Abstract
The central nervous system is plastic throughout life, but is most sensitive to the statistics of the sensory environment during critical periods of early postnatal development. In the auditory cortex, various forms of acoustic experience have been found to shape the formation of receptive fields and influence the overall rate of cortical organization. The synaptic mechanisms that control cortical receptive field plasticity are beginning to be described, particularly for frequency tuning in rodent primary auditory cortex. Inhibitory circuitry plays a major role in critical period regulation, and new evidence suggests that the formation of excitatory-inhibitory balance determines the duration of critical period plasticity for auditory cortical frequency tuning. Cortical inhibition is poorly tuned in the infant brain, but becomes co-tuned with excitation in an experience-dependent manner over the first postnatal month. We discuss evidence suggesting that this may be a general feature of the developing cortex, and describe the functional implications of such transient excitatory-inhibitory imbalance.
Keywords: auditory cortex, development, excitatory-inhibitory balance, plasticity, receptive fields
1. Introduction
The natural world is complex and dynamic. In order for an animal to survive and successfully navigate in such environments, the brain must be able to rapidly process and operate on a diverse range of sensory stimuli. Some components of the nervous system seem to be genetically specified and perinatally hard-wired, particularly in the peripheral sensory epithelium (Sobeih and Corfas, 2002; Harris and Rubel, 2006). More central regions, however, have been found to rely on electrical activity and sensory experience to instruct or control the development of synaptic transmission and the organization of receptive fields (Katz and Shatz, 1996; Sanes and Bao, 2009). This seems especially true in the primary auditory cortex (AI), where manipulations of early acoustic experience produce a range of profound and lasting effects on the structure and function of AI neurons and synapses.
Here we review the critical factors for developmental plasticity of AI synaptic receptive fields. We begin by summarizing important studies on the formation of the subcortical auditory system, as normative AI development presumably requires prior organization of the thalamus and other upstream regions. We then briefly review previous work on the establishment of AI tonotopy and spiking receptive fields, before describing the processes that shape the underlying synaptic receptive fields of AI neurons. We focus here on the postnatal development of excitatory-inhibitory balance for frequency tuning in rat AI. Although there are recent confusing data on the degree to which AI inhibitory inputs are tuned in neonatal AI (Dorrn et al., 2010; Sun et al., 2010), we aim to clarify this issue by discussing the findings and methods of these and other related studies in some detail. Collectively, these data suggest that various receptive field components or functional sectors of AI develop in distinct stages or at different rates, depending on position within the network and the computational complexity of the postnatal acoustic world.
2. Subcortical development
The rodent auditory system is altricious, developing throughout the first postnatal month (Sanes and Bao, 2009). Hearing onset in rodents such as rats and mice occurs around postnatal day (P) 11, although bone conduction-related events can be measured as early as P7 (Geal-Dor et al., 1993). For comparison, the human auditory system is functional in prenatal infants, and auditory responses can be evoked in utero as early as the 27th prenatal week (Moore and Linthicum, 2007). Regardless of the functional onset time, auditory development in most species studied is a protracted process. This extended and delayed maturation presumably allows central regions of the nascent auditory system to form connections and refine synaptic strengths in a manner that reflects the acoustical properties and behavioral significance of the sensory environment (Keuroghlian and Knudsen, 2007), while more peripheral areas develop precise connections independently of auditory experience (Rubel and Fritzsch, 2002).
Much of the rodent subcortical auditory system is mature by P11-P12. The cochlear microphonic can be recorded in rats as early as P8 (Uziel et al., 1981), and cochlear cells are spontaneously active from P0 to P10 (Tritsch and Bergles, 2010). Perhaps analogous to the hypothesized function of retinal waves (McLaughlin et al., 2003), this spontaneous activity is potentially important for pre-patterning the auditory periphery before hearing onset, and is suddenly curtailed in inner supporting cells upon hearing onset. Projections from the auditory brainstem to midbrain in rat are present at P4 and mature throughout P4-P12 (Fathke and Gabriele, 2009). A comparable process seems to occur at the same ages for thalamocortical connections from the ventral division of the medial geniculate nucleus into rodent AI (Lund and Mustari, 1977; Robertson et al., 1991). Neurogenesis and synapse formation in the inferior colliculus seems to occur early in perinatal life, and response properties of these midbrain neurons are largely mature soon after hearing onset (Brunso-Bechtold and Henkel, 2005), with lower thresholds and higher characteristic frequencies emerging at later ages (Aitkin and Moore, 1975), e.g., P13-P20 in the house mouse (Romand and Ehret, 1990). Therefore the subcortical circuitry is in place for robust tone-evoked responses to be detected in postnatal AI at ~P12, with refinement of receptive fields continuing through the first month of life in the rodent auditory system.
3. Development of AI maps and receptive fields
Despite this early wiring of the upstream auditory pathway, some physiological properties of AI remain immature throughout the first three postnatal weeks or longer. This is likely a consequence of the high level of plasticity inherent in AI: the auditory cortex is among the most plastic regions of the auditory system, rapidly re-tuning in response to changes of acoustic input (Buonomano and Merzenich, 1998). Plasticity seems greatest during neonatal critical periods, which are developmental epochs during which neural circuits are intrinsically sensitive to the acoustic parameters of the external environment (Hensch, 2005; Sanes and Bao, 2009). Critical periods in the auditory cortex usually last for a few days or weeks, beginning just after the onset of hearing. Recent compelling evidence suggests that various receptive field properties and distinct brain regions have different critical periods that are overlapping or staggered (Insanally et al., 2009; Popescu and Polley, 2010). In this way, lower level representations of the auditory world can be constructed, refined, and stabilized, enabling more complex stimuli to then be processed by cortical circuitry. As the excitatory inputs are mostly well-formed by hearing onset, we hypothesize that this protracted developmental process depends fundamentally on the delayed maturation of intracortical inhibitory circuitry (Dorrn et al., 2010), analogous to the development of visual cortical receptive field properties such as ocular dominance (Hensch, 2005).
Critical period development and plasticity of AI have been most thoroughly characterized in vivo at the level of spiking receptive fields and tonotopic map organization. In adult rats, AI is functionally defined as having short latency responses (5-20 msec from stimulus onset), with high reliability and well tuned to pure tones (Sally and Kelly, 1988, Polley et al., 2007). Prior to hearing onset (P11), tone-evoked responses cannot be detected in neonatal rat AI, except possibly via bone conduction (Geal-Dor et al., 1993). Immediately afterward, AI consists of a relatively small core region at P11, tuned to mid-range frequencies (de Villers-Sidani et al., 2007), and surrounded by a large responsive but untuned area (Zhang et al., 2001). At this young age, spike latencies can be longer (20-40 msec) and thresholds tend to be higher (50-60 dB SPL). After P11, the well-tuned sector of AI becomes progressively larger (Fig. 1A). By P13-P14, the size, tonotopic gradient, and responsiveness (including spike thresholds of ~20 dB SPL) of rat AI is equivalent to that in adult animals (de Villers-Sidani et al., 2007). However, response latencies can still be relatively long, taking until P20-P25 to reach mature levels. Similar patterns of postnatal cortical development can be observed in other mammalian species- e.g., cat (Brugge et al., 1988; Bonham et al., 2004), chinchilla (Pienkowski and Harrison, 2005), ferret (Mrsic-Flogel et al., 2006), and bat (Vater et al., 2010)- although there are important differences in the details of the development and mature organization of AI in each of these animal models, including the exact pre- and postnatal ages at which auditory system development occurs (Romand, 1997).
Figure 1.
Development and plasticity of rat AI spiking receptive fields and tonotopic organization. A, Characteristic frequency maps (top) and representative frequency-intensity receptive fields (bottom) recorded from four different animals at P11-P14. Each tile or symbol represents a different recording site, where the color of the tile indicates the characteristic frequency at that location. Xs denote unresponsive sites, Os denote untuned sites. B, Exposure to 7 kHz tones from P10-P14 leads to increase of 7 kHz representation in adult AI. Shown are maps from a control adult animal (left) and a 7 kHz-exposed animal (middle). On average, the 7 kHz region in control animals was 14.9 ± 1.9% of the total AI area, while this was almost doubled in exposed animals to 27.0 ± 4.6% of total AI area. Adapted from de Villers-Sidani et al., 2007.
This increase in effective size of rat AI is at least partially a consequence of how AI itself is physiologically defined: as previously unresponsive neurons, poorly-tuned cells, or cells with abnormally long latency take on aspects of mature AI cells, they become included within the experimentally-determined map of AI (Zhang et al., 2001). Therefore, tonotopic map formation necessarily develops in parallel with the organization of individual frequency-intensity receptive fields in rat AI (Fig. 1A). By ~P21, frequency-intensity receptive fields appear equivalent to those recorded in the adult brain. Prior to this date, some reports found that neurons were usually narrowly tuned (de Villers-Sidani et al., 2007; Insanally et al., 2009), while others observed that neonatal tuning was broad on average (Zhang et al., 2001). An examination of other statistics besides bandwidth (including latency, threshold, and overall area) reveals that the development of these properties of rat auditory cortical spiking receptive fields has been inconsistently reported in the field (compare Zhang et al., 2001; Chang et al., 2005; de Villers-Sidani et al., 2007; Insanally et al., 2009; Sun et al., 2010). The reasons for such heterogeneity are unclear, but could be related to differences in rodent neonatal auditory experience, or subtle variation in mapping, extracellular recording, and criteria for defining AI (see Section 6 below).
Other aspects of AI receptive fields may develop at different, slower rates. For example, the extent of sideband suppression in AI neurons seems to be larger in young animals than older animals, and the developmental progression of sideband suppression continues past the first postnatal month. Suppression can be measured by presenting a pair of pure tonal stimuli simultaneously; in this case, the responsive area of the frequency-intensity receptive field is reduced at the edges. The extent of simultaneous two-tone suppression remains broad until P45 (Chang et al., 2005). This developmentally-delayed suppression of AI responses is regulated to some degree by GABAergic inhibition, as iontophoretic application of bicuculline (a GABAA receptor antagonist) preferentially affected two-tone suppression in young (P20) animals to a greater extent than adults. A straightforward interpretation of these data is that excitatory input to AI neurons is fully developed soon after hearing onset, while the developmental sharpening of the effects of intracortical inhibition occurs later, being complete by P45, and perhaps requiring the prior tuning of excitation.
Sound localization abilities depend on cortical processing of binaural cues. Spatial receptive fields of AI neurons from ferrets develop gradually, following growth of the head and external ears (Mrsic-Flogel et al., 2003). Binaural tuning in AI also emerges over the first postnatal month (Razak and Fuzessery, 2007; Popescu and Polley, 2010), and can be impacted by transient hearing loss early in life. In particular, reversible monaural deprivation in rats (via unilateral ear canal ligation), when initiated within the first four postnatal weeks, leads to increases in responses to stimuli presented to the spared ear. In contrast, unilateral hearing loss affects AI tonotopic map structure only when deprivation begins at two weeks of age but not four weeks (Popescu and Polley, 2010). Thus sensory deprivation has a number of different effects, from reorganization of AI topography to changes in threshold and response amplitude, with each property seemingly having its own critical period in which deprivation is most effective.
There is good evidence that temporal processing takes considerably longer to develop than spectral tuning. Direction selectivity can be modified by exposure to downward frequency modulated (FM) sweeps even after P30 (Insanally et al., 2009), and intracortical inhibition plays a major role in shaping AI responses to FM sweeps (Zhang et al., 2003). Also, in response to trains of repetitive noise bursts, spiking activity from neurons in mature rat AI can reliably follow repetition rates up to 10-20 Hz. In comparison, neurons at P20 have difficulty following rates beyond 5 Hz (Kilgard and Merzenich, 1998). Not until after the first postnatal month do AI neurons achieve adult levels of performance (Chang et al., 2005). Similarly, EPSPs between layer 2/3 neurons in AI slices at P10-P14 exhibit pronounced paired-pulse and short-term depression at 10 Hz repetition rates, while cells in older animals (P19-P29) show little depression to 10 Hz trains and can more reliably follow faster rates of 20-40 Hz (Atzori et al., 2001; Oswald and Reyes, 2008).
4. Tonotopic map and receptive field plasticity
In general, repetitive exposure to patterned stimuli for longer periods of minutes to days rapidly and persistently alters AI, such that presented stimuli (salient features of the acoustic environment) become represented or over-represented by large numbers of neurons. Characteristic frequency maps in rat AI are profoundly changed if young animals are exposed to pulsed pure tones for a brief period between P11-P13 immediately after hearing onset, i.e., the same time window as tonotopic map organization (de Villers-Sidani et al., 2007). For example, animals exposed to 7 kHz tones in their home cage between P10 and P14 show unusually large sectors of AI tuned to 7 kHz tones when examined as late as P60 (Fig. 1B). Conversely, exposure to pulsed white noise stimuli early in life was found to degrade the tonotopic organization of rodent AI (Zhou and Merzenich, 2007). Therefore, exposure to either pulsed pure tones or white noise bursts has opposing effects on AI feature selectivity. In both cases, it seems that receptive fields are remodeled to match the statistics of the sensory environment.
Exposure to continual white noise, rather than periodic bursts of noise, has also been found to degrade cortical receptive fields, and prolongs the extent of the critical period into adulthood (Chang and Merzenich, 2003; Speechley et al., 2007). Continuous tonal exposure at a single unmodulated frequency also delays development and keeps the critical period open (Zhou et al., 2008). Collectively, these experiments indicate that the spectral structure of acoustic stimuli controls the formation of AI frequency tuning profiles, while the temporal pattern of sensory input regulates the overall duration of the AI critical period. Continual stimuli keep the critical period open, possibly because of the strong neuronal adaptation driven by unmodulated input at rates beyond 5 Hz. Pulsed or patterned inputs, in contrast, precociously close the critical period (Dorrn et al., 2010).
Unless played at very high, deafness-inducing intensity levels (Takesian et al., 2009), repetitive exposure to patterned stimuli is less effective at changing the adult cortex. Long-term adult cortical plasticity generally requires that exposed stimuli have some reliable behavioral context, such as reward prediction (Fritz et al., 2003; Bao et al., 2004; Weinberger, 2004). Alternatively, shifts of AI frequency tuning can be induced under anesthesia by pairing tones with direct activation of neuromodulatory nuclei in rat (Bakin and Weinberger, 1996; Kilgard and Merzenich, 1998; Froemke et al., 2007) and mouse (Yan and Zhang, 2005), in awake cats after prolonged passive exposure for months (Noreña et al., 2006), and after repetitive pairing of a non-preferred tone with a preferred tone in ferrets (Dahmen et al., 2008). It is still unknown whether adult cortical plasticity recapitulates developmental plasticity, in terms of the underlying mechanisms and spectrotemporal dynamics. As many phenomena can potentially influence spike generation, it is essential to look more carefully at the modifications of synaptic responses (and other elements such as ion channel expression or myelination) that could be induced after changes to the patterns of acoustic input (Rubenstein and Merzenich, 2003).
5. Synaptic receptive field plasticity in AI
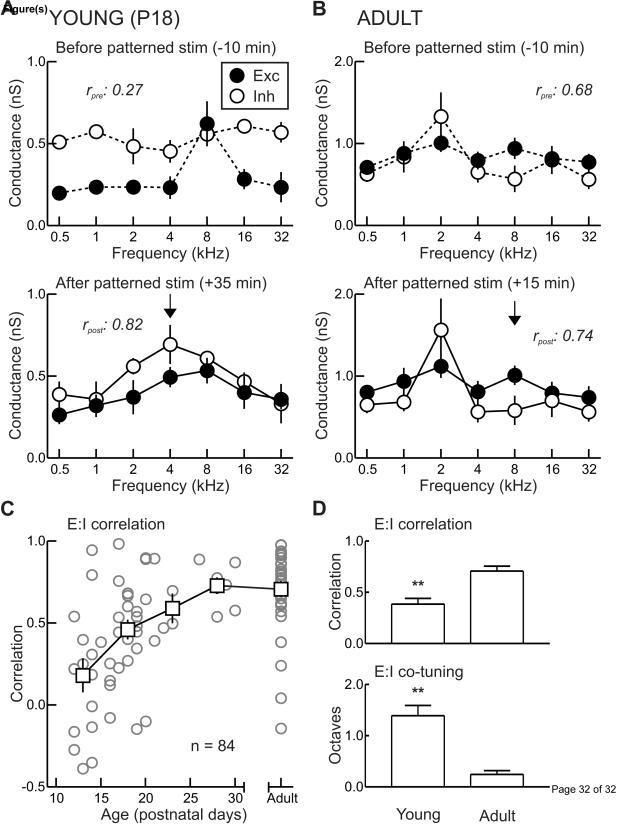
Corresponding forms of receptive field development and plasticity can also be observed at the synaptic level, which in turn controls the structure of spiking receptive fields (Tan et al., 2004). Experiments in slices of rat and mouse AI have documented the maturation of intrinsic and synaptic properties of excitatory neurons, showing that the most profound changes occur between P12-21 (Metherate and Aramakis, 1999; Oswald and Reyes, 2008), precisely along the same timeline as changes to rodent AI frequency tuning (Insanally et al., 2009). In vivo, excitatory inputs mature first and are tuned for sound frequency by approximately P14 (Dorrn et al., 2010). However, inhibitory inputs are potentially equally as strong in young versus adult AI (Dorrn et al., 2010; Sun et al., 2010), but on average exhibit little to no frequency tuning during the second postnatal week, resulting in imbalanced excitation and inhibition and erratic receptive field organization (Fig. 2A). After three postnatal weeks of relatively normal acoustic experience, though, cortical inhibition progressively becomes tuned to sound frequency, eventually matching and balancing excitatory inputs (Fig. 2B-D). By ‘balanced’, we mean that across different stimuli, the magnitude of sensory-evoked inhibitory responses generally scale with and are proportional to the magnitude of excitatory events evoked by the same stimuli, as measured by the linear correlation between excitation and inhibition (Wehr and Zador, 2003; Froemke et al., 2007; Dorrn et al., 2010), or the mean squared difference between the normalized amplitude of tone-evoked excitatory and inhibitory events (Sun et al., 2010).
Figure 2.
Development and plasticity of rat AI excitatory and inhibitory frequency tuning curves. A, In vivo whole-cell voltage-clamp recording from young (P18) AI neuron. Tone-evoked currents at different holding potentials were used to compute synaptic conductances. Top, before patterned stimulation, excitatory-inhibitory correlation is low (rpre: 0.27). Correlation (r) is the correlation coefficient of the linear fit to the mean values of excitation and inhibition across each of the seven tone frequencies used to measure synaptic tuning curves. Excitation indicated by filled symbols, inhibition indicated by open symbols. Bottom, synaptic frequency tuning in the same cell ~35 minutes after patterned stimulation with 4 kHz tones (arrow). Excitation and inhibition are both potentiated at 4 kHz, and the original best frequencies are depressed. Consequentially, excitatory-inhibitory correlation improves (rpost: 0.82). B, Recording from adult AI neuron. Top, before patterned stimulation, excitation and inhibition are balanced (rpre: 0.68). Bottom, patterned stimulation with 8 kHz tones has no lasting effect on synaptic strength or excitatory-inhibitory balance (rpost: 0.74). C, Correlation between excitatory and inhibitory frequency tuning profiles for all cells recorded between P12-P30 and from adults. Circles, individual recordings. Squares, averages at five day intervals. D, Comparison of developmental changes to synaptic frequency tuning between P12-P21 animals (‘Young’) and animals aged two months or older (‘Adult’). Top, excitatory-inhibitory balance (average linear correlation coefficient r). Bottom, co-tuning of best frequencies, in terms of the spectral difference (in octaves) between the best frequencies of excitation and inhibition. Adapted from Dorrn et al., 2010.
This experience-dependent process of inhibitory maturation can be affected in a similar manner to tonotopic maps. Repetitive tonal exposure accelerates balancing of excitation and inhibition, but only when performed between P12-P21 (Fig. 2A). Patterned stimulation leads to co-tuning of excitation and inhibition by a complex, orchestrated set of synaptic modifications across frequencies. Excitatory and inhibitory responses are potentiated at the presented frequency during patterned stimulation, with enhancements also spreading to neighboring frequencies within one octave on average. Responses at the original best frequencies are depressed, independent of the spectral distance between the best frequency and the presented frequency. Generally as a consequence of these changes, a new, single peak emerges in the excitatory and inhibitory frequency tuning curves, improving the correlation between them. Just a few minutes of patterned stimulation are required to induce these synaptic modifications, which are rapidly expressed within minutes and endure for over an hour. Once in place, further patterned stimulation has no effect on synaptic strength or excitatory-inhibitory balance (Dorrn et al., 2010). By definition, then, this manipulation closes the critical period for AI frequency tuning, at least for a few hours in absence of additional consolidation mechanisms.
Despite the correspondence between forms of plasticity at the spiking and synaptic levels, it is not always straightforward to determine how changes to synaptic transmission lead to changes in spike generation. In young adult and adult rats, integrate-and-fire models that utilize data on the kinetics and amplitudes of tone-evoked excitatory and inhibitory events make accurate predictions about the patterns of spike firing by AI neurons (Wehr and Zador, 2003; Tan et al., 2004). However, during the critical period for rat AI, tone-evoked spike firing is irregular and much less precise than observed in older animals. Even after episodes of patterned stimulation to increase excitatory-inhibitory balance to near-adult levels, spike firing in young rat AI is still imprecise (Dorrn et al., 2010), suggesting that the maturation of additional factors (such as myelination or K+ channel expression) might also be important for the emergence of adult types of spiking receptive fields.
The types of neonatal auditory experiences have diverse, profound, and lasting influences on AI synaptic transmission. Prior exposure to patterned tonal stimulation between P10-P14 leads to pre-balancing of excitation and inhibition, and prevents additional repetitive exposure from modifying synaptic tuning curves throughout P12-P21. White noise, presented either continuously or in brief pulses, does not balance excitation and inhibition at any age (Dorrn et al., 2010). Studies in vivo (Scholl and Wehr, 2008) and in brain slices (Kotak et al., 2008) have revealed that postnatal hearing loss, even to a partial degree, leads to persistent changes in the efficacy of AI synapses. Thus early in life, the patterns of acoustic experience- or lack thereof-can lead to rapid modifications of excitatory and inhibitory synaptic strength, which govern the organization of receptive fields, the output of cortical circuitry, and the perception of auditory stimuli.
What cell types and brain regions are directly affected by changes in acoustic input? Given that hearing loss and sensory exposure both affect responses within the entire auditory pathway, there are potentially many anatomical sites of developmental plasticity. In a seminal study, Sanes and Constantine-Paton (1983) observed that exposing young mice to click trains from P8 until P19-P24 lead to broadening of tuning curves recorded in the inferior colliculus. Many other studies have documented changes to auditory midbrain (Poon and Chen, 1992; Ma and Suga, 2005; Kotak et al., 2008) and brainstem (Tzounopoulos et al., 2004; Kandler et al., 2009) neurons, usually with extracellular recordings of receptive field properties in vivo, or with intracellular recordings in vitro to examine the potential for synaptic plasticity in these circuits.
While some forms of adult synaptic receptive field plasticity seem to be predominantly expressed at intracortical connections (Froemke et al., 2007), it is still unclear which connections in the auditory system are directly affected by developmental patterned sensory stimulation. Intracellular recordings in vivo of non-propagating, subthreshold synaptic events are generally required to localize which inputs have been fundamentally altered by changes to experience or activity patterns (Froemke et al., 2007). However, given that much of the subcortical auditory system is mature at relatively young ages (see Section 2), it is likely that some of these adjustments occur within auditory cortical circuits. Moreover, plasticity expressed at subcortical stations may be more transient or have different induction requirements than changes to cortical synapses and neurons. Recordings in big brown bat midbrain and AI revealed that pairing cholinergic modulation with intracortical microstimulation shifted tuning curves in both brain areas. Interestingly, changes to AI neurons persisted more than six hours, while changes to inferior colliculus neurons lasted less than three hours (Ma and Suga, 2005). Of course, changes to subcortical processing might be due indirectly to adjustments of cortical centers, communicated via descending corticofugal feedback projections (Suga et al., 2002).
The duration of the AI critical period for synaptic frequency tuning is identical to the time period over which inhibitory tuning balances excitation (P12-P21). As excitation is fully tuned at ~P14-P15, the dynamics of this developmental co-tuning is driven by the emergence and sharpening of tone-evoked inhibitory input. During this maturational stage, the imbalance between excitation and inhibition may be permissive for the induction of long-term synaptic plasticity. In particular, for local regions of AI in which the excitatory-inhibitory ratio favors excitation, we predict that sensory stimulation produces a strong depolarizing response, sufficient for engaging the mechanisms of spike-timing-dependent plasticity or other forms of long-term potentiation via NMDA receptor activation (Froemke et al., 2006; Feldman, 2009). These changes would be coordinated across excitatory and inhibitory synapses activated by the presented tone (Marsden et al., 2007; Lin et al., 2008), and trigger other mechanisms, such as heterosynaptic long-term depression (Royer and Paré, 2003), responsible for decreasing responses to the original best frequencies. In this way, initially-untuned inhibition can specifically regulate synaptic plasticity and become calibrated to match excitation across a diverse input range, analogous to models proposed for ocular dominance plasticity in the visual cortex (Hensch, 2005; Kuhlman et al., 2010).
In other sensory systems, inhibitory maturation is a developmentally-delayed phenomenon which corresponds to periods of critical period plasticity. In kitten visual cortex, receptive fields mature over postnatal weeks 2-4. Blockade of GABA receptors usually reduces selectivity, but sometimes unmasks responses in previously silent neurons (Wolf et al., 1986). In rat barrel cortex, sensory-evoked synaptic responses are mature at P12 in layer 4, but continue to be refined in layer 2/3 until as late as P20 (Stern et al., 2001). Finally, a strikingly analogous process to that described for rat AI (Dorrn et al., 2010) occurs in development of Xenopus tadpole retinotectal synaptic receptive fields. Excitatory and inhibitory spatial receptive fields are initially quite broad but sharpen over development, with inhibitory changes occurring later (Tao and Poo, 2005).
Anatomically, inhibitory synapse development is also delayed relative to excitation. While many GABAergic synapses are formed before glutamatergic synapses (Ben-Ari et al., 2004), the functional properties of these connections- and the overall development of cortical inhibitory circuitry- takes considerably longer to mature. In both the visual cortex and barrel cortex, excitatory synapses reach their peak numbers around the time of eye opening, but GABAergic synapse development unfolds over the subsequent weeks, paralleling critical periods and often unfolding in different phases (Winfield, 1981; Wolf et al., 1984; Fosse et al., 1989; Micheva and Beaulieu, 1997; Huang et al., 1999; Gao et al., 2000; Katagiri et al., 2007).
6. Potential pre-balancing of excitation and inhibition in rat AI
Given the progressive, drawn-out nature of inhibitory circuit maturation, throughout brain regions and across different species, inhibitory receptive fields might also take longer to develop and become co-tuned with excitatory receptive fields, as recently described for rat AI over the P12-P21 critical period (Dorrn et al., 2010). In contrast, Sun et al. (2010) reported that the excitatory and inhibitory synaptic frequency tuning profiles of rat AI neurons in layer 4 were essentially identical at 20 dB above threshold, even as early as P12-P14. While excitatory and inhibitory tuning were mismatched by about one octave at threshold, comparable to the extent of misalignment observed by Dorrn et al. (2010), this intensity dependence was not regulated with age. Therefore, Sun et al. (2010) concluded that excitation and inhibition are in some way pre-balanced prior to hearing onset.
What might account for the apparent discrepancy between the findings of Sun et al. (2010) and Dorrn et al. (2010)? One important methodological difference between these studies is the laminar position of the recordings. Dorrn et al. (2010) recorded from cortical layers 2-6, while Sun et al. (2010) restricted their recording depth to 450-650 μm below the surface (i.e., approximately within layer 4). Given that much of the thalamic input to AI targets this layer, and that thalamic circuitry is possibly mature itself by P12-P14, these results raise the exciting possibility that excitatory-inhibitory balance is regulated in a laminar-specific manner; in particular, that the first cortical station to be balanced in AI is layer 4. One caveat is that without histological verification of cell type and location, it is difficult to know precisely where recordings were made only judging by electrode depth, especially given that the rat cortex is growing in thickness by 20% or more during these ages (Diamond, 1987). In general, accurate determination of recording depth may be a major issue for electrophysiological studies of cortical organization. For example, Oviedo et al. (2010) reported that layer 2 but not layer 3 neurons in mouse auditory cortex were tonally responsive, suggesting that subtle variations in electrode position or laminar thickness might have profound consequences for investigation of cortical receptive field properties.
Also, as discussed elsewhere (Xiong et al., in this issue), there are significant differences in the sound intensity used during stimulus presentation in these two studies. Sun et al. (2010) examined a range of different intensities, while Dorrn et al. (2010) consistently presented tones at 70 dB SPL. Seven distinct tone frequencies at one intensity level were used as the stimulus set in Dorrn et al. (2010). This was because a central aim of that study was to characterize developmental plasticity of tone-evoked synaptic conductances and excitatory-inhibitory balance. Thus it was necessary to use a relatively small number of tonal stimuli, so that each stimulus could be presented enough times to get good estimates for both the mean and variability of tone-evoked responses. Sensory response variability is much higher in young animals than in adults (Yuan et al., 2010), necessitating a higher number of stimulus repetitions and therefore a lower total number of distinct tonal stimuli. Accurate measurement of the variance was required in order to assess whether forms of sensory experience such as patterned stimulation would significantly change synaptic tuning curves. Also, the developmental decrease in threshold rapidly improves from P11-P14, and is stable thereafter at ~20 dB SPL (de Villers-Sidani et al., 2007; but see Sun et al., 2010). Even in those recordings from P12-P14, tonal presentation at 70 dB SPL was then 20+ dB above the average threshold for spike generation.
There are other factors that could potentially affect synaptic receptive fields in developing AI. Tone-evoked synaptic responses can be modified in a remarkably short amount of time (Dorrn et al., 2010), suggesting that formation of excitatory-inhibitory balance in thalamorecipient layer 4 may not require much experience. Pre-balancing of layer 4 circuitry could then result from subtle variations in acoustic environments, housing conditions, or signals transmitted via bone conduction. In a similar manner, co-tuning of excitation and inhibition could arise from prolonged periods of auditory stimulation during the initial ‘mapping’ phase of each experiment, in which extracellular recording is used to first localize the position of the primary field within temporal cortex.
It is possible that various sectors or subregions of AI develop at different rates and/or with time courses. For example, there seems to be a core mid- to high-frequency region of the rat AI tonotopic map that initially develops (around P11-12) before lower frequency sites emerge a few days later (de Villers-Sidani et al., 2007). Just as layer 4 might express a high correspondence between excitation and inhibition before other cortical laminae (Sun et al., 2010), mature receptive field properties might emerge first within central areas of AI. As different receptive field properties are not entirely independent- e.g., bandwidth depends on characteristic frequency (Imaizumi and Schreiner, 2007)- some of the variability between reports of cortical development might be explained by differences between recording sites within neonatal AI maps.
Finally, inhibitory tuning has been found to be inherently variable at all ages. In the recordings summarized in Figure 2C, there is a considerable range of correlation values between excitation and inhibition in AI neurons recorded from both young (P12-P21) and adult animals. While the average correlation is low in critical period animals and higher in adults, at both age ranges, cells can be found that display very high (r > 0.7) or very low (r < 0.3) correspondence between these components of synaptic receptive fields. A surprisingly large fraction of cells (~20-30%) seem to have little to no tone-evoked inhibitory conductance relative to excitation (Wehr and Zador, 2003). Likewise, Sun et al. (2010) measured the frequency response range of excitatory inputs for a total of 40 cells, but measured inhibitory responses in just 27 cells. If tone-evoked inhibition is absent for a subset of tonal stimuli, this could strongly bias the correlation between excitation and inhibition towards lower values, irrespective of age and intensity level.
Despite this variation in excitatory-inhibitory balance in developing rat AI, both Sun et al. (2010) and Dorrn et al. (2010) agree that tone-evoked inhibitory responses can be robust shortly after hearing onset. In contrast to inhibition in the rodent visual cortex, which is progressively enhanced over development (Morales et al., 2002), the overall amplitudes of maximal tone-evoked excitatory and inhibitory responses in AI do not seem to dramatically change after hearing onset. Rather, precise refinements are made to existing connections, to balance AI synaptic inputs and emphasize salient features of the acoustic world.
7. Discussion
Synaptic inhibition controls information processing and plasticity in the young and adult brain. While the organization of excitatory inputs determines the overall potential responsiveness and output of sensory neurons, inhibition can sharpen tuning bandwidth, enhance spike timing precision, and prevent spurious NMDA receptor activation and induction of long-term synaptic plasticity (Artola and Singer, 1987; Wehr and Zador, 2003; Zhang et al., 2003). For these reasons it is essential that cortical networks have mechanisms for calibrating and coordinating excitatory and inhibitory synapses in some way, to meet certain set points of excitability, Ca2+ influx, or other downstream readouts of the absolute and relative levels of synaptic input.
There is consensus that, across different regions of the adult cortex, sensory-evoked excitatory and inhibitory responses are usually- but not always- proportional for different stimuli (Volkov and Galaziuk, 1991; Monier et al., 2003; Wehr and Zador, 2003; Zhang et al., 2003; Priebe and Ferster, 2005; Higley and Contreras, 2006; Froemke et al., 2007; Kenet et al., 2007), but the degree of correspondence between excitatory and inhibitory subfields or excitatory-inhibitory ratio can depend on cortical layer (Martinez et al., 2005; Adesnik and Scanziani, 2010). Due to the technical challenges of reliably recording synaptic events from young animals in vivo, far fewer studies have directly examined synaptic receptive fields and excitatory-inhibitory balance in neonatal cortex. Dorrn et al. (2010) and Sun et al. (2010) both observed that pure tones can evoke large excitatory and inhibitory responses in rat AI as early as P12, but these two reports differed on the amount of mismatch and co-tuning between excitatory and inhibitory frequency tuning profiles, as well as the degree to which synaptic excitation and inhibition fully predict spiking receptive fields in young AI. At hearing onset, excitatory-inhibitory balance may begin higher within layer 4, and increase in other layers with postnatal age and patterned, reliable auditory experience. Future studies focusing on laminar position, carefully controlling for the amount and form of early acoustic experience, will be required to resolve this important issue.
However, if cortical excitation and inhibition are pre-balanced before hearing onset at particular intensity levels, at least three other essential questions will then need to be addressed. First, what role does co-tuned inhibition play in determining the duration of the critical period for AI frequency tuning? Correspondingly, there must then be other cellular and network factors that contribute to plasticity in the young brain, and differentiate neonatal plasticity from that in adults. Second, there is abundant evidence that inhibitory synaptic development continues throughout the first postnatal month. If inhibitory synaptic receptive fields are fully formed and structured at hearing onset, how are developing GABAergic circuits precisely integrated and reorganized throughout postnatal ontogeny, to ensure that receptive fields are effectively unchanged by this extensive and protracted program of synaptogenesis and circuit remodeling? Finally, what mechanisms govern the change in threshold, and how is it that, as described by Sun et al. (2010), excitation and inhibition remain imbalanced near threshold throughout life, but are balanced at higher sound levels? Given the fundamental importance of excitatory-inhibitory balance for cortical processing, plasticity, and the prevention of pathological states such as epilepsy, much more effort is required to understand the nature of critical period plasticity at the level of synaptic circuitry, and reconcile these two views- developmentally precocious or delayed- of AI inhibitory frequency tuning.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adesnik H, Scanziani M. Lateral competition for cortical space by layer-specific horizontal circuits. Nature. 2010;464:1155–1160. doi: 10.1038/nature08935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitkin LM, Moore DR. Inferior colliculus. II. Development of tuning characteristics and tonotopic organization in central nucleus of the neonatal cat. J. Neurophysiol. 1975;38:1208–1216. doi: 10.1152/jn.1975.38.5.1208. [DOI] [PubMed] [Google Scholar]
- Artola A, Singer W. Long-term potentiation and NMDA receptors in rat visual cortex. Nature. 1987;330:649–652. doi: 10.1038/330649a0. [DOI] [PubMed] [Google Scholar]
- Atzori M, Lei S, Evans DI, Kanold PO, Phillips-Tansey E, McIntyre O, McBain CJ. Differential synaptic processing separates stationary from transient inputs to the auditory cortex. Nat. Neurosci. 2001;4:1230–1237. doi: 10.1038/nn760. [DOI] [PubMed] [Google Scholar]
- Bakin JS, Weinberger NM. Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis. Proc. Natl. Acad. Sci. USA. 1996;93:11219–11224. doi: 10.1073/pnas.93.20.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Chang EF, Woods J, Merzenich MM. Temporal plasticity in the primary auditory cortex induced by operant perceptual learning. Nat. Neurosci. 2004;7:974–981. doi: 10.1038/nn1293. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Khalilov I, Represa A, Gozlan H. Interneurons set the tune of developing networks. Trends Neurosci. 2004;27:422–427. doi: 10.1016/j.tins.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Bonham BH, Cheung SW, Godey B, Schreiner CE. Spatial organization of frequency response areas and rate/level functions in the developing AI. J. Neurophysiol. 91:841–854. doi: 10.1152/jn.00017.2003. [DOI] [PubMed] [Google Scholar]
- Brugge JF, Reale RA, Wilson GF. Sensitivity of auditory cortical neurons of kittens to monaural and binaural high frequency sound. Hear Res. 1988;34:127–140. doi: 10.1016/0378-5955(88)90100-1. 1988. [DOI] [PubMed] [Google Scholar]
- Brunso-Bechtold JK, Henkel CK. Development of auditory afferents to the central nucleus of the inferior colliculus. In: Winer JA, Schreiner CE, editors. The Inferior Colliculus. Springer; New York: 2005. pp. 537–558. [Google Scholar]
- Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu. Rev. Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- Chang EF, Bao S, Imaizumi K, Schreiner CE, Merzenich MM. Development of spectral and temporal response selectivity in the auditory cortex. Proc. Natl. Acad. Sci. U S A. 2005;102:16460–16465. doi: 10.1073/pnas.0508239102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang EF, Merzenich MM. Environmental noise retards auditory cortical development. Science. 2003;300:498–502. doi: 10.1126/science.1082163. [DOI] [PubMed] [Google Scholar]
- Dahmen JC, Hartley DE, King AJ. Stimulus-timing-dependent plasticity of cortical frequency representation. J. Neurosci. 2008;28:13629–13639. doi: 10.1523/JNEUROSCI.4429-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villers-Sidani E, Chang EF, Bao S, Merzenich MM. Critical period window for spectral tuning defined in the primary auditory cortex (A1) in the rat. J. Neurosci. 2007;27:180–189. doi: 10.1523/JNEUROSCI.3227-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond MC. Sex differences in the rat forebrain. Brain Res. 1987;434:235–240. doi: 10.1016/0165-0173(87)90014-2. [DOI] [PubMed] [Google Scholar]
- Dorrn AL, Yuan K, Barker AJ, Schreiner CE, Froemke RC. Developmental sensory experience balances cortical excitation and inhibition. Nat. Rev. Neurosci. 2010;11:536–537. doi: 10.1038/nature09119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathke RL, Gabriele ML. Patterning of multiple layered projections to the auditory midbrain prior to experience. Hear Res. 2009;249:36–43. doi: 10.1016/j.heares.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Feldman DE. Synaptic mechanisms for plasticity in neocortex. Annu. Rev. Neurosci. 2009;32:33–55. doi: 10.1146/annurev.neuro.051508.135516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosse VM, Heggelund P, Fonnum F. Postnatal development of glutamatergic, GABAergic, and cholinergic neurotransmitter phenotypes in the visual cortex, lateral geniculate nucleus, pulvinar, and superior colliculus in cats. J Neurosci. 1989;9:426–435. doi: 10.1523/JNEUROSCI.09-02-00426.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz J, Shamma S, Elhilali M, Klein D. Rapid task-related plasticity of spectrotemporal receptive fields in primary auditory cortex. Nat. Neurosci. 2003;6:1216–1223. doi: 10.1038/nn1141. [DOI] [PubMed] [Google Scholar]
- Froemke RC, Merzenich MM, Schreiner CE. A synaptic memory trace for cortical receptive field plasticity. Nature. 2007;450:425–429. doi: 10.1038/nature06289. [DOI] [PubMed] [Google Scholar]
- Froemke RC, Tsay IA, Raad M, Long JD, Dan Y. Contribution of individual spikes in burst-induced long-term synaptic modification. J. Neurophysiol. 2006;95:1620–1629. doi: 10.1152/jn.00910.2005. [DOI] [PubMed] [Google Scholar]
- Gao WJ, Wormington AB, Newman DE, Pallas SL. Development of inhibitory circuitry in visual and auditory cortex of postnatal ferrets: immunocytochemical localization of calbindin- and parvalbumin-containing neurons. J. Comp. Neurol. 2000;422:140–157. doi: 10.1002/(sici)1096-9861(20000619)422:1<140::aid-cne9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Geal-Dor M, Freeman S, Li G, Sohmer H. Development of hearing in neonatal rats: air and bone conducted ABR thresholds. Hear Res. 1993;69:236–242. doi: 10.1016/0378-5955(93)90113-f. [DOI] [PubMed] [Google Scholar]
- Harris JA, Rubel EW. Afferent regulation of neuron number in the cochlear nucleus: cellular and molecular analyses of a critical period. Hear Res. 2006:216–217. 127–137. doi: 10.1016/j.heares.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat. Rev. Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Higley MJ, Contreras D. Balanced excitation and inhibition determine spike timing during frequency adaptation. J. Neurosci. 2006;26:448–457. doi: 10.1523/JNEUROSCI.3506-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Imaizumi K, Schreiner CE. Spatial interaction between spectral integration and frequency gradient in primary auditory cortex. J. Neurophysiol. 2007;98:2933–2942. doi: 10.1152/jn.00511.2007. [DOI] [PubMed] [Google Scholar]
- Insanally MN, Köver H, Kim H, Bao S. Feature-dependent sensitive periods in the development of complex sound representation. J. Neurosci. 2009;29:5456–5462. doi: 10.1523/JNEUROSCI.5311-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler K, Clause A, Noh J. Tonotopic reorganization of developing auditory brainstem circuits. Nat. Neurosci. 2009;12:711–717. doi: 10.1038/nn.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri H, Fagiolini M, Hensch TK. Optimization of somatic inhibition at critical period onset in mouse visual cortex. Neuron. 2007;53:805–812. doi: 10.1016/j.neuron.2007.02.026. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kenet T, Froemke RC, Schreiner CE, Pessah IH, Merzenich MM. Perinatal exposure to a noncoplanar polychlorinated biphenyl alters tonotopy, receptive fields, and plasticity in rat primary auditory cortex. Proc. Natl. Acad. Sci. U S A. 2007;104:7646–7651. doi: 10.1073/pnas.0701944104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keuroghlian AS, Knudsen EI. Adaptive auditory plasticity in developing and adult animals. Prog. Neurobiol. 2007;82:109–121. doi: 10.1016/j.pneurobio.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279:1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- Kotak VC, Takesian AE, Sanes DH. Hearing loss prevents the maturation of GABAergic transmission in the auditory cortex. Cereb. Cortex. 2008;18:2098–2108. doi: 10.1093/cercor/bhm233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman SJ, Lu J, Lazarus MS, Huang ZJ. Maturation of GABAergic inhibition promotes strengthening of temporally coherent inputs among convergent pathways. PLoS Comput Biol. 2010 Jun 3;6(6):e1000797. doi: 10.1371/journal.pcbi.1000797. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Bloodgood BL, Hauser JL, Lapan AD, Koon AC, Kim TK, Hu LS, Malik AN, Greenberg ME. Activity-dependent regulation of inhibitory synapse development by Npas4. Nature. 2008;455:1198–204. doi: 10.1038/nature07319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund RD, Mustari MJ. Development of the geniculocortical pathway in rats. J. Comp. Neurol. 1977;173:289–306. doi: 10.1002/cne.901730206. [DOI] [PubMed] [Google Scholar]
- Ma X, Suga N. Long-term cortical plasticity evoked by electric stimulation and acetylcholine applied to the auditory cortex. Proc. Natl. Acad. Sci. USA. 2005;102:9335–9340. doi: 10.1073/pnas.0503851102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden KC, Beattie JB, Friedenthal J, Carroll RC. NMDA receptor activation potentiates inhibitory transmission through GABA receptor-associated protein-dependent exocytosis of GABA(A) receptors. J. Neurosci. 2007;27:14326–14337. doi: 10.1523/JNEUROSCI.4433-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez LM, Wang Q, Reid RC, Pillai C, Alonso JM, Sommer FT, Hirsch JA. Receptive field structure varies with layer in the primary visual cortex. Nat. Neurosci. 2005;8:372–379. doi: 10.1038/nn1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin D, Shapley R, Shelley MJ. Large-scale modeling of the primary visual cortex: influence of cortical architecture upon neuronal response. J. Physiol. Paris. 2003;97:237–252. doi: 10.1016/j.jphysparis.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Metherate R, Aramakis VB. Intrinsic electrophysiology of neurons in thalamorecipient layers of developing rat auditory cortex. Brain Res. Dev. Brain Res. 1999;115:131–144. doi: 10.1016/s0165-3806(99)00058-9. [DOI] [PubMed] [Google Scholar]
- Micheva KD, Beaulieu C. Development and plasticity of the inhibitory neocortical circuitry with an emphasis on the rodent barrel field cortex: a review. Can. J. Physiol. Pharmacol. 1997;75:470–478. [PubMed] [Google Scholar]
- Monier C, Chavane F, Baudot P, Graham LJ, Frégnac Y. Orientation and direction selectivity of synaptic inputs in visual cortical neurons: a diversity of combinations produces spike tuning. Neuron. 2003;37:663–680. doi: 10.1016/s0896-6273(03)00064-3. [DOI] [PubMed] [Google Scholar]
- Moore JK, Linthicum FH. The human auditory system: a timeline of development. Int. J. Audiol. 2007;46:460–478. doi: 10.1080/14992020701383019. [DOI] [PubMed] [Google Scholar]
- Morales B, Choi S-Y, Kirkwood A. Dark rearing alters the development of GABAergic transmission in visual cortex. J. Neurosci. 2002;22:8084–8090. doi: 10.1523/JNEUROSCI.22-18-08084.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrsic-Flogel TD, Schnupp JWH, King AJ. Acoustic factors govern developmental sharpening of spatial tuning in the auditory cortex. Nat. Neurosci. 2003;6:981–988. doi: 10.1038/nn1108. [DOI] [PubMed] [Google Scholar]
- Mrsic-Flogel TD, Versnel H, King AJ. Development of contralateral and ipsilateral frequency representations in ferret primary auditory cortex. Eur. J. Neurosci. 2006;23:780–792. doi: 10.1111/j.1460-9568.2006.04609.x. [DOI] [PubMed] [Google Scholar]
- Noreña AJ, Gourévitch B, Aizawa N, Eggermont JJ. Spectrally enhanced acoustic environment disrupts frequency representation in cat auditory cortex. Nat. Neurosci. 2006;9:932–939. doi: 10.1038/nn1720. [DOI] [PubMed] [Google Scholar]
- Oswald AM, Reyes AD. Maturation of intrinsic and synaptic properties of layer 2/3 pyramidal neurons in mouse auditory cortex. J. Neurophysiol. 2008;99:2998–3008. doi: 10.1152/jn.01160.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oviedo HV, Bureau I, Svoboda K, Zador AM. The functional asymmetry of auditory cortex is reflected in the organization of local cortical circuits. Nat. Neurosci. 2010;13:1413–1420. doi: 10.1038/nn.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pienkowski M, Harrison RV. Tone frequency maps and receptive fields in the developing chinchilla auditory cortex. J. Neurophysiol. 2005;93:454–466. doi: 10.1152/jn.00569.2004. [DOI] [PubMed] [Google Scholar]
- Polley DB, Read HL, Storace DA, Merzenich MM. Multiparametric auditory receptive field organization across five cortical fields in the albino rat. J. Neurophysiol. 2007;97:3621–3638. doi: 10.1152/jn.01298.2006. [DOI] [PubMed] [Google Scholar]
- Poon PW, Chen X. Postnatal exposure to tones alters the tuning characteristics of inferior collicular neurons in the rat. Brain Res. 1992;585:391–394. doi: 10.1016/0006-8993(92)91243-8. [DOI] [PubMed] [Google Scholar]
- Popescu MV, Polley DB. Monaural deprivation disrupts development of binaural selectivity in auditory midbrain and cortex. Neuron. 2010;65:718–731. doi: 10.1016/j.neuron.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priebe NJ, Ferster D. Direction selectivity of excitation and inhibition in simple cells of the cat primary visual cortex. Neuron. 2005;45:133–145. doi: 10.1016/j.neuron.2004.12.024. [DOI] [PubMed] [Google Scholar]
- Razak KA, Fuzessery ZM. Development of the functional organization of the pallid bat auditory cortex. Hear. Res. 2007;228:69–81. doi: 10.1016/j.heares.2007.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson RT, Mostamand F, Kageyama GH, Gallardo KA, Yu J. Primary auditory cortex in the rat: transient expression of acetylcholinesterase activity in developing geniculocortical projections. Brain Res. Dev. Brain Res. 1991;58:81–95. doi: 10.1016/0165-3806(91)90240-j. [DOI] [PubMed] [Google Scholar]
- Romand R, Ehret G. Development of tonotopy in the inferior colliculus. I. Electrophysiological mapping in house mice. Brain. Res. Dev. Brain Res. 1990;54:221–234. doi: 10.1016/0165-3806(90)90145-o. [DOI] [PubMed] [Google Scholar]
- Romand R. Modification of tonotopic representation in the auditory system during development. Prog. Neurobiol. 1997;51:1–17. doi: 10.1016/s0301-0082(96)00043-3. [DOI] [PubMed] [Google Scholar]
- Royer S, Paré D. Conservation of total synaptic weight through balanced synaptic depression and potentiation. Nature. 2003;422:518–522. doi: 10.1038/nature01530. [DOI] [PubMed] [Google Scholar]
- Rubel EW, Fritzsch B. Auditory system development: primary auditory neurons and their targets. Annu. Rev. Neurosci. 2002;25:51–101. doi: 10.1146/annurev.neuro.25.112701.142849. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sally SL, Kelly JB. Organization of auditory cortex in the albino rat: sound frequency. J. Neurophysiol. 1988;59:1627–1638. doi: 10.1152/jn.1988.59.5.1627. [DOI] [PubMed] [Google Scholar]
- Sanes DH, Constantine-Paton M. Altered activity patterns during development reduce neural tuning. Science. 1983;221:1183–1185. doi: 10.1126/science.6612332. [DOI] [PubMed] [Google Scholar]
- Sanes DH, Bao S. Tuning up the developing auditory CNS. Curr. Opin. Neurobiol. 2009;19:188–199. doi: 10.1016/j.conb.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl B, Wehr M. Disruption of balanced cortical excitation and inhibition by acoustic trauma. J. Neurophysiol. 2008;100:646–656. doi: 10.1152/jn.90406.2008. [DOI] [PubMed] [Google Scholar]
- Sobeih MM, Corfas G. Extracellular factors that regulate neuronal migration in the central nervous system. Int. J. Dev. Neurosci. 2002;20:349–357. doi: 10.1016/s0736-5748(02)00040-0. [DOI] [PubMed] [Google Scholar]
- Speechley WJ, Hogsden JL, Dringenberg HC. Continuous white noise exposure during and after auditory critical period differentially alters bidirectional thalamocortical plasticity in rat auditory cortex in vivo. Eur. J. Neurosci. 2007;26:2576–2584. doi: 10.1111/j.1460-9568.2007.05857.x. [DOI] [PubMed] [Google Scholar]
- Stern EA, Maravall M, Svoboda K. Rapid development and plasticity of layer 2/3 maps in rat barrel cortex in vivo. Neuron. 2001;31:305–315. doi: 10.1016/s0896-6273(01)00360-9. [DOI] [PubMed] [Google Scholar]
- Suga N, Xiao Z, Ma X, Ji W. Plasticity and corticofugal modulation for hearing in adult animals. Neuron. 2002;36:9–18. doi: 10.1016/s0896-6273(02)00933-9. [DOI] [PubMed] [Google Scholar]
- Sun YJ, Wu GK, Liu BH, Li P, Zhou M, Xiao Z, Tao HW, Zhang LI. Fine-tuning of pre-balanced excitation and inhibition during auditory cortical development. Nature. 2010;465:927–931. doi: 10.1038/nature09079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takesian AE, Kotak VC, Sanes DH. Developmental hearing loss disrupts synaptic inhibition: implications for auditory processing. Future Neurol. 2009;4:331–349. doi: 10.2217/FNL.09.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AY, Zhang LI, Merzenich MM, Schreiner CE. Tone-evoked excitatory and inhibitory synaptic conductances of primary auditory cortex neurons. J. Neurophysiol. 2004;92:630–643. doi: 10.1152/jn.01020.2003. [DOI] [PubMed] [Google Scholar]
- Tao HW, Poo MM. Activity-dependent matching of excitatory and inhibitory inputs during refinement of visual receptive fields. Neuron. 2006;45:829–836. doi: 10.1016/j.neuron.2005.01.046. [DOI] [PubMed] [Google Scholar]
- Tritsch NX, Bergles DE. Developmental regulation of spontaneous activity in the Mammalian cochlea. J. Neurosci. 2010;30:1539–1550. doi: 10.1523/JNEUROSCI.3875-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzounopoulous T, Kim Y, Oertel D, Trussell LO. Cell-specific, spike timing-dependent plasticities in the dorsal cochlear nucleus. Nat. Neurosci. 2004;7:719–725. doi: 10.1038/nn1272. [DOI] [PubMed] [Google Scholar]
- Uziel A, Gabrion J, Ohresser M, Legrand C. Effects of hypothyroidism on the structural development of the organ of Corti in the rat. Acta. Otolaryngol. 1981;92:469–480. doi: 10.3109/00016488109133286. [DOI] [PubMed] [Google Scholar]
- Vater M, Foeller E, Mora EC, Coro F, Russell IJ, Kössl M. Postnatal maturation of primary auditory cortex in the mustached bat, Pteronotus parnellii. J. Neurophysiol. 2010;103:2339–2354. doi: 10.1152/jn.00517.2009. [DOI] [PubMed] [Google Scholar]
- Volkov IO, Galaziuk AV. Formation of spike response to sound tones in cat auditory cortex neurons: interaction of excitatory and inhibitory effects. Neuroscience. 1991;43:307–321. doi: 10.1016/0306-4522(91)90295-y. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. Specific long-term memory traces in primary auditory cortex. Nat. Rev. Neurosci. 2004;5:279–290. doi: 10.1038/nrn1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfield DA. The postnatal development of synapses in the visual cortex of the cat and the effects of eyelid closure. Brain Res. 1981;206:166–171. doi: 10.1016/0006-8993(81)90110-4. [DOI] [PubMed] [Google Scholar]
- Wolf JR, Balcar VJ, Zetzsche T, Böttcher H, Schmechel OE, Chronwall BM. Development of GABAergic system in rat visual cortex. In: Lauder JM, Nelson P, editors. Gene Expression and Cell-Cell Interactions in the Developing Nervous System. Plenum; New York: 1984. [Google Scholar]
- Wolf W, Hicks TP, Albus K. The contribution of GABA-mediated inhibitory mechanisms to visual response properties of neurons in the kitten’s striate cortex. J. Neurosci. 1986;6:2779–2795. doi: 10.1523/JNEUROSCI.06-10-02779.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Zhang Y. Sound-guided shaping of the receptive field in the mouse auditory cortex by basal forebrain activation. Eur. J. Neurosci. 2005;21:563–576. doi: 10.1111/j.1460-9568.2005.03878.x. [DOI] [PubMed] [Google Scholar]
- Yuan K, Froemke RC, Schreiner CE. Developmental changes in the timing properties of auditory cortical synaptic inputs. Assoc. Res. Otolaryngol. Abstr. 2010;33:588. [Google Scholar]
- Zhang LI, Bao S, Merzenich MM. Persistent and specific influences of early acoustic environments on primary auditory cortex. Nat. Neurosci. 2001;4:1123–1130. doi: 10.1038/nn745. [DOI] [PubMed] [Google Scholar]
- Zhang LI, Tan AY, Schreiner CE, Merzenich MM. Topography and synaptic shaping of direction selectivity in primary auditory cortex. Nature. 2003;424:201–205. doi: 10.1038/nature01796. [DOI] [PubMed] [Google Scholar]
- Zhou X, Merzenich MM. Influences of un-modulated acoustic inputs on functional maturation and critical-period plasticity of the primary auditory cortex. Neuroscience. 2007;154:390–396. doi: 10.1016/j.neuroscience.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Nagarajan N, Mossop BJ, Merzenich MM. Enduring effects of early structured noise exposure on temporal modulation in the primary auditory cortex. Proc. Natl. Acad. Sci. USA. 2008;105:4423–4428. doi: 10.1073/pnas.0800009105. [DOI] [PMC free article] [PubMed] [Google Scholar]