Abstract
Chlamydia trachomatis and C pneumoniae are intracellular bacterial pathogens that have been shown to cause, or are strongly associated with, diverse chronic diseases. Persistent infections by both organisms are refractory to antibiotic therapy. The lack of therapeutic efficacy results from the attenuated metabolic rate of persistently infecting chlamydiae in combination with the modest intracellular drug concentrations achievable by normal delivery of antibiotics to the inclusions within which chlamydiae reside in the host cell cytoplasm. In this research, we evaluated whether nanoparticles formulated using the biodegradable poly(d-L-lactide-co-glycolide) (PLGA) polymer can enhance the delivery of antibiotics to the chlamydial inclusion complexes. We initially studied the trafficking of PLGA nanoparticles in Chlamydia-infected cells. We then evaluated nanoparticles for the delivery of antibiotics to the inclusions. Intracellular trafficking studies show that PLGA nanoparticles efficiently concentrate in inclusions in both acutely and persistently infected cells. Further, encapsulation of rifampin and azithromycin antibiotics in PLGA nanoparticles enhanced the effectiveness of the antibiotics in reducing microbial burden. Combination of rifampin and azithromycin was more effective than the individual drugs. Overall, our studies show that PLGA nanoparticles can be effective carriers for targeted delivery of antibiotics to intracellular chlamydial infections.
Keywords: Antibiotics, Chlamydiae, Polymeric Systems, Sustained Release, Targeted delivery, Intracellular trafficking, Nanoparticles
1.0 Introduction
Human infections by the intracellular bacterial pathogens Chlamydia trachomatis and C pneumoniae present enormous health care problems. C trachomatis is the most prevalent sexually transmitted bacterium, and is associated also with blinding trachoma; C pneumoniae is a respiratory pathogen responsible for a significant proportion of community-acquired pneumonia. In addition, both C trachomatis and C pneumoniae have been shown to cause, or are strongly associated with, diverse chronic diseases [1, 2]. Both C trachomatis and C pneumoniae are obligate intracellular bacteria and undergo a biphasic, intracellular developmental cycle. The developmental cycle is initiated when elementary bodies (EB), the infectious extracellular form of the organism, attach to the target host cell. Once bound, EB enter host cells and reside in a host cell membrane-bound cytoplasmic inclusion within which they spend their intracellular tenure. In the inclusion, EB develop into reticulate bodies (RB), the metabolically active growth form. Each RB undergoes 7–8 rounds of cell division, after which most dedifferentiate back to EB. Newly-formed EB are released by host cell lysis or exocytosis (see [3] for review). Many studies have demonstrated that both C trachomatis and C pneumoniae often disseminate widely from their sites of primary infection [4, 5]. At sites of their disseminated residence, both organisms enter an unusual biological state referred to as ‘persistence’ [5, 6]. In this state, a block in gene expression obviates the full completion of the normal developmental cycle, and the organisms display several unusual morphological, transcriptional and other properties [7, 8]. Importantly, persistently infecting Chlamydiae can elicit powerful immunopathogenic responses that can contribute to chronic diseases associated with the organism and thus present important therapeutic targets. However, persistent infections by both organisms have proved to be refractory to antibiotic therapy. The lack of therapeutic efficacy results from the attenuated metabolic rate of persistently infecting Chlamydiae in combination with the modest intracellular drug concentrations achievable by normal delivery of antibiotics to the inclusions within which Chlamydiae reside in the host cell cytoplasm. Thus, strategies that improve the penetration of antibiotics into the inclusions could potentially improve the treatment efficacy.
Recent studies show that Chlamydiae hijack the host cell machinery to obtain lipid, protein and other nutrients from extracellular sources. Specifically, it was shown that following chlamydial infection, cellular lipid nanoparticles, which are about 100 nm in size, are actively rerouted to the inclusions [9–11]. Previous studies showed that nanoparticles formulated using the biodegradable poly(d-L-lactide-co-glycolide) (PLGA) polymer and in the 100–300 nm size range can enter mammalian endothelial, epithelial, and smooth muscle cells efficiently and deliver encapsulated drug intracellularly at a sustained rate [12–14]. Based on this, we hypothesized that PLGA nanoparticles can be used as Trojan horses to enhance the delivery of antibiotics to the chlamydial inclusion complexes. We initially studied the trafficking of PLGA nanoparticles in C trachomatis-infected cells. We then evaluated nanoparticles for the delivery of antibiotics to the inclusions. Our studies show that nanoparticles target intracellular chlamydial inclusions and organism, and enhance the effectiveness of antibiotics in reducing microbial burden.
2.0 Material and Methods
2.1 Materials
PLGA was purchased from Absorbable Polymers (Pelham, AL). Glacial acetic acid (LC-MS grade) was procured from Alfa Aesar (Ward Hill, MA). Ammonium acetate crystals and sodium hydroxide was purchased from Mallinckrodt (Phillipsburg, NJ). Azithromycin was purchased from Astatech Inc (Bristol, PA). Rifampin, N-acetyl-L-cysteine (NAC), polyvinyl alcohol (PVA) (average Mw 30,000–70,000 Da), 6-coumarin and carboxy methyl cellulose sodium salt (CMC) (average Mw 90000 Da) were purchased from Sigma-Aldrich (St. Louis, MO). All the other chemicals were purchased from Fisher Scientific (Fair Lawn, NJ). Culture medium and additives were obtained from standard sources and are described below.
2.2 Methods
2.2.1 Fabrication of PLGA nanoparticles
PLGA (30 mg) and 6-coumarin (250 µg) were dissolved in 1 ml chloroform. An oil-in-water emulsion was formed by emulsifying the polymer-dye solution in 6 ml of 2.5% w/v aqueous PVA solution by sonication (Sonicator® XL, Misonix, NY) for 5 min over ice bath. The emulsion was stirred for 18 hrs at ambient conditions followed by for 2 hrs under vacuum to remove chloroform. Nanoparticles were recovered by ultracentrifugation (35,000 rpm for 35 min at 4° C, Optima™ LE-80K, Beckman, Palo Alta, CA) and washed three times with deionized water to remove excess PVA and unencapsulated dye. Nanoparticle suspension was then lyophilized (Labconco, FreeZone 4.5, Kansas City, MO). Extensive studies by several groups, including ours, have shown the usefulness of 6-coumarin as a fluorescent probe for polymeric nanoparticles for qualitative (microscopy) and quantitative studies in vitro and in vivo [14–18]. 6-coumarin is a highly lipophilic molecule and does not leach out of nanoparticles in the time frame of the study. Previous studies have shown that less than 0.1% w/w of the encapsulated molecule is released in 48 hrs [16].
Azithromycin- and rifampin-loaded nanoparticles were prepared as above but with a few modifications. The polymer (32 mg) and the drugs (ranging from 50 to 100% w/w of polymer) were dissolved in 1 ml of chloroform. The chloroform solution was emulsified in PVA solution and then processed as described above. PEG was introduced on the surface using the IAASF technique [19]. In brief, following the emulsification step as described above, a diblock copolymer of PLA-PEG (10 kDa/5 kDa; synthesis described in reference [19]) was added to the emulsion. This results in the formation of nanoparticles with PEG on the surface and is expected to improve the circulation half-life of the formulations when administered in vivo.
2.2.2. Characterization of nanoparticles
2.2.2.1. Particle size and zeta potential
Hydrodynamic diameter of nanoparticles was determined using dynamic light scattering (Brookhaven Instruments, Holtsville, NY). Nanoparticles (~1 mg/ml) were dispersed in distilled water using sonication prior to particle size determination. Mean hydrodynamic diameters were calculated based on size distribution by mass and the results were expressed as mean of five runs.
2.2.2.2. Drug loading and encapsulation efficiency
To determine azithromycin and rifampin loading, 5 mg of nanoparticles were extracted with 2 ml methanol for 15 hrs. The extract was centrifuged at 12,500 rpm for 20 min. The supernatant was analyzed for azithromycin and rifampin concentrations using a Beckman Coulter HPLC system (Fullerton, CA) equipped with System Gold 125 solvent module, System Gold 508 auto-injector, and System Gold 168 PDA UV/visible detector. For azithromycin, a C-18 column (4.6 mm × 25 cm) with 5 µm packing (Beckman) heated at 50° C was used. The mobile phase consisted of a mixture of mobile phases A and B in 75:25 ratio delivered at a flow rate of 1 ml/min; mobile phase A consisted of phosphate buffer (pH 8.5) and methanol (90:10), while mobile phase B consisted of acetonitrile and methanol (90:10). A 30 µl volume of the methanol extract was injected, and azithromycin was quantified by UV detection at 210 nm. Retention time of azithromycin was 8.7 min. For rifampin, the mobile phase consisted of phosphate buffer (pH 6.8) and acetonitrile (60:40) and the wavelength used for detection was 238 nm. Drug loading was defined as the amount of drug loaded in 100 mg of nanoparticles.
2.2.2.3. In vitro release
Drug release from nanoparticles was determined in phosphate buffer saline (PBS, 0.15 M, pH 7.4) containing 0.1% w/v Tween 80. Nanoparticle suspensions (1 mg/ml, 1 ml) were placed in several 1.5-ml centrifuge tubes and the centrifuge tubes placed in a water bath shaker set at 100 rpm and 37° C (Precision, Thermo Scientific, Marietta, OH). At predetermined time intervals, a set of three tubes were centrifuged at 12,500 rpm for 10 min and the supernatants were analyzed for azithromycin and rifampin concentrations by HPLC.
2.2.3 Growth of C trachomatis stock and in vitro infection
C trachomatis serovar K (UW-31) was used throughout this study. Standardized seed stock of C trachomatis elementary bodies was prepared by growth in HEp2 cells (human lung epithelial cells; ATCC, Manassas, VA) using our published methods [20]. Aliquots of the stock for all experiments were stored at −80°C. Infectious titers of the stocks were determined using standard methods with McCoy cells (mouse fibroblasts; kindly provided by Dr. J. Igietseme, CDC). McCoy cells were grown in Eagle’s Minimum Essential Medium with Earle’s salts (EMEM; Mediatech, Herndon, VA) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Atlanta Biologicals, Lawrenceville, GA), 0.2 µg of gentamicin/ml (Life Technologies, Carlsbad, CA) and 2mM L-glutamine (Sigma). HEp2 cells were grown in Iscove’s Modified Dulbecco’s Medium (Mediatech) with the same additives.
To induce acute infection, McCoy or HEp2 cell monolayers were grown in 2-chamber, chamber slides (5×105 or 2×105 cells/chamber, respectively) (Nunc, Rochester, NY). Cells were infected at a multiplicity of infection (m.o.i.) of 6 EBs/cell in infection media [MEM containing 10% heat-inactivated fetal bovine serum, 2mM L-glutamine, 0.06% glucose (Sigma) and 1 µg/ml of cyclohexamide (Sigma)]. Cells in chamber slides were incubated for a further 24 or 48 hrs before analyses.
To induce persistent chlamydial infection, growth medium was replaced with fresh growth medium containing Penicillin G (PenG; Sigma) at a concentration of 10 U/ml at 18 hrs post infection (hpi). These cells were pulsed with drug-nanoparticles 24–36 hrs later. In some experiments, HEp2 were used as these cells have somewhat different susceptibilities to azithromycin and rifampin compared to McCoy cells [21].
2.2.4 Nanoparticle trafficking to chlamydial inclusions
Chlamydiae develop and differentiate only intracellularly within a membrane bound vacuole termed an inclusion; the inclusion expands during the replicative cycle to accommodate increasing numbers of the organism. This intracellular localization requires antibiotics or nanoparticles to cross three membranes (the cell, inclusion, and chlamydial membranes). To observe the trafficking of nanoparticles to chlamydial inclusions, infected cell monolayers were pulsed with 50 µl of nanoparticle dispersion (100 – 300 µg/ml) at 37° C for 15 min. Cell monolayers were washed three times with Dulbecco’s PBS (DPBS; Mediatech). Chambers were removed and coverslips wet mounted over a volume of less than 10 µl PBS for live imaging on the RTM-3 microscope (Quorum Technologies, Inc., Guelph, Ontario, Canada). Duplicate monolayers were washed and fixed with glutaraldehyde using the formulation described earlier [22] or with absolute methanol for anti-chlamydial staining in the absence of nanoparticles to confirm adequate infection.
2.2.5 Efficacy studies with antibiotic-loaded nanoparticles
McCoy cells were seeded in 96-well microtiter plates at a concentration of 2×105 cells/well in complete Eagles MEM and allowed to attach overnight at 37° C and 5% CO2 atmosphere. The following day, cells were infected with C trachomatis at an m.o.i. of 1–10. Plates were centrifuged at 2500 rpm for 1 hr at room temperature, followed by incubation at 37° C for 1 hr to allow attachment and entry of chlamydial EBs into cells. The chlamydial inoculum was then aspirated and replaced with the appropriate two-fold dilution of free drug or drug encapsulated in nanoparticles prepared in antibiotic-free growth media containing 0.06% glucose and 1 µg/ml cyclohexamide. Stock solutions of azithromycin and rifampin were prepared at concentrations of 25 mg/ml in sterile water by adding glacial acetic acid dropwise until the compounds were completely dissolved as described previously [23]. The stocks were stored at −80° C in single use aliquots. Nanoparticle treatments were prepared fresh on the day of the treatment. Subsequent dilutions were prepared immediately prior to use in defined growth media without antibiotics. Cells were incubated in the presence of drug for 24 or 48 hrs post infection and then fixed with absolute methanol for 10 min. Chlamydial inclusions were detected by immunofluorescence using BioRad Pathfinder® FITC-labeled monoclonal anti-Chlamydial lipopolysaccharide (LPS) according to the manufacturer’s instructions. Plates were stored at 4° C protected from light. The mean inclusion number was plotted as a function of antibiotic concentration. The MIC50 was defined as the minimum drug concentration needed to reduce the total number of inclusions by 50%.
3.0 Results and Discussion
3.1 Nanoparticle formulation and characterization
PLGA nanoparticles labeled with a green fluorescent marker, 6-coumarin, were formulated with an average particle size of ~260 nm, which was in the range previously reported for PLGA nanoparticles [12, 15]. Nanoparticles loaded with the two antibiotics, rifampin and azithromycin, were also in similar size range (Table 1). While the extent of loading achieved here was less than optimal, especially for nanoparticles loaded with both the antibiotics, we proceeded to use these formulations, because both azithromycin and rifampin were potent, with MIC50 in the nM range [21].
Table 1.
Formulations used in this study
| Formulation | % Azithromycin Loading |
% Rifampin Loading |
Average particle size (nm) |
|---|---|---|---|
| 6-coumarin | - | - | 266 |
| Azithromycin | 4.2 ± 0.7 | - | 314 |
| Rifampin | - | 1.8 ± 0.0 | 173 |
| Azithromycin/Rifampin | 0.5 ± 0.1 | 0.1 ± 0.0 | 215 |
Rates of azithromycin and rifampin release from nanoparticles were determined using in vitro release studies. In the case of nanoparticles loaded with just azithromycin, the drug release was rapid (~80% in 24 hr, Fig. 1A). Rifampin release was slightly slower with about 60% released in the first 8 hrs, and the remaining being released over 7 days. In the case of nanoparticles loaded with both azithromycin and rifampin, the release was much more sustained (Fig. 1B). Only about 25% of the azithromycin and 12% of rifampin was released in 3 days. The slower release from the combination nanoparticles may be due to the significantly lower loading of both drugs in the combination nanoparticles compared to that in individual antibiotic loaded nanoparticles (Table 1).
Figure 1.
In vitro release of rifampin and azithromycin from (A) nanoparticles loaded with individual antibiotics and (B) nanoparticles loaded with both antibiotics in the same formulation. Nanoparticles were suspended in phosphate buffer saline (PBS, 0.15 M, pH 7.4) containing 0.1% w/v Tween 80 suspensions and incubated in a water bath shaker set at 100 rpm and 37° C.
3.2 Nanoparticle trafficking in Chlamydia-infected cells
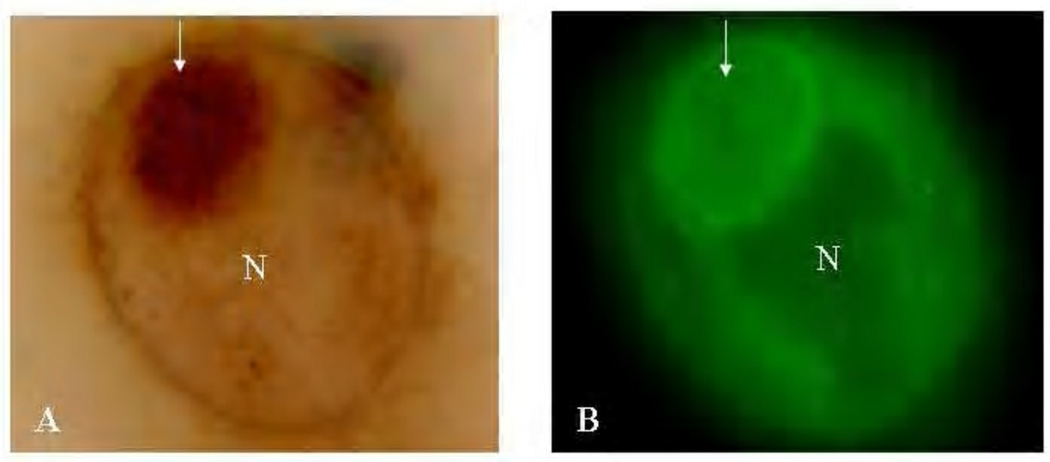
Nanoparticle uptake into live, infected cells was imaged on the RTM-3 microscope. Remarkably, nanoparticles homed quite rapidly into host cells and entered chlamydial inclusions within 15 min of nanoparticle addition to infected cell monolayers (Fig. 2). Fluorescence was concentrated at the inner inclusion membrane and at the membranes of RB resident at the internal side of that membrane as well as with differentiated EB deeper in the inclusion.
Figure 2.
Images demonstrate the homing of 6-coumarin-labeled nanoparticles into infected HEp-2 cells, viewed with the RTM-3 microscope. Panel A, visible light image of a 24 hr-infected HEp-2 cell pulsed with particles. Cells are not stained; the arrow points to the inclusion. Panel B, the same cells viewed under epifluorescence. The nucleus is the black area contiguous to the inclusion. Clearly, 6-coumarin is associated with the inclusion, RB and EB membranes. Original magnification 100X.
It is well established that chlamydial inclusions are neither acidified nor fusogenic with lysosomes. It has been shown that the inclusion, rather than interacting with the endocytic pathway, is fusogenic with exocytic vesicles containing sphingomyelin and cholesterol on route from the Golgi apparatus to the plasma membrane [24–26]. In our previous studies, we have shown that PLGA nanoparticles are actively endocytosed and exocytosed from mammalian cells [12]. Thus, the endocytic and exocytic pathways of nanoparticles could converge with the chlamydial inclusion, resulting in the accumulation of nanoparticles in the inclusion. While there is some disagreement in the literature on the role of lipid rafts in chlamydial entry into host cells (for example, [27]), Stuart et al showed that C pneumoniae, C psittaci, and C trachomatis serovars E and F enter host cells via cholesterol-rich lipid raft microdomains [28]. However, C trachomatis serovars A, 36B, and C, LGV, L2 and MoPn did not utilize lipid rafts for cellular entry. Previous studies by us and other groups have shown that PLGA nanoparticles may also utilize lipid rafts in a non-specific way to enter into cells [13, 14]. Because the inclusions (organism) may use the rafts to derive cholesterol and sphingolipids [24, 28, 29], we expect nanoparticles could also use the same pathway to traffic to inclusions. Interestingly, an association of C pneumoniae with LDL receptors has been reported [30, 31]. Alternatively, an active mechanism to transport host cell membrane components to inclusion (membranes) may account for the rapid movement of nanoparticles from the extracellular milieu to inclusions. Chlamydial infection may also increase membrane permeability of cells in addition to utilization of one or more host cell uptake pathways. Whatever the mechanism, nanoparticle uptake and arrival in inclusions occurs very rapidly, suggesting that PLGA nanoparticles can be utilized to target antibiotics to chlamydial inclusions.
Use of therapeutic antibiotic treatment for persistent chlamydial infections (eg, reactive arthritis [32] has been marginally effective until recently [33]. Therefore, we asked whether PLGA nanoparticles would enter host cells harboring persistent forms of C trachomatis. C trachomatis-infected cells were treated with Pen G 18 hrs after infection to induce persistence [34]. We show with this system that PLGA nanoparticles readily target aberrant RB in persistently infected cells as effectively as in acutely infected cells (Fig. 3). As in the previous experiment, fluorescence was concentrated at the inclusion membrane and at the membranes of large, aberrant RBs characteristic of persistent infections (arrows). This observation supported our hypothesis that antibiotic-loaded nanoparticles will effectively enter and treat persistently infected cells.
Figure 3.
Images showing the homing of labeled nanoparticles into inclusions of persistently infected cells. HEp2 cells were unstained and captured by real time microscopy using the RTM-3 microscope with Riveal Contrast (Quorum Technologies) (A), fluorescence mode (B), epifluorescence mode using FITC filter (C), and a black and white image of same inclusion (D). 6C-nanoparticles are associated with both the inclusion membrane (white arrow) and RB (yellow arrow). Red arrows in A, B indicate the inclusion which is viewed in C and D. N - Nucleus 100X original magnification
3.3 Effect of encapsulation in nanoparticles on antimicrobial activity of antibiotics
We initially determined the MIC50 of free antibiotics. McCoy cells were infected with C trachomatis and then treated immediately with serial two-fold dilutions of azithromycin or rifampin to a final concentration range of 31–2000 ng/ml and 1–64 ng/ml, respectively, and incubated for another 24 or 48 hrs. The MIC50 was ~40 ng/ml for azithromycin and ~5 ng/ml for rifampin (not shown). These data are consistent with previous sensitivity levels reported for both drugs [21, 35]. In addition, both azithromycin and rifampin showed no significant change in MIC50 with length of treatment.
We then evaluated if there was a difference in the effectiveness of free drug vs nanoparticles when the treatment occurred at 0, 24 or 48 hours post infection. McCoy cells (2×105/well) were infected with 104 IFU/well C trachomatis. Cells were treated at 0, 24 or 48 hours post infection with serial two-fold dilutions of azithromycin or rifampin. The cells were incubated for 24 hours in the presence of drug and then fixed. When free drug or encapsulated nanoparticles were added immediately following infection, the response was similar to that observed previously with an MIC50 of 5 ng/ml for rifampin and 40 ng/ml for azithromycin. In contrast, when free drug was added 24 or 48 hours post infection, a marked insensitivity was observed, reflected in a minimal decrease in inclusion number with increasing dose (Fig. 4A and B). Encapsulation of the drugs in nanoparticles improved the effectiveness of both drugs significantly (p<0.05), although complete inhibition was not seen in the dose range tested. This observation may relate to the biphasic developmental life cycle of Chlamydiae and different antibiotic susceptibilities depending upon metabolic and transcriptional rates. Alternatively, a single or few EBs may be more successfully eradicated at t0 than a large number of RBs found at t24–36. These possibilities remain to be distinguished in future experiments.
Figure 4.
Time of delivery of antibiotic-nanoparticles differentially affects the number of inclusions (infectious load). Free antibiotics (rifampin or azithromycin) were compared to nanoparticles loaded with the same antibiotic. (A) Rifampin, (B) Azithromycin. *, p < .05. Addition of free antibiotics at 24 or 48 hrs after infection was ineffective at reduction of infectious load. Encapsulated drugs significantly reduced inclusion numbers.
It was interesting to note that despite releasing only a fraction of the payload, nanoparticle-encapsulated antibiotics were more effective than free drugs. If delivered dosages are matched by taking into account the fraction of drug released, then nanoparticle encapsulated antibiotics are more potent than free antibiotics. This is shown in Fig. 5, using rifampin nanoparticles as an example (p < 0.03–0.001). Thus, sustained release of the antibiotics accounts only partially for the efficacy of antibiotic-loaded nanoparticles. We propose that enhanced drug delivery to the inclusions together with sustained drug release from nanoparticles accounts for the observed improved efficacy of nanoparticle-encapsulated antibiotics compared to free antibiotic.
Figure 5.
Delivered drug dose does not explain the efficacy in reduction of chlamydial inclusions (number of infected cells), because equivalent rifampin concentration delivered in nanoparticles results in significant reductions in inclusions at all doses tested (2.5–10ng/ml; p<.03–.001). *, p < .05. The number of inclusions was reduced by more than 50% by half the amount of encapsulated antibiotic, based on 50% release rate over the time of the experiment.
We also evaluated the effect of nanoparticle-encapsulated azithromycin on Pen G-induced persistent infections in HEp2 cells. Free azithromycin was not effective in reducing persistent infection, while nanoparticle-encapsulated azithromycin demonstrated a dose-dependent decrease in the area of infections (Fig. 6). We interpret this result to indicate that nanoparticles enhance the delivery of encapsulated antibiotics to persistent infections and thus, can more successfully reduce infectious load than free antibiotic even during persistent infection.
Figure 6.
Effect of encapsulated azithromycin (AZ) on persistent chlamydial infection. Encapsulated AZ significantly reduced the number of inclusions compared to free AZ. Whereas free AZ was ineffective over the broad dose range, encapsulated AZ successfully reduced the number of persistent inclusions at 32 ng/ml and higher.
3.4 Effect of combination antibiotic treatment on chlamydial viability
Previous studies have shown that a combination of antibiotics such as azithromycin and rifampin is more effective than the individual drugs against persistent chlamydial infections [33]. In addition, combination antibiotic therapies are less likely to result in antibiotic resistance. In our studies, we investigated the effect of combining the antibiotics, azithromycin and rifampin, on the MIC50 of the individual drugs. Combination therapy with free azithromycin and rifampin was significantly more effective than individual drugs (Fig. 7). In the presence of 4 ng/ml rifampin, the MIC50 of azithromycin dropped to 16 ng/ml, while in the presence of 16 ng/ml azithromycin, the MIC50 of rifampin was reduced to 2 ng/ml. This is consistent with other studies that have shown that combined treatment with both azithromycin and rifampin are more effective at treating chlamydial infections than individual free drugs [36].
Figure 7.
Combination drugs (azithromycin and rifampin) are more effective at elimination of chlamydia than single drug alone. Dose response experiments in the presence of a stable dose of the second antibiotic demonstrate the efficacy of in vitro combined drug therapy. (A), azithromycin at varying concentrations, fixed rifampin; (B) rifampin at varying concentrations and fixed Azithromycin. We also tested 16 ng/ml of encapsulated Azithromycin or 2 ng/ml of encapsulated Rifampin; the latter were not significantly better than no second drug (not shown). *, p < .05
We then compared the effectiveness of nanoparticle-encapsulated antibiotic combination with corresponding doses of free antibiotics. The doses of free antibiotics were initially determined based on the loading of the antibiotic combinations in nanoparticles. Encapsulation of the two antibiotics in nanoparticles enhanced their effectiveness marginally (Fig. 8A). However, when the data was adjusted for the amount of antibiotics released, significant differences were observed between nanoparticle-encapsulated and free antibiotics (Fig. 8B and 8C). These data provide additional evidence for effective delivery of nanoparticle-encapsulated payload to chlamydial inclusions.
Figure 8.
Effect of combination antibiotics on inclusion size (infectious load). Both azithromycin and rifampin were delivered simultaneously either as a free drug combination or inside the same nanoparticles. (A) Reduction in inclusion size without correction for drug release. The concentration of AZ and R are shown as AZ/R. Only small reductions are observed by delivery of combined drugs in nanoparticles. Wells with no drug had a mean inclusion size of 531 pixels as shown in 8B and 8C. (B) and (C) Reduction in inclusion sizes after correction for either (B) actual AZ release from combo nanoparticles or (C) actual R release from the same combo nanoparticles. The effective doses of both antibiotics are highly superior when delivered encapsulated in combination. Inclusion sizes expressed as mean pixels ± S.E.M.
4.0 Conclusions
Intracellular trafficking studies show that PLGA nanoparticles efficiently concentrate in inclusions in both acute and persistent C trachomatis infections. Encapsulation of antibiotics, azithromycin and rifampin, either singly or in combination, improved their effectiveness in reducing the infectious load of chlamydia based on inclusion size and numbers. Our studies suggest that enhanced intracellular and intra-inclusion accumulation, in combination with sustained drug release, contributes to the overall effectiveness of nanoparticle-encapsulated antibiotics. Clearly, further formulation optimization studies are needed to improve the efficiency of antibiotic encapsulation. Future studies will examine the effectiveness of PLGA nanoparticles in delivery of antibiotic combinations for effective elimination of persistent infections in vivo.
Acknowledgments
Funding from NIH (R01AI080928).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Honey E, Templeton A. Prevention of pelvic inflammatory disease by the control of C. trachomatis infection. Int J Gynaecol Obstet. 2002;78(3):257. doi: 10.1016/s0020-7292(02)00185-6. [DOI] [PubMed] [Google Scholar]
- 2.Wiesenfeld HC, Hillier SL, Krohn MA, Amortegui AJ, Heine RP, Landers DV, et al. Lower genital tract infection and endometritis: insight into subclinical pelvic inflammatory disease. Obstet Gynecol. 2002;100(3):456–463. doi: 10.1016/s0029-7844(02)02118-x. [DOI] [PubMed] [Google Scholar]
- 3.Hatch TP. In: Chlamydia - Intracellular biology, Pathogenesis, and Immunity. Stephens RS, editor. Washington DC: ASM Press; 1999. pp. 29–67. [Google Scholar]
- 4.Moazed TC, Kuo CC, Grayston JT, Campbell LA. Evidence of systemic dissemination of Chlamydia pneumoniae via macrophages in the mouse. J Infect Dis. 1998;177(5):1322–1325. doi: 10.1086/515280. [DOI] [PubMed] [Google Scholar]
- 5.Villareal C, Whittum-Hudson JA, Hudson AP. Persistent Chlamydiae and chronic arthritis. Arthritis Res. 2002;4(1):5–9. doi: 10.1186/ar382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hogan RJ, Mathews SA, Mukhopadhyay S, Summersgill JT, Timms P. Chlamydial persistence: beyond the biphasic paradigm. Infect Immun. 2004;72(4):1843–1855. doi: 10.1128/IAI.72.4.1843-1855.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gérard HC, Kohler L, Branigan PJ, Zeidler H, Schumacher HR, Hudson AP. Viability and gene expression in Chlamydia trachomatis during persistent infection of cultured human monocytes. Med Microbiol Immunol (Berl) 1998;187(2):115–120. doi: 10.1007/s004300050082. [DOI] [PubMed] [Google Scholar]
- 8.Gérard HC, Kráusse-Opatz B, Wang Z, Rudy D, Rao JP, Zeidler H, et al. Expression of Chlamydia trachomatis genes encoding products required for DNA synthesis and cell division during active versus persistent infection. Mol Microbiol. 2001;41(3):731–741. doi: 10.1046/j.1365-2958.2001.02550.x. [DOI] [PubMed] [Google Scholar]
- 9.Beatty WL. Trafficking from CD63-positive late endocytic multivesicular bodies is essential for intracellular development of Chlamydia trachomatis. J Cell Sci. 2006 15;119(Pt 2):350–359. doi: 10.1242/jcs.02733. [DOI] [PubMed] [Google Scholar]
- 10.Cocchiaro JL, Kumar Y, Fischer ER, Hackstadt T, Valdivia RH. Cytoplasmic lipid droplets are translocated into the lumen of the Chlamydia trachomatis parasitophorous vacuole. Proc Natl Acad Sci U S A. 2008;105(27):9379–9384. doi: 10.1073/pnas.0712241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cocchiaro JL, Valdivia RH. New insights into Chlamydia intracellular survival mechanisms. Cell Microbiol. 2009;11(11):1571–1578. doi: 10.1111/j.1462-5822.2009.01364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panyam J, Labhasetwar V. Dynamics of endocytosis and exocytosis of poly(D,L-lactide-co-glycolide) nanoparticles in vascular smooth muscle cells. Pharm Res. 2003;20(2):212–220. doi: 10.1023/a:1022219003551. [DOI] [PubMed] [Google Scholar]
- 13.Panyam J, Zhou WZ, Prabha S, Sahoo SK, Labhasetwar V. Rapid endo-lysosomal escape of poly(DL-lactide-co-glycolide) nanoparticles: implications for drug and gene delivery. FASEB J. 2002;16(10):1217–1226. doi: 10.1096/fj.02-0088com. [DOI] [PubMed] [Google Scholar]
- 14.Qaddoumi MG, Ueda H, Yang J, Davda J, Labhasetwar V, Lee VH. The characteristics and mechanisms of uptake of PLGA nanoparticles in rabbit conjunctival epithelial cell layers. Pharm Res. 2004;21(4):641–648. doi: 10.1023/b:pham.0000022411.47059.76. [DOI] [PubMed] [Google Scholar]
- 15.Panyam J, Sahoo SK, Prabha S, Bargar T, Labhasetwar V. Fluorescence and electron microscopy probes for cellular and tissue uptake of poly(D,L-lactide-co-glycolide) nanoparticles. Int J Pharm. 2003;262(1–2):1–11. doi: 10.1016/s0378-5173(03)00295-3. [DOI] [PubMed] [Google Scholar]
- 16.Davda J, Labhasetwar V. Characterization of nanoparticle uptake by endothelial cells. Int J Pharm. 2002;233(1–2):51–59. doi: 10.1016/s0378-5173(01)00923-1. [DOI] [PubMed] [Google Scholar]
- 17.Gao X, Chen J, Tao W, Zhu J, Zhang Q, Chen H, et al. UEA I-bearing nanoparticles for brain delivery following intranasal administration. Int J Pharm. 2007;340(1–2):207–215. doi: 10.1016/j.ijpharm.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 18.Zhao J, Liu CS, Yuan Y, Tao XY, Shan XQ, Sheng Y, et al. Preparation of hemoglobin-loaded nano-sized particles with porous structure as oxygen carriers. Biomaterials. 2007;28(7):1414–1422. doi: 10.1016/j.biomaterials.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 19.Patil YB, Toti US, Khdair A, Ma L, Panyam J. Single-step surface functionalization of polymeric nanoparticles for targeted drug delivery. Biomaterials. 2009;30(5):859–866. doi: 10.1016/j.biomaterials.2008.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campos M, Pal S, O'Brien TP, Taylor HR, Prendergast RA, Whittum-Hudson JA. A chlamydial major outer membrane protein extract as a trachoma vaccine candidate. Invest Ophthalmol Vis Sci. 1995;36(8):1477–1491. [PubMed] [Google Scholar]
- 21.Suchland RJ, Geisler WM, Stamm WE. Methodologies and cell lines used for antimicrobial susceptibility testing of Chlamydia spp. Antimicrob Agents Chemother. 2003;47(2):636–642. doi: 10.1128/AAC.47.2.636-642.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pietrasanta LI, Schaper A, Jovin TM. Imaging subcellular structures of rat mammary carcinoma cells by scanning force microscopy. J Cell Sci. 1994;107(Pt 9):2427–2437. doi: 10.1242/jcs.107.9.2427. [DOI] [PubMed] [Google Scholar]
- 23.Barry A, Bryskier A, Traczewski M, Brown S. Preparation of stock solutions of macrolide and ketolide compounds for antimicrobial susceptibility tests. Clin Microbiol Infect. 2004;10(1):78–83. doi: 10.1111/j.1469-0691.2004.00759.x. [DOI] [PubMed] [Google Scholar]
- 24.Carabeo RA, Mead DJ, Hackstadt T. Golgi-dependent transport of cholesterol to the Chlamydia trachomatis inclusion. Proc Natl Acad Sci USA. 2003;100(11):6771–6776. doi: 10.1073/pnas.1131289100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grassme HUC, Ireland RM, Vanputten JPM. Gonococcal opacity protein promotes bacterial entry-associated rearrangements of the epithelial cell actin cytoskeleton. Infect Immun. 1996;64(5):1621–1630. doi: 10.1128/iai.64.5.1621-1630.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scidmore MA, Fischer ER, Hackstadt T. Sphingolipids and glycoproteins are differentially trafficked to the Chlamydia trachomatis inclusion. J Cell Biol. 1996;134(2):363–374. doi: 10.1083/jcb.134.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gabel BR, Elwell C, van Ijzendoorn SC, Engel JN. Lipid raft-mediated entry is not required for Chlamydia trachomatis infection of cultured epithelial cells. Infect Immun. 2004;72(12):7367–7373. doi: 10.1128/IAI.72.12.7367-7373.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stuart ES, Webley WC, Norkin LC. Lipid rafts, caveolae, caveolin-1, and entry by Chlamydiae into host cells. Exp Cell Res. 2003;287(1):67–78. doi: 10.1016/s0014-4827(03)00059-4. [DOI] [PubMed] [Google Scholar]
- 29.Stuart ES, Wyrick PB, Choong J, Stoler SB, MacDonald AB. Examination of chlamydial glycolipid with monoclonal antibodies: cellular distribution and epitope binding. Immunol. 1991;74(4):740–747. [PMC free article] [PubMed] [Google Scholar]
- 30.Gérard HC, Whittum-Hudson JA, Hudson AP. C pneumoniae utilizes apoE and the LDL receptor family for host cell attachment. Eleventh International Symposium on Human Chlamydial Infections; Canada: Niagara-on-the-Lake; 2006. [Google Scholar]
- 31.Gérard HC, Whittum-Hudson JA, Hudson AP. C pneumoniae utilizes apoE and the LDL receptor family for host cell attachment. International Chlamydia Symposium; San Francisco. 2006. pp. 153–156. [Google Scholar]
- 32.Beutler AM, Hudson AP, Whittum-Hudson JA, Salameh WA, Gerard HC, Branigan PJ, et al. Chlamydia trachomatis can persist in joint tissue after antibiotic treatment in chronic Reiter's syndrome / reactive arthritis. J Clin Rheumatol. 1997;3(3):125–130. doi: 10.1097/00124743-199706000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Carter JD, Espinoza LR, Inman RD, Sneed KB, Ricca LR, Vasey FB, et al. Combination antibiotics as a treatment for chronic Chlamydia-induced reactive arthritis: a double-blind, placebo-controlled, prospective trial. Arthritis Rheum. 2010;62(5):1298–1307. doi: 10.1002/art.27394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wyrick PB, Knight ST. Pre-exposure of infected human endometrial epithelial cells to penicillin in vitro renders Chlamydia trachomatis refractory to azithromycin. J Antimicrob Chemother. 2004;54(1):79–85. doi: 10.1093/jac/dkh283. [DOI] [PubMed] [Google Scholar]
- 35.Kutlin A, Kohlhoff S, Roblin P, Hammerschlag MR, Riska P. Emergence of resistance to rifampin and rifalazil in Chlamydophila pneumoniae and Chlamydia trachomatis. Antimicrob Agents Chemother. 2005;49(3):903–907. doi: 10.1128/AAC.49.3.903-907.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolf K, Malinverni R. Effect of azithromycin plus rifampin versus that of azithromycin alone on the eradication of Chlamydia pneumoniae from lung tissue in experimental pneumonitis. Antimicrob Agents Chemother. 1999;43(6):1491–1493. doi: 10.1128/aac.43.6.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]