Abstract
Objective
To evaluate outcomes of cervical cancer screening within HIV care and treatment clinics in Kenya.
Methods
Beginning in October 2007, visual inspection with acetic acid (VIA), colposcopy, and loop electrosurgical excision procedure (LEEP) were added to the clinical services offered at Family AIDS Care and Education Services (FACES) clinics in Kisumu, Kenya, after a systematic campaign to build capacity and community awareness.
Results
From October 2007 to October 2010, 3642 women underwent VIA as part of routine HIV care. Cervical intraepithelial neoplasia 2/3 was identified in 259 (7.1%) women, who were offered excisional treatment by LEEP in the clinic. Among those women offered screening, uptake was 87%. Clinical staff reported a high level of satisfaction with training for and implementation of cervical cancer screening strategies.
Conclusion
Cervical cancer screening and prevention are feasible, acceptable, and effective within HIV care and treatment clinics. Screening test performance characteristics need to be defined for an HIV-positive population to determine the cost/benefit ratio of lower cost strategies that will ultimately be necessary to provide universal access to cervical cancer screening in low-resource settings.
Keywords: Cervical cancer screening, HIV, Kenya, Resource-limited setting
1. Introduction
Cervical cancer and HIV represent synergistic threats to women’s reproductive health and overall mortality in resource-limited countries. Biologically, HIV infection increases women’s risk of human papillomavirus (HPV) infection, cervical neoplasia, and invasive cervical cancer [1]. In addition, most global HIV infections occur in resource-limited settings where healthcare funding and infrastructure are inadequate for primary care and prevention programs such as cervical cancer screening, which substantially increases the vulnerability of HIV-infected women [2]. The synergistic effect of HIV and lack of resources on cervical cancer risk is particularly evident in sub-Saharan countries such as Kenya, where cervical cancer is the most common cancer diagnosed among women and the most common cause of cancer-related death [3].
Owing to a lack of surveillance programs, the exact incidence of cervical cancer in Kenya is unknown, but it has been estimated at approximately 29–200 cases per 100 000 women, with a 2- to 4-fold increase in incidence among HIV-infected women [4,5]. Historically, the large burden of disease has been attributed to a lack of national screening guidelines and funding for cervical cancer prevention programs [6]. In the past 2 decades, this has been augmented by the impact of the HIV epidemic in Kenya [5]. Overall HIV prevalence in Kenya was estimated to be approximately 8% at the end of 2008, and the prevalence in women aged 18–49 years was estimated at 8.9% [7,8]. Women of reproductive age are the fastest growing group to become newly infected with HIV, with a 5-fold greater risk than men, which will substantially increase the number of women at risk for cervical cancer and other HIV-related reproductive health effects over the next 5–10 years [7].
Alongside the individual impact, HIV presents immediate, severe challenges to the healthcare, social, and economic infrastructure in Kenya, reducing the overall life expectancy from 63 to 47 years [7]. An unprecedented amount of international donor funding from organizations such as the Global Fund to fight AIDS, Tuberculosis and Malaria, and the President’s Emergency Plan for AIDS Relief (PEPFAR) has helped Kenya and other hard-hit countries develop programs to provide HIV care and treatment, specifically highly active antiretroviral therapy (HAART).
In addition to supply, medication, and facility needs, one of the biggest challenges to building a strong healthcare infrastructure in sub-Saharan Africa has been the shortage of doctors and other skilled health workers. This challenge has been met through the targeted training of mid-level care providers, including clinical officers and nurses, in HIV care and prevention by using streamlined algorithmic approaches tailored to national and WHO guidelines. The result is that there has been an incredible increase in access to high-quality, standardized healthcare in areas where it would have been previously unimaginable in the past 5 years. This has increased survival in HIV-infected individuals and markedly reduced AIDS-related deaths in target countries [9].
While the provision of HAART has been a key factor in preventing much of the AIDS-related morbidity and mortality, HIV-infected individuals are still at risk for chronic and HIV-related illnesses. HAART has not been shown to reduce the risk of developing cervical dysplasia or cervical cancer, nor has it been shown to improve outcomes. The fact that more women are living longer on HAART suggests that the incidence of cervical cancer may increase in Kenya over the next few decades until effective HIV and cervical cancer prevention technologies are implemented. To meet the current and future needs of this population, a resource-appropriate, effective and sustainable strategy for cervical cancer screening and prevention (CCSP) needs to be defined and implemented.
The infrastructure and continuity of care necessary for the provision of HAART in HIV clinics represents an ideal opportunity for CCSP programs to reach this high-risk population. The Family AIDS Care and Education Services (FACES) program is a collaboration between the University of California, San Francisco, USA, and the Kenya Medical Research Institute, Nairobi, Kenya, funded by the Centers for Disease Control and Prevention. FACES provides comprehensive HIV care and treatment to over 80 000 individuals throughout the Nyanza province, which lies on the shores of Lake Victoria in western Kenya. The Nyanza province is the region hardest hit by HIV within Kenya, with infection rates ranging from 14% to 40% in different districts [8].
The aim of the present study was to report the results and details of a model of CCSP that has been implemented into an HIV care and treatment program in Kisumu, Nyanza province, Kenya. The program utilizes a low-cost, easily trainable, screening technique that capitalizes on the presence of mid-level providers and the clinic infrastructure in place for the regular visits that are an essential part of HIV care.
2. Materials and methods
A multi-faceted approach was used to implement CCSP in HIV clinics supported by the FACES program. Preparation for CCSP included a situation assessment, protocol development, clinical and laboratory capacity building, community and patient awareness, and implementation of a monitoring and evaluation strategy.
Relying on the infrastructure and continuity of care in place, the CCSP program was started on October 1, 2007, as an ongoing pilot project at the longest-running and largest FACES clinic located at Lumumba Hospital in Kisumu, Kenya. The Lumumba clinic has a clinical staff of approximately 25, including medical officers, clinical officers, nurses, and a fulltime phlebotomist. There are approximately 75 non-clinical staff, including receptionists, administrators, laboratory staff, clinical and community health workers (CCHAs), and peer educators. CCHAs and peer educators provide patient education through outreach work, home visits, treatment adherence counseling, and daily education talks.
Before implementing cervical cancer screening at FACES, a series of basic talks on gynecology, pelvic exams, and cervical cancer in HIV-infected women were given to the clinic staff. The content of the talks was tailored to meet the needs of the particular audience so that clinical staff received more clinically focused talks, whereas the outreach and counseling staff learned counseling and how to respond to patients’ questions about cervical cancer screening and treatment.
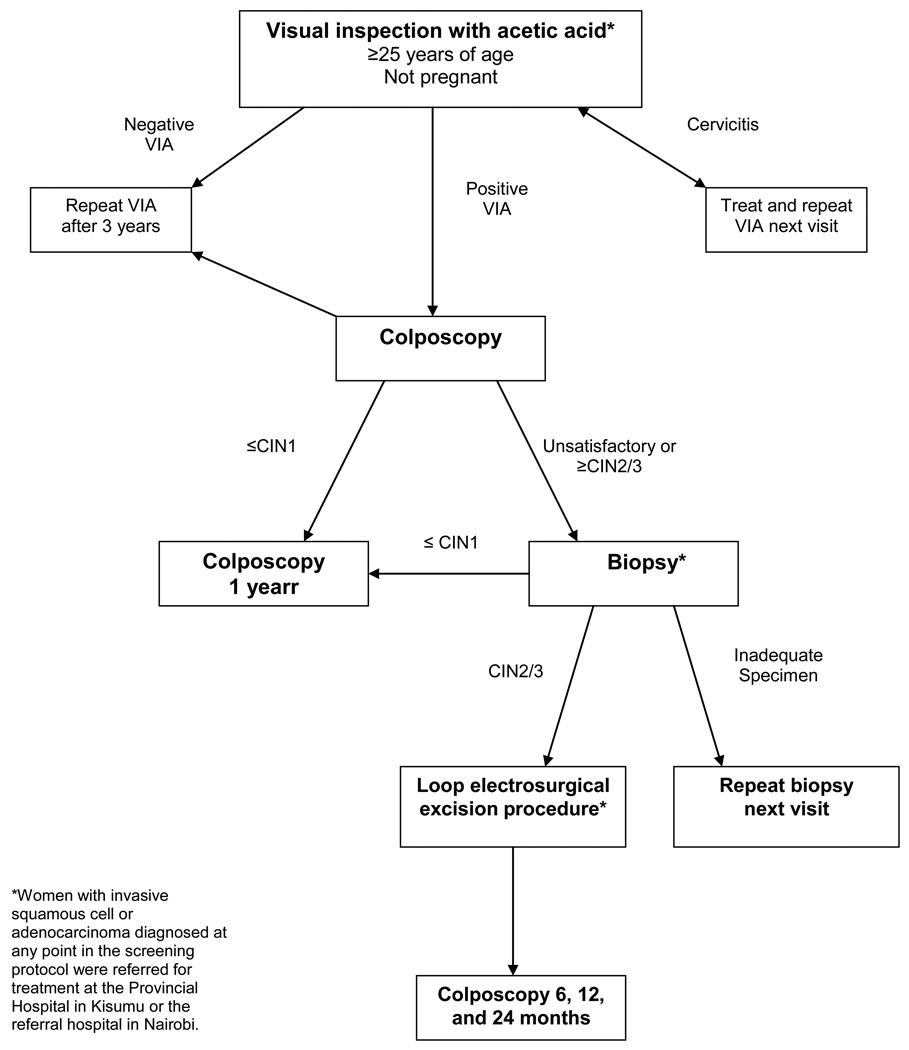
The clinical protocol was developed on the basis of recommendations from the WHO’s Comprehensive Guide to Cervical Cancer Control for resource-limited settings, coupled with relevant evidence from recent clinical trials [10–12]. The protocol uses visual inspection with 3–5% acetic acid (VIA) as a screening test, followed by colposcopy and biopsy to confirm cervical intraepithelial neoplasia 2/3 (CIN2/3), a precancerous lesion needing treatment [13] (Figure 1).
Figure 1.
Algorithm for cervical cancer screening and treatment at FACES.
In the CCSP program, screening is offered as part of comprehensive HIV care to all non-pregnant women aged over 25 years. Women with biopsy-proven CIN2/3 are offered treatment via loop electrosurgical excision procedure (LEEP), performed by clinical officers on-site.
Clinical officers undergo specific and ongoing training for competency in VIA and colposcopy. For both techniques, training includes a week of daily, 90-minute didactic sessions at the start or end of the clinical day. The curriculum covers cervical anatomy and histology; HPV epidemiology and role in carcinogenesis; and the theoretical basis of cytological, visual inspection, and biomarker-based screening programs. In addition, course materials include more than 100 slides depicting normal and abnormal VIA and colposcopy findings. At the end of the week, volunteers are brought in for a training demonstration. After completion of this portion of the training, clinical officers take a short test to assess their retention and understanding. This didactic training is followed by a predetermined number of observed VIA and colposcopic examinations. After satisfactory demonstration of competency, clinical officers receive certification in both techniques.
The screening protocol used at FACES relies on a confirmatory biopsy for treatment decisions. To facilitate histology interpretation and tracking of results, a local pathologist trains the laboratory staff at FACES in specimen processing. Pathology specimens are sent weekly to Nairobi with other clinical specimens, and the results are sent back via email to the CCSP coordinators at FACES, where they are entered into an electronic database.
To increase understanding of cervical cancer prevention strategies and to maintain a high screening uptake, an education and awareness campaign has been implemented that aims at clinical staff, patients, and the general community. The cornerstone of this campaign is the “CCSP team” and the peer educators working in the clinic. The CCSP team comprises representatives from each department at FACES, including clinical and non-clinical staff. Team members become experts in the components of the CCSP protocol that relate to their department, and serve as a liaison between their department and the CCSP staff. This enables them to answer colleagues’ questions, and to bring department-specific concerns to the core CCSP staff. Peer educators, who are HIV-infected FACES patients volunteering in the clinic, provide information and personalized advice to women who are contemplating screening.
Posters around the clinic advertise the CCSP program and encourage patients to talk to their providers about screening. HIV education talks given at the time of patient enrollment include information about basic gynecologic care, cervical cancer, and strategies for screening and prevention. This knowledge is reinforced through group health talks that take place daily while patients are waiting for their clinic visit. Nurses, peer educators, and clinical officers have laminated information sheets that include basic information and color illustrations explaining reproductive anatomy and the screening procedures. Women who are eligible for screening are identified when their vital signs are taken, and then counseled in a private setting by a nurse, along with the general and HIV-specific health counseling that is part of their regular clinic visit. When they reach the screening room, they are prepared for the exam and can address any additional concerns with the clinical officer at that time.
Clinical information from the cervical cancer screening visits is captured in the patients’ medical record and entered into an electronic database. Separate clinical encounter forms are created for women who undergo procedures, including colposcopy or LEEP. Summaries of the number of women screened and treated are reported to the clinical staff on a weekly, monthly, and quarterly basis. Nurses and CCHAs ask women who do not accept screening their reasons for declining: this information is captured in the medical record so that it may be readdressed at future visits. Clinicians undergo structured interviews after their initial training to assess perceived challenges and opportunities experienced during the integration of CCSP into clinical care. For this evaluation, the patient and clinician responses are transcribed into separate databases that are analyzed for common themes by 2 separate researchers.
To assess the impact of the CCSP project, data collected during the first 3 years of the program, from October 2007 to October 2010, have been reviewed in the present study. Data were collected at Lumumba Hospital from October 1, 2007, and at Kisumu District Hospital from April 16, 2009, when the program expanded to provide services at a second FACES-supported HIV-care and treatment center. Publication of results from the CCSP project was approved by ethical review boards at the University of California, San Francisco, and the Kenya Medical Research Institute.
3. Results
From October 2007 to October 2010, 3642 women receiving HIV care at FACES-supported HIV care and treatment clinics in Kisumu were screened for cervical neoplasia with VIA as part of their routine care—representing 87% of the 4186 women offered screening. Among women who accepted screening, 531 (15%) underwent colposcopy for either positive or unsatisfactory VIA. CIN2/3 was diagnosed and confirmed histologically in 259 women (7.1%) and 243 LEEPs were performed, with no serious adverse events requiring treatment or referral.
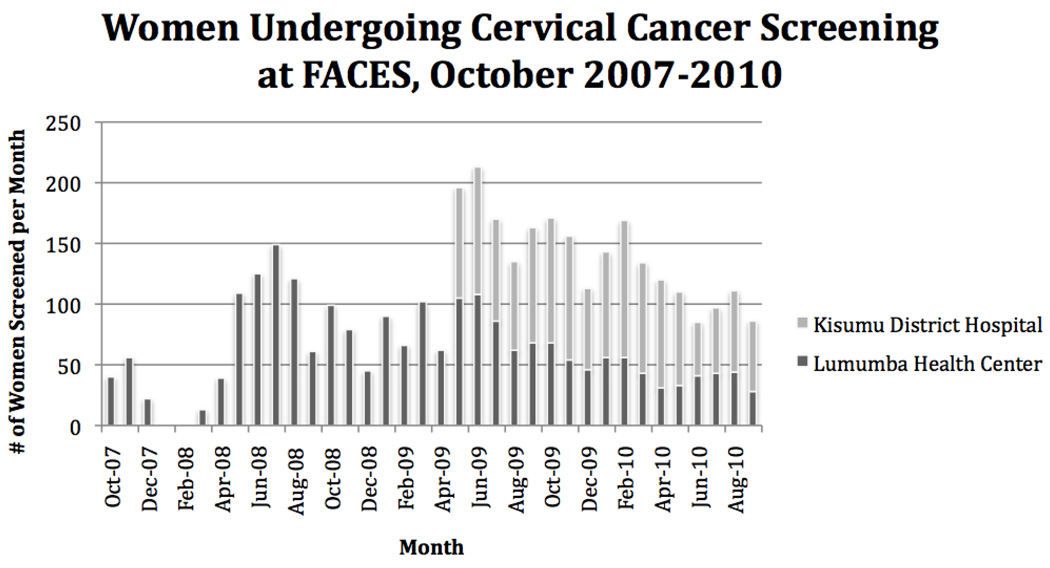
Eight women (0.1%) were diagnosed with invasive cancer. Two women had stage IIIB cervical cancer and were referred for radiation therapy, only available at the national hospital Nairobi. The remaining 6 women were diagnosed with stage I cervical cancer and were referred for surgery at the Provincial Hospital in Kisumu. In April 2009, the program expanded to provide services at a second FACES-supported HIV-care and treatment center located in the local district hospital, substantially increasing the number of women screened daily (Figure 2).
Figure 2.
Women undergoing cervical cancer screening at FACES from October 2007 to October 2010.
Among the 87% of women screened for cervical cancer, almost all accepted screening during the current visit (3496, 96%); the remaining women underwent screening on a first or second follow-up visit. Reasons for declining screening included “needing to talk with their husband”, “being on their menses”, “needing to think about it”, and expressing fear of the speculum exam. Only 18 women (<1%) who were approached reported having had screening in the past.
During the first 3 years of the program, 28 (90%) clinical officers underwent training in VIA and colposcopy. Three medical officers and 5 clinical officers were trained in LEEP. Staff reported a high level of satisfaction with their training and their role in implementing cervical cancer screening in the clinic (Table 1). The main challenges reported were related to infrastructure limitations (lack of water, electricity and supplies; and long waits in the clinic) and perceived patient barriers.
Table 1.
Clinician reported challenges and successes related to the training for and implementation of CCSP in FACES clinics (n=23)
| Challenges | No. (%) |
|---|---|
| Infrastructure limitations: lack of supplies, or power and/or water interruptions | 18 (78) |
| Not enough time: clinic too busy, or patient has waited too long | 10 (43) |
| Perceived patient discomfort: unfamiliar with and/or afraid of exam, or on menses |
5 (22) |
| Patient has child or children with her | 2 (9) |
| Successes | |
| More confident in performing pelvic exams | 23 (100) |
| More knowledgeable about cervical cancer | 23 (100) |
| VIA training will help future career | 21 (91) |
| Learning process and/or mentoring enjoyable | 19 (83) |
| Able to provide valuable service to patients | 18 (78) |
4. Discussion
Cervical cancer screening coupled with outpatient treatment of precancerous lesions has the potential to prevent the development of a large number of cervical cancers among HIV-infected women. Our experience demonstrates that cervical cancer screening is feasible and acceptable within the setting of an HIV care and treatment clinic. Measures of our success include the high acceptance of cervical cancer screening, a detection rate for cervical dysplasia similar to that seen in other HIV-infected populations, and an extremely low rate of post-LEEP adverse events.
Clinical officers with specialization in HIV care were successfully trained in cervical cancer screening and treatment techniques, and reported an overall high level of satisfaction with learning these new skills and incorporating them into comprehensive HIV care. VIA was chosen for cervical cancer screening because it has been shown to detect CIN2/3 with a sensitivity of 60–86% and a specificity ranging from 64% to 94%, similar to the values achieved by cytology screening. However, it has a relatively low positive predictive value (9–27%) and has not been validated specifically in HIV-infected populations [14]. Although we are confident that VIA will perform similarly among HIV-infected women, we feel that the implications of overtreatment in this population deserve special consideration, including the potential to increase HIV infectiousness through the presence of increased amounts of virus in blood or genital secretions after treatment. In addition, LEEP was chosen for treatment of cervical cancer because it has been shown to be safe and has significantly better outcomes in HIV-infected women than cryotherapy—an alternative outpatient treatment—with minimal differences in adverse events [15]. Moreover, LEEP falls within the scope of practice of clinical officers working in Kenya.
Staff acceptance of and enthusiasm for the program is essential for the continued success of the project. The multi-disciplinary CCSP team meets monthly to discuss the needs, challenges, and successes as they relate to different disciplines. This has fostered a sense of program-wide ownership of cervical cancer screening. In addition, feedback from the clinical staff interviews is incorporated into training programs and implementation strategies in an ongoing fashion.
This program was funded through a seed grant, and therefore depends heavily on the existing infrastructure of the HIV clinic. Universal access to cervical cancer screening will require programs that can be scaled up to more remote areas that have even fewer resources and lower levels of staffing. Sustainability will depend on the validation of low-cost screening tests among HIV-infected women to determine whether the time, expense, and infrastructure requirements of colposcopy can be removed, and screening can be paired with same-day treatment to reach more remote sites. As organizations begin to recognize fully the importance of reproductive health and the burden of cervical cancer in HIV-infected women, low-cost screening programs based in HIV care and treatment clinics may become the model of low-cost, high-impact, public health strategies. Ultimately, this will generate more public awareness and increase the pool of trained clinical staff so that universal access to screening and treatment becomes available in resource-limited settings.
Acknowledgments
This project was supported through the donation of an electrosurgical generator by Cooper-Surgical. While working on this project, M.J.H. was supported through a National Institutes of Health career development award (KL2 RR024130-04).
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors have no conflicts of interest.
References
- 1.Palefsky J. Human papillomavirus-related disease in people with HIV. Curr Opin HIV AIDS. 2009;4(1):52–56. doi: 10.1097/COH.0b013e32831a7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Parkin DM, Ferlay J, Hamdi-Cherif M. Cancer in Africa: Epidemiology and Prevention. Vol. 268. Lyon, France: IARC Press; 2003. Cervix Cancer; pp. 268–276. [Google Scholar]
- 4.Goldie SJ, Gaffikin L, Goldhaber-Fiebert JD, Gordillo-Tobar A, Levin C, Mahé C, et al. Cost-effectiveness of cervical-cancer screening in five developing countries. N Engl J Med. 2005;353(20):2158–2168. doi: 10.1056/NEJMsa044278. [DOI] [PubMed] [Google Scholar]
- 5.Castellsagué X, de Sanjosé S, Aguado T, Louie KS, Bruni L, Muñoz J, et al. HPV and Cervical Cancer in the World. 2007 Report. WHO/ICO Information Centre on HPV and Cervical Cancer (HPV Information Centre) www.who.int/hpvcentre http://www.who.int/hpvcentre/publications/HPVReport2007.pdf? Published 2007.
- 6.Tsu VD, Levin CE. Making the case for cervical cancer prevention: what about equity? Reprod Health Matters. 2008;16(32):104–112. doi: 10.1016/S0968-8080(08)32411-2. [DOI] [PubMed] [Google Scholar]
- 7.UNAIDS, WHO. AIDS Epidemic Update. www.unaids.orgPublished 2010.
- 8.National AIDS and STI Control Programme, Ministry of Health, Kenya. Kenya AIDS Indicator Survey. Preliminary Report. www.aidskenya.org. http://www.aidskenya.org/public_site/webroot/cache/article/file/KAIS__Preliminary_Report.pdf. Published 2008.
- 9.Bendavid E, Bhattacharya J. The President’s Emergency Plan for AIDS Relief in Africa: an evaluation of outcomes. Ann Intern Med. 2009;150(10):688–695. doi: 10.7326/0003-4819-150-10-200905190-00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO. Comprehensive cervical cancer control: a guide to essential practice. www.who.int. http://whqlibdoc.who.int/publications/2006/9241547006_eng.pdf.Published 2006. [PubMed]
- 11.Denny L, Kuhn L, De Souza M, Pollack AE, Dupree W, Wright TC., Jr Screen-and-treat approaches for cervical cancer prevention in low-resource settings: a randomized controlled trial. J Am Med Assoc. 2005;294(17):2173–2181. doi: 10.1001/jama.294.17.2173. [DOI] [PubMed] [Google Scholar]
- 12.Sankaranarayanan R, Esmy PO, Rajkumar R, Muwonge R, Swaminathan R, Shanthakumari S, et al. Effect of visual screening on cervical cancer incidence and mortality in Tamil Nadu, India: a cluster-randomised trial. Lancet. 2007;370(9585):398–406. doi: 10.1016/S0140-6736(07)61195-7. [DOI] [PubMed] [Google Scholar]
- 13.Bm RH, Woo W, Walker P. Internal Federation for Cervical Pathology and Colposcopy nomenclature and treatment for cervical preinvasive disease. J Low Genit Tract Dis. 2006;10(1):51–54. doi: 10.1097/01.lgt.0000192698.38915.1a. [DOI] [PubMed] [Google Scholar]
- 14.Gaffikin L, Lauterbach M, Blumenthal PD. Performance of visual inspection with acetic acid for cervical cancer screening: a qualitative summary of evidence to date. Obstet Gynecol Surv. 2003;58(8):543–550. doi: 10.1097/01.OGX.0000079632.98372.26. [DOI] [PubMed] [Google Scholar]
- 15.Chirenje ZM, Rusakaniko S, Akino V, Mlingo M. A randomised clinical trial of loop electrosurgical excision procedure (LEEP) versus cryotherapy in the treatment of cervical intraepithelial neoplasia. J Obstet Gynaecol. 2001;21(6):617–621. doi: 10.1080/01443610120085618. [DOI] [PubMed] [Google Scholar]