Abstract
Purpose
We assessed risk factors for end stage renal disease (ESRD) in Wilms Tumor (WT) patients without known WT1-related syndromes. We hypothesized that patients with characteristics suggestive of a WT1 etiology (early onset, stromal predominant histology, intralobar nephrogenic rests) would have a higher risk of ESRD due to chronic renal failure (CRF). We predicted a high risk of ESRD due to progressive bilateral WT (PBWT) in patients with metachronous bilateral disease.
Methods
ESRD was ascertained in 100 of 7,950 non-syndromic patients enrolled on a National Wilms Tumor Study during 1969-2002. Risk factors were evaluated with cumulative incidence curves and proportional hazard regressions.
Results
The cumulative incidence of ESRD due to CRF 20 years after WT diagnosis was 0.7%. For ESRD due to PBWT, it was 4.0% at 3 years post-WT diagnosis in patients with synchronous BWT and 19.3% in patients with metachronous BWT. For ESRD due to CRF, stromal predominant histology had a hazard ratio (HR) of 6.4 relative to mixed (95% CI [3.4 11.9], p <.001); intralobar rests had a HR of 5.9 relative to no rests (95% CI[2.0, 17.3], p = .001); and WT diagnosis <24 months had a HR of 1.7 relative to 24-48 months and 2.8 relative to >48 months (p=.003 for trend).
Conclusions
Metachronous BWT is associated with high rates of ESRD due to surgery for progressive WT. Characteristics associated with a WT1 etiology markedly increased the risk of ESRD due to CRF, despite a low risk in non-WT1 syndromic patients overall.
Keywords: Kidney Failure, Chronic; Nephrectomy; Wilms Tumor; Genes, Wilms Tumor
Introduction
Treatment advances for Wilms tumor (WT) have greatly improved survival, with cure rates now approaching 90%.1 Survivors of WT are at increased risk of additional health problems, however, due to direct effects of treatment and to genetic conditions associated with the disease. End-stage renal disease (ESRD) is a particularly serious outcome, and identification of risk factors for ESRD in the WT patient population is a clinically important goal.
A prior National Wilms Tumor Study (NWTS) report estimated that unilateral WT patients had a 20-year cumulative incidence of ESRD of 1.3%; it was 15% for bilateral WT (BWT).2 High rates of ESRD were found among patients with the Wilms tumor-aniridia (WAGR) and Denys-Drash (DDS) syndromes and those with associated male genitourinary (GU) anomalies, all caused by germline WT1 mutations.3,4 Over 70% of DDS patients developed early onset ESRD, while approximately 40% of WAGR patients developed ESRD by young adulthood.
The present study investigates risk factors for ESRD in patients without WT1 associated syndromes, using an expanded NWTS cohort with longer follow-up. We consider separately ESRD due to (1) progressive BWT (PBWT) and (2) chronic renal failure (CRF), the latter encompassing ESRD attributable to other or unspecified etiologies. BWT may be synchronous or metachronous. There is a greater chance of saving part of one kidney and thus preventing renal failure in patients with synchronous disease, who have 2 kidneys when the BWT is diagnosed. Moreover, tumor cells that develop in the contralateral kidney while the patient is undergoing primary treatment may be less likely to respond to further treatment than those responsible for synchronous bilateral disease.5 We hypothesized, therefore, that patients with metachronous BWT would experience higher rates of treatment failure leading to ESRD from PBWT than those with synchronous BWT.
Host factors may predispose individuals to non-malignant kidney pathology, and thus to ESRD due to CRF, even if it is an apparent consequence of radiation or chemotherapy. Early age at diagnosis, stromal predominant histology and the presence of intralobar nephrogenic rests (ILNR) are strongly associated in the NWTS cohort.6 Since these 3 characteristics are also associated with germline WT1 mutations,7,8 we suggested earlier that they could be markers for a WT1 etiology.9 Here we further hypothesized that these factors were predictors of ESRD due to CRF among patients who did not have known WT1 related syndromes. We also considered how clinical risk factors were related to ESRD due to PBWT.
Our earlier ESRD study limited the GU anomaly category to hypospadias and cryptorchidism.2 These two anomalies were used here as a basis for exclusion due to the presence of a WT1 associated syndrome. However, we also examined the association between ESRD due to CRF and other genital anomalies, specifically streak ovaries and disorders of sexual differentiation (DSD), that are associated with WT1 mutations and may represent a forme fruste of DDS.10
Methods
Study and analysis cohort
The complete cohort consisted of 9,237 patients enrolled by North American institutions on 1 of 5 NWTS protocol studies between October, 1969 and April, 2002.11 The closing date for data acquisition was December 31, 2008. Patients with WAGR syndrome (n=69), DDS (n=37) or male GU anomalies (n=217) were excluded from the non-WT1-syndromic cohort,. Additional exclusion factors, in order, included WT in an extra-renal site (n=22), a missing histology record (n=276) or a histologic type recorded as rhabdoid tumor of the kidney, clear cell sarcoma of the kidney or as another rare, indeterminant or non-WT type (n=665). The final non-WT1-syndromic cohort consisted of 7,950 subjects. Brief results are reported separately for the syndromic patients and those with rare histologic types.
Definition of endpoint
WT patients were considered to have ESRD if they required long-term dialysis or kidney transplant, or if they died of renal failure before treatment for ESRD was initiated (n=7, of which 5 WAGR and 1 DDS). ESRD was categorized as due to PBWT if the patient required surgical removal of all kidney tissue as a consequence of PBWT. ESRD occurring in patients with unilateral WT, or in those with BWT who had been in complete remission for at least 1 year with no intervening relapse, was categorized as due to CRF.
Classification of risk factors
Age at WT diagnosis was categorized as 0-23, 24-47, and 48+ months. Favorable histology tumors were classified as blastemal, epithelial or stromal predominant if a corresponding histologic pattern comprised more than 2/3 of the available tumor sections, otherwise as mixed. The few tumors with anaplastic histology were classified as anaplastic regardless of the mixture of cell types. Nephrogenic rests were classified as intralobar, perilobar or none; patients with both types were assigned to intralobar. This factor was available only for patients on NWTS studies 3-5 who had normal kidney tissue available for pathology evaluation.
Statistical methods
Cumulative incidence of ESRD of specific type as a function of time since WT diagnosis was estimated for various patient subgroups, treating death and ESRD due to the other cause as competing events.12,13 Cox regression was used to examine relationships between risk factors and ESRD rates, with event times censored by death, loss to follow-up, termination of the study or development of ERSD of the other type. The risk factor analyses for ESRD due to CRF were stratified by unilateral WT vs. BWT; for metachronous BWT the stratification was time-dependent. Stratified regressions of ESRD due to PBWT were restricted to individuals with synchronous BWT or metachronous disease after contralateral relapse. Both single and multiple regressions were examined to determine marginal and adjusted relationships between risk factors and ESRD rates. Tests of proportionality of ESRD rates were reported as significant for p <.10. Instantaneous hazards were estimated from cumulative hazards by kernel smoothing with a 5 year bandwidth. All statistical analyses were conducted with R 2.11.14
Results
Patients with WT1 syndromes
Table 1 shows estimates of cumulative incidence of ESRD at 20 years since WT onset in patient anomaly groups. Thirty of 37 subjects with DDS developed ESRD, 6 prior to or at the same time as their diagnosis of WT. Among GU anomaly patients, those with both cryptorchidism and hypospadias had the highest cumulative incidence; however, a test for differences in hazard rates across the 3 GU anomaly subgroups was not significant (p=.25).
Table 1. Cumulative incidence of ESRD at 20 years since WT onset in WT1 syndromic patients.
| No. Patients | Cases of ESRD PBWT | CRF | Total | Cumulative Incidence | 95% CI | |
|---|---|---|---|---|---|---|
| DDS | 37 | 1 | 29 | 30 | 82.7 | (60.5, 92.4) |
| WAGR | 69 | 1 | 18 | 19 | 43.3 | (20.8, 59.5) |
| GU Anomalies | 217 | 6 | 14 | 20 | 9.4 | (4.2, 14.4) |
| Cryptorchidism only | 139 | 4 | 10 | 14 | 10.5 | (3.5, 17.1) |
| Hypospadias only | 46 | 0 | 1 | 1 | 0.0 | (0.0, 0.0) |
| Both | 32 | 2 | 3 | 5 | 16.6 | (0.0, 31.1) |
Subjects with unusual or missing histologic types
Eleven cases of ESRD occurred among 941 patients with rare or missing histologic types. Three cases of ESRD due to PBWT occurred in 9 of 45 patients with teratoid histology who had synchronous (n=7) or metachronous (n=2) BWT. Thirteen patients with intralobar rests, but only 2 with perilobar rests and none with both types, were identified among the 34 patients with teratoid histology who were evaluable for nephrogenic rests.
The non-WT1-syndromic cohort
The main analysis cohort consisted of 512 patients with synchronous BWT at diagnosis, 7,351 patients with unilateral WT, and 87 patients with metachronous BWT. One unilateral WT patient diagnosed with ESRD due to hemolytic uremic syndrome 197 days before WT was excluded from all further analyses, bringing the main cohort size to 7,949. We identified 100 cases of ESRD in the main cohort, 45 classified as due to PBWT and 55 to CRF. Table 2 tabulates patient ESRD outcomes by risk factors. Additional demographic information and putative causes of ESRD for patients with CRF is available as supplementary material.*
Table 2. ESRD outcomes by risk factors in the non-WT1 syndromic cohort.
| ESRD Status | ||||||
|---|---|---|---|---|---|---|
| None | PBWT | CRF | ||||
| Age at WT Diagnosis (months) | N | % | N | % | N | % |
| 0-23 | 2171 | 27.7 | 28 | 62.2 | 29 | 52.7 |
| 24-47 | 2578 | 32.8 | 10 | 22.2 | 16 | 29.1 |
| >48 | 3100 | 39.5 | 7 | 15.6 | 10 | 18.2 |
| Histology | ||||||
| Mixed | 3734 | 47.6 | 29 | 64.4 | 28 | 50.9 |
| Blastemal | 2394 | 30.5 | 5 | 11.1 | 9 | 16.4 |
| Epithelial | 845 | 10.8 | 3 | 6.7 | 3 | 5.5 |
| Stromal | 208 | 2.7 | 3 | 6.7 | 15 | 27.3 |
| Anaplastic | 668 | 8.5 | 5 | 11.1 | 0 | 0 |
| Nephrogenic Rests* | ||||||
| No rests detected | 3219 | 59.3 | 5 | 16.7 | 6 | 27.3 |
| Perilobar | 1121 | 20.7 | 10 | 33.3 | 2 | 9.1 |
| Intralobar or both | 1087 | 20.0 | 15 | 50.0 | 14 | 63.6 |
| Radiation Dose to Contralateral Kidney (Gy) | ||||||
| None | 6904 | 88 | 34 | 75.6 | 44 | 80.0 |
| 0.1-14.9 | 723 | 9.2 | 5 | 11.1 | 5 | 9.1 |
| 15.0+ | 221 | 2.8 | 6 | 13.3 | 6 | 10.9 |
Intralobar and perilobar nephrogenic rests were not evaluable in 2470 subjects.
Median follow-up for surviving patients in the main cohort was 14.2 years; 4,537 patients were still under observation at 10 years from WT diagnosis, 2,082 at 20 years. The average annual loss-to-follow-up rate was 1.5% for the first 10 years and 1.8% for the next 10.
Cumulative incidence of ESRD
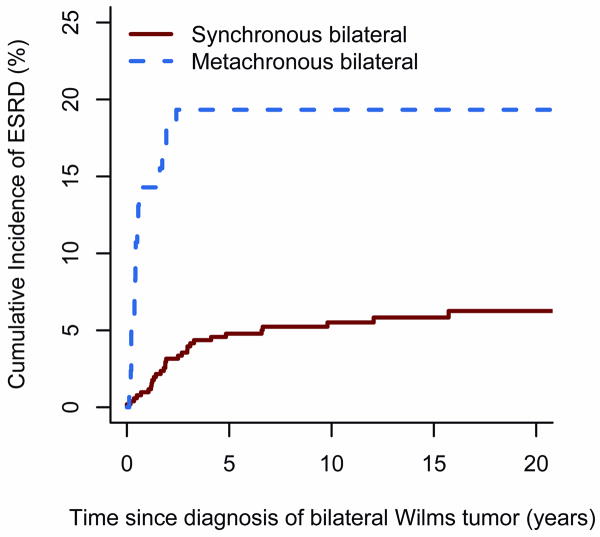
The 20-year cumulative incidence of ESRD of either type in non-syndromic patients was 1.7% (95% CI[1.3,2.1]). Figure 1 plots the cumulative incidence of ESRD due to PBWT for patients with synchronous and metachronous BWT. Twenty nine patients with synchronous BWT developed ESRD due to PBWT, as did 16 with metachronous disease. The percentages of patients who survived and developed ESRD due to PBWT within 3 years of bilateral diagnosis were 4.0% (95% CI[2.3, 5.7]) for synchronous and 19.3% (95% CI[10.7,27.9]) for metachronous BWT. Nine cases of ESRD due to PBWT occurred 3 or more years after synchronous BWT diagnosis. All but one of these late ESRD events coincided with the date of nephrectomy, either for persistent WT (n=1), or a first (n=5), second (n=1) or third (n=1) relapse in the residual kidney. One patient, who developed renal failure 9 years after partial bilateral nephrectomy and a subsequent relapse, was found to have WT regrowth in the remaining kidney prior to transplant, indicating PBWT as the cause of ESRD. The hazard ratio for patients with metachronous vs. synchronous BWT was 4.9 (95% CI[2.6, 9.0], p<.001).
Figure 1.
Cumulative incidence of ESRD due to PBWT by time since diagnosis of BWT for patients with synchronous vs. metachronous BWT.
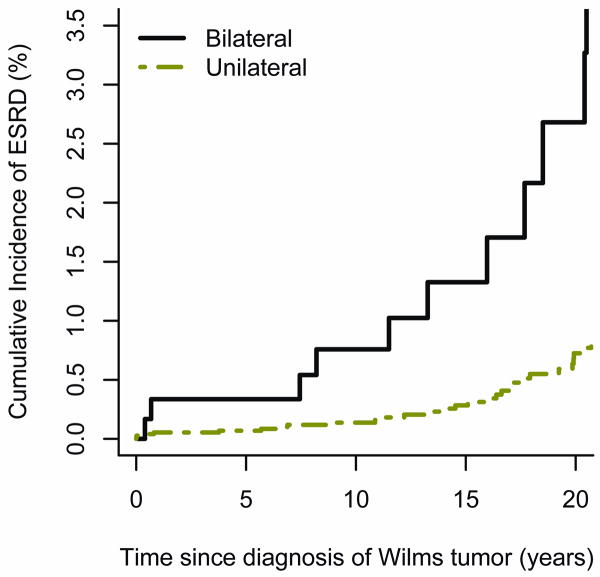
Figure 2 plots the cumulative incidence of ESRD due to CRF for patients with unilateral vs. bilateral disease. Forty patients with unilateral WT developed ESRD due to CRF, as did 14 with synchronous and 1 with metachronous BWT. The cumulative incidence among patients with unilateral WT was 0.7 % (95% CI[0.4, 1.0]) at 20 years; it was 3.1 % (95% CI[0.9, 5.3]) for those with BWT. The estimated hazard ratio was 5.9 (95% CI[3.2,10.6], p<.001) for BWT vs. unilateral WT. In both groups the cumulative incidence rose steadily from diagnosis for 20 years and beyond.
Figure 2.
Cumulative incidence of ESRD due to CRF by time since WT diagnosis for patients with unilateral vs. bilateral disease.
Table 3 shows the results of fitting separate univariate regression models, each of which will be discussed in turn.
Table 3. Single predictor Cox regression analyses of ESRD due to PBWT and CRF.
| ESRD status | ||||||
|---|---|---|---|---|---|---|
| CRF | PBWT | |||||
| HR | 95% CI | p | HR | 95% CI | p | |
| Age at WT diagnosis (months) | 0.003 | 0.05 | ||||
| >48 (ref) | ||||||
| 0-23 | 2.8 | (1.4, 5.6) | 0.004 | 2.1 | (0.9, 4.9) | 0.08 |
| 24-48 | 1.6 | (0.7, 3.6) | 0.24 | 1.1 | (0.4, 2.8) | 0.92 |
| HR | 95% CI | p | HR | 95% CI | p | |
| Histologic Type | <.001 | 0.11 | ||||
| Mixed (ref) | ||||||
| Blastemal | 0.6 | (0.3, 1.2) | 0.12 | 0.3 | (0.1, 0.9) | 0.02 |
| Epithelial | 0.4 | (0.1, 1.3) | 0.14 | 0.3 | (0.1, 1.1) | 0.07 |
| Stromal | 6.4 | (3.4, 11.9) | <.001 | 0.9 | (0.3, 2.7) | 0.82 |
| Anaplastic | 0.8 | (0.3, 2.1) | 0.68 | |||
| HR | 95% CI | p | HR | 95% CI | p | |
| Nephrogenic Rests* | <.001 | 0.10 | ||||
| No rests detected (ref) | ||||||
| Intralobar or both | 5.9 | (2, 17.3) | 0.001 | 1.4 | (0.6, 3.7) | 0.45 |
| Perilobar | 0.5 | (0.1, 2.8) | 0.43 | 0.6 | (0.2, 1.6) | 0.31 |
| HR | 95% CI | p | HR | 95% CI | p | |
| NWTS Study Cohort | 0.94 | 0.95 | ||||
| I (ref) | ||||||
| II | 0.8 | (0.3, 1.9) | 0.57 | 0.7 | (0.1, 4.1) | 0.73 |
| III | 0.6 | (0.3, 1.5) | 0.28 | 1.0 | (0.3, 3.5) | 0.97 |
| IV | 1.0 | (0.4, 2.5) | 0.96 | 1.3 | (0.4, 4.4) | 0.63 |
| V | 0.9 | (0.2, 3.8) | 0.91 | 0.9 | (0.2, 3.1) | 0.83 |
| HR | 95% CI | p | HR | 95% CI | p | |
| Radiation Dose to Contralateral Kidney (Gy) | 0.18 | 0.06 | ||||
| None (ref) | ||||||
| 0.1-14.9 | 0.9 | (0.3, 2.2) | 0.76 | 0.8 | (0.3, 2) | 0.59 |
| 15.0+ | 2.3 | (0.9, 5.7) | 0.08 | 4.2 | (1.6, 10.7) | 0.003 |
HR: hazard ratio; p: Wald p-value.
Overall p-values are from group tests for categorical variables and tests of the variable as a linear predictor for ordinal variables.
Intralobar and perilobar nephrogenic rests were not evaluable in 2470 subjects.
Age at WT diagnosis
Diagnosis of WT prior to 24 months of age was more common in those with ESRD due to PBWT than in unaffected patients (Table 2) and was associated with a 2-fold increase in the rate of ESRD due to PBWT (p=.05 for trend with age category after stratification). In an analysis limited to the 28 cases of ESRD due to PBWT that occurred among 240 BWT patients diagnosed before 24 months, the hazard of ESRD due to PBWT decreased 10% with each additional month of age at WT diagnosis (per-month HR .90, 95% CI[0.84, .97], p=.003 after stratification), suggesting the highest rates occurred in the very young.
Over half of CRF patients were diagnosed with WT before 24 months (Table 2). Early WT diagnosis predicted a 70% increase in rate of ESRD due to CRF relative to those diagnosed between 24-47 months and a 2.8 fold increase relative to those diagnosed after 48 months (p=.003 for test of trend with age category after stratification). However, among those diagnosed before 24 months, the ESRD hazard did not decrease with increasing age at diagnosis (per-month HR 1.04, 95% CI[.99,1.10], p=.09).
Histologic type
Strikingly, 27% of patients with ESRD due to CRF had stromal predominant histology compared with 7% for ESRD due to PBWT and 3% for no ESRD (Table 2). Among patients who did not develop ESRD, blastemal and epithelial predominant histology were relatively frequent. Mixed histology was the most common type among all patients (48%, n=3791).
In a regression stratified on bilateral status, rates of ESRD due to CRF in those with stromal predominant histology were 6.4 times those for mixed histology (p <.001) and more than 10 times those for blastemal and epithelial predominant histology (Table 3). For ESRD due to PBWT, by contrast, the HR for ESRD among those with stromal predominant histology relative to mixed was under 1.0 and not statistically significant (p=.82).
Nephrogenic rests
The occurrence of ILNR, whether or not perilobar rests were also present, was 2-3 times more common among patients who later developed ESRD due to PBWT (50%, n=15) and CRF (64%, n=14) versus no ESRD (20%, n=1087) (Table 2). Associations between nephrogenic rests and ESRD due to PBWT, however, reflect the association between nephrogenic rests and bilaterality: patients with BWT were much more likely to have nephrogenic rests of either type [(52%, n=208) with perilobar only and 32% (n=124) with intralobar or both] compared to unilateral patients [(18%, n=926) with perilobar only and 20% (n=992) with intralobar or both]. For patients with BWT, stratified regressions indicated that neither perilobar nor intralobar rests were significantly predictive of a higher hazard of ESRD due to PBWT (Table 3).
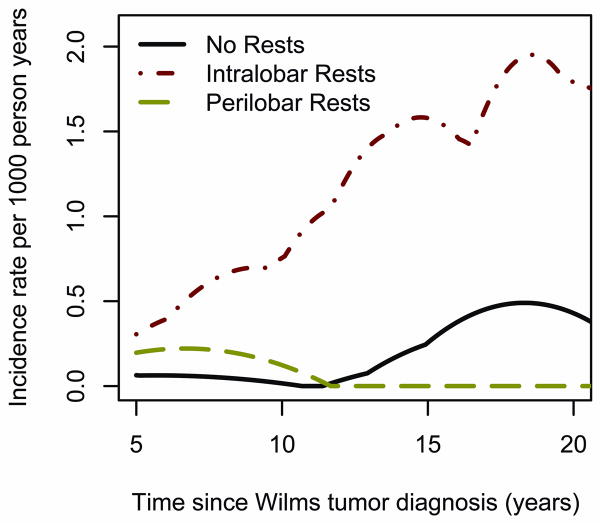
By contrast, stratified regression indicated ILNR predicted 6-fold higher rates of ESRD due to CRF (p=.001). The hazard rate for patients with ILNR increased with time since unilateral WT diagnosis (Figure 3), although non-proportionality tests were not significant.
Figure 3.
Smoothed hazard rates for ESRD due to CRF among patients with unilateral WT classified according to presence or absence of nephrogenic rests.
Other GU anomalies
In the non-syndromic cohort, 5 patients had potentially WT1-related genital anomalies, two children with DSD and three with streak ovaries not related to radiation treatment or other anomaly syndromes (e.g., Turner syndrome). Stromal histology was present in 2/5 patients; the rest had mixed histology. Three patients had nephrogenic rests; one had ILNR while 2 were diagnosed before nephrogenic rests were distinguished by type. All 5 patients had WT before 2 years of age.
Two of the 5 patients were diagnosed with ESRD due to CRF. The child with ovotesticular DSD had unilateral WT with stromal predominant histology and developed ESRD due to “chronic kidney disease, etiology unknown” 23 years after WT diagnosis. One girl with “streak gonads” had unilateral WT with mixed histology and developed ESRD due to focal-segmental glomerulosclerosis 24 years after WT diagnosis. Neither had evidence of nephrogenic rests. While the risk of ESRD was much higher in this small group than among other “non-syndromic” patients, precise estimates of the HR were not possible due to the small numbers.
The significant univariate associations between WT1-related predictors and ESRD due to CRF were not much changed if these patients were excluded: ILNR had a HR of 5.9 (95% CI [2.0,17.3], p < .001) and stromal predominant histology had a HR of 6.2 (95% CI[3.2,11.7], p<.001).
Other Predictors
Stratified univariate regressions did not suggest differences in rates of ESRD due to PBWT or CRF by study cohort (Table 3). Radiation of 15 Gy or more to the contralateral kidney was associated with an increased risk of ESRD, particularly of ESRD due to PBWT for which the HR was 4.2 (p=.003).
Multiple regressions for CRF and PBWT ESRD
Results of multiple Cox regression analyses are shown in Table 4. Although restricted to patients from NWTS 3-5 for whom nephrogenic rest status was evaluable, and with different reference categories for some factors as a result of having collapsed factor levels, the results are broadly consistent with those obtained from the separate univariate regressions. The exclusion resulted in a loss of 33 cases of ESRD due to CRF since the earlier studies had the longest follow-up and incidence rises sharply with increasing follow-up. Confidence limits widened accordingly. For ESRD due to CRF, the hazard ratios associated with stromal predominant histology and young age at diagnosis increased somewhat in the multiple regression analysis, while those associated with intralobar rests decreased. Nonetheless, both stromal histology and ILNR remained statistically significant. The multiple predictor model for ESRD due to PBWT was similar to the univariate regressions in that young age at diagnosis and high radiation dose predicted increased risk.
Table 4. Multiple predictor Cox regression analyses of ESRD due to CRF and PBWT.
| ESRD status | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| CRF | PBWT | ||||||||
| No. Patients* | Cases | HR | 95% CI | p | Cases | HR | 95% CI | p | |
| Age at WT diagnosis (months) | |||||||||
| >24 (ref) | 3999 | 7 | 11 | ||||||
| 0-23 | 1480 | 15 | 2.0 | (0.7, 5.9) | 0.22 | 19 | 2.5 | (1.0, 5.9) | 0.04 |
| Histologic type | |||||||||
| All others (ref) | 5331 | 14 | 28 | ||||||
| Stromal | 148 | 8 | 7.8 | (3.0, 20.6) | <.001 | 2 | 1.4 | (0.4, 5.4) | 0.62 |
| Nephrogenic rests | 0.04 | 0.56 | |||||||
| None identified (ref) | 3230 | 6 | 5 | ||||||
| Intralobar or both | 1133 | 2 | 3.8 | (1.2, 11.8) | 0.02 | 10 | 1.0 | (0.4, 2.5) | 0.92 |
| Perilobar rests | 1116 | 14 | 0.7 | (0.1,3.7) | 0.67 | 15 | 0.6 | (0.2, 1.8) | 0.37 |
| Radiation Dose to Contralateral Kidney (Gy)** | 0.3 | (.1, 1.7) | 0.17 | 1.8 | (0.9, 3.8) | 0.12 | |||
| None | 4859 | 20 | 24 | ||||||
| 0.1-14.9 | 533 | 2 | 4 | ||||||
| 15.0+ | 87 | 0 | 2 | ||||||
HR: hazard ratio; p: Wald p-value.
Patients limited to those with evaluable nephrogenic rests.
Radiation Dose to contralateral kidney was treated as a grouped linear predictor in the regression.
Discussion
The results in the present study confirm that patients with congenital anomaly syndromes associated with WT1 mutations accounted for a large fraction of ESRD cases, especially in patients with unilateral WT. By 20 years from WT diagnosis, the cumulative incidence of ESRD was 83% in patients with DDS, 43% in those with WAGR syndrome and 9% in those with associated GU anomalies.
Our prior study2 considered separately ESRD occurring in patients with unilateral vs. bilateral disease because of the obvious risk of ESRD due to PBWT. With increasing follow-up came the recognition that this dichotomy was no longer adequate. Several cases of ESRD occurred in patients who had long been cured of their BWT and had been functioning without even a single intact kidney. It seemed reasonable to suppose that the subsequent decline in their renal function might be influenced by the same factors as those operating in patients with unilateral WT. Consequently, all ESRD due to factors other than PBWT was grouped under the rubric “ESRD due to CRF”.
Since the high risk of ESRD in patients with WT1 related syndromes and anomalies was becoming well known, our attention shifted to the much larger cohort of non-syndromic patients, in whom an increasing fraction of the ESRD cases occurred as follow-up lengthened. Results of the statistical analyses confirmed the hypotheses specified a priori: once the risk of PBWT had passed, ESRD occurred preferentially in patients whose WT phenotype was consistent with a WT1 etiology.
ESRD due to progressive bilateral Wilms tumor
The cumulative incidence curves for ESRD due to PBWT showed a rapid accumulation of cases shortly after the diagnosis of BWT, whether synchronous or metachronous. All of these cases became anephric following removal of their cancerous kidneys. The earliest cases occurred in patients who never achieved remission from their BWT. Later cases occurred in patients who had one or more periods of remission followed by relapse leading to ESRD.
Three years from the diagnosis of BWT, the cumulative incidence of ESRD due to PBWT was nearly 5 fold higher in patients with metachronous compared to synchronous BWT. This dramatic finding is consistent with our expectation that aggressive disease developing after initial treatment in a solitary remaining kidney may require surgical excision of all remaining kidney tissue to prevent further progression and metastasis.
Radiation doses to the contralateral kidney of 15 Gy and above predicted higher rates of ESRD due to PBWT, possibly reflecting more aggressive surgical and adjuvant treatments for patients with more severe disease. We found no association of NWTS study with ESRD due to PBWT. However, treatment for BWT was not specified by study protocols and varied substantially, from complete bilateral nephrectomy to treatment with chemotherapy and radiation only.15
A diagnosis of WT under the age of 2 years increased the risk of ESRD due to PBWT, and younger children in this age range had even higher risk. This effect persisted in multiple regressions that adjusted for histology and nephrogenic rests. It is possibly a chance finding, however, given lack of prior hypotheses or adjustment for multiple testing.
ESRD due to CRF
The cumulative incidence of ESRD due to CRF at 20 years from WT diagnosis was low: 3.1% for patients with BWT and under 1% for those with unilateral WT. Monitoring of renal function was not part of the original study design, and we are unable to report the stages of chronic kidney disease as defined by the National Kidney Foundation. It is likely, however, that the cumulative incidence of chronic renal insufficiency (CRI) may be considerably higher than that of ESRD in the non-syndromic WT patient population. Rates of CRI are elevated in adult cancer patients with unilateral and bilateral nephrectomies,16,17 CRI predicts serious health outcomes, and may eventually evolve into ESRD.18 With longer follow-up, we will likely see the cumulative incidence of ESRD due to CRF continue to steadily but gradually increase.
Our results provide circumstantial support for the hypothesis that germline WT1 mutations leading to WT may predispose to renal failure and ESRD due to CRF, even in patients without the classic WT1 syndromes. In univariate regression analyses a stromal predominant histology increased the incidence rates more than 6 fold compared to mixed favorable histology, even more so in comparison to blastemal or epithelial predominant histology, while the presence of ILNR increased them nearly 6 fold. Diagnosis of WT before 2 years of age predicted a 2-fold increase in rates of ESRD relative to those diagnosed between 2 and 4. Estimates of the frequency of germline WT1 mutations among WT patients are generally on the order of 5% or less overall, and only 2% in the non-syndromic subgroup.19,20, 21 Thus a large fraction of the cases of ESRD due to CRF may occur in a relatively small subset of the WT population.
Molecular analysis has revealed a variety of WT1 mutations that are associated with a diversity of WT phenotypes.8 WT1 mutations may be associated with kidney pathology in patients who lack all criteria for specific syndromes.22 WT1 mutations with or without genital anomalies and WT may lead to steroid resistant nephrotic syndrome23 and diffuse mesangial sclerosis characteristic of DDS.24
We found two cases of ESRD due to CRF in patients with DSD who did not fall into the DDS or WAGR categories. In both cases ESRD occurred in young adulthood, which is characteristic of ESRD in patients with WAGR or Frasier syndromes rather than DDS.25 Their clinico-pathologic characteristics were also consistent with a WT1 mutation. These GU anomalies should likely be considered together with cryptorchidism and hypospadias as WT1-related risk factors of ESRD due to CRF in WT patients.
The putative causes of ESRD due to CRF mentioned on flow sheets and other clinical records were diverse, and may obscure a more direct relationship to WT1 mutation. WT1 expression plays a crucial role in normal kidney development as well as in maintenance of adult kidney function, where it continues to be expressed in the podocytes.26 Studies in mice show that disruption of the activity of WT1 may lead to fewer functional nephrons at birth,27 and histologic studies of WAGR patients indicate a reduction in size of glomeruli that is presumably related to the WT1 deletion.28 Reductions in nephron and podocyte number and mass could increase an individual's susceptibility to renal failure, particular in those with unilateral or partial bilateral nephrectomies.29,30 Reduced expression of WT1 in adult podocytes may reduce the glomerular filtration rate and eventually lead to glomerular sclerosis.31
Implications for Renal-sparing Surgery
Renal-sparing surgery has long been advocated for BWT. Tumor resections that leave the surrounding tissue intact aim to prevent or delay the onset of ESRD, thereby improving quality of life. On the other hand, radical nephrectomy is more successful at preventing relapse. A prior review of NWTS-4 BWT patients who had renal sparing procedures under modern surgical practices found that 8% had recurrence of WT, compared to 2% of those with radical bilateral nephrectomies.32
Some have suggested performing renal sparing surgery for children with unilateral WT, with the primary motivation of preventing renal failure.33,34 However, only the occasional child with WT will present with a lesion small enough to allow partial nephrectomy at diagnosis, e.g., tumors detected due to screening studies. Most WT are too large at diagnosis to allow partial nephrectomy and therefore require preoperative chemotherapy. Moreover, as with BWT, there is increased risk for local recurrence after partial nephrectomy for unilateral disease,35,36 and patients that develop intra abdominal relapse have a markedly decreased survival.37 Results of the present study will help identify unilateral patients most at risk of ESRD. Prospective studies can then clarify the role of renal sparing surgery in this select group of patients.
Conclusion
In summary, we hypothesize that in patients treated for WT, many, though not all, cases of ESRD due to CRF are related to underlying WT1 mutations, even without other phenotypic characteristics of WT1-related syndromes. To ground our hypothesis in more than circumstantial evidence, one would ideally conduct a retrospective molecular analysis using stored biological material to determine if patients who developed ESRD, especially ESRD due to CRF, were more likely to have WT1 mutations than the WT patient population in general. Results of such a study would help to quantify the associations between WT1 mutation and such phenotypic characteristics as stromal predominant histology and the presence of ILNR. Whether or not the hypothesis is borne out, we recommend that patients with these characteristics should undergo increased surveillance for chronic kidney disease and ESRD.
Supplementary Material
Key to Abbreviations
- ESRD
end stage renal disease
- WT
Wilms Tumor
- CRF
chronic renal failure
- PBWT
progressive bilateral Wilms Tumor
- BWT
bilateral Wilms Tumor
- WAGR
Wilms tumor-aniridia
- DDS
Denys-Drash syndrome
- GU
genitourinary
- DSD
disorder of sexual differentiation
- NWTS
National Wilms Tumor Study
Footnotes
References
- 1.Stiller CA, Parkin DM. International variations in the incidence of childhood renal tumours. Br J Cancer. 1990;62:1026. doi: 10.1038/bjc.1990.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breslow NE, Collins AJ, Ritchey ML, et al. End stage renal disease in patients with Wilms tumor: results from the National Wilms Tumor Study Group and the United States Renal Data System. J Urol. 2005;174:1972. doi: 10.1097/01.ju.0000176800.00994.3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diller L, Ghahremani M, Morgan J, et al. Constitutional WT1 mutations in Wilms' tumor patients. J Clin Oncol. 1998;16:3634. doi: 10.1200/JCO.1998.16.11.3634. [DOI] [PubMed] [Google Scholar]
- 4.Scott RH, Stiller CA, Walker L, Rahman N. Syndromes and constitutional chromosomal abnormalities associated with Wilms tumour. J Med Genet. 2006;43:705. doi: 10.1136/jmg.2006.041723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beckwith JB, Zuppan CE, Browning NG, et al. Histological analysis of aggressiveness and responsiveness in Wilms' tumor. Med Pediatr Oncol. 1996;27:422. doi: 10.1002/(SICI)1096-911X(199611)27:5<422::AID-MPO6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 6.Beckwith JB, Kiviat NB, Bonadio JF. Nephrogenic rests, nephroblastomatosis, and the pathogenesis of Wilms' tumor. Pediatr Pathol. 1990;10:1. doi: 10.3109/15513819009067094. [DOI] [PubMed] [Google Scholar]
- 7.Beckwith JB. Nephrogenic rests and the pathogenesis of Wilms tumor: developmental and clinical considerations. Am J Med Genet. 1998;79:268–273. doi: 10.1002/(sici)1096-8628(19981002)79:4<268::aid-ajmg7>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 8.Royer-Pokora B, Beier M, Henzler M, et al. Twenty-four new cases ofWT1 germline mutations and review of the literature: genotype/phenotype correlations for Wilms tumor development. Am J Med Genet. 2004;127A:249–257. doi: 10.1002/ajmg.a.30015. [DOI] [PubMed] [Google Scholar]
- 9.Breslow NE, Beckwith JB, Perlman EJ, et al. Age distributions, birth weights, nephrogenic rests, and heterogeneity in the pathogenesis of Wilms tumor. Pediatr Blood Cancer. 2006;47:260–267. doi: 10.1002/pbc.20891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schumacher V, Scharer K, Wuhl E, et al. Spectrum of early onset nephrotic syndrome associated with WT1 missense mutations. Kidney Int. 1998;53:1594–1600. doi: 10.1046/j.1523-1755.1998.00948.x. [DOI] [PubMed] [Google Scholar]
- 11.Dome JS, Perlman EJ, Ritchey ML, et al. Renal tumors. In: Pizzo PA, Poplack DG, editors. Principles and Practice of Pediatric Oncology, chapter 30. Lippincott, Williams & Wilkins; Philadelphia: 2005. pp. 905–932. [Google Scholar]
- 12.Gooley TA, Leisenring W, Crowley J, et al. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 13.Satagopan JM, Ben-Porat L, Berwick M, et al. A note on competing risks in survival data analysis. Br J Cancer. 2004;91:1229–1235. doi: 10.1038/sj.bjc.6602102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.R Development Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2010. [Google Scholar]
- 15.Hamilton TE, Ritchey ML, Argani P, et al. Synchronous bilateral Wilm's tumor with complete radiographic response managed without surgical resection: a report from the National Wilm's Tumor Study 4. J Pediatr Surg. 2008;43:1982. doi: 10.1016/j.jpedsurg.2008.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song C, Bang JK, Park HK, et al. Factors Influencing Renal Function Reduction After Partial Nephrectomy. J Urol. 2009;181:48. doi: 10.1016/j.juro.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 17.Simmons MN, Brandina R, Hernandez AF, et al. Surgical management of bilateral synchronous kidney tumors: functional and oncological outcomes. J Urol. 2010;184:865. doi: 10.1016/j.juro.2010.05.042. [DOI] [PubMed] [Google Scholar]
- 18.Menon V, Wang X, Sarnak MJ, Hunsicker LH, et al. Long-term outcomes in nondiabetic chronic kidney disease. Kidney Int. 2008;73:1310. doi: 10.1038/ki.2008.67. [DOI] [PubMed] [Google Scholar]
- 19.Pritchard-Jones K. Molecular genetic pathways to Wilms tumor. Crit Rev Oncog. 1997;8:1. doi: 10.1615/critrevoncog.v8.i1.10. [DOI] [PubMed] [Google Scholar]
- 20.Huff V. Wilms tumor genetics. Am J Med Genet. 1998;79:260. doi: 10.1002/(sici)1096-8628(19981002)79:4<260::aid-ajmg6>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 21.Little SE, Hanks SP, King-Underwood L, et al. Frequency and heritability of WT1 mutations in nonsyndromic Wilms' tumor patients: a UK Children's Cancer Study Group Study. J Clin Oncol. 2004;22:4140. doi: 10.1200/JCO.2004.02.136. [DOI] [PubMed] [Google Scholar]
- 22.Niaudet P, Gubler MC. WT1 and glomerular diseases. Pediatr Nephrol. 2006;21:1653. doi: 10.1007/s00467-006-0208-1. [DOI] [PubMed] [Google Scholar]
- 23.Mucha B, Ozaltin F, Hinkes BG, et al. Mutations in the Wilms' tumor 1 gene cause isolated steroid resistant nephrotic syndrome and occur in exons 8 and 9. Pediatr Res. 2006;59:325. doi: 10.1203/01.pdr.0000196717.94518.f0. [DOI] [PubMed] [Google Scholar]
- 24.Jeanpierre C, Denamur E, Henry I, et al. Identification of constitutional WT1 mutations, in patients with isolated diffuse mesangial sclerosis, and analysis of genotype/phenotype correlations by use of a computerized mutation database. Am J Hum Genet. 1998;62:824. doi: 10.1086/301806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klamt B, Koziell A, Poulat F, et al. Frasier syndrome is caused by defective alternative splicing of WT1 leading to an altered ratio of WT1 +/-KTS splice isoforms. Hum Mol Genet. 1998;7:709. doi: 10.1093/hmg/7.4.709. [DOI] [PubMed] [Google Scholar]
- 26.Menke AL, Schedl A. WT1 and glomerular function. Semin Cell Dev Biol. 2003;14:233. doi: 10.1016/s1084-9521(03)00026-0. [DOI] [PubMed] [Google Scholar]
- 27.Guo JK, Menke AL, Gubler MC, et al. WT1 is a key regulator of podocyte function: reduced expression levels cause crescentic glomerulonephritis and mesangial sclerosis. Hum Mol Genet. 2002;11:651. doi: 10.1093/hmg/11.6.651. [DOI] [PubMed] [Google Scholar]
- 28.Dahan K, Kamal M, Noel LH, et al. Small glomeruli inWAGR (Wilms Tumor, Aniridia, Genitourinary Anomalies and Mental Retardation) syndrome. Am J Kidney Dis. 2007;49:793. doi: 10.1053/j.ajkd.2007.02.275. [DOI] [PubMed] [Google Scholar]
- 29.Macconi D, Bonomelli M, Benigni A, et al. Pathophysiologic implications of reduced podocyte number in a rat model of progressive glomerular injury. Am J Pathol. 2006;168:42. doi: 10.2353/ajpath.2006.050398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoy WE, Hughson MD, Bertram JF, et al. Nephron number, hypertension, renal disease, and renal failure. J Am Soc Nephrol. 2005;16:2557. doi: 10.1681/ASN.2005020172. [DOI] [PubMed] [Google Scholar]
- 31.Menke AL, IJpenberg A, Fleming S, et al. The wt1-heterozygous mouse; a model to study the development of glomerular sclerosis. J Pathol. 2003;200:667. doi: 10.1002/path.1390. [DOI] [PubMed] [Google Scholar]
- 32.Davidoff AM, Giel DW, Jones DP, et al. The feasibility and outcome of nephron-sparing surgery for children with bilateral Wilms tumor. The St Jude Children's Research Hospital experience: 1999-2006. Cancer. 2008;112:2060. doi: 10.1002/cncr.23406. [DOI] [PubMed] [Google Scholar]
- 33.Horwitz J, Ritchey ML, Moksness J, et al. Renal salvage procedures in patients with synchronous bilateral tumors: a report of the NWTSG. J Pediatr Surg. 1996;31:1020. doi: 10.1016/s0022-3468(96)90077-9. [DOI] [PubMed] [Google Scholar]
- 34.Zani A, Schiavetti A, Gambino M, et al. Long-term outcome of nephron sparing surgery and simple nephrectomy for unilateral localized Wilms tumor. J Urol. 2005;173:946–8. doi: 10.1097/01.ju.0000152580.90861.d3. [DOI] [PubMed] [Google Scholar]
- 35.Linni K, Urban C, Lackner CH, et al. Nephron-sparing procedures in 11 patients with Wilms tumor. Pediatr Surg Int. 2003;19:457. doi: 10.1007/s00383-003-0957-x. [DOI] [PubMed] [Google Scholar]
- 36.Haecker FM, vonSchweinitz D, Harms D, et al. Partial nephrectomy for unilateral Wilms tumor: result of study SIOP 93-01/GPOH. J Urol. 2003;170:939. doi: 10.1097/01.ju.0000073848.33092.c7. [DOI] [PubMed] [Google Scholar]
- 37.Shamberger RC, Guthrie KA, Ritchey ML, et al. Surgery-Related Factors and Local Recurrence of Wilms Tumor in National Wilms Tumor Study 4. Ann Surg. 1998;229:292. doi: 10.1097/00000658-199902000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.