Abstract
Introduction and hypothesis
To investigate the urethral compensatory mechanisms to maintain urinary continence after pudendal nerve injury.
Methods
In naive, acute pudendal nerve transection (PNT) and 4 weeks after PNT (PNT-4w) female rats, leak point pressures (LPPs) during bladder compression were measured before and after the application of hexamethonium (C6), propranolol and Nωnitro-L-arginine methyl ester (L-NAME), or terazosin and atropine. Responses to carbachol and phenylephrine of proximal and middle urethral muscle strips from naive and PNT-4w rats were also examined.
Results
LPPs were significantly decreased in PNT rats but not in PNT-4w rats. LPPs in PNT rats were significantly increased by C6 or L-NAME while LPPs in PNT-4w rats were significantly decreased by C6, or terazosin and atropine. Excitatory responses to carbachol and phenylephrine in the proximal urethral muscle were significantly larger in PNT-4w rats.
Conclusions
These results suggest that α1-adrenoceptor and muscarinic receptor-mediated contractility is upregulated in the proximal urethra 4 weeks after PNT.
Keywords: urinary incontinence, compensation, pudendal nerve injury
Introduction
Over 200 million people worldwide have urinary incontinence [1]. It has been reported that the prevalence of urinary incontinence in women increases during young adult life (20% to 30%), reaches a broad peak around middle age (30% to 40%), and then steadily increases in elderly (30% to 50%) [2]. In all age groups, stress urinary incontinence (SUI) is most common (49%) followed by mixed incontinence (29%) and pure urge incontinence (21%) [3]. Risk factors for female SUI include parity, aging and obesity, as well as parturition-associated injuries to muscles, connective tissues and nerves.
SUI after childbirth is common as approximately 30% of mothers become urinary incontinent after their first vaginal delivery; however, it is usually short-lasting and spontaneously disappears in most women [4]. On the other hand, damage of pelvic floor nerves and musculature induced by pregnancy or parturition seems to continue for lifetime in women. It has been reported that a prolonged pudendal motor terminal latency to the external anal sphincter occurred in women 2 days postpartum, and this prolonged latency was present when the women were re-investigated after 5 years despite the recovery from SUI 2 months postpartum [5]. In other reports, more pronounced pudendal nerve injury induced by pregnancy or childbirth was found in women with SUI than in those who were continent [6, 7]. Women with SUI also exhibited a age-dependent decline in maximum vaginal surface electromyogram activity, which was not seen in healthy women of the same age group with comparable parity [8]. Taken together, it is possible that age-dependent deterioration of pelvic floor neuromuscular function might be involved in overt SUI after a symptom-free period in women who have given birth to their children 10-to-30 years earlier. However, an etiologically based explanation for the delay from the time of pregnancy or childbirth to the appearance of SUI in middle age has not been provided.
The urethra consists of three smooth muscle layers which are the inner and outer longitudinal layers and the middle circular layer, as well as the striated muscle layer comprising the external urethral sphincter (EUS) located at the middle urethra [9]. These urethral muscles are controlled by three sets of peripheral nerves; sacral parasympathetic nerves (the pelvic nerves) and thoracolumbar sympathetic nerves (the hypogastric nerves) innervating urethral smooth muscles, and sacral somatic nerves (the pudendal nerves) innervating urethral striated muscles including EUS and pelvic floor muscles. The pudendal nerves and pelvic nerves also carry postganglionic axons from caudal sympathetic chain ganglia, indicating that both somatic nerves and sympathetic nerves could be damaged by pudendal nerve injury after childbirth [10]. Urethral closure mechanisms under stress conditions consist of passive mechanisms involving connective tissues, fascia and/or ligaments in the pelvis and active mechanisms mediated by these three sets of nerves. We have previously reported that active urethral closure during intravesical pressure elevation involves both smooth and striated muscle contractions and is mediated by the hypogastric, pelvic and pudendal nerves [11].
It is well known that α1-adrenoceptor (AR) is a predominant receptor to increase urethral pressure during activation of the hypogastric nerves and nerves from sympathetic chain ganglia [10, 12-14]. The activation of muscarinic receptors (MRs) mediated by the pelvic nerves also can induce urethral pressure elevation [15]. On the other hand, nitric oxide (NO) seems to be a predominant neurotransmitter to produce urethral smooth muscle relaxation during micturition [10, 16]. The activation of β-ARs mediated by the hypogastric nerves and nerves from sympathetic chain ganglia also can decrease urethral pressure [17]. However, there are no previous reports regarding the involvement of NO release and β-ARs activation in urinary continence mechanisms.
These findings led us to the hypothesis that urethral smooth muscle contraction mediated by autonomic nerves (the pelvic and hypogastric nerves) might compensate urethral sphincter deficiency induced by pudendal nerve injury to maintain urinary continence, which might contribute to the symptom-free period before the appearance of SUI. Thus, to clarify the urethral compensatory mechanisms after pudendal nerve injury, acute and chronic nonparous female SUI rats with bilateral pudendal nerve transection were examined using leak point pressure (LPP) measurements during bladder compression and urethral muscle strip studies. Changes in LPPs with or without hexamethonium (C6) application were also investigated to determine the autonomic nerve-sensitive components in LPP in response to bladder compression. It is assumed that the administration of C6 can block ganglionic transmission of the sympathetic and parasympathetic efferent pathways contained in the hypogastric and pelvic nerves including nerves from sympathetic chain ganglia. According to the results of C6 administration, in which LPP is either decreased or increased, we tested the effects of an α1-AR antagonist and a MR antagonist, or a β-AR antagonist and a NO synthase (NOS) inhibitor, respectively, to identify the excitatory or inhibitory transmitters involved in autonomic nerve-mediated urethral responses.
Materials and methods
Animals preparation
Forty-six nonparous female Sprague-Dawley rats (12-14 weeks old) weighing 250-280g were used. To examine LPP, 30 rats were divided into three groups of naive (n=6), acute pudendal nerve transection (acute PNT; n=12) and 4 weeks after PNT (PNT-4w; n=12) rats. We used PNT-4w rats to investigate the urethral compensatory mechanisms because the changes in LPPs with or without chemical autonomic nerve blockade were more consistent in PNT-4w rats compared with rats 2 weeks after PNT in our preliminary experiments. The remaining 16 rats were divided into naive (n=8) and PNT-4w (n=8) groups and used for the urethral muscle strip study because the contractile properties of urethral muscle strips in acute PNT rats were similar to those in naive rats in our preliminary experiments. All experiments were conducted in accordance with institutional guidelines and approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Pudendal nerve transection (PNT)
Under isoflurane (Hospira, Lake Forest, IL, USA) anesthesia, the pudendal nerves were transected bilaterally near the internal iliac vessels through a lower midline abdominal incision [18] in 32 rats. An approximately 3 mm segment of the nerves was removed to prevent nerve regeneration. Twenty PNT-4w rats that underwent LPP testing (n=12) or urethral muscle strip study (n=8) were treated for 3 days postoperatively with subcutaneous (s.c.) injection of ampicillin (100 mg/kg, Fort Dodge, IA, USA) and buprenorphine (0.5 mg/kg, Reckitt Benckiser, Richmond, VA, USA).
Leak point pressure (LPP) measurement
Under isoflurane anesthesia, the spinal cord was transected at the T8-9 level after laminectomy to eliminate reflex voiding mediated by spino-bulbo-spinal pathways passing through a micturition center in the pons. This manipulation does not interfere with urethral continence reflexes which are predominantly organized in the lumbosacral spinal cord [15]. After the bladder was exposed through a lower midline abdominal incision, a polyethylene (PE) -50 catheter (Clay Adams, Parsippany, NJ, USA) was inserted into the bladder through the bladder dome to monitor intravesical pressure, and a PE-10 catheter was implanted into the right jugular vein to inject test drugs. Feces were removed from the distal colon through a small incision in the colon wall. After the surgery, isoflurane anesthesia was turned off and replaced with urethane (Sigma, St. Louis, MO, USA) anesthesia (1.2 g/kg, s.c.). The animals were placed in the supine position without suturing the lower abdominal wall. The bladder catheter was connected to a pressure transducer (Transbridge 4M, World Precision Instruments, Sarasota, FL, USA) and a syringe pump (Harvard Apparatus, Holliston, MA, USA) via a three-way stopcock. Saline solution (0.4 ml) containing Evans blue (100 μg/ml, Sigma) was infused into the bladder at 0.02 ml per minute. A gentle pressure was then manually applied to the bladder wall while the intravesical pressure was monitored using a data-acquisition software (sampling at 10 Hz, Chart, ADInstruments, Castle Hill, NSW, Australia) on a computer system equipped with an analog-to-digital converter (PowerLab, ADInstruments). We directly manually compressed the bladder wall because lower abdominal wall compression might stimulate abdominal wall muscles and/or the colon to induce spinal reflexes other than the bladder-to-urethral reflex. The gentle pressure was slowly applied for about ten seconds until fluid leakage occurred from the urethral orifice, and then immediately removed (Fig. 1). The pressure at which leakage occurred was regarded as LPP, and the average of three-to-five consecutive LPPs was taken as a data point. The leaked fluid around the urethral orifice was removed after each leakage.
Fig. 1.
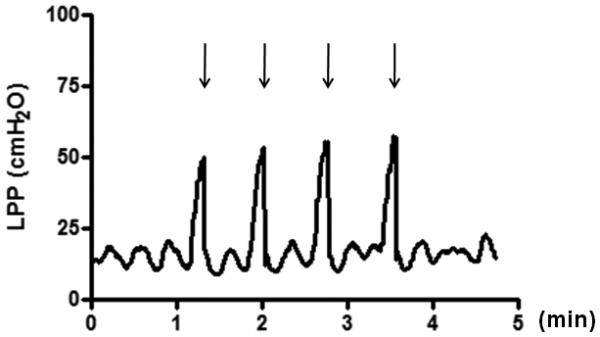
Representative leak point pressure (LPP) testing during bladder compression.
Gentle pressure was slowly applied for approximately ten seconds until fluid leakage occurred at the urethral orifice, and then immediately removed. Arrows indicate the timing of fluid leakage. The pressure at fluid leakage was regarded as LPP, and three-to-five consecutive LPPs were averaged to obtain each data point.
Experiment 1: Changes in LPPs with or without chemical autonomic nerve blockade in naive, acute PNT and PNT-4w rats
In naive (n=6), acute PNT (n=6) and PNT-4w rats (n=6), LPPs were measured before and after the intravenous (i.v.) application of C6 (25 mg/kg, Sigma). In other acute PNT rats (n=6), to identify the neurotransmitters responsible for the C6-sensitive portion of LPPs, the changes in LPPs were examined following the consecutive i.v. application of propranolol (1 mg/kg, Sigma), a β-AR antagonist, Nω-nitro-L-arginine-methyl ester (L-NAME; 20 mg/kg, Sigma), a NOS inhibitor, and C6 (25 mg/kg) at 10-minute intervals. Furthermore, in other PNT-4w rats (n=6), the changes in LPPs were examined following the consecutive i.v. application of terazosin (0.3 mg/kg, Tocris Cookson, Ellisville, MO, USA), an α1-AR antagonist, atropine (0.5 mg/kg, Sigma), a MR antagonist, and C6 (25 mg/kg) at 10-minute intervals. All the measurements of LPPs were performed within 10 minutes after drug administration.
All test drugs were dissolved in saline solution and administered i.v. in 0.5 ml/kg volume followed by a flush of 0.1 ml saline solution. Drug concentrations were selected based on previous studies [15, 19-21] and our preliminary experiments.
Experiment 2: Changes in proximal and middle urethral contractile responses of muscle strips in naive and PNT-4w rats
The proximal and middle urethra of naive (n=8) and PNT-4w (n=8) female rats were used because we have previously reported that urethral contractile responses to intravesical pressure elevation were more remarkable in the proximal and middle urethra compared with the distal urethra in female rats [11]. Transverse muscle strips (1.5 × 4 mm) of the proximal and middle urethra were prepared in a cold Krebs-Henseleit (K-H) solution composed of 118 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM MgSO4, 25 mM NaHCO3, 1.2 mM KH2PO4 and 10 mM glucose. The muscle strips were then suspended in a 30 ml organ bath filled with K-H solution at 37°C and gassed with a 95% O2 and 5% CO2 mixture. Each strip was adjusted to a tension of 0.5g and then allowed to equilibrate for at least 60 minutes [19]. The final resting tension was 0.1-0.2 g. After eliciting responses to 80 mM KCl, cumulative, concentration-dependent contractions induced by carbachol (10−7 to 10−4 M, Sigma), a MR agonist, or phenylephrine (10−7 to 10−4 M, Sigma), an α1-AR agonist, were recorded in a stepwise manner after the response to the previous concentration had reached a plateau. All the data were recorded using a Chart software on a PowerLab system (sampling at 40 Hz, ADInstruments). The contractile responses were expressed as a percent of the responses to 80 mM KCl, and EC50 values (concentration required to produce 50% of maximal contractile response) were obtained from contractile response curves with an iterative non-linear least square curve-fitting using a Prism software (GraphPad Software, San Diego, CA, USA).
Data analyses
All data are represented as mean values ± standard error of the mean (SEM). A statistical analysis software (Prism, GraphPad Software) was used to compare LPPs and urethral contractile responses of muscle strips. The differences in LPPs with or without C6 application and urethral contractile responses of muscle strips in naive, acute PNT and PNT-4w rats were analyzed using unpaired Student t-test. The changes in LPPs of acute PNT and PNT-4w rats before and after test drugs were compared using paired Student t-test. Values of p<0.05 were considered significant.
Results
Experiment 1: Changes in LPPs with or without chemical autonomic nerve blockade
LPPs in acute PNT rats were significantly lower (p<0.01) than those in naive rats. Following C6 application, LPPs in naive rats were not altered from 84.2 ± 4.5 (range: 74.0 – 103.0) to 84.7 ± 4.9 (range: 72.5 – 101.0) cmH2O while LPPs in acute PNT rats were significantly increased from 66.2 ± 2.5 (range: 58.9 – 73.5) to 77.8 ± 1.9 (range: 71.3 – 83.7) cmH2O (p<0.01), indicating that activation of autonomic nerves induces relaxing responses to bladder compression in urethral smooth muscle in acute PNT rats. In addition, post-C6 LPPs in acute PNT rats were significantly lower than those in naive rats (p<0.05) (Fig. 2). In other acute PNT rats, LPPs were not changed by propranolol compared with the predrug values, but were significantly increased by L-NAME compared with the post-propranolol values (p<0.01). The post-L-NAME LPPs were not altered by C6 (Fig. 3A). These results indicate that, in acute PNT rats, autonomic nerve-mediated urethral relaxation during bladder compression is induced by NO.
Fig. 2.

Comparison of leak point pressures (LPPs) before and after hexamethonium (C6) application in naive, acute pudendal nerve transection (acute PNT) and 4 weeks after PNT (PNT-4w) female rats.
LPPs in acute PNT rats were significantly lower than LPPs in naive rats, whereas LPPs in PNT-4w rats were equal to those in naive rats but significantly higher than those in acute PNT rats. After C6 administration, LPPs in naive rats were not changed while LPPs in acute PNT and PNT-4w rats were significantly increased and decreased, respectively. Furthermore, the post-C6 LPPs in PNT-4w rats were significantly lower compared with those in naive and acute PNT rats. *p<0.05, **p<0.01, N.S.: not significant.
Fig. 3.

Changes in leak point pressures (LPPs) after serial application of chemical autonomic efferent nerve blockade in acute pudendal nerve transection (acute PNT) and 4 weeks after PNT (PNT-4w) female rats.
(A) Changes in LPPs after serial application of propranolol (Pro), Nω-nitro-Larginine-methyl ester (L-NAME) and hexamethonium (C6) in acute PNT rats. LPPs were not changed by propranolol compared with the predrug values, but were significantly increased by L-NAME compared with the post-propranolol values. The post-L-NAME LPPs were not altered by C6. (B) Changes in LPPs after serial application of terazosin (Ter), atropine (Atr) and C6 in PNT-4w rats. LPPs were significantly decreased by the application of terazosin compared with the predrug values and atropine compared with the post-terazosin values. The post-atropine LPPs were not altered by C6. **p<0.01, N.S.: not significant.
On the other hand, LPPs in PNT-4w rats were equal to those in naive rats but significantly higher than those in acute PNT rats (p<0.01). After C6 application, LPPs in PNT-4w rats were, however, significantly decreased from 85.1 ± 4.9 (range: 70.9 – 100.7) to 61.0 ± 3.2 (range: 53.5 – 75.0) cmH2O (p<0.05), indicating that activation of autonomic nerves induces contractile responses to bladder compression in urethral smooth muscle in PNT-4w rats. Furthermore, the post-C6 LPPs in PNT-4w rats were significantly lower than those in naive rats (p<0.01) and in acute PNT rats (p<0.01) (Fig. 2). In other PNT-4w rats, LPPs were significantly decreased by the application of terazosin compared with the predrug value (p<0.01) and atropine compared with the post-terazosin value (p<0.01). The post-atropine LPPs were not altered by C6 (Fig. 3B). These results indicate that, in PNT-4w rats, autonomic nerve-mediated urethral contraction during bladder compression is induced by α1-AR and MR activation.
Experiment 2: Changes in the contractile responses of proximal and middle urethral muscle strips
The urethral contractile responses to muscarinic and adrenergic agonists were expressed as a percentage of the response induced by 80 mM KCl. When compared to the responses in tissues from naive rats the contractile responses to low concentrations of carbachol in PNT-4w rats were significantly increased in the proximal urethra (p<0.01 and p<0.05) (Fig. 4A) but not altered at the middle urethra. The contractile responses to low concentrations of phenylephrine in PNT-4w rats were also significantly increased in the proximal urethra (p<0.05) (Fig. 4B) but not altered at the middle urethra. The EC50 values of proximal and middle urethra in naive and PNT-4w rats are shown in Table 1. EC50 values of the proximal urethra in responses to carbachol and phenylephrine in PNT-4w rats were significantly lower than those in naive rats (p<0.01 and p<0.05, respectively). However, there were no significant differences in EC50 values of the middle urethra between PNT-4w and naive rats.
Fig. 4.

Comparison of proximal urethral muscle contractile responses to carbachol and phenylephrine in naive and 4 weeks after pudendal nerve transection (PNT-4w) female rats.
Note that the response curves of carbachol (A) and phenylephrine (B) in PNT-4w rats were shifted to the left compared with naive rats. Contractile responses are expressed as a percent of the responses to 80 mM KCl. *p<0.05, **p<0.01.
Table 1.
Contractile responses of proximal and middle urethral muscle strips to carbachol and phenylephrine in naive and 4-week pudendal nerve transection (PNT-4w) female rats.
| EC50 (μM) |
|||
|---|---|---|---|
| Proximal urethra | Middle urethra | ||
| Carbachol | Naive | 3.8 ± 1.3 (1.3-8.7) | 2.6 ± 1.2 (1.2-6.5) |
| PNT-4w | 1.5 ± 1.2** (0.6-3.2) | 1.8 ± 1.2 (0.7-5.0) | |
| Phenylephrine | Naive | 7.6 ± 1.3 (3.0-16.1) | 2.8 ± 1.3 (1.2-11.1) |
| PNT-4w | 2.8 ± 1.3* (1.6-8.5) | 2.6 ± 1.2 (1.2-7.2) | |
EC50 values are concentrations required to produce 50% of the maximal contractile response. (n=8 rats /group). Mean ± SEM and the range of values in parentheses are shown.
p<0.05
p<0.01 compared with naive rats.
Discussion
This study used measurements of leak point pressure (LPP), acute transection of the pudendal nerves and administration of drugs that block neurotransmitter receptors to examine spinal somatic and autonomic reflex mechanisms contributing to urinary continence during bladder compression. Changes in these mechanisms after chronic (4 weeks) transection of the pudendal nerves were also studied. The experiments have revealed that manual compression of the bladder stimulates afferent nerves that in turn activate lumbosacral reflex pathways controlling LPP. With these methods it is not possible to identify the relative contribution of the urethra and the bladder neck to the LPP measurements; however it is clear that autonomic regulation of smooth muscle is an important component of the spinal reflex control of LPP.
Our results in spinal cord transected, urethane anesthetized female rats indicate that: (1) urethral striated muscles are involved in urethral continence reflex during bladder compression because after blocking autonomic pathways with C6 LPPs are lower in acute PNT rats than in naive rats; (2) NO induces urethral smooth muscle relaxation during bladder compression because L-NAME increased LPPs in acute PNT rats; (3) in PNT-4w rats, LPPs during bladder compression are elevated to the level measured in naive rats due to an increase in the α1-AR and MR-mediated contractile responses of urethral smooth muscles and (4) the sensitivity of α1-ARs and MRs in the proximal, but not middle urethra is enhanced in PNT-4w rats. Thus it appears that, 4 weeks after PNT, urethral continence mechanisms are dramatically altered in response to somatic nerve and sympathetic nerve denervation of urethral striated and smooth muscles which occurs after transecting the pudendal nerves. The major change is a switch from NO-mediated relaxation to α1-AR and MR-mediated contraction of proximal urethral smooth muscles.
In this study, we found that C6 application increased LPPs during bladder compression after acute PNT, indicating that bladder compression induces autonomic nerve-mediated urethral relaxation. Since L-NAME, but not propranorol, mimics the effect of C6 on LPPs, NO-mediated urethral smooth muscle relaxation seems to be responsible for the C6-sensitive component of LPPs in acute PNT rats (Fig. 5B). NO is a predominant neurotransmitter to induce urethral relaxation during micturition in female rats [10, 16]. In the major pelvic ganglia (MPG), which receive preganglionic inputs from the hypogastric and pelvic efferent nerves, all NOS-positive cells are stained for choline acetyltransferase [22], suggesting that NO is released from parasympathetic postganglionic nerves originating from the MPG [23]. It has also been reported that electrical stimulation of the hypogastric nerves decreases intraurethral pressure and that this relaxing response is mediated by NO in female rats [24]. Thus, it is assumed that NO released from parasympathetic and sympathetic nerves carried through the pelvic and hypogastric nerves, respectively, induces urethral smooth muscle relaxation during bladder compression in acute PNT rats, which is blocked by L-NAME or C6 (Figs. 3A and 5B).
Fig. 5.

Hypothetical scheme of the urethral closure mechanisms to maintain urinary continence in female rats.
(A) In the normal state (naive), leak point pressure (LPP) during bladder compression is maintained positively by the pudendal nerves (Pud-N), which contain somatic (Som) and sympathetic (Sym) nerves, and negatively by sympathetic (Sym) and parasympathetic (Para) nerves in the hypogastric (Hg-N) and pelvic nerves (Pel-N), respectively, both of which release NO to induce urethral smooth muscle relaxation. Since the removal of autonomic nerve activity by hexamethonium (C6) did not alter LPPs in naive rats, activation of sympathetic nerves in the pudendal nerves is assumed to offset the NO-mediated smooth muscle relaxation. The + and − signs indicate the contractile and relaxing effects on LPP, respectively.
(B) After acute pelvic nerve transection (acute PNT), the excitatory inputs from the pudendal nerves are lost, resulting in the lowered LPP (LPP↓). NO released from sympathetic (Sym) and parasympathetic (Para) nerves in the hypogastric (Hg-N) and pelvic nerves (Pel-N), respectively, induces urethral smooth muscle relaxation during bladder compression, which is antagonized by C6 or L-NAME application.
(C) Four weeks after PNT (PNT-4w), activation of sympathetic (Sym) and parasympathetic (Para) nerves in the hypogastric (Hg-N) and pelvic nerves (Pel-N) induces urethral smooth muscle contraction via α1-adrenoceptors (α1-AR) and muscarinic receptors (MR), respectively. These urethral smooth muscle contractile responses compensate urethral sphincter deficiency induced by PNT, thereby normalizing the LPP.
In addition, because LPPs in naive rats were not altered by C6 application in this study, NO-mediated urethral relaxation seen in acute PNT rats seems to be negated by activation of the pudendal nerves in the normal condition (Fig. 5A). Since the pudendal nerves contain not only somatic nerves, but also postganglionic axons from caudal sympathetic chain ganglia [10], urethral smooth muscle contraction induced by sympathetic nerve activation in the pudendal nerves might offset NO-mediated urethral smooth muscle relaxation during bladder compression, resulting in no apparent effects of C6 on LPPs in naive rats (Fig. 5A). Further studies are needed to clarify this point.
The most interesting finding of this study is that the urethral smooth muscle response during bladder compression changed from NO-mediated relaxation to α1-AR and MR-mediated contraction which restored LPPs to a normal level 4 weeks after PNT (Fig. 5C). It has been reported that the percentage of urethral striated muscle volume is significantly decreased 2 weeks after PNT, but the percentage of urethral smooth muscle volume is not altered [25]. Thus, urethral smooth muscles in PNT-4w rats might compensate for the deficient urethral sphincter function by the activation of α1-ARs and MRs to maintain urinary continence during bladder compression (Fig. 5C). Similar changes have been reported in the α1-AR-mediated urethral smooth muscle response of aged parous female dogs where intraurethral pressure in the aged dogs was significantly increased by α1-AR agonists at the proximal, but not middle urethra compared with young nonparous dogs [26].
To clarify the urethral compensatory mechanisms that might maintain urinary continence after childbirth, a SUI animal model induced by PNT was used in this study. Pudendal nerve crush which has also been used as a SUI model in female rats [27-29] produces the lowest LPPs (tested by abdominal wall compression) at 4 days after injury, and then the LPPs gradually recover with increasing time after the crush even though only about 50% of the crushed pudendal nerves are regenerated 3 months after injury. These findings might also suggest that the urethral compensatory changes to enhance urethral smooth muscle activity could occur after pudendal nerve injury to maintain urinary continence during Valsalva-like stress conditions. On the other hand, we have previously reported that LPPs in PNT-4w female rats were significantly decreased compared with LPPs in sham-operated rats [30, 31]. Because LPPs in the latter experiments were measured in the vertical position and evoked by saline infusion into the bladder, the deficient urethral striated muscle function may have been more prominent than in the present experiments.
In conclusion, this study has demonstrated urethral compensatory mechanisms after pudendal nerve injury in female rats. To compensate for the deficient striated muscle function induced by pudendal nerve injury, urethral smooth muscle activity in the proximal urethra seems to be enhanced by switching from the NO-mediated relaxation to the α1-AR and MR-mediated contractile responses 4 weeks after injury. These compensatory mechanisms could contribute to the temporal recovery of urinary continence seen in patients with SUI after childbirth.
Brief summary.
α1-adrenoceptor and muscarinic receptor-mediated contractility is upregulated in the proximal urethra 4 weeks after pudendal nerve transection (PNT) to offset PNT-induced urethral sphincter deficiency.
Acknowledgements
This study was supported by National Institutes of Health grants (DK067226, AR049398 and DK055387).
Footnotes
Furuta A : Project development, Data collection, Data analysis, Manuscript writing
Suzuki Y : Project development, Manuscript editing
Asano K : Project development
de Groat WC : Manuscript editing
Egawa S : Manuscript editing
Yoshimura N : Project development, Data analysis, Manuscript editing
References
- 1.Norton P, Brubaker L. Urinary incontinence in women. Lancet. 2006;367:57–67. doi: 10.1016/S0140-6736(06)67925-7. [DOI] [PubMed] [Google Scholar]
- 2.Hunskaar S, Burgio K, Clark A, et al. Epidemiology of urinary (UI) and faecal (FI) incontinence and pelvic organ prolapse (POP) In: Abrams P, Cardozo L, Khoury S, et al., editors. Incontinence 3rd International Consultation on Incontinence. Health Publications; United Kingdom: 2005. pp. 2046–2078. [Google Scholar]
- 3.Nitti VW, Blaivas JG. Urinary incontinence: epidemiology, pathophysiology, evaluation, and management overview. In: Wein AJ, Kavoussi LR, Novick AC, et al., editors. Cambell-Walsh Urology. 9th Edition WB Saunders Elsevier; Philadelphia, USA: 2007. pp. 2046–2078. [Google Scholar]
- 4.Meyer S, Schreyer A, De Grandi P, Hohlfeld P. The effects of birth on urinary continence mechanisms and other pelvic-floor characteristics. Obstet Gynecol. 1998;92:613–618. doi: 10.1016/s0029-7844(98)00248-8. [DOI] [PubMed] [Google Scholar]
- 5.Snooks SJ, Swash M, Mathers SE, Henry MM. Effect of vaginal delivery on the pelvic floor: a 5-year follow-up. Br J Surg. 1990;77:1358–1360. doi: 10.1002/bjs.1800771213. [DOI] [PubMed] [Google Scholar]
- 6.Clark MH, Scott M, Vogt V, Benson JT. Monitoring pudendal nerve function during labor. Obstet Gynecol. 2001;97:637–639. doi: 10.1016/s0029-7844(00)01207-2. [DOI] [PubMed] [Google Scholar]
- 7.Tetzschner T, Sorensen M, Jonsson L, Lose G, Christiansen J. Delivery and pudendal nerve function. Acta Obstet Gynecol Scand. 1997;76:324–331. doi: 10.1111/j.1600-0412.1997.tb07986.x. [DOI] [PubMed] [Google Scholar]
- 8.Gunnarsson M, Mattiasson A. Female stress, urge, and mixed urinary incontinence are associated with a chronic and progressive pelvic floor/vaginal neuromuscular disorder: An investigation of 317 healthy and incontinent women using vaginal surface electromyography. Neurourol Urodyn. 1999;18:613–621. doi: 10.1002/(sici)1520-6777(1999)18:6<613::aid-nau11>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 9.Schroder HD, Reske-Nielsen E. Fiber types in the striated urethral and anal sphincters. Acta Neuropathol (Berl) 1983;60:278–282. doi: 10.1007/BF00691877. [DOI] [PubMed] [Google Scholar]
- 10.de Groat WC. Integrative control of the lower urinary tract: preclinical perspective. Br J Pharmacol. 2006;147(Suppl 2):S25–40. doi: 10.1038/sj.bjp.0706604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamo I, Cannon TW, Conway DA, et al. The role of bladder-to-urethral reflexes in urinary continence mechanisms in rats. Am J Physiol Renal Physiol. 2004;287:F434–441. doi: 10.1152/ajprenal.00038.2004. [DOI] [PubMed] [Google Scholar]
- 12.Danuser H, Bemis K, Thor KB. Pharmacological analysis of the noradrenergic control of central sympathetic and somatic reflexes controlling the lower urinary tract in the anesthetized cat. J Pharmacol Exp Ther. 1995;274:820–825. [PubMed] [Google Scholar]
- 13.Danuser H, Thor KB. Inhibition of central sympathetic and somatic outflow to the lower urinary tract of the cat by the alpha 1 adrenergic receptor antagonist prazosin. J Urol. 1995;153:1308–1312. [PubMed] [Google Scholar]
- 14.Kaiho Y, Kamo I, Chancellor MB, Arai Y, de Groat WC, Yoshimura N. Role of noradrenergic pathways in sneeze-induced urethral continence reflex in rats. Am J Physiol Renal Physiol. 2007;292:F639–646. doi: 10.1152/ajprenal.00226.2006. [DOI] [PubMed] [Google Scholar]
- 15.Kakizaki H, Fraser MO, De Groat WC. Reflex pathways controlling urethral striated and smooth muscle function in the male rat. Am J Physiol. 1997;272:R1647–1656. doi: 10.1152/ajpregu.1997.272.5.R1647. [DOI] [PubMed] [Google Scholar]
- 16.Bennett BC, Kruse MN, Roppolo JR, Flood HD, Fraser M, de Groat WC. Neural control of urethral outlet activity in vivo: role of nitric oxide. J Urol. 1995;153:2004–2009. [PubMed] [Google Scholar]
- 17.Takeda H, Matsuzawa A, Igawa Y, et al. Functional characterization of beta-adrenoceptor subtypes in the canine and rat lower urinary tract. J Urol. 2003;170:654–658. doi: 10.1097/01.ju.0000074622.50255.a8. [DOI] [PubMed] [Google Scholar]
- 18.Manzo J, Vazquez MI, Cruz MR, Hernandez ME, Carrillo P, Pacheco P. Fertility ratio in male rats: effects after denervation of two pelvic floor muscles. Physiol Behav. 2000;68:611–618. doi: 10.1016/s0031-9384(99)00219-x. [DOI] [PubMed] [Google Scholar]
- 19.Furuta A, Jankowski RJ, Pruchnic R, Egawa S, Yoshimura N, Chancellor MB. Physiological effects of human muscle-derived stem cell implantation on urethral smooth muscle function. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:1229–1234. doi: 10.1007/s00192-008-0608-9. [DOI] [PubMed] [Google Scholar]
- 20.Thor KB, Katofiasc MA. Effects of duloxetine, a combined serotonin and norepinephrine reuptake inhibitor, on central neural control of lower urinary tract function in the chloralose-anesthetized female cat. J Pharmacol Exp Ther. 1995;274:1014–1024. [PubMed] [Google Scholar]
- 21.Torimoto K, Hirao Y, Matsuyoshi H, de Groat WC, Chancellor MB, Yoshimura N. alpha1-Adrenergic mechanism in diabetic urethral dysfunction in rats. J Urol. 2005;173:1027–1032. doi: 10.1097/01.ju.0000146268.45662.36. [DOI] [PubMed] [Google Scholar]
- 22.Persson K, Alm P, Uvelius B, Andersson KE. Nitrergic and cholinergic innervation of the rat lower urinary tract after pelvic ganglionectomy. Am J Physiol. 1998;274:R389–397. doi: 10.1152/ajpregu.1998.274.2.R389. [DOI] [PubMed] [Google Scholar]
- 23.Persson K, Johansson K, Alm P, Larsson B, Andersson KE. Morphological and functional evidence against a sensory and sympathetic origin of nitric oxide synthase-containing nerves in the rat lower urinary tract. Neuroscience. 1997;77:271–281. doi: 10.1016/s0306-4522(96)00443-5. [DOI] [PubMed] [Google Scholar]
- 24.Kontani H, Shiraoya C. Sex differences in urethral pressure response to electrical stimulation of the hypogastric nerves in rats. J Urol. 2000;163:1364–1368. [PubMed] [Google Scholar]
- 25.Heidkamp MC, Leong FC, Brubaker L, Russell B. Pudendal denervation affects the structure and function of the striated, urethral sphincter in female rats. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9:88–93. doi: 10.1007/BF01982215. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi S, Moriyama N, Yamazaki R, Kawabe K. Urodynamic analysis of age-related changes of alpha 1-adrenoceptor responsiveness in female beagle dogs. J Urol. 1996;156:1485–1488. [PubMed] [Google Scholar]
- 27.Ahn H, Lin DL, Esparza N, Damaser MS. Short-term timecourse of bilateral pudendal nerve injury on leak-point pressure in female rats. J Rehabil Res Dev. 2005;42:109–114. [PubMed] [Google Scholar]
- 28.Damaser MS, Broxton-King C, Ferguson C, Kim FJ, Kerns JM. Functional and neuroanatomical effects of vaginal distention and pudendal nerve crush in the female rat. J Urol. 2003;170:1027–1031. doi: 10.1097/01.ju.0000079492.09716.43. [DOI] [PubMed] [Google Scholar]
- 29.Kerns JM, Damaser MS, Kane JM, et al. Effects of pudendal nerve injury in the female rat. Neurourol Urodyn. 2000;19:53–69. doi: 10.1002/(sici)1520-6777(2000)19:1<53::aid-nau7>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 30.Kwon D, Kim Y, Pruchnic R, et al. Periurethral cellular injection: comparison of muscle-derived progenitor cells and fibroblasts with regard to efficacy and tissue contractility in an animal model of stress urinary incontinence. Urology. 2006;68:449–454. doi: 10.1016/j.urology.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 31.Lee JY, Cannon TW, Pruchnic R, Fraser MO, Huard J, Chancellor MB. The effects of periurethral muscle-derived stem cell injection on leak point pressure in a rat model of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2003;14:31–37. doi: 10.1007/s00192-002-1004-5. [DOI] [PubMed] [Google Scholar]


