Abstract
Background
We describe institutional vasopressor usage, and examine the effect of vasopressors on hemodynamics: heart rate (HR), mean arterial blood pressure (MAP), intracranial pressure (ICP), cerebral perfusion pressure (CPP), brain tissue oxygenation (PbtO2), and jugular venous oximetry (SjVO2) in adults with severe traumatic brain injury (TBI).
Methods
We performed a retrospective analysis of 114 severely head injured patients who were admitted to the neurocritical care unit of Level 1 trauma center and who received vasopressors (phenylephrine, norepinephrine, dopamine, vasopressin or epinephrine) to increase blood pressure
Results
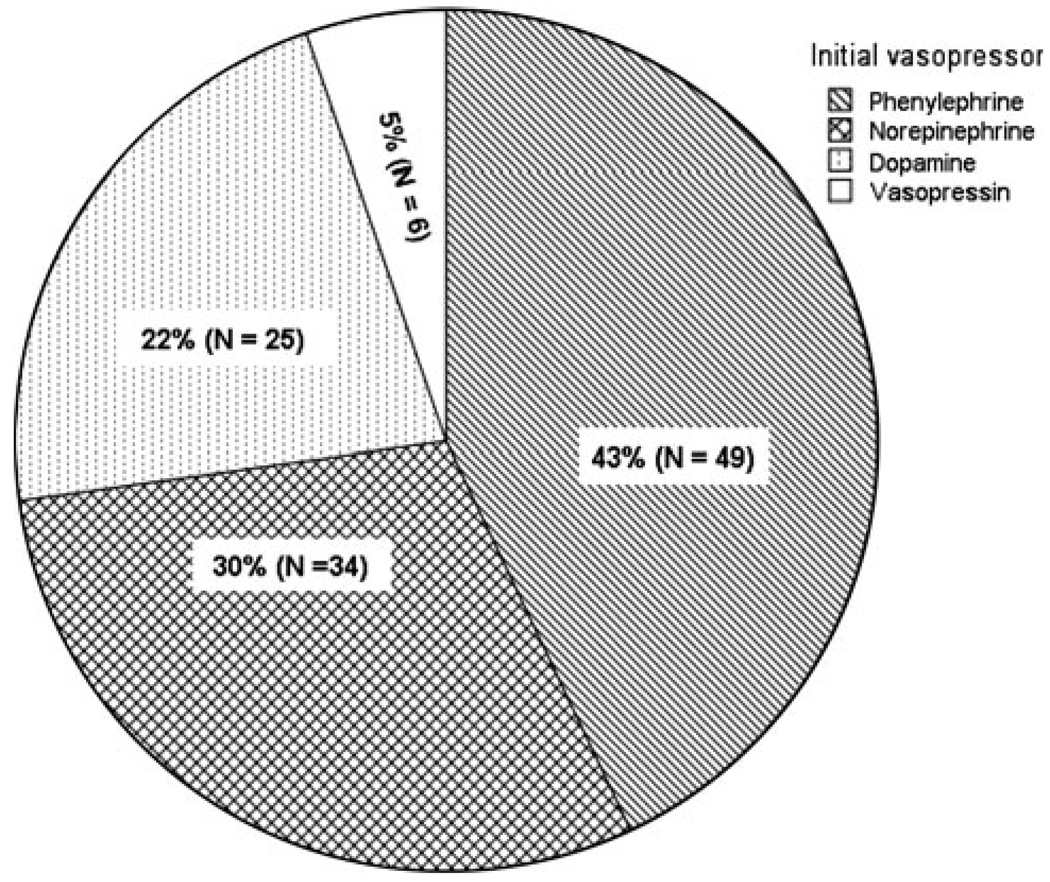
Phenylephrine was the most commonly used vasopressor (43%), followed by norepinephrine (30%), dopamine (22%), and vasopressin (5%). Adjusted for age, gender, injury severity score, vasopressor dose, baseline blood pressure, fluid administration, propofol sedation, and hypertonic saline infusion, phenylephrine use was associated with 8 mmHg higher mean arterial pressure (MAP) than dopamine (P = 0.03), and 12 mmHg higher cerebral perfusion pressure (CPP) than norepinephrine (P = 0.02) during the 3 h after vasopressor start. There was no difference in ICP between the drug groups, either at baseline or after vasopressor treatment.
Conclusions
Most severe TBI patients received phenylephrine. Patients who received phenylephrine had higher MAP and CPP than patients who received dopamine and norepinephrine, respectively.
Keywords: Traumatic brain injury, Vasopressor, Phenylephrine, Norepinephrine, Dopamine, Cerebral perfusion pressure
Introduction
Traumatic brain injury (TBI) is a major global public health problem [1, 2]. After TBI, systemic hypotension can compromise cerebral hemodynamics and cause cerebral ischemia [3–5]. Therefore, blood pressure management after TBI, including the choice of vasopressor, is of paramount importance. Brain Trauma Foundation guidelines for the management of TBI recommend avoiding hypotension (systolic blood pressure <90 mmHg) [6] and maintaining cerebral perfusion pressure (CPP) between 50 and 70 mmHg [7]. Vasopressors are commonly administered to augment CPP [7, 8]. However, there are only a few studies comparing the effectiveness of commonly used vasopressors in TBI and results of these studies are conflicting. Human data explicitly comparing vasopressors are limited to three small (N = 19, N = 10, N = 11) prospective, randomized, crossover trials comparing sequential effectiveness between norepinephrine and dopamine. Despite no difference in mean cerebral blood flow velocity [9, 10] and cerebral oxygenation or metabolism [11] between two vasopressors, norepinephrine had more predictable and consistent effect [10] while dopamine use led to higher ICP [9]. Although phenylephrine has been used to augment CPP in patients with TBI [5], it has not been compared to other vasopressors for effectiveness. Therefore, the purpose of our study was to (1) describe institutional vasopressor use, and (2) compare the effect of commonly used vasopressors on MAP (mean arterial blood pressure), ICP and CPP after severe TBI.
Materials and Methods
Study Design
After Institutional Review Board approval, a retrospective cohort study involving patients admitted to Harborview Medical Center (HMC; Level 1 trauma center), Seattle, Washington, between 1 January 2002 and 31 December 2007 was performed.
Study Population
Eligibility criteria included: (1) patients aged ≥18 years; (2) severe TBI defined as Glasgow Coma Scale (GCS) score ≤8 on admission to the intensive care unit (ICU), Head Abbreviated Injury Scale (AIS) score ≥3, and TBI ICD-9 codes (800–801.9, 803–804.9, 850–854.1, or 959.01), and (3) patients who received vasopressors (phenylephrine, norepinephrine, dopamine, epinephrine, or vasopressin) for at least 3 h after TBI in ICU for the explicit purpose of increasing blood pressure. Patients with polytrauma and who received multiple vasopressors were included. During the study period, there was no documented institutional protocol for choice of vasopressor or level of MAP when the second vasopressor should be added. In general, failure to meet clinically relevant blood pressure goals despite one vasopressor resulted in the addition of a second vasopressor.
Data sources were the HMC Trauma Registry, billing data, and electronic medical records. First, a list of eligible patients was generated using the HMC Trauma registry. Then, HMC billing data were used to identify a subset of HMC trauma registry patients who were charged for intravenous vasopressor therapy after ICU admission.
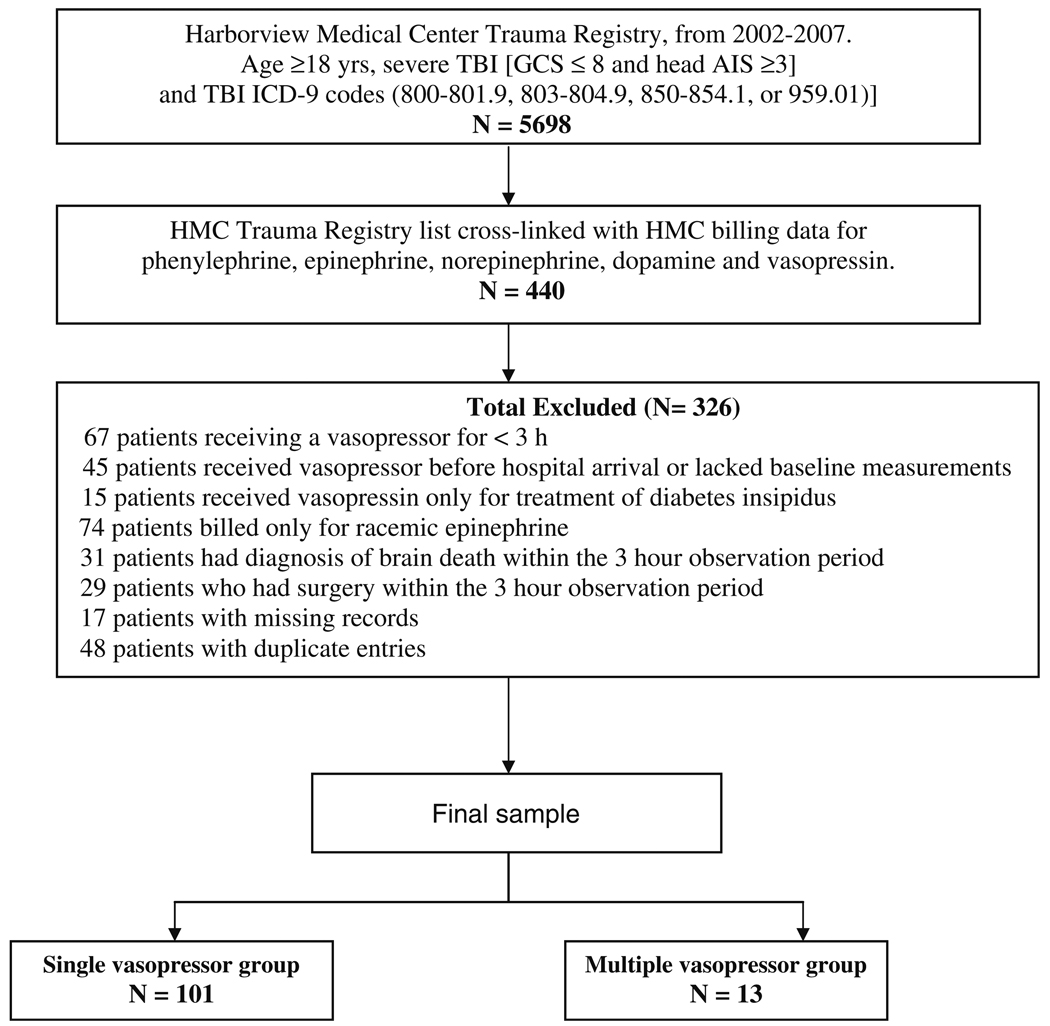
Exclusion criteria were vasopressor therapy for less than 3 h, vasopressor therapy before hospital arrival or lack of baseline MAP (prior to vasopressor start), vasopressin therapy only for treatment of diabetes insipidus, diagnosis of brain death, and surgery within 3 h of starting vasopressor therapy. Additionally, patients who had duplicate entries or missing records were also excluded. Figure 1 details the derivation of the final sample.
Fig. 1.
Study design and derivation of final sample
Standard neurointensive care: During the study period, patients were resuscitated according to institutional practice, which is consistent with the current 2007 Brain Trauma Foundation Guidelines [10]. Relevant to this study, practice includes ICP monitoring via camino, or ventriculostomy, maintaining intracranial pressure (ICP) <20 mmHg, CPP 50–70 mmHg, PaCO2 35–40 mmHg, SaO2 >90% and maintaining core body temperature between 35 and 37.5°C with antipyretics, cooling/warming blankets, or intravascular cooling devices if needed. During the study period, measures of cerebral oxygenation, including brain tissue oxygenation and jugular venous oximetry (PbtO2 and SjVO2, respectively) were patient specific and there was no standard treatment practice pertaining to vasopressor choice.
Data Abstracted
The following patient level characteristics were abstracted: baseline data were demographic data (age, gender, weight), Glasgow Coma Scale (GCS) score on ICU admission, presence of polytrauma, Injury severity score (ISS), Head AIS (Head Abbreviated Injury Score), and in-hospital mortality. Relevant physiologic data (heart rate (HR), MAP, ICP, CPP, PbtO2, and SjVO2), fluid balance, blood products received, and concurrent medications (propofol, hypertonic saline, mannitol, furosemide, pentobarbital, and vasopressin for treatment of diabetes insipidus during 3 h before and 3 h after vasopressor initiation were recorded.
Blood pressure was measured by non-invasive (cuff) and/or invasive measurements. When both non-invasive and invasive blood pressure measurements were recorded, we used the invasive measurements. We abstracted hourly MAP, ICP, and CPP for the 3 h period both preceding and following vasopressor therapy started.
Bradycardia was defined as HR <60 beats per minute (bpm) and tachycardia was defined as HR >100 bpm.
Outcomes
While the current guidelines recommend ICP monitoring for patients with severe TBI, ICP monitoring may not occur in all patients with severe TBI because of patient level factors such as coagulopathy, or hemodynamic instability, and/or organizational level factors such as institutional decision not to use ICP monitoring to guide blood pressure management [12], and in this case, MAP is used to estimate CPP and provide cerebral perfusion. Therefore, main outcome measures were intentionally broad and were: (1) initial vasopressor use, and (2) MAP, ICP, and CPP during 3 h after initiation of a single vasopressor. Secondarily, we also examined changes in measures of cerebral oxygenation when these data were available and the association between vasopressor use and bradycardia as well as vasopressor use and patient level outcomes such as length of stay in ICU and hospital and in-hospital mortality.
Statistical Analysis
All statistical analyses were performed by statistical software (SPSS 16.0, Chicago, Illinois). Descriptive statistics were used to detail the study cohort, patients who received single versus multiple vasopressors, and patients by vasopressor category (phenylephrine, norepinephrine, dopamine, and vasopressin). For univariate analyses, data are presented as median and standard error of the mean (SEM) for non-parametric distribution, and as percentage for binary variables.
To compare patient level characteristics of the single vasopressor vs. the multiple vasopressor groups, and one vasopressor vs. the others (phenylephrine vs. norepinephrine, phenylephrine vs. dopamine, and norepinephrine vs. dopamine), the Mann–Whitney U test was used for continuous variables, and Chi-square test or Fischer’s exact test was used for binary variables.
Mean arterial blood pressure, ICP, and CPP before and after initiation of vasopressor therapy were examined in those patients who received single vasopressor treatment using the Wilcoxon signed-rank test. To determine the baseline MAP for each patient, we first compared the median hemodynamic values to the lowest values during the 3 h preceding start of vasopressor therapy. We used median values to describe our data since our sample size was small and because these data had a non-parametric distribution. Since there was no difference between the median and lowest values, we chose to define baseline MAP, ICP, and CPP as lowest recorded MAP in the 1 h period prior to initiation of a vasopressor. We then examined change in baseline (prior to vasopressor start) and median MAP, ICP, CPP, and HR during the 3 h after start of vasopressor therapy. The Kruskal–Wallis test was used to examine differences among vasopressor groups in median MAP, ICP, CPP, and HR within 3 h of starting a vasopressor. For MAP data that were not recorded at the 3 h mark after vasopressor start, MAP data closest to and within 30 min of the 3 h mark was recorded (i.e., range 2.5–3.5 h after vasopressor start). When ICP data were available during the period of vasopressor therapy as defined above, baseline and 3 h ICP and CPP data were similarly recorded.
Multivariate linear regression analysis was used to examine the effect of vasopressor on MAP, CPP, and ICP, in patients not requiring addition of a second vasopressor during the first 3 h after treatment initiation. The independent variable was drug category, which was treated as a dummy variable. The reference drug group was norepinephrine when comparing differences between norepinephrine vs. phenylephrine and norepinephrine vs. dopamine, and the reference group was dopamine when comparing dopamine vs. phenylephrine and dopamine vs. norepinephrine. The reference drug group was norepinephrine or dopamine as these were the second and third most common vasopressors used in our population. The dependent variable was the median of MAP within 3 h of infusion, and we adjusted for the baseline MAP. In addition, we adjusted for patient age, gender, ISS, vasopressor dose, fluid administration, propofol, and hypertonic saline infusion during 3 h of observation, if they were significant confounders.
The coefficients with 95% confidence interval (CI) from the regression model were interpreted as the difference in median of MAP during 3 h of the vasopressor as compared to the reference category (norepinephrine or dopamine). Significance was set as P < 0.05.
Results
Final Sample
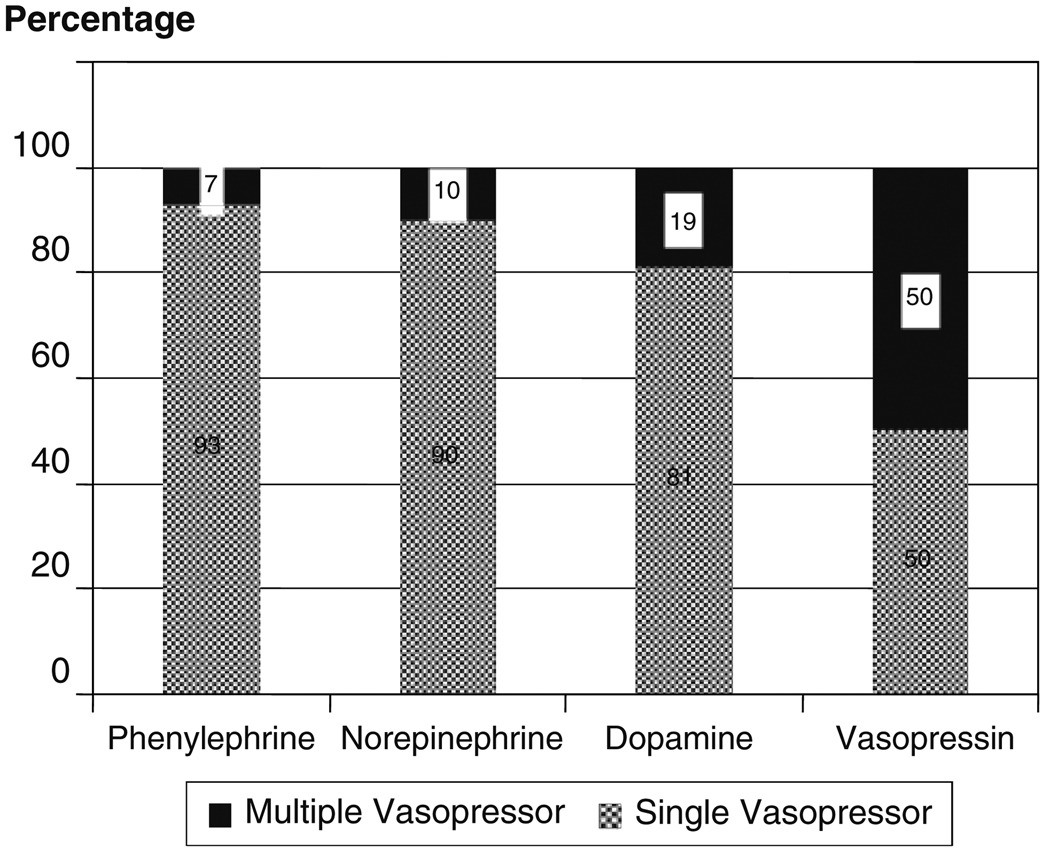
Of the 114 patients who met study inclusion criteria (Fig. 1), the majority (n = 101) received a single vasopressor and 13 patients received multidrug therapy. The most common initial drug used was phenylephrine (43%; Fig. 2). The proportion of patients with each vasopressor agent who required a second vasopressor are shown in Fig. 3. The majority (79%) of patients in the single vasopressor group received vasopressors within the first 3 days after hospital admission, 8% received vasopressors between days 4 and 7, and 13%, received vasopressors more than 7 days after hospital admission. One hundred and one patients contributed MAP data and 56 patients contributed CPP data.
Fig. 2.
Initial vasopressor used to augment blood pressure patients with severe traumatic brain injury (n = 114)
Fig. 3.
Proportion of patients with second vasopressor added by initial vasopressor category (n = 114)
Baseline Clinical Differences Between Patients Who Received Single Vs. Multiple Vasopressors
There were no differences between single vasopressor and the multiple vasopressor groups with regards to age, gender, weight, GCS at ICU admission, head AIS, and polytrauma (Table 1). During the 3 h before and 3 h after vasopressor treatment, the proportion of patients who received blood products and the number of units of blood transfusion were higher in the multiple vasopressor group than single vasopressor group; (70 vs. 21%, P = 0.001, and 3 + 0.1 vs. 0 + 0.4 units, P = 0.001), respectively. Heart rate was higher in the multiple vasopressor group (120 + 8 vs. 93 + 2 bpm, P = 0.001). Both groups had an equivalent (8 ± 2 ml/kg) positive fluid balance during the evaluation period.
Table 1.
Descriptive statistics of all patients (single and multiple vasopressor; n = 114) data as median ± SEM and percentage
| Single vasopressor (n = 101) |
Second vasopressor (n = 13) |
P | |
|---|---|---|---|
| Baseline characteristics | |||
| Age (years) | 40 ± 2 | 26 ± 4 | 0.29 |
| Male gender | 78% | 77% | 1 |
| Weight (kg) | 77 ± 2 | 83 ± 6 | 0.79 |
| ICU GCS | 3 ± 0 | 3 ± 0 | 1 |
| ISS | 35 ± 1 | 43 ± 5 | 0.14 |
| Head AIS | 5 ± 0 | 5 ± 0 | 0.17 |
| Polytrauma | 70% | 61% | 0.54 |
| Fluid balance and blood products requirement during 3 h before and 3 h after VP started | |||
| Fluid balance (ml/kg) | 8 ± 2 | 8.5 ± 2 | 0.84 |
| % Blood products requirement | 21% | 70% | 0.001 |
| Number of RBC units transfused | 0 ± 0 | 3 ± 0 | 0.001 |
| Physiologic data | |||
| HR at 3 h, bpm (n = 112) | 93 ± 2 (N = 99) | 120 ± 8 (N = 13) | 0.001 |
| MAP at 3 h, mmHg (n = 114) | 81 ± 1 (N = 101) | 78 ± 5 (N = 13) | 0.18 |
| ICP at 3 h, mmHg (n = 61) | 17 ± 2 (N = 58) | 16 ± 7 (N = 3) | – |
| CPP at 3 h, mmHg (n = 59) | 66 ± 2 (N = 56) | 48 ± 20 (N = 3) | – |
| PbtO2 at 3 h, mmHg (n = 6) | 20 ± 3 (N = 5) | 11 (N = 1) | – |
| In-hospital mortality | 55% | 85% | 0.07 |
ICU GCS GCS score at the intensive care unit admission, ISS Injury severity score, Head AIS Head Abbreviated Injury Scale Score, HR heart rate (bpm), MAP mean arterial pressure (mmHg), ICP intracranial pressure (mmHg), CPP cerebral perfusion pressure (mmHg), PbtO2 brain tissue oxygenation (mmHg)
Baseline Clinical Differences Between Vasopressor Groups
One hundred and one patients received single vasopressor treatment (Table 2). Since there was small number of patients who received vasopressin, we did not formally test for differences between vasopressin and the other groups. There were no differences in age, weight, ICU GCS, head AIS, and polytrauma between the phenylephrine, norepinephrine, and dopamine groups (Table 2). Although there was no difference in ISS between phenylephrine vs. norepinephrine, or between phenylephrine vs. dopamine groups, ISS was higher in the norepinephrine vs. the dopamine group (41 + 2 vs. 30 + 3, P = 0.04).
Table 2.
Clinical characteristics by initial vasopressor in patients who received single vasopressor (n = 101)
| PHE (n = 46) | NE (n = 31) | DA (n = 21) | AVP (n = 3) | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age (years) | 35 ± 2 | 42 ± 3 | 48 ± 4 | 34 ± 9 |
| Male (%) | 80 | 80 | 76 | 33 |
| Weight (kg) | 77 ± 2 | 78 ± 4 | 77 ± 4 | 63 ± 6 |
| ICU admission GCS | 3 ± 0 | 3 ± 0 | 3 ± 0 | 3 ± 0 |
| ISS | 35 ± 2 | 41 ± 2** | 29.5 ± 3 | 38 ± 3 |
| Head AIS | 5 ± 0 | 5 ± 0 | 5 ± 0 | 5 ± 0 |
| Polytrauma (%) | 70 | 74 | 57 | 100 |
| Baseline CPP < 50 mmHg [n = 56; PHE (34), NE (13), DA (9)] | 35% (n = 12) | 38% (n = 5) | 44% (n = 4) | – |
| Fluid balance and blood products requirement during 3 h before and 3 h after vasopressor started | ||||
| Fluid administered (ml/kg) | 12 ± 4 | 17 ± 4 | 4.7 ± 12 | −0.5 ± 15 |
| % Patients who received blood products (%) | 17 | 22 | 28 | – |
| Number of RBC unit transfused | 0 ± 0 | 0 ± 0 | 0 ± 1 | – |
| Dose during 3 h | 0.7 ± 0.1 (0.14–2.8) mcg/kg/min |
0.1 + 0.04 (0.02 + 1.2) mcg/kg/min |
4.5 ± 3 (0.14–75) mcg/kg/min |
0.04 ± 0.01 (0.01–0.04) unit/min |
| Concurrent medications during 3 h before and 3 h after vasopressor started | ||||
| Propofol (%) | 67 | 42* | 38 | 33 |
| Hypertonic saline (%) | 30 | 32 | 14 | 0 |
| Mannitol (%) | 27 | 3* | 23 | 0 |
| Lasix (%) | 0 | 3 | 0 | 0 |
| Pentobarbital (%) | 0 | 0 | 0 | 0 |
| Patient outcomes | ||||
| ICU length of stay | 10 ± 1 | 4 ± 2* | 8 ± 3 | 1 ± 5 |
| Hospital length of stay | 17 ± 2 | 5 ± 3* | 8 ± 4 | 1 ± 21 |
| Discharge GCS | 14 ± 0 (n = 26) | 15 ± 1 (n = 10) | 14 ± 0 (n = 8) | 15 (n = 1) |
| In-hospital mortality (%) | 43 | 67* | 62 | 67 |
Data as median ± SEM (range) and percentage
PHE phenylephrine, NE norepinephrine, DA dopamine, AVP vasopressin, GCS at ICU Glasgow Coma Scale score at the intensive care unit, ISS Injury severity score, Head AIS Head Abbreviated Injury Scale Score
P < 0.05; phenylephrine vs. norepinephrine
P < 0.05; norepinephrine vs. dopamine
Difference in MAP, ICP, and CPP Between Vasopressor Groups
There was no difference in baseline MAP, ICP or CPP among the phenylephrine, norepinephrine, and dopamine groups. Baseline CPP was less than 50 mmHg in 21 patients; 12 (57%) received phenylephrine, 5 (24%) received norepinephrine, and 4 (19%) received dopamine. Despite this, average baseline CPP exceeded 50 mmHg for all groups. There was no statistically significant difference in the incidence of CPP less than 50 mmHg between the vasopressor groups (phenylephrine vs. norepinephrine, P = 1.0; phenylephrine vs. dopamine, P = 0.7; norepinephrine vs. dopamine, P = 1.0). Unadjusted, only the phenylephrine group had a higher MAP (86 + 2 vs. 74 + 3 mmHg; P = 0.001) and CPP (67 ± 3 vs. 57 ± 4 mmHg; P < 0.001) during 3 h after vasopressor start than baseline. During the 3 h after treatment, patients in the phenylephrine and dopamine groups had higher MAP (86 + 2 and 81 + 3 vs. 78 + 2 mmHg; P = 0.01) and higher CPP (67 + 3 and 64 + 5 vs. 53 + 4 mmHg; P = 0.03) than patients in the norepinephrine group (Table 3).
Table 3.
Descriptive statistics of hemodynamics 3 h before (baseline) and during 3 h after vasopressor start by initial vasopressor and percent change in patients who received single vasopressor (n = 101)
| Worst hemodynamics 1 h prior to vasopressor start |
Hemodynamics during 3 h after vasopressor start |
Pa | Hemodynamics at 3 h after vasopressor start |
Pb | |
|---|---|---|---|---|---|
| HR (bpm; n = 99) | |||||
| PHE (n = 45) | 86 ± 3 | 80 ± 2.4 | 0.005** | 79 ± 3 | 0.002*** |
| NE (n = 31) | 105 ± 3 | 101 ± 4 | 0.07 | 100 ± 4 | 0.09 |
| DA (n = 21) | 92 ± 7 | 100 ± 5 | 0.1 | 101 ± 5 | 0.2 |
| AVP (n = 2) | 96 ± 10 | 88 ± 1 | – | 118 ± 18 | – |
| P | 0.003* | <0.001* | <0.001* | ||
| MAP (mmHg; n = 101) | |||||
| PHE (n = 46) | 74 ± 3 | 86 ± 2 | 0.001** | 83 ± 2 | 0.009*** |
| NE (n = 31) | 73 ± 2 | 78 ± 2 | 0.07 | 78 ± 2 | 0.06 |
| DA (n = 21) | 75 ± 4 | 81 ± 3 | 0.33 | 82 ± 4 | 0.34 |
| AVP (n = 3) | 87 ± 12 | 89 ± 10 | – | 95 ± 7 | – |
| P | 0.47 | 0.01* | 0.07 | ||
| ICP (mmHg; n = 58) | |||||
| PHE (n = 36) | 18 ± 2 | 16 ± 3 | 0.86 | 17 ± 3 | 0.63 |
| NE (n = 13) | 25 ± 4 | 28 ± 4 | 0.64 | 23 ± 5 | 0.94 |
| DA (n = 9) | 17 ± 6 | 17 ± 8 | 0.53 | 18 ± 8 | 0.53 |
| P | 0.08 | 0.35 | 0.26 | ||
| CPP (mmHg; n = 56) | |||||
| PHE (n = 34) | 56 ± 4 | 67 ± 3 | <0.001** | 68 ± 3 | <0.001*** |
| NE (n = 13) | 53 ± 5 | 53 ± 4 | 0.15 | 58 ± 5 | 0.44 |
| DA (n = 9) | 57 ± 7 | 64 ± 5 | 0.67 | 66 ± 5 | 0.41 |
| P | 0.58 | 0.03* | 0.04* | ||
| PbtO2 (mmHg; n = 5) | |||||
| PHE (n = 4) | 10.5 ± 5 | 22 ± 3 | – | 20 ± 4 | – |
| NE (n = 1) | 23 | 26 | – | 27 | – |
| P | – | – | |||
Data present as median ± SEM
PHE phenylephrine, NE norepinephrine, DA dopamine, AVP vasopressin, GCS at ICU Glasgow Coma Scale score at the intensive care unit, ISS injury severity score, Head AIS Head Abbreviated Injury Scale Score, HR heart rate (bpm), MAP mean arterial pressure (mmHg), ICP intracranial pressure (mmHg), CPP cerebral perfusion pressure (mmHg), PbtO2 brain tissue oxygenation (mmHg). There was no difference in baseline hemodynamics whether defined as median or lowest before start of vasopressor therapy
P < 0.05; phenylephrine vs. norepinephrine vs. dopamine
P < 0.05; baseline vs. during 3 h
P < 0.05; baseline vs. 3 h
Baseline vs. median values during 3 h vs. vasopressor start
Baseline vs. values at 3 h mark vs. vasopressor start
Adjusted for confounders, including baseline values, the phenylephrine group had 8 mm Hg higher MAP than dopamine (p = 0.03) and the phenylephrine group tended to have a higher MAP than norepinephrine (5 mmHg; P = 0.06) during the 3 h after vasopressor start. In the subgroup of 56 patients who had CPP data, patients who received phenylephrine had a 12 mmHg higher median CPP compared to patients who received norepinephrine (P = 0.02).
There was no difference in ICP between patients who received phenylephrine and norepinephrine (P = 0.5), between phenylephrine and dopamine (P = 0.25) or between dopamine and norepinephrine (P = 0.15) during the 3 h after vasopressor start.
Difference in Adverse Effects Between Vasopressor Groups
Heart rate at baseline and during 3 h after vasopressor start was lowest in the phenylephrine group (Table 3). The incidence of bradycardia was also highest in patients who received phenylephrine (phenylephrine 18%, vs. dopamine 5%, vs. norepinephrine 0%; P = 0.04). The incidence of tachycardia was highest in the dopamine, followed by norepinephrine and phenylephrine groups (57 vs. 51 vs. 18%; P = 0.001).
Patient Outcomes
Overall mortality was 55%. Crude in-hospital mortality was lower in phenylephrine compared to norepinephrine group (43 vs. 67%, P = 0.04) and ICU and hospital lengths of stay was longer in phenylephrine compared to norepinephrine group (10 + 1 vs. 4 + 2 days, P = 0.02; 17 + vs. 5 + 3 days, P = 0.03). There was no difference discharge GCS between the vasopressor groups (Table 2).
Vasopressor Dosage (Table 2)
During 3 h after vasopressor start, median phenylephrine dose was 0.7 + 0.1 (range 0.14–2.8) mcg/kg/min, norepinephrine dose was 0.1 + 0.04 (range 0.02–1.18) mcg/kg/min, dopamine dose was 4.5 + 3 (range 0.14–75) mcg/kg/min, and vasopressin dose was 0.04 + 0.01 (range 0.01–0.04) units/min.
Discussion
The main findings of this study are that among patients requiring vasopressors after severe TBI, (1) most received single vasopressor therapy to augment MAP or CPP, (2) while the most common initial vasopressor used was phenylephrine, there was significant variability in vasopressor choice, and (3) patients who received phenylephrine had the greatest increase in MAP and CPP from baseline during the 3 h after vasopressor start regardless of the dose. We also observed that there was no difference in ICP between the vasopressor groups after initiating vasopressor treatment. These data add to previous work examining the effect of vasopressors on cerebral hemodynamics by providing new information on the patterns of clinical use and effectiveness of commonly used vasopressors, including phenylephrine, for blood pressure augmentation after severe adult TBI.
Pharmacologic elevation of blood pressure is frequently utilized in patients with severe TBI to prevent or treat cerebral ischemia by either treating hypotension or augmenting blood pressure to achieve the Brain Trauma Foundation goals of CPP between 50 and 70 mmHg in TBI. However, there are no guidelines addressing how this should best be achieved, nor are there data on prevalent clinical practices regarding vasopressor use, especially comparisons of phenylephrine with other vasopressors. In this sample, most patients required a single vasopressor to augment blood pressure, and possessed similar clinical characteristics to patients requiring multiple vasopressors, except for a higher heart rate and blood product requirement in the multiple vasopressor group. Although there was a comparable proportion of patients in both single and multiple vasopressor groups with polytrauma, it seems likely that, on the basis of the higher ISS and the greater heart rate and blood product requirement, there was more extracranial injury, with consequently increased blood loss, transfusion and requirement of multiple vasopressors in the multiple vasopressor group. In this series, the most common initial choice of vasopressor was phenylephrine. One explanation for this local preference may be a desirable effect observed at the bedside with phenylephrine. However, despite previous reports of variable effects on ICP, both norepinephrine and dopamine were used frequently, showing that there is still clinical equipoise in vasopressor selection for blood pressure augmentation in patient with severe TBI.
The overall effects of vasopressors on the brain are probably a consequence of their systemic and cerebral effects but are also influenced by other factors such as injury to the brain, integrity of blood brain barrier and status of cerebral autoregulation [13–16]. While phenylephrine is a selective α1-adrenergic agonist, norepinephrine has mixed effects on α and β-adrenergic receptors and causes potent vasoconstriction to increase venous return and improved cardiac preload. Dopamine directly stimulates dopaminergic and β-adrenergic receptor at low to moderate doses and α-adrenergic receptors at higher doses. In the intracerebral vessels, although alpha receptor densities are low, α1 and α2-adreneric receptors mediate vasoconstriction while β1 and β2 receptors mediate vasodilatation [17].
Patients who received phenylephrine in this series had significantly higher MAP and CPP during the 3 h after initiation of infusion compared to baseline than patients who received either norepinephrine or dopamine. We decided to use the 3 h time period after vasopressor start to examine MAP, ICP, and CPP data because desirable hemodynamic targets are typically expected to have been achieved by this time and to examine the effect of vasopressors on sustained changes in systemic and cerebral hemodynamics. Although increase in MAP with both norepinephrine and dopamine increases cerebral perfusion, studies have suggested that the effect may be more sustained with norepinephrine, possibly due to a metabolically driven increase in cerebral perfusion with norepinephrine [18, 19]. Results of this study, however, suggest that phenylephrine may be more desirable when considering MAP and CPP as target endpoints during the first 3 h after initiation of vasopressor treatment.
Whether improved MAP/CPP with vasopressor use translated into improved cerebral blood flow or oxygenation is unclear. In a randomized, cross-over study of head injured patients, augmentation of CPP using norepinephrine resulted in more predictable and consistent increases in transcranial Doppler cerebral blood flow velocity when compared to dopamine [10]. Such data for phenylephrine are currently not available. Norepinephrine, but not dopamine, has been shown to consistently and predictably increase global and regional cerebral oxygenation and decrease the regional oxygen extraction fraction and ischemic brain volume in TBI [11]. One animal study evaluating the effect of phenylephrine has shown improvement in CPP and cerebral oxygenation after TBI [20]. We do not have transcranial Doppler or cerebral metabolism data and hence, cannot comment on global cerebral blood flow or metabolic changes. Brain tissue oxygenation (PbtO2) data were available for only five patients in this study, thereby limiting our examination of the effect of vasopressors on tissue oxygenation. However, in four of these patients who received phenylephrine, PbtO2 doubled during the 3 h after start of infusion. Only one patient was monitored using jugular venous oximetry (SjVO2) before vasopressors started, but no SjVO2 data available at 3 h after start of infusion.
Our results indicate higher in-hospital mortality in patients who received norepinephrine compared to those who received phenylephrine and while we adjusted for injury severity, ISS in patients who received norepinephrine was higher than those who received dopamine, and CPP after start of vasopressor treatment was lower in the norepinephrine group compared to the other two groups, suggesting that patients who received norepinephrine were likely more critically ill. Moreover, the higher mortality in norepinephrine group may explain the shorter length of ICU and hospital stay compared to the phenylephrine group. We do not have GOS score or long term outcome data, as this information was not available in the medical records. Consequently, we cannot comment on the effects of vasopressor choice on patient level outcomes and this merits further investigation.
Vasopressor use is associated with adverse effects, such as increased ICP and bradycardia. Using vasopressors to elevate CPP in an injured brain may increase ICP [21–23] and cause secondary brain edema [24, 25], and or result in unwanted hemodynamic effects. In the present study, neither administration of phenylephrine, norepinephrine nor dopamine increased ICP compared with baseline, consistent with previously published work [26, 27]. However, as expected, patients who received phenylephrine had significantly lower heart rate after vasopressor start compared to baseline, as well as a higher incidence of bradycardia. Again as expected, the norepinephrine and dopamine groups had more tachycardia than the phenylephrine cohort. The lower incidence of tachycardia in the phenylephrine group compared to norepinephrine and dopamine groups suggests that patients with ischemic heart disease might benefit from phenylephrine. No arrhythmias were recorded for any patients but this may be a limitation of a retrospective study.
Induced hypertension in treatment of TBI has also been associated with adult respiratory distress syndrome and lung edema [28], but we do not have those data.
Our study has some limitations. First, this was a retrospective study. Second, we may not have been able to adjust for injury severity differences between the vasopressor groups. Third, reasons for vasopressor choices were not known. Finally, this is a single center study and our results may not be generalizable to all centers.
In summary, while we observed considerable variability in vasopressor use after severe adult TBI, phenylephrine was the preferred vasopressor and these patients had the greatest increase in MAP and CPP after start of infusion, compared to patients who received norepinephrine or dopamine. Prospective comparison of phenylephrine with other vasopressors is required to further understand the benefits of one vasopressor over another in severe TBI.
Acknowledgment
Funding This work was funded by Departmental Sources.
Contributor Information
Pimwan Sookplung, Department of Anesthesiology and Pain Medicine, University of Washington, Seattle, WA, USA.
Arunotai Siriussawakul, Department of Anesthesiology and Pain Medicine, University of Washington, Seattle, WA, USA.
Amin Malakouti, Department of Anesthesiology and Pain Medicine, University of Washington, Seattle, WA, USA.
Deepak Sharma, Department of Anesthesiology and Pain Medicine, University of Washington, Seattle, WA, USA.
Jin Wang, Pediatrics and Neurological Surgery, University of Washington, Seattle, WA, USA.
Michael J. Souter, Department of Anesthesiology and Pain Medicine, University of Washington, Seattle, WA, USA
Randall M. Chesnut, Pediatrics and Neurological Surgery, University of Washington, Seattle, WA, USA
Monica S. Vavilala, Department of Anesthesiology and Pain Medicine, University of Washington, Seattle, WA, USA Harborview Injury Prevention and Research Center, Seattle, WA, USA; Department of Anesthesiology and Pediatrics, Harborview Medical Center, 325 Ninth Avenue, Box 359724, Seattle, WA 98104, USA, vavilala@u.washington.edu.
References
- 1.Selassie AW, Zaloshnja E, Langlois JA, et al. Incidence of long-term disability following traumatic brain injury hospitalization, United States, 2003. J Head Trauma Rehabil. 2008;23(2):123–131. doi: 10.1097/01.HTR.0000314531.30401.39. [DOI] [PubMed] [Google Scholar]
- 2.Rutland-Brown W, Langlois JA, Thomas KE, et al. Incidence of traumatic brain injury in the United States, 2003. J Head Trauma Rehabil. 2006;21(6):544–548. doi: 10.1097/00001199-200611000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Vavilala MS, Lee LA, Lam AM. Cerebral blood flow and vascular physiology. Anesthesiol Clin North America. 2002;20(2):247–264. doi: 10.1016/s0889-8537(01)00012-8. [DOI] [PubMed] [Google Scholar]
- 4.Jünger EC, Newell DW, Grant GA, et al. Cerebral autoregulation following minor head injury. J Neurosurg. 1997;86(3):425–432. doi: 10.3171/jns.1997.86.3.0425. [DOI] [PubMed] [Google Scholar]
- 5.Sahuquillo J, Munar F, Baguena M, et al. Evaluation of cerebrovascular CO2-reactivity and autoregulation in patients with post-traumatic diffuse brain swelling (diffuse injury III) Acta Neurochir Suppl. 1998;71:233–236. doi: 10.1007/978-3-7091-6475-4_67. [DOI] [PubMed] [Google Scholar]
- 6.Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons, Joint Section on Neurotrauma and Critical Care, AANS/CNS. Bratton SL, Chestnut RM, Ghajar J, et al. Guidelines for the management of severe traumatic brain injury. I. Blood pressure and oxygenation. J Neurotrauma. 2007;24:S7–S13. doi: 10.1089/neu.2007.9995. [DOI] [PubMed] [Google Scholar]
- 7.Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons, Joint Section on Neurotrauma and Critical Care, AANS/CNS. Bratton SL, Chestnut RM, Ghajar J, et al. Guidelines for the management of severe traumatic brain injury. IX. Cerebral perfusion thresholds. J Neurotrauma. 2007;24:S59–S64. doi: 10.1089/neu.2007.9987. [DOI] [PubMed] [Google Scholar]
- 8.Bouma GJ, Muizelaar JP. Relationship between cardiac output and cerebral blood flow in patients with intact and with impaired autoregulation. J Neurosurg. 1990;73(3):368–374. doi: 10.3171/jns.1990.73.3.0368. [DOI] [PubMed] [Google Scholar]
- 9.Ract C, Vigué B. Comparison of the cerebral effects of dopamine and norepinephrine in severely head-injured patients. Intensive Care Med. 2001;27(1):101–106. doi: 10.1007/s001340000754. [DOI] [PubMed] [Google Scholar]
- 10.Steiner LA, Johnston AJ, Czosnyka M, et al. Direct comparison of cerebrovascular effects of norepinephrine and dopamine in head-injured patients. Crit Care Med. 2004;32(4):1049–1054. doi: 10.1097/01.ccm.0000120054.32845.a6. [DOI] [PubMed] [Google Scholar]
- 11.Johnston AJ, Steiner LA, Chatfield DA, et al. Effect of cerebral perfusion pressure augmentation with dopamine and norepinephrine on global and focal brain oxygenation after traumatic brain injury. Intensive Care Med. 2004;30(5):791–797. doi: 10.1007/s00134-003-2155-7. [DOI] [PubMed] [Google Scholar]
- 12.Bulger EM, Nathens AB, Rivara FP, et al. Management of severe head injury: institutional variations in care and effect on outcome. Crit Care Med. 2002;30:1870–1876. doi: 10.1097/00003246-200208000-00033. [DOI] [PubMed] [Google Scholar]
- 13.Drummond JC, Shapiro HM. Cerebral physiology. In: Miller RD, editor. Anesthesia. Edinburgh: Churchill Livingstone; 1994. pp. 689–730. [Google Scholar]
- 14.Rosner MJ, Rosner SD, Johnson AH, et al. Cerebral perfusion pressure: management protocol and clinical results. J Neurosurg. 1995;83(6):949–962. doi: 10.3171/jns.1995.83.6.0949. [DOI] [PubMed] [Google Scholar]
- 15.Bevan JA, Duckworth J, Laher I, et al. Sympathetic control of cerebral arteries: specialization in receptor type, reserve, affinity, and distribution. FASEB J. 1987;1(3):193–198. doi: 10.1096/fasebj.1.3.2887477. [DOI] [PubMed] [Google Scholar]
- 16.Starkey K, Docherty J. Alpha 1 and alpha 2 adrenoreceptors: pharmacology and clinical implications. J Cardiovasc Pharmacol. 1981;3 Suppl 1:514–516. [PubMed] [Google Scholar]
- 17.Guimarães S, Moura D. Vascular adrenoceptors: an update. Pharmacol Rev. 2001;53(2):319–356. [PubMed] [Google Scholar]
- 18.Kroppenstedt SN, Sakowitz OW, Thomale UW, et al. Norepinephrine is superior to dopamine in increasing cortical perfusion following controlled cortical impact injury in rats. Acta Neurochir Suppl. 2002;81:225–227. doi: 10.1007/978-3-7091-6738-0_58. [DOI] [PubMed] [Google Scholar]
- 19.Kroppenstedt SN, Sakowitz OW, Thomale UW, et al. Influence of norepinephrine and dopamine on cortical perfusion, EEG activity, extracellular glutamate, and brain edema in rats after controlled cortical impact injury. J Neurotrauma. 2002;19(11):1421–1432. doi: 10.1089/089771502320914651. [DOI] [PubMed] [Google Scholar]
- 20.Dudkiewicz M, Proctor KG. Tissue oxygenation during management of cerebral perfusion pressure with phenylephrine or vasopressin. Crit Care Med. 2008;36(9):2641–2650. doi: 10.1097/CCM.0b013e3181847af3. [DOI] [PubMed] [Google Scholar]
- 21.Stubbe HD, Greiner C, Westphal M, et al. Cerebral response to norepinephrine compared with fluid resuscitation in ovine traumatic brain injury and systemic inflammation. Crit Care Med. 2006;34(10):2651–2657. doi: 10.1097/01.CCM.0000239196.17999.B7. [DOI] [PubMed] [Google Scholar]
- 22.Cherian L, Chacko G, Goodman JC, et al. Cerebral hemodynamic effects of phenylephrine and L-arginine after cortical impact injury. Crit Care Med. 1999;27(11):2512–2517. doi: 10.1097/00003246-199911000-00031. [DOI] [PubMed] [Google Scholar]
- 23.Ract C, Vigué B, Bodjarian N, et al. Comparison of dopamine and norepinephrine after traumatic brain injury and hypoxichypotensive insult. J Neurotrauma. 2001;18:1247–1254. doi: 10.1089/089771501317095287. [DOI] [PubMed] [Google Scholar]
- 24.Malhotra AK, Schweitzer JB, Fox JL, et al. Cerebral perfusion pressure directed therapy following traumatic brain injury and hypotension in swine. J Neurotrauma. 2003;20(9):827–839. doi: 10.1089/089771503322385764. [DOI] [PubMed] [Google Scholar]
- 25.Beaumont A, Hayasaki K, Marmarou A, et al. Contrasting effects of dopamine therapy in experimental brain injury. J Neurotrauma. 2001;18(12):1359–1372. doi: 10.1089/08977150152725650. [DOI] [PubMed] [Google Scholar]
- 26.Feinstein AJ, Patel MB, Sanui M, et al. Resuscitation with pressors after traumatic brain injury. J Am Coll Surg. 2005;201(4):536–545. doi: 10.1016/j.jamcollsurg.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 27.Kroppenstedt SN, Thomale UW, Griebenow M, et al. Effects of early and late intravenous norepinephrine infusion on cerebral perfusion, microcirculation, brain-tissue oxygenation, and edema formation in brain-injured rats. Crit Care Med. 2003;31(8):2211–2221. doi: 10.1097/01.CCM.0000080482.06856.62. [DOI] [PubMed] [Google Scholar]
- 28.Robertson CS, Valadka AB, Hannay HJ, et al. Prevention of secondary ischemic insults after severe head injury. Crit Care Med. 1999;27(10):2086–2095. doi: 10.1097/00003246-199910000-00002. [DOI] [PubMed] [Google Scholar]