Abstract
Osteoblastic differentiation is an important landmark for bone formation, bone repair and regeneration, however it is a very complex process controlled by different signaling mechanisms. Several groups have reported that the cyclic adenosine monophosphate (cAMP) signaling system is responsible for regulating osteoblast cell differentiation. Nonetheless, to date, the principle role of the cAMP molecules related to this process remains controversial. Moreover, the underlying cAMP-dependent signaling cascade governing the osteoblastic differentiation has not been clarified. In this report, we investigated the roles of the cAMP-dependent protein kinase A (PKA) signaling in proliferation, differentiation and mineralization of osteoblast-like MC3T3-E1 cells using the PKA-specific small molecule cAMP analogue, 6-Bnz-cAMP, at 100 µM. Alkaline phosphatase (ALP) activity, runt transcription factor 2 (Runx2), osteopontin (OPN) and osteocalcin (OCN) protein expressions were used as osteoblast-specific markers to demonstrate osteoblastic differentiation. Further, calcium measurement of the extracellular matrix was employed as the hallmark of matrix mineralization or calcification. We report here that activation of PKA by the small molecule 6-Bnz-cAMP induces osteoblastic differentiation and matrix mineralization of osteoblast-like MC3T3-E1 cells. Moreover, 6-Bnz-cAMP does not induce cytotoxicity to the cells as revealed by our cell proliferation studies. Therefore, based on these findings, we propose that the PKA-specific small molecule 6-Bnz-cAMP may serve as a novel bone-inducing growth factor for repairing and regenerating bone tissues during bone regenerative engineering.
Keywords: cAMP, osteoblast, small molecules, differentiation, bone tissue engineering, growth factors, PKA signaling cascade, osteogenesis
1 Introduction
Growth factors are a driving force for many cellular processes, including proliferation, differentiation and gene expression. Therefore, for years scientists have actively searched for factors that stimulate bone regeneration and repair for bone regenerative engineering technologies. Traditionally, growth factors are usually a large recombinant protein, and several groups of protein have been shown to be osteoinductive, i.e. they have the capability to induce bone formation in ectopic sites. For instance, bone morphogenetic proteins (BMPs) (Urist, 1965), platelet-derived growth factor (PDGF) (Hollinger et al., 2008), transforming growth factor beta (TGF-β) (Ripamonti et al., 2009), and fibroblast growth factor-2 (FGF-2) (Gong et al., 2001) have shown great potential for use in bone regeneration and repair. Unfortunately, there are several shortcomings associated with using protein growth factors for bone disease treatment. For instance, protein instability, low solubility, high cost, supraphysiologic dose and immunogenicity are the common limitations in protein-based therapeutic strategy (Boyne et al., 1997; Geesink et al., 1999; Hwang et al., 2009; Saito et al., 2001). Therefore, an alternative form of bone growth factor is needed to obviate the drawbacks.
Small molecules with osteoinductive capacity have recently gained much attention in the field because of their inherent physical properties that minimize the limitations observed in the protein growth factors. For instance, phenamil, a small molecule BMP inducer, has been recently shown to stimulate osteoblast differentiation and mineralization via the BMP/Smad signaling cascade (Park et al., 2009). Compared to the recombinant BMPs, phenamil is an inexpensive stable small molecule. More importantly, low concentrations of phenamil are sufficient to induce bone formation via the BMP/Smad signaling pathway, and therefore the need for high doses of recombinant BMP can be bypassed (Park et al., 2009). Similarly, several other novel small molecules that also express osteoinductive capabilities at a sub-micromolar range of concentrations have been recently discovered through chemical screens (Han et al., 2009). Certain small molecules can serve as a second messenger in a number of cell signaling cascades. Typically, the small molecules trigger intracellular signaling pathways by binding to specific protein sensors, which then lead to certain gene transcriptions and expressions. One such second messenger that controls diverse cellular properties is cyclic adenosine monophosphate (cAMP), a well-known signaling small molecule that is found ubiquitously in mammalian cells (Beavo and Brunton, 2002; Daniel et al., 1998) cAMP-dependent protein kinase A (PKA) is one of the intracellular receptors responsible for cAMP level elevation. The activated form of PKA results in the phosphorylation of a large variety of downstream target proteins including various transcription factors which, in turn, ultimately regulate numerous cellular events (Taylor et al., 1990). Cyclic AMP-dependent pathways, such as the cAMP/PKA signaling cascade, have been suggested for regulating osteogenesis in a number of cell types including osteoblast-like MC3T3-E1 cells. However, the ultimate effect is still controversial, i.e. whether the cAMP/PKA pathway promotes or inhibits osteogenesis (Ghayor et al., 2009; Siddappa et al., 2009).
Cell-permeable cAMP analogs have been developed for decades and routinely used as biological tools for investigating various cAMP mediated signal transduction pathway mechanisms (Poppe et al., 2008). Recently, a research group suggested the use of a cAMP analogue, N6,2'-O-dibutyryladenosine 3':5' cyclic monophosphate (db-cAMP), as a novel growth factor for osteoblastic differentiation and bone formation (Siddappa et al., 2008). db-cAMP is first generation cell permeable non-specific cAMP analogue. One of the drawbacks of using db-cAMP is the generation of butyrate as a side product during hydrolysis (Swislocki, 1970). It has been reported in the literature that the butyrate released from db-cAMP can alter the PKA activity, and also functions as a stimulator of gene expression and differentiation in a number of cell types including osteoblasts and mesenchymal stem cells (Aukema et al., 1997; Chen et al., 2007; Katono et al., 2008). These observations raise the possibility that the osteoinductive impact of db-cAMP might partially be due to effects from the generated the side product butyrate. Moreover, the unspecific targeting of db-cAMP and more recent developed cAMP analog, 8-bromo-cAMP (8-Br-cAMP), not only activates the PKA signaling pathway, but also other cAMP-dependent signaling cascades such as exchange protein activated by cAMP (Epac) and cAMP-gated ion channel (Herness et al., 1997; Somekawa et al., 2005). Thus, such non-specific cAMP analogs cannot be used in elucidating the influences of cAMP-mediated PKA-independent signaling mechanisms on osteogenesis regulation. In addition, it has been shown that these non-specific cAMP analogs could negatively affect the proliferation of mescenchymal stem cells and osteoblast-like cells (Siddappa et al., 2008; Tintut et al., 1998). Based on these findings, the principle roles of the cAMP molecules and the PKA signaling mechanism governing osteogenesis still remains inconclusive. Moreover, the small molecule non-specific db-cAMP may not be an ideal candidate for serving as a bone-inducing growth factor for bone regeneration and repair purposes.
In this study, we investigated the principle effect of small molecule cAMP analogue on the osteogenesis of osteoblast-like MC3T3-E1 cells. We employed a newly developed cAMP analogue, N6-benzoyladenosine-3’,5’-cyclic monophosphate (6-Bnz-cAMP), which is a butyrate-free PKA-specific cAMP analogue, to study the proliferation, differentiation and mineralization of osteoblast-like MC3T3-E1 cells in vitro. 6-Bnz-cAMP is a target-specific cAMP analogue which exclusively activates the PKA signaling, as validated in the literature (Poppe et al., 2008). Our data indicated that 6-Bnz-cAMP supports cell proliferation and effectively induces osteoblastic differentiation and mineralization of osteoblast-like MC3T3-E1 cells. Our experimental evidences also suggest the involvement of PKA/CREB signaling in the osteoblast-like cell differentiation event. These results suggest that the small molecule cAMP analogue, 6-Bnz-cAMP, may represent a novel osteogenic molecule for bone regeneration and repair.
2 Materials and Methods
2.1. Reagents
N6-benzoyladenosine-3’,5’-cyclic monophosphate (6-Bnz-cAMP) was purchased from Biolog Life Science Institute (Bremen, Germany); anti-Runx2 antibody was purchased from Invitrogen (Camsrillo, CA); anti-phosho-CREB (1B6), anti-phospho-Erk1/2, anti-Erks antibodies were purchased from Cell Signaling Technology Inc (Temecula, CA); anti-β-tubulin (clone AA2) antibody was purchased from Millipore (Billerica, MA); anti-osteopontin antibody (AKm2A1), anti-osteocalcin antibody (FL-95) and Protein A/G agarose were purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). Horseradish peroxidase (HRP)-labeled affinity purified antibodies to mouse IgG and rabbit IgG were purchased from KPL (Gaithersburg, MD). Chemiluminescent substrate for detection of HRP and X-Ray films were purchased from Thermo Scientific (Rockford, IL).
2.2. Cell Culture
MC3T3-E1 osteoblast-like cells subclone 4 (American Type Culture Collection, Manassas, VA) (passage 21 to 30) were used to study the effects of 6-Bnz-cAMP on cell differentiation and mineralization. The cells were maintained in tissue culture flasks containing regular growth medium, i.e. alpha minimal essential medium (Invitrogen, Carlsbad, CA) supplemented with 10% FBS and 1% of antibiotic (100U/ml penicillin G and 100mg/ml streptomycin). Cells were incubated at 37 °C in a humidified incubator containing 5 % carbon dioxide. To investigate the osteogenic effects of the cAMP analogue, cells were cultured in regular growth medium with or without 6-Bnz-cAMP. Cells cultured in mineralization medium (alpha minimal essential medium supplemented with 10% FBS, 1% of antibiotic, 3 mM β-glycerolphosphate, and 10 µg/ml ascorbic acid) served as a positive control for osteoblastic differentiation (Beck et al., 2000). To clarify which media were being used in the experimental studies in each figure, the term “Control” refers to regular growth medium, “6-Bnz-cAMP” refers to regular growth medium supplemented with 6-Bnz-cAMP, “Mineralization” refers to mineralization medium, and “6-Bnz-cAMP + Mineralization” refers to mineralization medium supplemented with 6-Bnz-cAMP.
2.3. Cell Proliferation and Viability Assay
Cell proliferation and viability studies were performed using PicoGreen dsDNA assay kit (Molecular Probes, Eugene, OR) and trypan blue viability assay, respectively. The procedure of the PicoGreen dsDNA assay was performed according to the manufacturer’s instructions. Briefly, 5 × 104 cells ml−1 were seeded in a well of a 24-well tissue culture plate. 100 µM of 6-Bnz-cAMP was added to the regular growth medium during cell seeding. The regular growth medium and 6-Bnz-cAMP (100 µM) were replaced every 3 to 4 days. At day 7, 14, and 21, media were removed and cells were washed with phosphate buffered saline (PBS) prior to the procedures of the assays. For trypan blue viability assay method, at day 7, 14, and 21, cells were collected by trypsinization and stained with trypan blue dye (MP Biochemicals LLC, Solon, OH). The cells were then numbered in a hemacytometer (Hausser Scientific, Horsham, PA) under a light microscopy (Olympus Corporation, Tokyo, Japan). Cell stained blue was scored a dead cell, while unstained cell was scored a live cell. The results are presented in % of viability.
2.4. Polyacrylamide Gel Electrophoresis (PAGE) and Western Blotting
Osteogenic protein markers were analyzed by SDS-PAGE and Western Blotting. Protein samples were resolved by SDS-PAGE on precast 12.5% polyvinylidene-Tris gels and transferred electrophoretically onto polyvinylidene difluoride membranes according to the manufacturer’s instructions (Bio-Rad, Hercules, CA). Membranes were blocked with skim milk and then probed with respective antibodies. Protein bands on blots were visualized by an enhanced chemiluminescent detection kit.
2.5. Immunoprecipitation
The cell culture media after 18 days incubation were collected in microcentrifuge tubes. The extracellullar protein osteocalcin and osteopontin in the culture media was individually isolated by incubating with anti-osteocalcin antibody and anti-osteopontin antibody, respectively. Antibody-bound proteins were then precipitated by incubating with Protein A/G agarose for another 2 h at 4 °C. Immune complexes were washed 4 times with PBS to eliminate non-specific bindings. The resulting pellets were resuspended in Laemmli SDS sample buffer (Bio-Rad, Hercules, CA) for polyacrylamide gel electrophoresis and Western Blot analysis.
2.6. Alkaline phosphatase activity (ALP)
ALP activities were measured using ALP assay kit according to the manufacturer’s instructions (Bio-Rad, Hercules, CA). Briefly, at day 7 and 14, cells were collected and washed 4 times with PBS and then lysed with 0.1 % TX-100. Cell lysates were collected and mixed with the ALP substrate solution for 1h at 37 °C incubation. The colorimetric change due to the presence of ALP activity was measured by a spectrophotometric plate reader at 405 nm. The absorbance values were then normalized to cellular DNA.
2.7. Matrix Mineralization assay
The osteoblast-like MC3T3-E1 cells were incubated in mineralization medium and supplemented with 6-Bnz-cAMP (100 µM) during cell seeding. The mineralization medium and 6-Bnz-cAMP (100 µM) were replaced every 3 to 4 days. For the qualitative determination of calcified matrix mineralization, at day 21 media were removed and cells were rinsed with calcium-free PBS for 3 times. The precipitated calcium of mineralized matrix was dissolved overnight in 0.6N hydrochloric acid at 4°C. The extracted calcium was then measured with a calcium assay kit (Calcium Liquicolor, Stanbio Laboratory, Boerne, TX) according to the manufacturer’s instructions. The absorbance values were normalized to cellular DNA.
2.8. Statistical Analysis
Significant values (P < 0.05) were determined by using the two-tailed Student's t-test. An asterisk denotes a significant (P < 0.05) in the figures.
3 Results
3.1. Cellular proliferation in 6-Bnz-cAMP treated osteoblast-like MC3T3-E1 cells
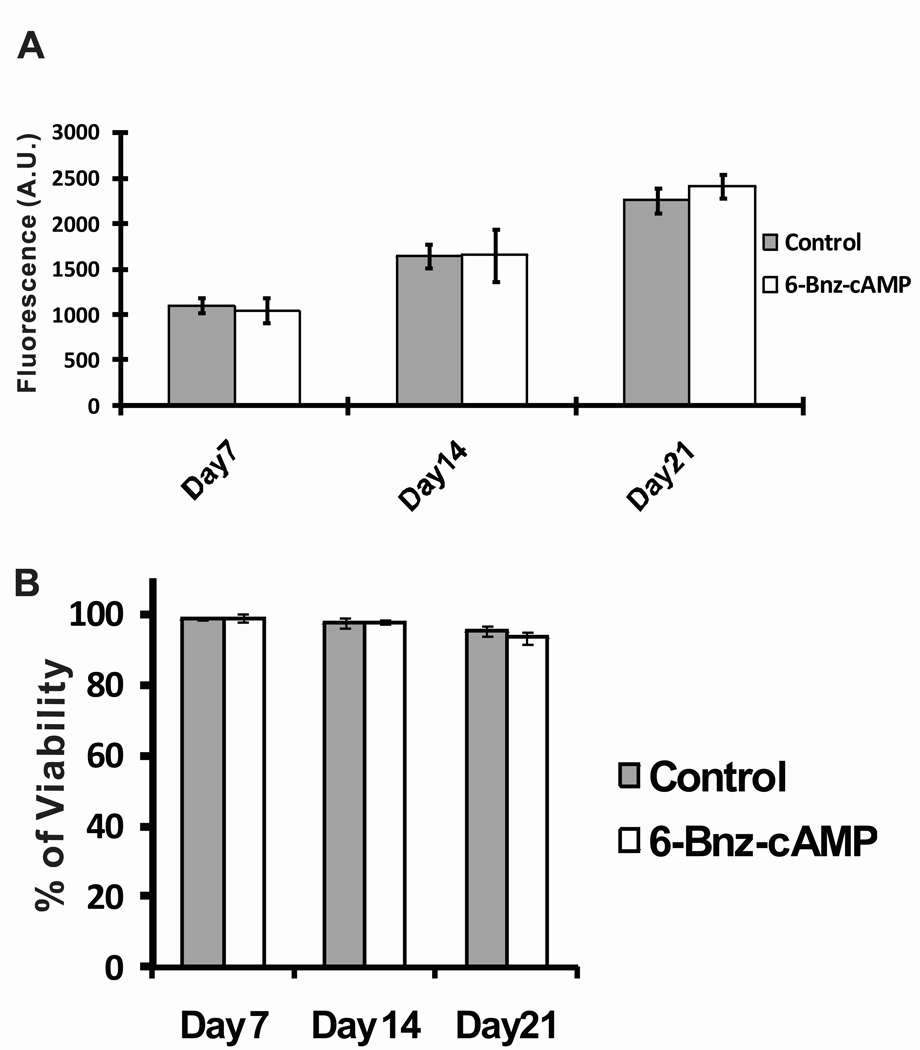
The viability and cell proliferation of osteoblast-like MC3T3-E1 cells in the presence or absence of 6-Bnz-cAMP were determined by cell proliferation assay using the PicoGreen fluorescence dsDNA. PicoGreen is an ultrasensitive fluorescent stain for the quantification of dsDNA in solution. Since it provided a good linear correlation between cell number and fluorescence (data not shown), the fluorescent signals generated from the PicoGreen assay served as a surrogate for cell numbers. The data from Figure 1A indicated that both untreated control and 6-Bnz-cAMP treated cells showed statistically significant increase in cell proliferation from day 7 to day 21. However, there were no significant differences in cell proliferation among the cells treated with 6-Bnz-cAMP when compared to the untreated control group at day 7, 14, and 21. We then used trypan blue stain to access the viability of cells in the presence of 6-Bnz-cAMP. Figure 1B demonstrated that both 6-Bnz-cAMP treated and untreated control groups maintained greater than 90% of the cell viability throughout the study period. Moreover, there were no significant changes in cell viability among the cells treated with 6-Bnz-cAMP when compared to the untreated control at any time point. Taken together, these observations indicated that 6-Bnz-cAMP (100 µM) does not induce cytotoxicity to MC3T3-E1 cells. Consistent with these observations, our previously published data demonstrated that 6-Bnz-cAMP supported MC3T3-E1 cell proliferation using nonradioactive cell proliferation (MTS) assay method (Lo et al., 2010).
Figure 1.
Effect of 6-Bnz-cAMP on the proliferation and viability of osteoblast-like MC3T3-E1 cells. (A) Cell proliferation was assessed by PicoGreen dsDNA assay. The assay demonstrated that 6-Bnz-cAMP treated and untreated groups supported cell proliferation from day 7 to day 21. (B) Cell viability was measured by trypan blue viability stain. No significant differences were found when comparing between the 6-Bnz-cAMP treated and the untreated control cells at each time point, indicating that the viability of MC3T3-E1 cells is not affected by the 6-Bnz-cAMP treatment.
3.2. 6-Bnz-cAMP upregulates the expression of osteoblast-specific transcription factor, Runx2
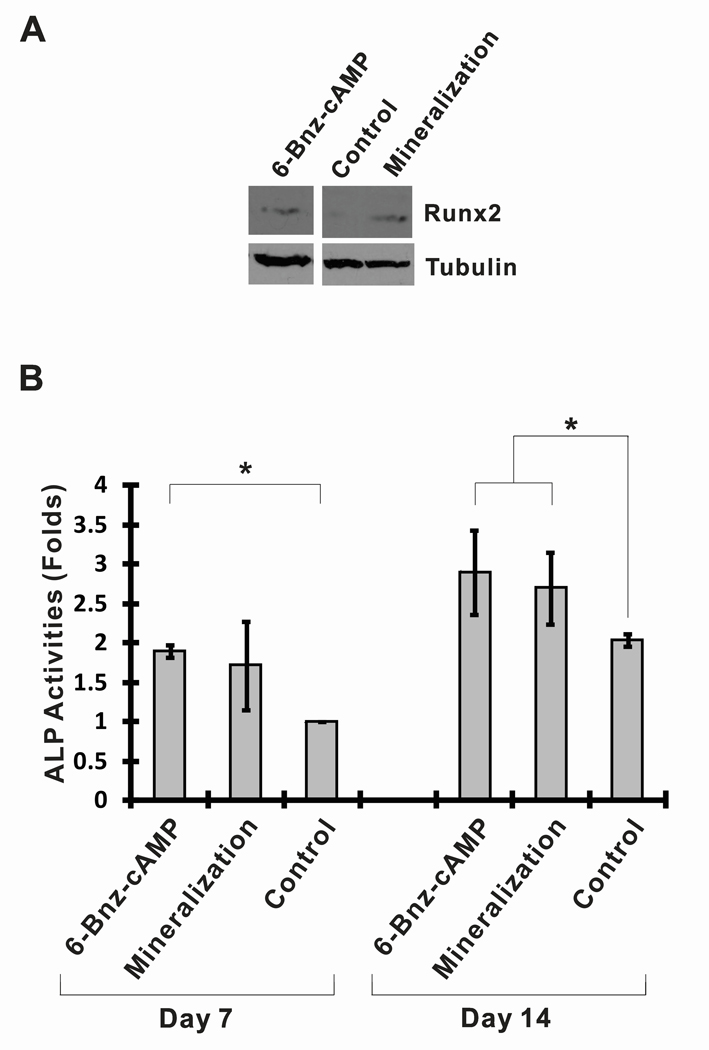
It is generally believed that Runx2 is strongly associated with osteoblastic differentiation and it is one of the early-stage osteoblastic differentiation markers (Ducy et al., 1997). Runx2 increases bone formation by stimulating the transcription of bone-marker genes such as ALP and osteopontin, and osteocalcin (Phimphilai et al., 2006). To investigate whether the small molecule 6-Bnz-cAMP could induce Runx2 protein expression, osteoblast-like MC3T3-E1 cells cultured in regular growth medium were exposed to 6-Bnz-cAMP for 7 days. At day 7, cells were lysed with laemmli sample buffer and the whole cell lysates were used for Runx2 protein expression analysis (Figure 2A). Runx2 protein was detected in the MC3T3-E1 cell lysates when cultured in regular growth medium supplemented with 6-Bnz-cAMP (100 µM) for 7 days. In contrast, Runx2 protein was not detected when cells were grown in the regular growth medium alone. However, as expected, Runx2 protein was detected by Western Blot in MC3T3-E1 cell lysates after 7 days of culture in mineralization medium (positive control). These observations suggested that treatment of MC3T3-E1 cells with 6-Bnz-cAMP in regular growth medium is sufficient to induce Runx2 expression.
Figure 2.
6-Bnz-cAMP induces osteoblastic differentiation of osteoblast-like MC3T3-E1 cells. (A) 6-Bnz-cAMP promotes Runx2 expression. Cells cultured in the mineralization medium served as a positive control for the Runx2 expression. The blot “tubulin” was used as a control for equal protein loading. (Note that the blots “Runx2” and “tubulin” were adapted from the same X-ray films with the equal exposure times) (B) 6-Bnz-cAMP increases ALP activity. ALP activities of MC3T3-E1 cells were induced by 6-Bnz-cAMP at day 7 and further increased at day 14. To facilitate the comparison of different experimental settings, the ALP activity of the cells cultured in the regular growth medium at day 7 was set to one relative unit.
3.3. 6-Bnz-cAMP enhances ALP activity
To further assess the osteoinductive capabilities of 6-Bnx-cAMP, we next tested whether 6-Bnz-cAMP increased ALP activity, an early/intermediate stage marker of osteoblastic differentiation. As shown in Figure 2B, in comparision with the untreated “Control,” ALP activity in osteoblast-like MC3T3-E1 cells was significantly enhanced by the “6-Bnz-cAMP” treatment at day 7. On the other hand, ALP activity of the cells cultured in mineralization medium was not statistically significantly enhanced in comparision with the “Control,” indicating that 6-Bnz-cAMP was able to promote ALP activity at earlier time point. At day 14, cells cultured in mineralization medium or in “6-Bnz-cAMP” condition were found to increase ALP activities significantly in MC3T3-E1 cells. Consistent with the data from the Runx2 protein expression studies, these results indicate that the small molecule 6-Bnz-cAMP is able to induce osteogenic differentiation of MC3T3-E1 cells.
3.4. 6-Bnz-cAMP promotes OCN and OPN extracellular protein productions
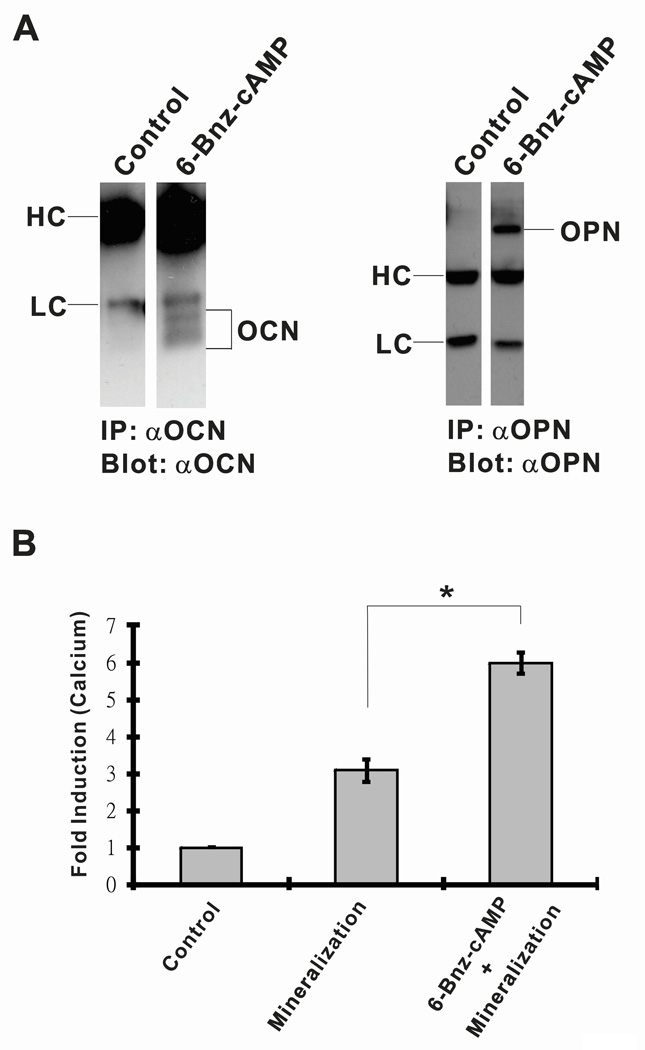
OCN and OPN are secretory extracellular proteins which constitute major noncollagenous matrix proteins of bone (Young et al., 1992) and are considered the late-stage markers for osteoblast differentiation (Beck et al., 2000). To investigate the roles of 6-Bnz-cAMP in extracellular OCN and OPN protein productions, osteoblast-like MC3T3-E1 cells were cultured in regular growth medium with or without 6-Bnz-cAMP. At day 18, the culture media were collected and the extracellular content of OCN and OPN proteins was determined by the individual isolation and concentration of the proteins by the immunoprecipitation method. Following, western blot analysis indicated that in the presence of 6-Bnz-cAMP, both extracellular OCN and OPN proteins were individually immunoprecipitated by the corresponding antibodies. However, both OCN and OPN proteins were undetectable when the cells were cultured in the regular growth media without 6-Bnz-cAMP treatment (Figure 3A). Both OPN and OCN proteins were undetected in our assay system at earlier stage, i.e. at day 10 (data not shown). These data is consistent with the published data that OPN and OCN were only expressed near or at the time of mineralization, i.e. day 21, when MC3T3-E1 cells had been induced to differentiate (Beck et al., 2000).
Figure 3.
(A) 6-Bnz-cAMP promotes osteogenic markers OCN and OPN extracellular proteins productions. The OCN and OPN proteins were detected exclusively in the “6-Bnz-cAMP” treated samples (note that blots in the left panels were from the same X-ray film with the equal exposure time of 5 min and the blots in the right panels were from the same film with the exposure time of 3 min). The immunoprecipitate components antibody heavy (H) and light (L) chains are indicated. (B) 6-Bnz-cAMP promotes osteoblastic mineralization. After 21 days of culture of osteoblast-like MC3T3-E1 cells in mineralization medium with or without 6-Bnz-cAMP, quantitative calcium levels were determined. Calcium levels were significantly elevated in osteoblasts cultured in mineralization medium. Osteoblast-like MC3T3-E1 cells cultured in mineralization medium supplemented with 6-Bnz-cAMP further enhanced calcium deposition when compared to the cells cultured in the mineralization medium alone. To facilitate the comparison of different experimental settings, the level of calcium measurement of the “Control” cells cultured in the regular growth medium was set to one relative unit.
3.5. 6-Bnz-cAMP promotes in vitro mineralization
Our findings of increased ALP activity and unregulated Runx2, OCN, and OPN expression in response to 6-Bnz-cAMP treatment prompted us to investigate the role of 6-Bnz-cAMP on matrix mineralization in MC3T3-E1 cells. Extracellular matrix mineralization or calcification is a definitive hallmark of osteoblastic differentiation (Declercq et al., 2005). MC3T3-E1 cells were cultured in mineralization medium with or without 6-Bnz-cAMP for 21 days. We used mineralization media instead of using regular growth medium in this instance because the one component, beta-glycerophosphate, is essential in providing the inorganic phosphate group for mineralized matrices (Bellows et al., 1992). Moreover, both beta-glycerophosphate and ascorbic acid are essential to induce the productions of proteoglycan-degrading metalloproteinases favoring matrix calcification (Dean et al., 1994). The mineralized matrix of the cultured osteoblasts was quantified by colorimetric determination of total calcium deposited in the matrix. In the Figure 3B, cells exposed to the “6-Bnz-cAMP + mineralization” condition exhibited a significant increase in calcium level as compared to cells cultured in the mineralization medium alone, suggesting that 6-Bnz-cAMP further promotes matrices mineralization of osteoblast-like MC3T3-E1 cells. Cells cultured in the regular growth medium “Control” served as the baseline for the mineralization study.
3.6. The involvement of cAMP/PKA/CREB signaling cascade in 6-Bnz-cAMP induced osteoblastic differentiation of osteoblast-like MC3T3-E1 cells
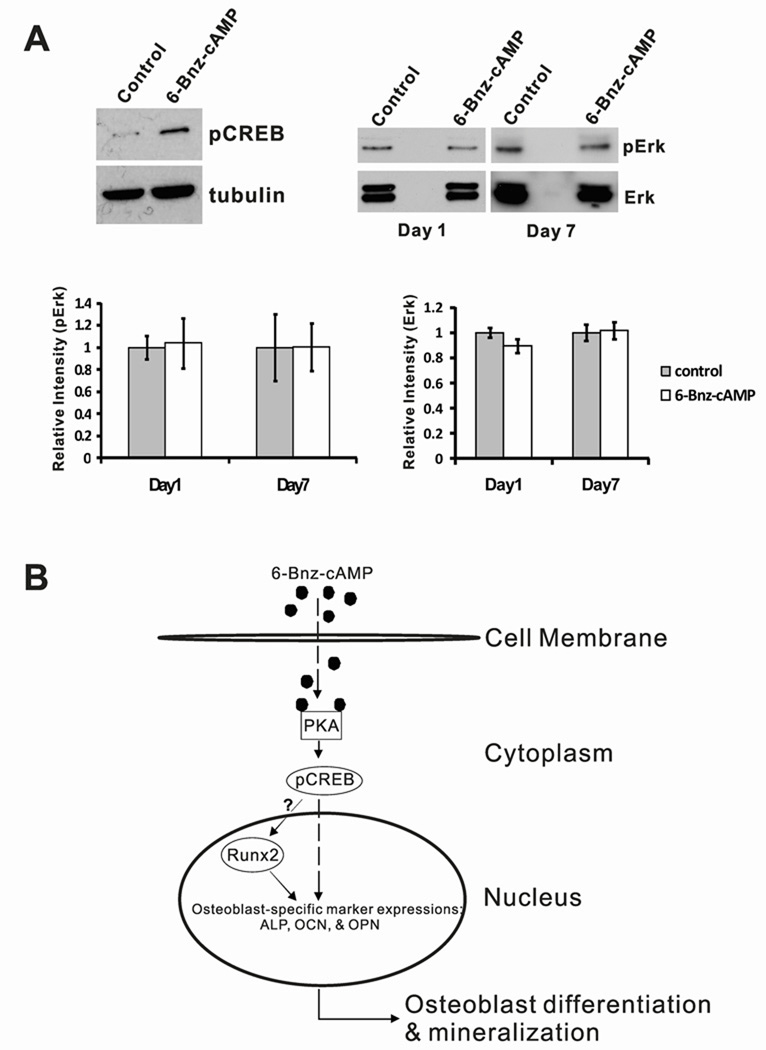
Since 6-Bnz-cAMP is specially designed for specifically activating PKA, we next sought to identify the downstream protein target(s) of the activated PKA that supported the function of osteoinduction. cAMP response element-binding protein (CREB) is the most attractive candidate because it is the well-known direct target protein of PKA (Rangarajan et al., 2003; Siddappa et al., 2008). Moreover, phosphorylation of CREB (pCREB) by PKA has been shown to be involved in regulating osteoblast differentiation (Yang et al., 2008). As hypothesized, in comparison to the untreated control, pCREB level was obviously upregulated in MC3T3-E1 cells upon treatment with 6-Bnz-cAMP, suggesting that 6-Bnz-cAMP-induced osteoblastic differentiation is associated with the CREB activation in osteoblast-like MC3T3-E1 cells (Figure 4A, upper left panel).
Figure 4.
Proposed molecular mechanism of 6-Bnz-cAMP induced in vitro osteogenesis of osteoblast-like MC3T3-E1 cells. (A) Compared to the untreated “Control,” 6-Bnz-cAMP activates the phosphorylation of CREB in osteoblast-like MC3T3-E1 cells (upper left panel). Note that the faint band in the “Control” lane was likely due to the endogenous pCREB protein. To demonstrate that 6-Bnz-cAMP induced osteoblastic differentiation of MC3T3-E1 cells is independent of Erk, MC3T3-E1 cells were stimulated with 6-Bnz-cAMP (100 µM) for 1 day and 7 days. The cell lysates were analyzed by Western Blots (upper right panel) for pErk and total Erk proteins (note that the blots are a representative data from 3 independent experiments). The bar graphs represent densitometry analyses (Adobe Photoshop 7.0) and statistical evaluations of the pErk (lower left panel) and Erk (lower right panel) Western Blots from 3 independent experiments and are presented as relative intensity. To facilitate the comparison of the intensity differences, the intensity levels of all the “Control” were set to one relative unit. (B) Schematic representation of the small molecule 6-Bnz-cAMP signaling pathway involved in osteogenesis of osteoblast-like MC3T3-E1 cells. The cell permeable 6-Bnz-cAMP small molecules pass through the cell membrane and specially activate PKA in cytoplasm. Activation of PKA will lead to modification of transcription factors such as phosphorylation of CREB. pCREB will translocate from cytoplasm to nucleus. pCREB may then (1) directly modulate the osteoblast-specific gene expression and/or (2) indirectly induce the expressions of osteoblast-specific genes via Runx2 and its cofactors.
Phosphorylated extracellular signal-regulated kinase (pErk) is also known to play an important role in osteoblast differentiation through the regulation of Runx2 expression (Cheung et al., 2006; Xiao et al., 2002). The phosphorylation of Erk is regulated by the PKA activation, and the process is mediated by Crk SH3 domain guanine nucleotide exchange factor and its downstream target substrate, Ras-proximate-1. In concert, these factors constitute the signaling cascade of the cAMP-mediated PKA-dependent activation of Erk (Gerdin and Eiden, 2007). These observations prompted us to investigate whether 6-Bnz-cAMP executes its osteogenic effect through the activation of Erk. Cells were cultured in the regular growth medium with or without 6-Bnz-cAMP for 1 and 7 days. In the presence of 6-Bnz-cAMP, no obvious upregulation of pErk were observed in the western blots at day 1 and day 7 (Figure 4A, upper right panel). Moreover, statistical analyses of the pixel intensity levels of the pErk and the total Erk proteins confirmed that no significant differences of pErk and total Erk protein levels among the cells treated with 6-Bnz-cAMP compared to control groups at day 1 and day 7 (Figure 4A, lower panel), indicating that 6-Bnz-cAMP/PKA induced osteoblastic differentiation of MC3T3-E1 cells independent of Erk.
4 Discusion
The objective of this study was to investigate the osteoinductivity of a PKA-specific cAMP analogue, 6-Bnz-cAMP, using osteoblast-like MC3T3-E1 cells as a study model. Osteoblast-like MC3T3-E1 cell line was recruited in our study because they display classical osteogenic markers when stimulated to differentiate (Beck et al., 2000). Our data demonstrated that 6-Bnz-cAMP in vitro induces various osteogenic marker protein expressions from early to late stages of differentiation, and ultimately leads to calcium precipitation in osteoblast-like MC3T3-E1 cells via the PKA signaling cascade. These results suggested that the small molecule 6-Bnz-cAMP may potentially function as an alternative growth factor for bone repair and regeneration.
As an initial step in characterizing the bone-inducing capability of the small molecule 6-Bnz-cAMP, we examined the role of 6-Bnz-cAMP in regulating cell proliferation. It has been reported that non-specific cAMP analogs such as db-cAMP and 8-Br-cAMP inhibits cell proliferation in a number of cell types including osteoblast-like MC3T3-E1 cells (Siddappa et al., 2008; Siddappa et al., 2009) (our unpublished observations). Interestingly, we did not observe the anti-proliferative effects of 6-Bnz-cAMP in our proliferation assays at any time point in our evaluation of MC3T3-E1 cells (Figure 1). Since 6-Bnz-cAMP is a PKA-selective activator, these observations suggested that cAMP-mediated PKA-independent signaling pathways stimulated by the non-specific cAMP analogs might negatively regulate the proliferation of MC3T3-E1 cells. For instance, it was recently shown that the cAMP/Epac signaling pathway inhibited cell proliferations in human prostate carcinoma cells and human airway smooth muscle cells (Grandoch et al., 2009; Kassel et al., 2008). Our viability study also suggests that 6-Bnz-cAMP does not induce cytotoxicity to MC3T3-E1 cells at the concentration used in this study (Figure 1B).
The cAMP/PKA signaling pathway has been shown to be involved in regulating either anabolic or catabolic effects on bone cells in various cell and animal models including osteoblast-like MC3T3-E1 cells (Hock, 2001; Schiller et al., 1999) (Yang et al., 2008). The ultimate effect depends on the type and dosage of the cAMP activator, treatment duration, and culture condition (Schiller et al., 1999). For instance, a low and intermittent dose of parathyroid hormone (PTH), a cAMP PKA-dependent stimulating hormone, has been shown to be essential for osteoblast differentiation in rat (Krishnan et al., 2003). In contrast, continuous administration of PTH results in a catabolic effect on rat bone (Uzawa et al., 1995). Intriguingly, a recent report demonstrated that a repetitive regimen of continuous treatments of PTH followed by periodic PTH withdrawals induces bone formation (Etoh and Yamaguchi). In our study, continuous stimulation of the PKA signaling pathway by administrating the PKA-specific cAMP analogue continuously in osteoblast-like MC3T3-E1 cells resulted in increased ALP activity, up-regulation of Runx2, OPN and OCN protein productions, and increased matrix mineralization or calcification. To our knowledge, this study provides the first evidence that prolonged activation of PKA signaling cascade using PKA-selective cAMP analogue is able to promote differentiation of osteoblast-like MC3T3-E1 cells in vitro.
Our studies provided a clear connection between 6-Bnz-cAMP, PKA, pCREB, and osteoblast-specific protein expressions. We thus propose that the 6-Bnz-cAMP/PKA/pCREB signaling cascade directly and/or indirectly regulates the expression osteoblast-specific genes in osteoblast-like MC3T3-E1 cells (Figure 4B). However, a detailed look at the underlying mechanism of pCREB regulation in the expression of osteoblast-specific genes remains to be addressed. Interestingly, it has been reported that activating transcription factor 4 (ATF4) promotes Runx2-dependent osteoblast-specifc gene expression and the activity of ATF4 is regulated by the cAMP/PKA/pCREB signaling pathway (Luo et al., 2009; Xiao et al., 2005). Thus, it is reasonable for us to speculate that the 6-Bnz-cAMP/PKA/CREB signaling may regulate osteoblast-specific gene expression through co-factors including ATF4 and Runx2. Future experiments such as systematic knockdown studies with small interfering RNAs to CREB, ATF4, and Runx2 will provide the unique opportunity to test our proposed model.
The osteogenic effect and lack of cytotoxicity of 6-Bnz-cAMP suggest that this small molecule can be transferrable to bone regenerative engineering applications using a biodegradable polymeric scaffold as a delivery vector. Such delivery vehicles have been previously reported for full length proteins such as recombinant BMPs (Bessa et al., 2008). However, these techniques always require the maintenance of protein tertiary structures in order to ensure protein bioactivities (Fu et al., 2000). The use of osteogenic small molecules such as 6-Bnz-cAMP can bypass the need of maintaining such structural integrities. Moreover, due to the small molecular size of 6-Bnz-cAMP, it may facilitate release kinetic from a tissue-engineered polymeric scaffold system. It is also worth noting that 6-Bnz-cAMP can promote not only differentiation and mineralization, but also initial cell adhesion (Lo et al., 2010). This novel property of 6-Bnz-cAMP is of great interest since cell adhesion is an important pre-requisite for subsequent cell specialization (Marquis et al., 2009). Therefore, the increased cell adhesion induced by 6-Bnz-cAMP might also contribute to downstream cellular responses important for differentiation and mineralization of osteoblast-like MC3T3-E1 cells. Taken together, the results generated from our studies of 6-Bnz-cAMP warrant further research to evaluate the feasibility of engineering biodegradable polymeric scaffolds containing the small molecule 6-Bnz-cAMP for bone regenerative engineering applications.
Acknowledgements
We would like to thank Mr. James Veronick and Miss Ashley Amini for comments. Dr. Laurencin was the recipient of a Presidential Faculty Fellowship Award from NSF. This work was supported by National Science Foundation (NSF-EFRI 0736002) and National Institute of Health (NIH-AR052536).
References
- Aukema HM, Davidson LA, Pence BC, et al. Butyrate alters activity of specific cAMP-receptor proteins in a transgenic mouse colonic cell line. J Nutr. 1997;127:18–24. doi: 10.1093/jn/127.1.18. [DOI] [PubMed] [Google Scholar]
- Beavo JA, Brunton LL. Cyclic nucleotide research -- still expanding after half a century. Nat Rev Mol Cell Biol. 2002;3:710–718. doi: 10.1038/nrm911. [DOI] [PubMed] [Google Scholar]
- Beck GR, Jr, Zerler B, Moran E. Phosphate is a specific signal for induction of osteopontin gene expression. Proc Natl Acad Sci U S A. 2000;97:8352–8357. doi: 10.1073/pnas.140021997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellows CG, Heersche JN, Aubin JE. Inorganic phosphate added exogenously or released from beta-glycerophosphate initiates mineralization of osteoid nodules in vitro. Bone Miner. 1992;17:15–29. doi: 10.1016/0169-6009(92)90707-k. [DOI] [PubMed] [Google Scholar]
- Bessa PC, Casal M, Reis RL. Bone morphogenetic proteins in tissue engineering: the road from laboratory to clinic, part II (BMP delivery) J Tissue Eng Regen Med. 2008;2:81–96. doi: 10.1002/term.74. [DOI] [PubMed] [Google Scholar]
- Boyne PJ, Marx RE, Nevins M, et al. A feasibility study evaluating rhBMP-2/absorbable collagen sponge for maxillary sinus floor augmentation. Int J Periodontics Restorative Dent. 1997;17:11–25. [PubMed] [Google Scholar]
- Chen TH, Chen WM, Hsu KH, et al. Sodium butyrate activates ERK to regulate differentiation of mesenchymal stem cells. Biochem Biophys Res Commun. 2007;355:913–918. doi: 10.1016/j.bbrc.2007.02.057. [DOI] [PubMed] [Google Scholar]
- Cheung WM, Ng WW, Kung AW. Dimethyl sulfoxide as an inducer of differentiation in preosteoblast MC3T3-E1 cells. FEBS Lett. 2006;580:121–126. doi: 10.1016/j.febslet.2005.11.062. [DOI] [PubMed] [Google Scholar]
- Daniel PB, Walker WH, Habener JF. Cyclic AMP signaling and gene regulation. Annu Rev Nutr. 1998;18:353–383. doi: 10.1146/annurev.nutr.18.1.353. [DOI] [PubMed] [Google Scholar]
- Dean DD, Schwartz Z, Bonewald L, et al. Matrix vesicles produced by osteoblast-like cells in culture become significantly enriched in proteoglycan-degrading metalloproteinases after addition of beta-glycerophosphate and ascorbic acid. Calcif Tissue Int. 1994;54:399–408. doi: 10.1007/BF00305527. [DOI] [PubMed] [Google Scholar]
- Declercq HA, Verbeeck RM, De Ridder LI, et al. Calcification as an indicator of osteoinductive capacity of biomaterials in osteoblastic cell cultures. Biomaterials. 2005;26:4964–4974. doi: 10.1016/j.biomaterials.2005.01.025. [DOI] [PubMed] [Google Scholar]
- Ducy P, Zhang R, Geoffroy V, et al. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- Etoh M, Yamaguchi A. Repetition of continuous PTH treatments followed by periodic withdrawals exerts anabolic effects on rat bone. J Bone Miner Metab. 2010 doi: 10.1007/s00774-010-0181-4. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Fu K, Klibanov AM, Langer R. Protein stability in controlled-release systems. Nat Biotechnol. 2000;18:24–25. doi: 10.1038/71875. [DOI] [PubMed] [Google Scholar]
- Geesink RG, Hoefnagels NH, Bulstra SK. Osteogenic activity of OP-1 bone morphogenetic protein (BMP-7) in a human fibular defect. J Bone Joint Surg Br. 1999;81:710–718. doi: 10.1302/0301-620x.81b4.9311. [DOI] [PubMed] [Google Scholar]
- Gerdin MJ, Eiden LE. Regulation of PC12 cell differentiation by cAMP signaling to ERK independent of PKA: do all the connections add up? Sci STKE. 2007;2007:pe15. doi: 10.1126/stke.3822007pe15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghayor C, Ehrbar M, San Miguel B. cAMP enhances BMP2-signaling through PKA and MKP1-dependent mechanisms. Biochem Biophys Res Commun. 2009;381:247–252. doi: 10.1016/j.bbrc.2009.02.032. [DOI] [PubMed] [Google Scholar]
- Gong Z, Zhou S, Cao J. Effects of recombinant human basic fibroblast growth factor on cell proliferation during mandibular fracture healing in rabbits. Chin J Traumatol. 2001;4:110–112. [PubMed] [Google Scholar]
- Grandoch M, Rose A, ter Braak M, et al. Epac inhibits migration and proliferation of human prostate carcinoma cells. Br J Cancer. 2009;101:2038–2042. doi: 10.1038/sj.bjc.6605439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han CY, Wang Y, Yu L, et al. Small molecules with potent osteogenic-inducing activity in osteoblast cells. Bioorg Med Chem Lett. 2009;19:1442–1445. doi: 10.1016/j.bmcl.2009.01.025. [DOI] [PubMed] [Google Scholar]
- Herness MS, Sun XD, Chen Y. cAMP and forskolin inhibit potassium currents in rat taste receptor cells by different mechanisms. Am J Physiol. 1997;272:C2005–C2018. doi: 10.1152/ajpcell.1997.272.6.C2005. [DOI] [PubMed] [Google Scholar]
- Hock JM. Anabolic actions of PTH in the skeletons of animals. J Musculoskelet Neuronal Interact. 2001;2:33–47. [PubMed] [Google Scholar]
- Hollinger JO, Hart CE, Hirsch SN, et al. Recombinant human platelet-derived growth factor: biology and clinical applications. J Bone Joint Surg Am. 2008;90 Suppl 1:48–54. doi: 10.2106/JBJS.G.01231. [DOI] [PubMed] [Google Scholar]
- Hwang CJ, Vaccaro AR, Lawrence JP, et al. Immunogenicity of bone morphogenetic proteins. J Neurosurg Spine. 2009;10:443–451. doi: 10.3171/2009.1.SPINE08473. [DOI] [PubMed] [Google Scholar]
- Kassel KM, Wyatt TA, Panettieri RA., Jr Inhibition of human airway smooth muscle cell proliferation by beta 2-adrenergic receptors and cAMP is PKA independent: evidence for EPAC involvement. Am J Physiol Lung Cell Mol Physiol. 2008;294:L131–L138. doi: 10.1152/ajplung.00381.2007. [DOI] [PubMed] [Google Scholar]
- Katono T, Kawato T, Tanabe N, et al. Sodium butyrate stimulates mineralized nodule formation and osteoprotegerin expression by human osteoblasts. Arch Oral Biol. 2008;53:903–909. doi: 10.1016/j.archoralbio.2008.02.016. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Moore TL, Ma YL, et al. Parathyroid hormone bone anabolic action requires Cbfa1/Runx2-dependent signaling. Mol Endocrinol. 2003;17:423–435. doi: 10.1210/me.2002-0225. [DOI] [PubMed] [Google Scholar]
- Lo KWH, Ashe KM, Kan HM, et al. Activation of Cyclic AMP/Protein Kinase A Signaling Pathway Enhances Osteoblast Cell Adhesion on Biomaterials for Regenerative Engineering. Journal of Orthopaedic Research. 2010 doi: 10.1002/jor.21276. n/a. doi: 10.1002/jor.21276. [DOI] [PubMed] [Google Scholar]
- Luo J, Zhou W, Zhou X, et al. Regulation of bone formation and remodeling by G-protein-coupled receptor 48. Development. 2009;136:2747–2756. doi: 10.1242/dev.033571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis ME, Lord E, Bergeron E, et al. Bone cells-biomaterials interactions. Front Biosci. 2009;14:1023–1067. doi: 10.2741/3293. [DOI] [PubMed] [Google Scholar]
- Park KW, Waki H, Kim WK, et al. The small molecule phenamil induces osteoblast differentiation and mineralization. Mol Cell Biol. 2009;29:3905–3914. doi: 10.1128/MCB.00002-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phimphilai M, Zhao Z, Boules H, et al. BMP signaling is required for RUNX2-dependent induction of the osteoblast phenotype. J Bone Miner Res. 2006;21:637–646. doi: 10.1359/JBMR.060109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppe H, Rybalkin SD, Rehmann H, et al. Cyclic nucleotide analogs as probes of signaling pathways. Nat Methods. 2008;5:277–278. doi: 10.1038/nmeth0408-277. [DOI] [PubMed] [Google Scholar]
- Rangarajan S, Enserink JM, Kuiperij HB, et al. Cyclic AMP induces integrin-mediated cell adhesion through Epac and Rap1 upon stimulation of the beta 2-adrenergic receptor. J Cell Biol. 2003;160:487–493. doi: 10.1083/jcb.200209105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripamonti U, Ferretti C, Teare J, et al. Transforming growth factor-beta isoforms and the induction of bone formation: implications for reconstructive craniofacial surgery. J Craniofac Surg. 2009;20:1544–1555. doi: 10.1097/SCS.0b013e3181b09ca6. [DOI] [PubMed] [Google Scholar]
- Saito N, Okada T, Horiuchi H, et al. A biodegradable polymer as a cytokine delivery system for inducing bone formation. Nat Biotechnol. 2001;19:332–335. doi: 10.1038/86715. [DOI] [PubMed] [Google Scholar]
- Schiller PC, D'Ippolito G, Roos BA, et al. Anabolic or catabolic responses of MC3T3-E1 osteoblastic cells to parathyroid hormone depend on time and duration of treatment. J Bone Miner Res. 1999;14:1504–1512. doi: 10.1359/jbmr.1999.14.9.1504. [DOI] [PubMed] [Google Scholar]
- Siddappa R, Martens A, Doorn J, et al. cAMP/PKA pathway activation in human mesenchymal stem cells in vitro results in robust bone formation in vivo. Proc Natl Acad Sci U S A. 2008;105:7281–7286. doi: 10.1073/pnas.0711190105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddappa R, Mulder W, Steeghs I, et al. cAMP/PKA signaling inhibits osteogenic differentiation and bone formation in rodent models. Tissue Eng Part A. 2009;15:2135–2143. doi: 10.1089/ten.tea.2008.0512. [DOI] [PubMed] [Google Scholar]
- Somekawa S, Fukuhara S, Nakaoka Y, et al. Enhanced functional gap junction neoformation by protein kinase A-dependent and Epac-dependent signals downstream of cAMP in cardiac myocytes. Circ Res. 2005;97:655–662. doi: 10.1161/01.RES.0000183880.49270.f9. [DOI] [PubMed] [Google Scholar]
- Swislocki NI. Decomposition of dibutyryl cyclic AMP in aqueous buffers. Anal Biochem. 1970;38:260–269. doi: 10.1016/0003-2697(70)90175-2. [DOI] [PubMed] [Google Scholar]
- Taylor SS, Buechler JA, Yonemoto W. cAMP-dependent protein kinase: framework for a diverse family of regulatory enzymes. Annu Rev Biochem. 1990;59:971–1005. doi: 10.1146/annurev.bi.59.070190.004543. [DOI] [PubMed] [Google Scholar]
- Tintut Y, Parhami F, Bostrom K, et al. cAMP stimulates osteoblast-like differentiation of calcifying vascular cells. Potential signaling pathway for vascular calcification. J Biol Chem. 1998;273:7547–7553. doi: 10.1074/jbc.273.13.7547. [DOI] [PubMed] [Google Scholar]
- Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- Uzawa T, Hori M, Ejiri S, et al. Comparison of the effects of intermittent and continuous administration of human parathyroid hormone(1–34) on rat bone. Bone. 1995;16:477–484. doi: 10.1016/8756-3282(95)90194-9. [DOI] [PubMed] [Google Scholar]
- Xiao G, Jiang D, Ge C, et al. Cooperative interactions between activating transcription factor 4 and Runx2/Cbfa1 stimulate osteoblast-specific osteocalcin gene expression. J Biol Chem. 2005;280:30689–30696. doi: 10.1074/jbc.M500750200. [DOI] [PubMed] [Google Scholar]
- Xiao G, Jiang D, Gopalakrishnan R, et al. Fibroblast growth factor 2 induction of the osteocalcin gene requires MAPK activity and phosphorylation of the osteoblast transcription factor, Cbfa1/Runx2. J Biol Chem. 2002;277:36181–36187. doi: 10.1074/jbc.M206057200. [DOI] [PubMed] [Google Scholar]
- Yang DC, Tsay HJ, Lin SY, et al. cAMP/PKA regulates osteogenesis, adipogenesis and ratio of RANKL/OPG mRNA expression in mesenchymal stem cells by suppressing leptin. PLoS One. 2008;3:e1540. doi: 10.1371/journal.pone.0001540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MF, Kerr JM, Ibaraki K, et al. Structure, expression, and regulation of the major noncollagenous matrix proteins of bone. Clin Orthop Relat Res. 1992:275–294. [PubMed] [Google Scholar]