreatic tumors [58, 59]. These data show the feasibility of using 19F MRI for intravital monitoring of nanodroplet location. Further experiments are required to identify the origin of the voxels with the accumulated nanodroplets in order to discern signals coming from the liver tissue, liver metastases, and tumor.
Fig. 10.
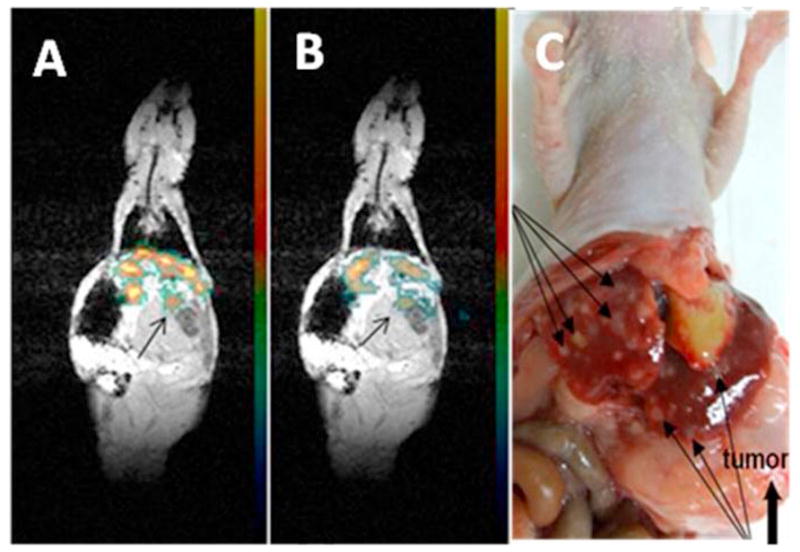
(A) and (B) – two coronal slices in the 19F MR images superimposed on the low resolution proton anatomic images of a pancreatic tumor bearing mouse. A 2% PFCE nanoemulsion stabilized with PEG-PCL copolymer was systemically injected every two hours, 200 μl each. Four injections were given, for a total of the PFCE dose of 2 mmol/kg. Images were recorded an hour after the last nanodroplet injection (seven hours after the start). Imaging parameters: Proton imaging: GRE (Gradient Recalled Echo) pulse sequence, resolution 1×1×3mm, TR/TE=400/4.24, matrix 128×128, average 12; the 19F imaging: GRE pulse sequence, resolution 2×2×3 mm, TR/TE=400/4.24, matrix 64×64, average 32. (C)- Multiple liver metastases (arrows) are revealed at the necropsy of the mouse (indicated by long thing arrows). Transparent large tumor is indicated by a thick arrow. Organs could be displaced at necropsy.
Measurement of nanodroplet concentration in tumor tissue by 19F MRI is complicated by a strong dependence of the fluorine nucleus relaxation time T2 on the local oxygen concentration, i.e. tumor oxygenation. Due to this effect, hypoxic areas of tumors produce very low, if any MRI signal. Therefore fluorine signals visualized in the tumor tissue depend not only on the concentration of fluorine nuclei but also on oxygen concentration, i.e. on the geometry of tumor vascularization. This makes fluorine MRI a poor quantitative indicator of the intratumoral uptake of nanodroplets, especially for poorly vascularized tumors such as pancreatic cancer. For such tumors, information provided by 19F MR images may suffer from the underestimation of the intratumoral nanodroplet uptake and distribution. This problem is not imminent to 19F MR images of highly vascularized organs such as the liver, spleen, heart, or lung.
3.2.3. Pilot experiment on the ultrasound-mediated chemotherapy of a breast cancer tumor xenograft using paclitaxel-loaded PFCE nanoemulsion combined with 1-MHz focused therapeutic ultrasound
In these experiments, breast cancer MDA MB231 tumor was treated by four systemic injections of paclitaxel loaded 1% PFCE/0.25% PEG-PCL nanoemulsion and focused 1-MHz CW ultrasound; treatment was given twice weekly for two weeks as described in the Methods section. Paclitaxel dose was 40 mg/kg. Ultrasound pressure at the first treatment was 2.74 MPa. It was reduced to 2.0 MPa in subsequent treatments due to some skin burn that required treatment with antibiotic ointment.
Complete tumor regression was observed as shown in Fig. 11. No tumor monitoring period. Dramatic therapeutic effect observed in this study encouraged testing lower ultrasound energies generated by unfocused ultrasound transducer in subsequent larger scale experiments using orthotopic pancreatic cancer model. The results of this study are presented below.
Fig. 11.
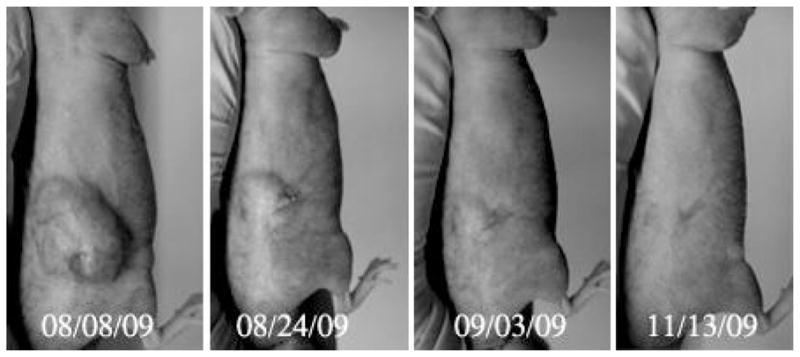
Dramatic regression of a breast cancer MDA MB231 tumor treated by four systemic injections of paclitaxel loaded 1% PFCE/0.25% PEG-PCL nanoemulsion and focused 1-MHz CW ultrasound applied for 60 s as described in Methods section. Paclitaxel dose was 40 mg/kg. The tumor of an anesthetized mouse was inserted into a water tank and sonicated by ultrasound generated by the HIFU transducer. During the first treatment, sonication was performed in water maintained at room temperature, which caused significantly extended period of anesthesia; peak rarefactional pressure was 2.74 MPa; this caused some skin burn that were treated by antibiotic; for subsequent treatments, the pressure was reduced to 2.0 MPa and water temperature was maintained at 33 °C.
3.2.4. Ultrasound-mediated chemotherapy of the orthotopic pancreatic cancer xenografts using paclitaxel-loaded PFCE nanoemulsion combined with 1-MHz unfocused therapeutic ultrasound
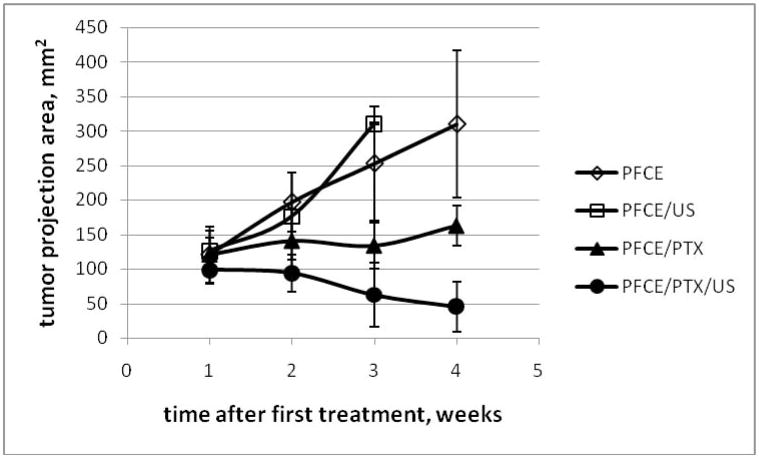
The results obtained in the experiments presented en Figure 12 are qualitatively similar to those obtained earlier with pfp nanodroplets. No therapeutic effect was observed for empty nanodroplets with or without ultrasound (empty symbols in figure 12). Paclitaxel loaded nanodroplets caused disease stabilization (closed triangles) while combining paclitaxel loaded nanodroplets with tumor sonication resulted in tumor regression (closed circles). All animals treated with empty droplets (N = 4 in each group) developed metastases and ascites while no metastases or ascites was observed in animals treated with drug-loaded nanodroplets with or without ultrasound (N = 5). These data indicate unambiguously that the therapeutic effect was caused by drug and not by ultrasound. The same was observed earlier in our experiments with drug-loaded micelles or pfp nanodroplets [5, 30, 60].Tthe action of the micellar or nanodroplet encapsulated drug was enhanced by ultrasound, as was also indicated by the data presented in figure 7; the difference between the sonicated and non-sonicated drug loaded groups was statistically significant in a two-tail equal variance student’s test (p < 0.05).
Fig. 12.
Tumor growth/regression curves for pancreatic cancer bearing mice treated with either empty (open symbols) or PTX-loaded (closed symbols) 1% PFCE/5% PEG-PDLA nanodroplets. PTX dose was 40 mg/kg. N = 4 (empty symbols); N = 5 (filled symbols).
4. Discussion
The results presented above show that block copolymer stabilized PFCE nanodroplets effectively encapsulate chemotherapeutic drugs (e.g. paclitaxel), generate ultrasound and 19F MRI contrast, and are susceptible to reversible acoustic droplet vaporization and stable cavitation of formed microbubbles. These qualities make PFCE nanoemulsions excellent drug carriers for ultrasound-mediated, image-guided drug delivery.
Effective drug targeting to tumors requires prolonged nanodroplet circulation time and uptake suppression by the RES cells. To accomplish this, it is necessary to diminish the protein adsorption onto the particle surfaces. This is usually achieved by coating the nanoparticles with polyethylene oxide chains. The efficiency of this approach depends on a number of factors, including the degree of surface coating, PEG chains length and motion, and nanoparticle size [62, 63]. In particular for nanodroplets, the degree of surface coating and PEG chain dynamics on the surface will depend on nanoparticle size, concentration of stabilizing block copolymer, length of PEG blocks, and PEG interactions with other components of the formulation, including the core-forming perfluorocarbon compound, hydrophobic blocks of the copolymer, and the drug. While this problem goes beyond the scope of the current study, it is noteworthy that the accumulation seen in the liver after systemic injections was usually higher for PEG-PCL coated nanodroplets (PEG molecular weight 2,000 Da; copolymer concentration 0.25%) than PEG-PDLA coated nanodroplets (PEG molecular weight 5000 Da; copolymer concentration 5.0%) (Figure 9). This result demonstrates that the nanodroplet uptake by the RES system cells can be modulated by optimizing shell composition. One advantage of using PEG-PDLA as a droplet shell forming copolymer is that it allows for a higher concentration in the aqueous environment when compared to the PEG-PLLA or PEG-PCL copolymer. Further studies are needed for developing optimal nanodroplet shell compositions.
4.1. Acoustic vaporization of PFCE nanodroplets: anticipated mechanism
It is noteworthy that PFCE droplets are extremely thermally stable (boiling temperature of 146 °C at atmospheric pressure); however, despite thermal stability, PFCE nanodroplets undergo acoustic vaporization. Ultrasound pressures that trigger the vaporization of PFCE nanodroplets are only slightly higher for PFCE nanodroplets than for PFP nanodroplets (boiling temperature of 29 °C at atmospheric pressure) (Table 1). A similar phenomenon was observed earlier by others for different types of perfluorocarbon emulsions [57]. Note that due to a high Laplace pressure inside the nanodroplets, boiling temperatures of nanodroplet cores are expected to be higher than those presented above at atmospheric pressure [29, 30]. For instance, for PFP droplets with a 500 nm diameter, boiling temperatures estimated using the Antoine equation were found to be between 68 °C and 80 °C, depending on the value of the surface tension at the droplet/water interface [29, 30]. For PFCE, the parameters of the Antoine equation are not known but the trend is expected to be the same. The high boiling temperature of perfluorocarbon nanodroplets raises a question regarding the driving force of droplet-to-bubble transition under the ultrasound action. The data shown in Fig. 3 indicates that the droplet-to-bubble transition in PFCE nanodroplets occurs not only under continuous wave ultrasound but also under pulsed ultrasound with a 20% duty cycle that causes negligible sample heating with respect to thermal vaporization. This suggests that the droplet-to-bubble transition in PFCE nanodroplets has a non-thermal mechanism. One possible factor involved in acoustically triggered droplet-to-bubble transition in PFCE nanodroplets is the high solubility of gases, particularly oxygen. This feature has allowed using perfluorocarbon emulsions as blood substitutes [64]. Note that according to Henry’s law, the solubility of gases increases with pressure. We hypothesize that during the rarefactional phase of ultrasound, the evolution of dissolved oxygen into a gas phase occurs inside the nanodroplet shell, followed by the rectified diffusion of dissolved gases into the nanobubble formed. Though some gases are probably released from bubbles during the ultrasound compression phase, the degree of release is expected to be less than the uptake due to higher gas solubility under pressure and smaller surface area of the compressed bubbles. According to this hypothesis, PFCE bubbles contain a mixture of oxygen and other ambient gases as well as some PFCE vapor in equilibrium with the PFCE liquid phase. When ultrasound is turned off, equilibrium corresponding to the ambient pressure is restored; gases with super-equilibrium concentrations diffuse out, thus restoring PFCE nanodroplets. This hypothesis has been corroborated by the fact that degassing a PFCE nanoemulsion by pumping out dissolved gases inhibited droplet-to-bubble transition; the latter was restored after the contact with air was re-established. The suggested mechanism of bubble formation is different from real vaporization of PFCE droplets. However, biological implications of bubble formation associated with bubble cavitation do not depend on the specific mechanism of droplet-to-bubble transition.
The droplet-to-bubble transition in PFCE nanoemulsions is completely reversible. The reversibility of PFCE bubble formation reduces the risk of embolism, which makes PFCE formulations safe for clinical applications. This favorably differentiates PFCE from PFP nanodroplets whose vaporization may become irreversible if microbubbles larger than a threshold size are formed [27, 29, 30].
4.2. Anticipated mechanism of ultrasound-mediated chemotherapy
Bubbles formed from PFCE nanodroplets undergo acoustic cavitation, which is considered to be the most important biologically relevant process. Droplet-to-bubble transition and bubble oscillations result in absorption of ultrasound energy and sample heating (Figures 5, 6B). This suggests that PFCE nanodroplets may be used for a number of heat-associated applications such as hyperthermia-based chemotherapy, catalysis of drug release from temperature-responsive carriers [8, 26, 65, 66], or catalysis of tumor ablation. In addition to thermal mechanisms, the mechanical component of ultrasound (bubble cavitation) triggers drug release from a variety of carrier types (polymeric micelles, lipososmes, nanoemulsions) and enhances intracellular drug uptake [5, 30]. It can be hypothesized that the strong therapeutic effects observed in refs. [42, 43, 48, 50, 51] with drug loaded, phospholipid stabilized perfluorooctylbromide nanoemulsions could be associated, at least partly, with transient droplet-to-bubble transition and bubble cavitation.
While the mechanical and thermal ultrasound action on a drug carrier is essential, the biological effects of ultrasound at the body and cellular levels should also be considered. Ultrasound helps to overcome various biological barriers the drug encounters on the way to its target. First, the ultrasound radiation force pushes nanoparticles through blood capillary walls thus enhancing extravasation of nanoparticle drug carries or macromolecular drugs [12, 66–69]. Second, even a moderate temperature increase may significantly increase the permeability of blood capillaries [70, 71]. On the cellular level, a temperature increase may lead to cell membrane fluidization [72, 73]. This effect may be accompanied by mechanical permeabilization (sonoporation) of cell membranes. The thermal and mechanical action of ultrasound increases the intracellular uptake of nanoparticles, genes, and drugs [5, 7, 8, 19, 30, 74–91]. In addition, ultrasound affects the intracellular drug trafficking by overcoming the barrier created by nuclear membranes [92]. Biological effects of ultrasound may be also associated with enhancing the immune response to the tumor [93–100]. Ultimately, the various effects of ultrasound on drug carriers and biological objects result in significantly enhanced therapeutic action of chemotherapeutic drugs as illustrated in Figures 7, 11, and 12.
4.3. Guiding therapy by ultrasound and MR imaging
Ultrasound-mediated drug delivery, similar to other energy-based tumor treatment modalities, requires imaging for both guiding and monitoring the therapy. PFCE nanodroplets fill this requirement due to their ultrasound and 19F MRI contrast properties. There is a long history of using perfluorocarbon emulsions as contrast agents [101–103]. The emulsions employed in the earlier studies were stabilized by phospholipids and did not have protective polyethylene oxide chains on their surfaces; therefore the droplets were mostly taken up by RES system cells. The information obtained in the early research was ambiguous. While CT scans using perfluorooctylbromide or iodide nanoemulsions showed preferential accumulation in the spleen and Kupfer cells of the liver thus providing negative contrast to liver metastases, ultrasonography manifested preferential accumulation of nanodroplets in liver metastases [101–103]. During the last decade, this field has made a key step forward due to works by the Wickline and Lanza research group. Actively targeted perfluorocarbon nanoemulsions have been developed and successfully used as molecularly targeted imaging and therapeutic agents [41–52]. The phospholipid stabilized perfluorooctylbromide nanoemulsions developed in these studies have recently entered clinical trials as molecularly targeted drug delivery and imaging agents [43, 44]. It is worth mentioning that as blood substitutes, perfluorocarbon nanoemulsions have been clinically used at much higher concentrations [104] than those suggested for current application in drug delivery.
In the present study, the feasibility of using 19F MR imaging for monitoring the biodistribution of PFCE nanodroplets has been demonstrated. However, this approach is feasible only for highly oxygenated tumors because obtaining quantitative measurements of the PFCE biodistribution is complicated due to the strong dependence of the T2 relaxation time (and therefore MR signal strength) on the local oxygen concentration. This dependence causes hypoxic areas of tumors to produce very low, if any MRI signal. In other words, fluorine signals visualized in the tumor tissue depend not only on the concentration of fluorine nuclei but also on the geometry of tumor vascularization [105–108]. In the experiments presented in ref. [106], PFCE nanodroplet accumulation in the tumor was not visualized at normoxic conditions while a strong fluorine signal in the tumor was clearly seen after a mouse breathed Carbogen (comprising 95% oxygen) for 15 minutes. This approach may be used to study intratumoral distribution of functional blood vessels [106, 108, 109] but prevents the use of 19F MRI as an indicator of the intratumoral uptake of nanodroplets, especially for poorly vascularized tumors such as pancreatic cancer. In that application, the information provided by 19F MR images may suffer from underestimation of the nanodroplet uptake. This is not the case for 19F MR imaging of highly vascularized organs such as the liver, spleen, heart, or lung.
Conclusions
Biodegradable block copolymer stabilized PFCE nanoemulsions are effective theranostic formulations with high potential for use in image-guided, ultrasound-mediated drug delivery.
Acknowledgments
The project was supported by the grants R56EB001033 and R01EB001033 to NR from the National Institute of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Biomedical Imaging And Bioengineering or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rapoport N. Physical stimuli-responsive polymeric micelles for anti-cancer drug delivery. Prog Polym Sci. 2007;32:962–990. [Google Scholar]
- 2.Iyer AK, Khaled G, Fang J, Maeda H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today. 2006;11:812–818. doi: 10.1016/j.drudis.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, Jain RK. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci U S A. 1998;95:4607–4612. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell RB. Tumor physiology and delivery of nanopharmaceuticals. Anticancer Agents Med Chem. 2006;6:503–512. doi: 10.2174/187152006778699077. [DOI] [PubMed] [Google Scholar]
- 5.Rapoport N, Gao Z, Kennedy A. Multifunctional nanoparticles for combining ultrasonic tumor imaging and targeted chemotherapy. J Natl Cancer Inst. 2007;99:1095–1106. doi: 10.1093/jnci/djm043. [DOI] [PubMed] [Google Scholar]
- 6.Gao Z, Kennedy AM, Christensen DA, Rapoport NY. Drug-loaded nano/microbubbles for combining ultrasonography and targeted chemotherapy. Ultrasonics. 2008;48:260–270. doi: 10.1016/j.ultras.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrara K, Pollard R, Borden M. Ultrasound microbubble contrast agents: fundamentals and application to gene and drug delivery. Annu Rev Biomed Eng. 2007;9:415–447. doi: 10.1146/annurev.bioeng.8.061505.095852. [DOI] [PubMed] [Google Scholar]
- 8.Ferrara KW. Driving delivery vehicles with ultrasound. Adv Drug Deliv Rev. 2008;60:1097–1102. doi: 10.1016/j.addr.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Unger EC, Porter T, Culp W, Labell R, Matsunaga T, Zutshi R. Therapeutic applications of lipid-coated microbubbles. Adv Drug Deliv Rev. 2004;56:1291–1314. doi: 10.1016/j.addr.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Qin S, Caskey CF, Ferrara KW. Ultrasound contrast microbubbles in imaging and therapy: physical principles and engineering. Phys Med Biol. 2009;54:R27–57. doi: 10.1088/0031-9155/54/6/R01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng H, Kruse DE, Stephens DN, Ferrara KW, Sutcliffe P, Gardner E. A sensitive ultrasonic imaging method for targeted contrast microbubble detection. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:5290–5293. doi: 10.1109/IEMBS.2008.4650408. [DOI] [PubMed] [Google Scholar]
- 12.Dayton PA, Zhao S, Bloch SH, Schumann P, Penrose K, Matsunaga TO, Zutshi R, Doinikov A, Ferrara KW. Application of ultrasound to selectively localize nanodroplets for targeted imaging and therapy. Mol Imaging. 2006;5:160–174. [PMC free article] [PubMed] [Google Scholar]
- 13.Greenleaf WJ, Bolander ME, Sarkar G, Goldring MB, Greenleaf JF. Artificial cavitation nuclei significantly enhance acoustically induced cell transfection. Ultrasound Med Biol. 1998;24:587–595. doi: 10.1016/s0301-5629(98)00003-9. [DOI] [PubMed] [Google Scholar]
- 14.Kost J, Mitragotri S, Gabbay RA, Pishko M, Langer R. Transdermal monitoring of glucose and other analytes using ultrasound. Nat Med. 2000;6:347–350. doi: 10.1038/73213. [DOI] [PubMed] [Google Scholar]
- 15.Taniyama Y, Tachibana K, Hiraoka K, Namba T, Yamasaki K, Hashiya N, Aoki M, Ogihara T, Yasufumi K, Morishita R. Local delivery of plasmid DNA into rat carotid artery using ultrasound. Circulation. 2002;105:1233–1239. doi: 10.1161/hc1002.105228. [DOI] [PubMed] [Google Scholar]
- 16.Unger EC, Hersh E, Vannan M, McCreery T. Gene delivery using ultrasound contrast agents. Echocardiography. 2001;18:355–361. doi: 10.1046/j.1540-8175.2001.00355.x. [DOI] [PubMed] [Google Scholar]
- 17.Mitragotri S. Healing sound: the use of ultrasound in drug delivery and other therapeutic applications. Nat Rev Drug Discov. 2005;4:255–260. doi: 10.1038/nrd1662. [DOI] [PubMed] [Google Scholar]
- 18.Sheikov N, McDannold N, Sharma S, Hynynen K. Effect of focused ultrasound applied with an ultrasound contrast agent on the tight junctional integrity of the brain microvascular endothelium. Ultrasound Med Biol. 2008;34:1093–1104. doi: 10.1016/j.ultrasmedbio.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hynynen K. Ultrasound for drug and gene delivery to the brain. Adv Drug Deliv Rev. 2008;60:1209–1217. doi: 10.1016/j.addr.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDannold N, Vykhodtseva N, Hynynen K. Effects of acoustic parameters and ultrasound contrast agent dose on focused-ultrasound induced blood-brain barrier disruption. Ultrasound Med Biol. 2008;34:930–937. doi: 10.1016/j.ultrasmedbio.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaw GJ, Meunier JM, Huang SL, Lindsell CJ, McPherson DD, Holland CK. Ultrasound-enhanced thrombolysis with tPA-loaded echogenic liposomes. Thromb Res. 2009;124:306–310. doi: 10.1016/j.thromres.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kopechek JA, Abruzzo TM, Wang B, Chrzanowski SM, Smith DA, Kee PH, Huang S, Collier JH, McPherson DD, Holland CK. Ultrasound-mediated release of hydrophilic and lipophilic agents from echogenic liposomes. J Ultrasound Med. 2008;27:1597–1606. doi: 10.7863/jum.2008.27.11.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith DA, Porter TM, Martinez J, Huang S, MacDonald RC, McPherson DD, Holland CK. Destruction thresholds of echogenic liposomes with clinical diagnostic ultrasound. Ultrasound Med Biol. 2007;33:797–809. doi: 10.1016/j.ultrasmedbio.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 24.Datta S, Coussios CC, Ammi AY, Mast TD, de Courten-Myers GM, Holland CK. Ultrasound-enhanced thrombolysis using Definity as a cavitation nucleation agent. Ultrasound Med Biol. 2008;34:1421–1433. doi: 10.1016/j.ultrasmedbio.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller DL, Averkiou MA, Brayman AA, Everbach EC, Holland CK, Wible JH, Jr, Wu J. Bioeffects considerations for diagnostic ultrasound contrast agents. J Ultrasound Med. 2008;27:611–632. doi: 10.7863/jum.2008.27.4.611. quiz 633-616. [DOI] [PubMed] [Google Scholar]
- 26.Tartis MS, Kruse DE, Zheng H, Zhang H, Kheirolomoom A, Marik J, Ferrara KW. Dynamic microPET imaging of ultrasound contrast agents and lipid delivery. J Control Release. 2008;131:160–166. doi: 10.1016/j.jconrel.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rapoport N, Christensen DA, Kennedy AM, Nam KH. Cavitation properties of block copolymer stabilized phase-shift nanoemulsions used as drug carriers. Ultrasound Med Biol. 2010;36:419–429. doi: 10.1016/j.ultrasmedbio.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rapoport N, Kennedy AM, Shea JE, Scaife CL, Nam KH. Ultrasonic nanotherapy of pancreatic cancer: lessons from ultrasound imaging. Mol Pharm. 2010;7:22–31. doi: 10.1021/mp900128x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rapoport NY, Efros AL, Christensen DA, Kennedy AM, Nam KH. Microbubble generation in phase-shift nanoemulsions used as anticancer drug carriers. Bub Sci Eng Tech. 2009;1:31–39. doi: 10.1179/175889709X446516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rapoport NY, Kennedy AM, Shea JE, Scaife CL, Nam KH. Controlled and targeted tumor chemotherapy by ultrasound-activated nanoemulsions/microbubbles. J Control Release. 2009;138:268–276. doi: 10.1016/j.jconrel.2009.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nam KH, Christensen DA, Kennedy AM, Rapoport N. Acoustic droplet vaporization, cavitation, and therapeutic properties of copolymer-stabilized perfluorocarbon nanoemulsions. Am Inst Phys Conf Proc. 2009;1113:124–128. [Google Scholar]
- 32.Kripfgans OD, Fabiilli ML, Carson PL, Fowlkes JB. On the acoustic vaporization of micrometer-sized droplets. J Acoust Soc Am. 2004;116:272–281. doi: 10.1121/1.1755236. [DOI] [PubMed] [Google Scholar]
- 33.Kripfgans OD, Fowlkes JB, Miller DL, Eldevik OP, Carson PL. Acoustic droplet vaporization for therapeutic and diagnostic applications. Ultrasound Med Biol. 2000;26:1177–1189. doi: 10.1016/s0301-5629(00)00262-3. [DOI] [PubMed] [Google Scholar]
- 34.Lo AH, Kripfgans OD, Carson PL, Rothman ED, Fowlkes JB. Acoustic droplet vaporization threshold: effects of pulse duration and contrast agent. IEEE Trans Ultrason Ferroelectr Freq Control. 2007;54:933–946. doi: 10.1109/tuffc.2007.339. [DOI] [PubMed] [Google Scholar]
- 35.Hernot S, Klibanov AL. Microbubbles in ultrasound-triggered drug and gene delivery. Adv Drug Deliv Rev. 2008;60:1153–1166. doi: 10.1016/j.addr.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mehier-Humbert S, Yan F, Frinking P, Schneider M, Guy RH, Bettinger T. Ultrasound- mediated gene delivery: influence of contrast agent on transfection. Bioconjug Chem. 2007;18:652–662. doi: 10.1021/bc0602432. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki R, Takizawa T, Negishi Y, Hagisawa K, Tanaka K, Sawamura K, Utoguchi N, Nishioka T, Maruyama K. Gene delivery by combination of novel liposomal bubbles with perfluoropropane and ultrasound. J Control Release. 2007;117:130–136. doi: 10.1016/j.jconrel.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 38.Ahrens ET, Flores R, Xu H, Morel PA. In vivo imaging platform for tracking immunotherapeutic cells. Nat Biotechnol. 2005;23:983–987. doi: 10.1038/nbt1121. [DOI] [PubMed] [Google Scholar]
- 39.Yu JX, Kodibagkar VD, Cui W, Mason RP. 19F: a versatile reporter for non-invasive physiology and pharmacology using magnetic resonance. Curr Med Chem. 2005;12:819–848. doi: 10.2174/0929867053507342. [DOI] [PubMed] [Google Scholar]
- 40.Noth U, Morrissey SP, Deichmann R, Jung S, Adolf H, Haase A, Lutz J. Perfluoro-15- crown-5-ether labelled macrophages in adoptive transfer experimental allergic encephalomyelitis. Artif Cells Blood Substit Immobil Biotechnol. 1997;25:243–254. doi: 10.3109/10731199709118914. [DOI] [PubMed] [Google Scholar]
- 41.Caruthers SD, Cyrus T, Winter PM, Wickline SA, Lanza GM. Anti-angiogenic perfluorocarbon nanoparticles for diagnosis and treatment of atherosclerosis. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1:311–323. doi: 10.1002/wnan.9. [DOI] [PubMed] [Google Scholar]
- 42.Kaneda MM, Caruthers S, Lanza GM, Wickline SA. Perfluorocarbon nanoemulsions for quantitative molecular imaging and targeted therapeutics. Ann Biomed Eng. 2009;37:1922–1933. doi: 10.1007/s10439-009-9643-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lanza GM, Winter PM, Caruthers SD, Hughes MS, Hu G, Schmieder AH, Wickline SA. Theragnostics for tumor and plaque angiogenesis with perfluorocarbon nanoemulsions. Angiogenesis. 2010 doi: 10.1007/s10456-010-9166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pan D, Caruthers SD, Chen J, Winter PM, Senpan A, Schmieder AH, Wickline SA, Lanza GM. Nanomedicine strategies for molecular targets with MRI and optical imaging. Future Med Chem. 2010;2:471–490. doi: 10.4155/fmc.10.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pan D, Lanza GM, Wickline SA, Caruthers SD. Nanomedicine: perspective and promises with ligand-directed molecular imaging. Eur J Radiol. 2009;70:274–285. doi: 10.1016/j.ejrad.2009.01.042. [DOI] [PubMed] [Google Scholar]
- 46.Soman NR, Baldwin SL, Hu G, Marsh JN, Lanza GM, Heuser JE, Arbeit JM, Wickline SA, Schlesinger PH. Molecularly targeted nanocarriers deliver the cytolytic peptide melittin specifically to tumor cells in mice, reducing tumor growth. J Clin Invest. 2009;119:2830–2842. doi: 10.1172/JCI38842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Southworth R, Kaneda M, Chen J, Zhang L, Zhang H, Yang X, Razavi R, Lanza G, Wickline SA. Renal vascular inflammation induced by Western diet in ApoE-null mice quantified by (19)F NMR of VCAM-1 targeted nanobeacons. Nanomedicine. 2009;5:359–367. doi: 10.1016/j.nano.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tran TD, Caruthers SD, Hughes M, Marsh JN, Cyrus T, Winter PM, Neubauer AM, Wickline SA, Lanza GM. Clinical applications of perfluorocarbon nanoparticles for molecular imaging and targeted therapeutics. Int J Nanomedicine. 2007;2:515–526. [PMC free article] [PubMed] [Google Scholar]
- 49.Wickline SA, Neubauer AM, Winter PM, Caruthers SD, Lanza GM. Molecular imaging and therapy of atherosclerosis with targeted nanoparticles. J Magn Reson Imaging. 2007;25:667–680. doi: 10.1002/jmri.20866. [DOI] [PubMed] [Google Scholar]
- 50.Winter PM, Cai K, Caruthers SD, Wickline SA, Lanza GM. Emerging nanomedicine opportunities with perfluorocarbon nanoparticles. Expert Rev Med Devices. 2007;4:137–145. doi: 10.1586/17434440.4.2.137. [DOI] [PubMed] [Google Scholar]
- 51.Winter PM, Caruthers SD, Zhang H, Williams TA, Wickline SA, Lanza GM. Antiangiogenic synergism of integrin-targeted fumagillin nanoparticles and atorvastatin in atherosclerosis. JACC Cardiovasc Imaging. 2008;1:624–634. doi: 10.1016/j.jcmg.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou HF, Chan HW, Wickline SA, Lanza GM, Pham CT. Alphavbeta3-targeted nanotherapy suppresses inflammatory arthritis in mice. FASEB J. 2009;23:2978–2985. doi: 10.1096/fj.09-129874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim SC, Kim DW, Shim YH, Bang JS, Oh HS, Wan Kim S, Seo MH. In vivo evaluation of polymeric micellar paclitaxel formulation: toxicity and efficacy. J Control Release. 2001;72:191–202. doi: 10.1016/s0168-3659(01)00275-9. [DOI] [PubMed] [Google Scholar]
- 54.De Poorter J, De Wagter C, De Deene Y, Thomsen C, Stahlberg F, Achten E. Noninvasive MRI thermometry with the proton resonance frequency (PRF) method: in vivo results in human muscle. Magn Reson Med. 1995;33:74–81. doi: 10.1002/mrm.1910330111. [DOI] [PubMed] [Google Scholar]
- 55.Scaife CL, Shea JE, Dai Q, Firpo MA, Prestwich GD, Mulvihill SJ. Synthetic extracellular matrix enhances tumor growth and metastasis in an orthotopic mouse model of pancreatic adenocarcinoma. J Gastrointest Surg. 2008;12:1074–1080. doi: 10.1007/s11605-007-0425-3. [DOI] [PubMed] [Google Scholar]
- 56.Torgenson MJ, Shea JE, Firpo MA, Dai Q, Mulvihill SJ, Scaife CL. Natural history of pancreatic cancer recurrence following “curative” resection in athymic mice. J Surg Res. 2008;149:57–61. doi: 10.1016/j.jss.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 57.Giesecke T, Hynynen K. Ultrasound-mediated cavitation thresholds of liquid perfluorocarbon droplets in vitro. Ultrasound Med Biol. 2003;29:1359–1365. doi: 10.1016/s0301-5629(03)00980-3. [DOI] [PubMed] [Google Scholar]
- 58.Katz MH, Takimoto S, Spivack D, Moossa AR, Hoffman RM, Bouvet M. A novel red fluorescent protein orthotopic pancreatic cancer model for the preclinical evaluation of chemotherapeutics. J Surg Res. 2003;113:151–160. doi: 10.1016/s0022-4804(03)00234-8. [DOI] [PubMed] [Google Scholar]
- 59.Katz MH, Takimoto S, Spivack D, Moossa AR, Hoffman RM, Bouvet M. An imageable highly metastatic orthotopic red fluorescent protein model of pancreatic cancer. Clin Exp Metastasis. 2004;21:7–12. doi: 10.1023/b:clin.0000017160.93812.3b. [DOI] [PubMed] [Google Scholar]
- 60.Rapoport N. Combined cancer therapy by micellar-encapsulated drug and ultrasound. In: Amiji M, editor. Nanotechnology for cancer therapy. CRC Press; Boca Raton (FL): 2006. pp. 417–437. [Google Scholar]
- 61.Kim SO, Lee SW, Jeong SW, Jung YA, Kang SY, Kumar S, Oh HS. Superior antitumor efficacy of Genexol-PM®, a biodegradable polymeric micelle-based formulation of paclitaxel (Genexol®) compared with Gemzar® (gemcitabine) and Taxol® in human pancreatic cancer cells in vitro and in vivo. Experimental and Molecular Therapeutics 10: Drug Targeting. Proc Amer Assoc Cancer Res. 2005;46 Abstract 1440. [Google Scholar]
- 62.Bohner M, Ring TA, Rapoport N, Caldwell KD. Fibrinogen adsorption by PS latex particles coated with various amounts of a PEO/PPO/PEO triblock copolymer. J Biomater Sci Polym Ed. 2002;13:733–746. doi: 10.1163/156856202320269184. [DOI] [PubMed] [Google Scholar]
- 63.Tan JS, Butterfield DE, Voycheck CL, Caldwell KD, Li JT. Surface modification of nanoparticles by PEO/PPO block copolymers to minimize interactions with blood components and prolong blood circulation in rats. Biomaterials. 1993;14:823–833. doi: 10.1016/0142-9612(93)90004-l. [DOI] [PubMed] [Google Scholar]
- 64.Cohn CS, Cushing MM. Oxygen therapeutics: perfluorocarbons and blood substitute safety. Crit Care Clin. 2009;25:399–414. doi: 10.1016/j.ccc.2008.12.007. Table of Contents. [DOI] [PubMed] [Google Scholar]
- 65.Seo JW, Zhang H, Kukis DL, Meares CF, Ferrara KW. A novel method to label preformed liposomes with 64Cu for positron emission tomography (PET) imaging. Bioconjug Chem. 2008;19:2577–2584. doi: 10.1021/bc8002937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stieger SM, Caskey CF, Adamson RH, Qin S, Curry FR, Wisner ER, Ferrara KW. Enhancement of vascular permeability with low-frequency contrast-enhanced ultrasound in the chorioallantoic membrane model. Radiology. 2007;243:112–121. doi: 10.1148/radiol.2431060167. [DOI] [PubMed] [Google Scholar]
- 67.Dayton P, Klibanov A, Brandenburger G, Ferrara K. Acoustic radiation force in vivo: a mechanism to assist targeting of microbubbles. Ultrasound Med Biol. 1999;25:1195–1201. doi: 10.1016/s0301-5629(99)00062-9. [DOI] [PubMed] [Google Scholar]
- 68.Ferrara KW. Driving delivery vehicles with ultrasound. Adv Drug Deliv Rev. 2008;60:1097–1102. doi: 10.1016/j.addr.2008.03.002. Epub 2008 Mar 1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Holland CK, McPherson DD. Echogenic Lipsomes for Targeted Drug Delivery. Proc IEEE Int Symp Biomed Imaging. 2009;2009:755–758. [PMC free article] [PubMed] [Google Scholar]
- 70.Kong G, Braun RD, Dewhirst MW. Hyperthermia enables tumor-specific nanoparticle delivery: effect of particle size. Cancer Res. 2000;60:4440–4445. [PubMed] [Google Scholar]
- 71.Kong G, Braun RD, Dewhirst MW. Characterization of the effect of hyperthermia on nanoparticle extravasation from tumor vasculature. Cancer Res. 2001;61:3027–3032. [PubMed] [Google Scholar]
- 72.Hayat H, Friedberg I. Heat-induced alterations in cell membrane permeability and cell inactivation of transformed mouse fibroblasts. Int J Hyperthermia. 1986;2:369–378. doi: 10.3109/02656738609004967. [DOI] [PubMed] [Google Scholar]
- 73.Krupka T, Dremann D, Exner A. Time and dose dependence of pluronic bioactivity in hyperthermia-induced tumor cell death. Exp Biol Med (Maywood) 2009;234:95–104. doi: 10.3181/0807-RM-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qin S, Caskey CF, Ferrara KW. Ultrasound contrast microbubbles in imaging and therapy: physical principles and engineering. Phys Med Biol. 2009;54:R27–57. doi: 10.1088/0031-9155/54/6/R01. Epub 2009 Feb 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hynynen K. Macromolecular delivery across the blood-brain barrier. Methods Mol Biol. 2009;480:175–185. doi: 10.1007/978-1-59745-429-2_13. [DOI] [PubMed] [Google Scholar]
- 76.Hynynen K. MRI-guided focused ultrasound treatments. Ultrasonics. 2009;50:221–229. doi: 10.1016/j.ultras.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 77.Hynynen K, McDannold N, Vykhodtseva N, Raymond S, Weissleder R, Jolesz FA, Sheikov N. Focal disruption of the blood-brain barrier due to 260-kHz ultrasound bursts: a method for molecular imaging and targeted drug delivery. J Neurosurg. 2006;105:445–454. doi: 10.3171/jns.2006.105.3.445. [DOI] [PubMed] [Google Scholar]
- 78.Laing ST, McPherson DD. Cardiovascular therapeutic uses of targeted ultrasound contrast agents. Cardiovasc Res. 2009;83:626–635. doi: 10.1093/cvr/cvp192. Epub 2009 Jul 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pua EC, Zhong P. Ultrasound-mediated drug delivery. IEEE Eng Med Biol Mag. 2009;28:64–75. doi: 10.1109/MEMB.2008.931017. [DOI] [PubMed] [Google Scholar]
- 80.Rao R, Nanda S. Sonophoresis: recent advancements and future trends. J Pharm Pharmacol. 2009;61:689–705. doi: 10.1211/jpp.61.06.0001. [DOI] [PubMed] [Google Scholar]
- 81.Stride E. Physical principles of microbubbles for ultrasound imaging and therapy. Cerebrovasc Dis. 2009;27:1–13. doi: 10.1159/000203122. Epub 2009 Apr 2016. [DOI] [PubMed] [Google Scholar]
- 82.Stride EP, Coussios CC. Cavitation and contrast: the use of bubbles in ultrasound imaging and therapy. Proc. 224:171–191. doi: 10.1243/09544119JEIM622. [DOI] [PubMed] [Google Scholar]
- 83.Wells DJ. Electroporation and ultrasound enhanced non-viral gene delivery in vitro and in vivo. Cell Biol Toxicol. 2009;26:21–28. doi: 10.1007/s10565-009-9144-8. [DOI] [PubMed] [Google Scholar]
- 84.Yoon CS, Park JH. Ultrasound-mediated gene delivery. Expert. 7:321–330. doi: 10.1517/17425241003596329. [DOI] [PubMed] [Google Scholar]
- 85.Chopra R, Vykhodtseva N, Hynynen K. Influence of exposure time and pressure amplitude on blood-brain-barrier opening using transcranial ultrasound exposures. Acs. 1:391–398. doi: 10.1021/cn9000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Colucci V, Strichartz G, Jolesz F, Vykhodtseva N, Hynynen K. Focused ultrasound effects on nerve action potential in vitro. Ultrasound Med Biol. 2009;35:1737–1747. doi: 10.1016/j.ultrasmedbio.2009.05.002. Epub 2009 Aug 1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McDannold N, Vykhodtseva N, Hynynen K. Effects of acoustic parameters and ultrasound contrast agent dose on focused-ultrasound induced blood-brain barrier disruption. Ultrasound Med Biol. 2008;34:930–937. doi: 10.1016/j.ultrasmedbio.2007.11.009. Epub 2008 Feb 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McDannold NJ, Vykhodtseva NI, Hynynen K. Microbubble contrast agent with focused ultrasound to create brain lesions at low power levels: MR imaging and histologic study in rabbits. Radiology. 2006;241:95–106. doi: 10.1148/radiol.2411051170. [DOI] [PubMed] [Google Scholar]
- 89.Treat LH, McDannold N, Vykhodtseva N, Zhang Y, Tam K, Hynynen K. Targeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultrasound. Int J Cancer. 2007;121:901–907. doi: 10.1002/ijc.22732. [DOI] [PubMed] [Google Scholar]
- 90.Vykhodtseva N, McDannold N, Hynynen K. Induction of apoptosis in vivo in the rabbit brain with focused ultrasound and Optison. Ultrasound Med Biol. 2006;32:1923–1929. doi: 10.1016/j.ultrasmedbio.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 91.Vykhodtseva N, McDannold N, Hynynen K. Progress and problems in the application of focused ultrasound for blood-brain barrier disruption. Ultrasonics. 2008;48:279–296. doi: 10.1016/j.ultras.2008.04.004. Epub 2008 Apr 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mohan P, Rapoport N. Doxorubicin as a molecular nanotheranostic agent: effect of doxorubicin encapsulation in micelles or nanoemulsions on the ultrasound-mediated intracellular delivery and nuclear trafficking. Mol Pharmaceutics. 2010 doi: 10.1021/mp100269f. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Deng J, Zhang Y, Feng J, Wu F. Dendritic cells loaded with ultrasound-ablated tumour induce in vivo specific antitumour immune responses. Ultrasound. 36:441–448. doi: 10.1016/j.ultrasmedbio.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 94.Lu P, Zhu XQ, Xu ZL, Zhou Q, Zhang J, Wu F. Increased infiltration of activated tumor-infiltrating lymphocytes after high intensity focused ultrasound ablation of human breast cancer. Surgery. 2009;145:286–293. doi: 10.1016/j.surg.2008.10.010. Epub 2009 Jan 2025. [DOI] [PubMed] [Google Scholar]
- 95.Wu F, Wang ZB, Cao YD, Zhou Q, Zhang Y, Xu ZL, Zhu XQ. Expression of tumor antigens and heat-shock protein 70 in breast cancer cells after high-intensity focused ultrasound ablation. Ann Surg Oncol. 2007;14:1237–1242. doi: 10.1245/s10434-006-9275-6. Epub 2006 Dec 1224. [DOI] [PubMed] [Google Scholar]
- 96.Wu F, Zhou L, Chen WR. Host antitumour immune responses to HIFU ablation. Int J Hyperthermia. 2007;23:165–171. doi: 10.1080/02656730701206638. [DOI] [PubMed] [Google Scholar]
- 97.Zhang Y, Deng J, Feng J, Wu F. Enhancement of antitumor vaccine in ablated hepatocellular carcinoma by high-intensity focused ultrasound. World. 16:3584–3591. doi: 10.3748/wjg.v16.i28.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hu Z, Yang XY, Liu Y, Sankin GN, Pua EC, Morse MA, Lyerly HK, Clay TM, Zhong P. Investigation of HIFU-induced anti-tumor immunity in a murine tumor model. J Transl Med. 2007;5:34. doi: 10.1186/1479-5876-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu F, Hu Z, Qiu L, Hui C, Li C, Zhong P, Zhang J. Boosting high-intensity focused ultrasound-induced anti-tumor immunity using a sparse-scan strategy that can more effectively promote dendritic cell maturation. J. 8(7) doi: 10.1186/1479-5876-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xing Y, Lu X, Pua EC, Zhong P. The effect of high intensity focused ultrasound treatment on metastases in a murine melanoma model. Biochem Biophys Res Commun. 2008;375:645–650. doi: 10.1016/j.bbrc.2008.08.072. Epub 2008 Aug 2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Behan M, O’Connell D, Mattrey RF, Carney DN. Perfluorooctylbromide as a contrast agent for CT and sonography: preliminary clinical results. AJR Am J Roentgenol. 1993;160:399–405. doi: 10.2214/ajr.160.2.8424361. [DOI] [PubMed] [Google Scholar]
- 102.Mattrey RF, Hajek PC, Gylys-Morin VM, Baker LL, Martin J, Long DC, Long DM. Perfluorochemicals as gastrointestinal contrast agents for MR imaging: preliminary studies in rats and humans. AJR Am J Roentgenol. 1987;148:1259–1263. doi: 10.2214/ajr.148.6.1259. [DOI] [PubMed] [Google Scholar]
- 103.Mattrey RF, Strich G, Shelton RE, Gosink BB, Leopold GR, Lee T, Forsythe J. Perfluorochemicals as US contrast agents for tumor imaging and hepatosplenography: preliminary clinical results. Radiology. 1987;163:339–343. doi: 10.1148/radiology.163.2.3550878. [DOI] [PubMed] [Google Scholar]
- 104.Castro CI, Briceno JC. Perfluorocarbon-based oxygen carriers: review of products and trials. Artif. 34:622–634. doi: 10.1111/j.1525-1594.2009.00944.x. [DOI] [PubMed] [Google Scholar]
- 105.Mason RP, Antich PP, Babcock EE, Constantinescu A, Peschke P, Hahn EW. Non-invasive determination of tumor oxygen tension and local variation with growth. Int J Radiat Oncol Biol Phys. 1994;29:95–103. doi: 10.1016/0360-3016(94)90231-3. [DOI] [PubMed] [Google Scholar]
- 106.McCoy CL, McIntyre DJ, Robinson SP, Aboagye EO, Griffiths JR. Magnetic resonance spectroscopy and imaging methods for measuring tumour and tissue oxygenation. Br J Cancer Suppl. 1996;27:S226–231. [PMC free article] [PubMed] [Google Scholar]
- 107.McNab JA, Yung AC, Kozlowski P. Tissue oxygen tension measurements in the Shionogi model of prostate cancer using 19F MRS and MRI. Magma. 2004;17:288–295. doi: 10.1007/s10334-004-0083-3. Epub 2004 Dec 2016. [DOI] [PubMed] [Google Scholar]
- 108.Xia M, Kodibagkar V, Liu H, Mason RP. Tumour oxygen dynamics measured simultaneously by near-infrared spectroscopy and 19F magnetic resonance imaging in rats. Phys Med Biol. 2006;51:45–60. doi: 10.1088/0031-9155/51/1/004. Epub 2005 Dec 2015. [DOI] [PubMed] [Google Scholar]
- 109.Dardzinski BJ, Sotak CH. Rapid tissue oxygen tension mapping using 19F inversion-recovery echo-planar imaging of perfluoro-15-crown-5-ether. Magn Reson Med. 1994;32:88–97. doi: 10.1002/mrm.1910320112. [DOI] [PubMed] [Google Scholar]