Abstract
Wnt signalling pathways have been shown to play key roles in both normal development and tumorigenesis. Progression of many human cancers is associated with defined mutations in Wnt pathway components that result in dysregulated β-catenin-mediated gene transcription. Although Wnt pathway mutations are rare in epithelial ovarian cancer (with the exception of the endometrioid histotype), accumulating evidence supports a role for Wnt signalling in ovarian tumorigenesis in the absence of genetic mutations. The present review summarizes evidence in support of activated Wnt signalling in ovarian tumours and discusses alternative mechanisms for Wnt pathway activation in the ovarian tumour microenvironment.
Keywords: β-catenin, endothelin, integrin, lysophosphatidic acid, microenvironment, ovarian cancer, T-cell factor/lipoprotein receptor-related (Tcf/Lef), Wnt
Abbreviations: APC, adenomatous polyposis coli; COX2,cyclo-oxygenase 2; CTGF, connective tissue growth factor; Dkk, Dickkopf; Dvl, Dishevelled 1; E-cadherin, epithelial cadherin; EGF, epidermal growth factor; ETAR, ET type A receptor; ETBR, ET type B receptor; EGFR, EGF receptor; EOC, epithelial ovarian carcinoma; ET, endothelin; FRAT1, frequently rearranged in advanced T-cell lymphomas 1; Fzd, Frizzled; GPCR, G-protein-coupled receptor; GSK3β, glycogen synthase kinase 3β; IDAX, inhibitor of the Dvl and Axin complex; IGF-1, insulin-like growth factor-1; IL, interleukin; ILK, integrin-linked kinase; Lef, lymphoid enhancer factor; LPA, lysophosphatidic acid; LPAR, LPA receptor; LRP, lipoprotein receptor-related protein; MCA, multi-cellular aggregate; MMP, matrix metalloproteinase; NF-κB, nuclear factor κB; PI3K, phosphoinositide 3-kinase; PTEN, phosphatase and tensin homologue deleted on chromosome 10; SFRP, secreted Fzd-related protein; Tcf, T-cell factor; TGF, transforming growth factor; VEGF, vascular endothelial growth factor; VEGFR, vascular EGFR; WIF-1, Wnt-inhibitory factor 1
INTRODUCTION
Overview of canonical Wnt signalling
Wnt signalling regulates a diversity of processes fundamental to embryogenesis, including proliferation, differentiation, polarity, adhesion and motility [1–3]. The highly conserved and complex Wnt pathway transduces signals from the extracellular environment through transmembrane receptors and co-receptors to have an impact on cytoskeletal rearrangements and gene expression changes and thereby modulate cell behaviour. In the adult organism, aberrant activation of these same biological processes can induce neoplasia and promote tumour progression [4]. There are two distinct pathways for transduction of Wnt signals: the canonical Wnt/β-catenin pathway (Figure 1) and the non-canonical β-catenin-independent pathway. The latter can be subdivided further into Wnt/PCP (planar cell polarity) and Wnt/Ca2+ signalling pathways. Many excellent reviews have been recently published that detail both the canonical and non–canonical Wnt pathways, thus the present review will provide only a brief summary of the canonical pathway as it relates to EOC (epithelial ovarian carcinoma).
Figure 1. Canonical Wnt signalling and nuclear β-catenin in ovarian tissue.
(A) In the absence of Wnt pathway activation, β-catenin is either associated with the cytoplasmic tail of E-cadherin or is targeted to an Axin/APC/GSK3β complex whereupon it is phorphorylated and targeted for degradation (left-hand panel). Activation of Wnt signalling, for example by binding of Wnt to the Fzd receptor, results in association of the co-receptors LRP-5/6 with Fzd and recruitment of Dvl to Fzd at the plasma membrane. Binding of Axin to this complex disrupts the β-catenin degradation complex, enabling accumulation of β-Catenin. β-Catenin can then translocate to the nucleus, bind to Tcf/Lef transcription factors and transcriptional co-activators (not shown) to activate transcription of Wnt target genes. Wnt signalling can be blocked by Dkk that binds Kremen and inhibits the interaction between Fzd and LRP-5/6, or by sequestration of Wnt ligands via WIF or SFRP (right-hand panel). (B) Example of a serous ovarian tumour exhibiting nuclear β-catenin staining. Magnification ×200.
Canonical Wnt signalling is commonly activated by secreted proteins in the Wnt family, which currently consists of 19 members [1–3,5]. Wnts bind to transmembrane Fzd (Frizzled) GPCRs (G-protein-coupled receptors). Interaction of ligated Fzd with co-receptors designated LRP (lipoprotein receptor-related protein)-5 or LRP-6, members of the low-density LRP family, initiates Wnt signalling. In the absence of Wnt signalling, β-catenin functions as a structural component of E-cadherin (epithelial cadherin) junctions and is complexed with the cytoplasmic tail of E-cadherin. In normal epithelial cells, the majority of β-catenin is associated with E-cadherin at cell–cell junctions, and the levels are maintained at low concentrations in the cytoplasm by phosphorylation-dependent degradation of β-catenin. Cytoplasmic β-catenin is targeted to a complex comprised of Axin, APC (adenomatous polyposis coli) and GSK3β (glycogen synthase kinase 3β), resulting in phosphorylation of β-catenin that targets it for degradation through the ubiquitin–proteasome pathway (Figure 1A). Activation of Wnt signalling leads to phosphorylation of LRP-5/6, recruitment of Axin and Dvl (Dishevelled 1) to the plasma membrane, and functional disruption of the β-catenin degradation complex. This in turn enables accumulation of cytoplasmic β-catenin, which can then translocate to the nucleus, bind proteins in the Tcf (T-cell factor)/Lef (lymphoid enhancer factor) family, and activate transcription of Wnt target genes. Wnt signalling can be inhibited by sequestration of Wnt ligands via interaction with SFRP (secreted Fzd-related protein) or WIF (Wnt-inhibitory factor)-1 as well as by blocking co-receptor binding between Kremen and LRP-5/6 via Dkk (Dickkopf).
Accumulation of non-junctional β-catenin, and in particular nuclear translocation, is used as a surrogate marker for activation of the Wnt/β-catenin pathway. This is observed in a number of human cancers, as summarized below, and often results from mutations in Wnt pathway components. With the exception of the endometrioid histotype, Wnt pathway mutations are rare in ovarian cancer [6,7]. However, accumulating evidence suggests a role for Wnt signalling in ovarian tumorigenesis in the absence of genetic mutations, suggesting alternative mechanisms for Wnt pathway activation in EOC (Figure 1B) [8–10].
Ovarian tumours inhabit a unique metastatic niche
EOC is a leading cause of death from gynaecologial malignancy. Worldwide, each year approximately 204000 women are diagnosed with EOC, and 125000 die due to complications from the disease. Incidence is highest in the United States and Northern Europe and lowest in Asia and Africa. The majority of women are initially diagnosed after the primary tumour has already metastasized, resulting in a 5-year survival of <30%. The normal ovarian surface epithelium is mesenchymally derived and tissue cohesion is provided by the mesenchymal N-cadherin (neural cadherin). In contrast with most carcinomas that lose epithelial characteristics with tumour progression, EOCs undergo a mesenchymal–epithelial transition, acquire a more differentiated phenotype and gain expression of E-cadherin (reviewed in [11]). During progression, tumours acquire differentiated characteristics reminiscent of specialized Mullerian duct epithelia, resulting in various histotypes of EOC. Thus serous EOC resemble fallopian tubes, endometrioid EOC have characteristics of the endometrium, mucinous EOC is similar to the endocervix and clear-cell EOC tumours resemble vaginal tissue (Figures 2A–2D).
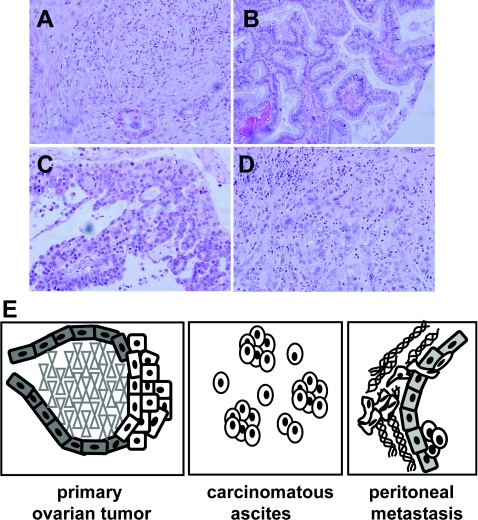
Figure 2. Ovarian tumours and the metastatic niche.
Examples of epithelial ovarian carcinoma histotypes after haematoxylin and eosin staining at ×200 magnification: serous (A), endometrioid (B), mucinous (C) and clear cell (D). (E) Model of ovarian cancer metastastatic niche. The model depicts a primary ovarian tumour arising from malignant transformation of ovarian surface epithelium. Single cells and MCAs are shed from the primary tumour into the peritoneal cavity. Accumulation of carcinomatous ascites is commonly observed, particularly in women with advanced disease. Metastasis is the result of multiple intraperitoneal adhesive events, whereupon tumour cells attach to peritoneal mesothelium, disrupt mesothelial cell–cell contacts and migrate into the submesothelial matrix to anchor secondary lesions on the bowel, diaphragm, omentum and other sites.
Clinically, tumours often involve the ovary and omentum, with diffuse multi-focal intraperitoneal metastasis and malignant ascites [12]. Malignant cells are exfoliated as single cells and MCAs (multi-cellular aggregates) from the primary tumour into the peritoneal cavity (Figure 2E), wherein distribution is facilitated by peritoneal fluid. Formation of malignant ascites is believed to result from shed tumour-cell blockage of peritoneal lymphatics and may further facilitate metastatic dissemination. Shed tumour cells interact with mesothelial cells lining the peritoneal cavity, induce mesothelial cell retraction and anchor in the collagen-rich submesothelial matrix to establish secondary lesions [13]. Adhesion of EOC cells to collagen types I and III in the submesothelial matrix is mediated by α2β1 and α3β1 integrins [14–16]. This affinity for interstitial collagens is reflective of the mesenchymal origin of the ovarian surface epithelium. β1 Integrin-mediated adhesion represents an important early event in EOC metastatic dissemination, and adhesion-mediated integrin signalling probably contributes to metastatic success.
As EOC dissemination occurs largely within the peritoneal cavity, a unique microenvironmental niche is established comprised of tumour cells, inflammatory cells and soluble factors secreted by both the tumour and host cells including bioactive lipids, growth factors, ECM (extracellular matrix) proteins and inflammatory mediators. The contribution of the unique ovarian carcinoma microenvironment to the development and progression of metastatic disease is an area of active investigation. Aberrant activation of numerous signalling pathways has been observed in human EOC that result in stimulated proliferation or dysregulated cell death and most have been reviewed elsewhere. For example, activation of receptor tyrosine kinases such as the EGFR [EGF (epidermal growth factor) receptor] family and the VEGFR (vascular EGFR) family is frequently observed in EOC and regulates cell proliferation, survival, motility and metastasis [17,18]. Moreover, activation of VEGFR also alters vascular permeability, thereby contributing to build-up of ascites in EOC patients [19].
Activation of PI3K (phosphoinositide 3-kinase) signalling is observed in the majority of ovarian cancers as a result of gene amplification, activating mutations or activation downstream of receptor tyrosine kinases [20]. Signalling through the IL (interleukin)-6 receptor is also observed in EOC and results in nuclear translocation of STAT3 (signal transducer and activator of transcription 3) and corresponding stimulation of proliferation and angiogenesis [21]. Cytokine-mediated activation of NF-κB (nuclear factor κB) is also commonly detected in ovarian tumours, leading to up-regulation of anti-apoptotic and antioxidant gene products [22]. Lipid signalling via interaction of LPA (lysophosphatidic acid) with LPA GPCRs is a potent means of microenvironmental regulation, as LPA can be produced by tumour cells as well as stromal components, including mesothelial and inflammatory cells [23]. LPA binds to surface GPCRs, leading to activation of diverse signalling pathways depending on the LPA receptor complement and the specific G-proteins expressed by the cell. For example, LPA stimulation of Gα12/13 leads to activation of the small GTPase RhoA, resulting in cytoskeletal changes and cell rounding [24]. In addition, secondary effects of LPA occur as a result of induction of VEGF (vascular endothelial growth factor), TGF (transforming growth factor) α, TGFβ, IL-8, IL-6 and NF-κB expression, thereby contributing to activation of additional signalling pathways [25,26].
WNT SIGNALLING IN EOC
Progression of most cancers has been associated with activation of Wnt signalling acquired through two major routes: mutations in key components of the pathway or through mutation-independent aberrant gene expression. For example, the majority of colon, uterine and bladder tumours are strongly associated with APC, Axin or β-catenin mutations [27–31]. Interestingly, many cancers, including breast, prostate, lung, thyroid and pancreatic cancers (reviewed in [32–37]), do not depend on mutations in APC, Axin or β-catenin for activation of Wnt signalling.
Examples of Wnt signalling activation independent of mutations in key Wnt signalling genes
The presence of nuclear or cytoplasmic β-catenin in human breast cancer specimens is considered to be a strong indicator of activated Wnt signalling in this malignancy [38]. Multiple pathways, such as phosphorylation of β-catenin by EGFR/HER2 (human EGFR) [39], regulation of GSK3β activity by insulin, IGF-1 (insulin-like growth factor-1) [40], ILK (integrin-linked kinase) [41] or PI3K/Akt [42] and loss of PTEN (phosphatase and tensin homologue deleted on chromosome 10) [43] or p53 [44,45], have been associated with Wnt activation in breast cancers. Interestingly, activation of Wnt signalling in prostate cancer also depends on PTEN, PI3K/Akt [42] and IGF-1 [46], and can additionally be regulated by the androgen receptor [47]. In lung carcinoma, activation of Wnt signalling probably occurs through routes involving overexpression of Dvl [48] and repression of Wnt antagonists, such as WIF-1 and Dkk [49,50]. Activation of Wnt signalling in pancreatic adenocarcinoma has been related to overexpression of Wnt-1 and Fzd-2, which promotes stabilization of β-catenin [37].
Histotype-dependent Wnt signalling activation in ovarian carcinoma
Mechanisms for activation of Wnt signalling in ovarian carcinoma exhibit histotype dependence. Thus only endometrioid EOC is strongly associated with activating mutations in β-catenin leading to constitutively active Wnt signalling. The majority of low-grade endometrioid ovarian carcinomas often display nuclear immunoreactivity for β-catenin (70% of cases), and these cases often harbour mutations in the β-catenin gene at codons that encode for residues phosphorylated by GSK3β (54% of cases) [51]. Several studies confirmed the predominance of nuclear β-catenin and frequent β-catenin gene mutations in endometrioid EOC as well as in cell lines derived from this histotype [52–54]. Nuclear β-catenin in low-grade endometrioid EOC also associates with squamous differentiation and correlates with good prognosis and lack of relapse [51,55–57]. Moreover, expression of Wnt target genes including FGF9 (fibroblast growth factor 9) has been described, suggesting that this pathway is active in endometrioid carcinomas [55–57]. Mutations in Axin in cell lines of endometrioid EOC have also been reported [54]; however, a distinct study found no mutations in either APC or Axin in human endometrioid EOC [58]. Furthermore, high-grade endometrioid ovarian carcinomas do not display nuclear β-catenin immunoreactivity and progression is not associated with β-catenin mutations [51]. This evidence supports the existence of two distinct subtypes of endometrioid EOC that may originate from different sources, (reviewed in [59]) based on differences in molecular pathobiology.
In contrast with endometrioid EOC, ovarian carcinomas of serous, clear-cell and mucinous histotypes have only rarely been associated with activating mutations in the key proteins of the Wnt signalling pathway. One report identified cases of clear-cell EOC positive for nuclear β-catenin [60]. Another study identified mucinous EOC positive for mutations in the β-catenin gene in the GSK3β-binding region [61]. Nevertheless, several lines of evidence implicate activation of Wnt/β-catenin signalling in serous EOC in the absence of activating mutations in either APC, Axin or β-catenin. The strongest evidence is the presence of nuclear β-catenin (Figure 1B). A broad range (3–59%) of serous EOCs have been reported to contain nuclear and cytoplasmic β-catenin [60,62,63]. It is noteworthy that a significantly higher percentage of high-grade (23%) serous EOC correlated with the presence of nuclear β-catenin compared with low grade (2.1%) [63], opposite from trends observed for endometrioid EOC [51]. Together, these observations suggest that additional factors initiate Wnt signalling activation in serous EOC progression.
Potential mechanisms of β-catenin stabilization in serous EOC
Nuclear factors
As described above, the results of several studies indicate that ovarian carcinoma cells contain nuclear β-catenin, although the percentage of positive tissues and positive cells within each tested case vary widely. In the transcriptional activation complex, β-catenin partners with Tcf/Lef, Legless and Pygopus to initiate transcription. The presence of cytoplasmic Lef-1 has been reported in serous EOC and in NIH:OVCAR-3 (a serous EOC cell line). These authors also demonstrated co-immunoprecipitation of Lef-1 and β-catenin in poorly differentiated stage III serous EOC and NIH:OVCAR-3, but not in the normal ovary [9]. Comparison of Lef-1 expression in ten specimens of normal ovary and 23 specimens of serous EOC revealed no differences between the two groups [9]. The transcriptional co-activator Pygopus2 was found to be widely expressed in ovarian carcinoma tissues and cell lines of all histotypes, and was demonstrated to be required for tumour cell growth [64]. Furthermore, an additional co-activator designated Legless (BCL9) is widely expressed in both human EOC and normal ovary specimens at the RNA level (Table 1). Thus there are no apparent barriers for formation of the transcriptional activation complex, as the key players are expressed in serous EOC. Nevertheless, Wnt signalling is not constitutively activated in the majority of serous EOC cases or in the majority of individual cells in positive cases. However, it is important to note that currently available approaches do not enable detection of transient activation of Wnt signalling, suggesting that evaluation of Wnt target gene expression may provide potential evidence with which to evaluate signalling pathway activation.
Table 1. RNA expression of IDAX, BCL9 and RPL19 in EOC and normal ovary human specimens.
Real-time reverse transcription–PCR was used to detect the levels of IDAX and BCL9 mRNAs in samples from non-cancerous ovarian tissues and ovarian carcinomas (Origene), according to the manufacturer's suggestions. Expression of RPL19 was detected as a positive control. Real-time PCR was carried out with the ABI Prizm (Applied Biosystems) and the Ct (threshold cycle) values were obtained according to the manufacturer's instructions. SYBR Green was used for quantitative PCR as a double-stranded DNA-specific fluorophore. The following primers were used for detection of IDAX, BCL9 and RPL19 mRNAs: IDAX, forward 5′-CAGCCAAGAAGAAGAGGA-3′ and reverse 5′-GGGAACAGGTGTTCTCTCTA3-′; BCL9, forward 5′-ACGACCTCAGAGCAGAGTAT-3′ and reverse 5′-GACAAGACAGTGCTGAAGAG-3′; RPL19, forward 5′-CAATGAAATCGCCAATGCCAACTC-3′ and reverse 5′-TGGACCGTCACAGGCTTGC-3′. *Information obtained from the manufacturer (OriGene) regarding Ovarian Carcinoma Panel I; †information on stage is related to ovarian carcinoma specimens only; ‡positive expression is defined by Ct value below 35, whereas negative expression (neg) is defined as the absence of a detectible signal or Ct value equal or higher than 35. FIGO, International Federation of Gynecology and Obstetrics; AJCC, American Joint Committee on Cancer.
| Ct values‡ | |||||
|---|---|---|---|---|---|
| Diagnosis* | Tumour grade* | Stage† | BCL9 | IDAX | RPL19 |
| Adenocarcinoma of endometrium, papillary serous | FIGO G3: poorly differentiated | Non-cancerous | 27.7 | 29.3 | 20 |
| Carcinoma of cervix, squamous cell | FIGO G3: poorly differentiated | Non-cancerous | 28.7 | 27.4 | 20 |
| Abscess of tissue | Not reported | Non-cancerous | 30.7 | 30.4 | 20 |
| Endometriosis | Not reported | Non-cancerous | 33.1 | 30.9 | 20 |
| Endometriosis | Not reported | Non-cancerous | 29.8 | 28.9 | 20 |
| Endometriosis | Not reported | Non-cancerous | 33.2 | 32.5 | 20 |
| Endometriosis | Not reported | Non-cancerous | 32.1 | 31.4 | 20 |
| Carcinoma of ovary, endometrioid | FIGO G2: moderately differentiated | I | 34.4 | 33.4 | 20 |
| Adenocarcinoma of ovary, papillary serous | FIGO G2: moderately differentiated | IA | 30.4 | 33.0 | 20 |
| Tumour of ovary, papillary serous, borderline | AJCC GB: borderline malignancy | IA | 31.7 | 33.0 | 20 |
| Tumour of ovary, papillary serous, borderline | AJCC GB: borderline malignancy | IA | 33.2 | 30.6 | 20 |
| Carcinoma of ovary, endometrioid | FIGO G1: well differentiated | IA | 28.9 | neg | 20 |
| Tumour of ovary, serous, borderline | AJCC GB: borderline malignancy | IA | 30.3 | 30.3 | 20 |
| Tumour of ovary, borderline | AJCC GB: borderline malignancy | IA | 32.2 | 33.0 | 20 |
| Tumour of ovary, mucinous, borderline | AJCC GB: borderline malignancy | IA | 33.3 | 31.0 | 20 |
| Adenocarcinoma of ovary, mucinous | FIGO G3: poorly differentiated | IB | 30.7 | 34.0 | 20 |
| Adenocarcinoma of ovary, endometrioid | FIGO G3: poorly differentiated | IB | 33.6 | neg | 20 |
| Tumour of ovary, borderline | Not reported | IB | 31.8 | 31.4 | 20 |
| Tumour of ovary, mucinous, borderline | AJCC GB: borderline malignancy | IC | 30.7 | 28.4 | 20 |
| Tumour of ovary, serous, borderline | AJCC GB: borderline malignancy | IC | 31.0 | neg | 20 |
| Tumour of ovary, serous, borderline | AJCC GB: borderline malignancy | IC | 32.2 | 33.0 | 20 |
| Adenocarcinoma of ovary, mucinous | FIGO G2: moderately differentiated | IC | 32.1 | 34.9 | 20 |
| Adenocarcinoma of ovary, endometrioid, sq. feat. | FIGO G2: moderately differentiated | IC | 30.0 | 31.9 | 20 |
| Adenocarcinoma of ovary, serous | FIGO G3: poorly differentiated | IIB | 31.4 | 31.7 | 20 |
| Adenocarcinoma of ovary, endometrioid | FIGO G3: poorly differentiated | IIB | 30.9 | neg | 20 |
| Adenocarcinoma of ovary, endometrioid | FIGO G1: well differentiated | IIC | 31.4 | 31.3 | 20 |
| Adenocarcinoma of ovary, papillary serous | FIGO G2: moderately differentiated | III | 29.2 | 32.0 | 20 |
| Adenocarcinoma of ovary, serous | FIGO G3: poorly differentiated | III | 28.7 | neg | 20 |
| Carcinoma of ovary, endometrioid | FIGO G2: moderately differentiated | IIIA | 33.9 | 30.3 | 20 |
| Tumour of ovary, serous, borderline | AJCC GB: borderline malignancy | IIIA | 31.6 | 31.7 | 20 |
| Adenocarcinoma of ovary, papillary serous | FIGO G3: poorly differentiated | IIIB | 30.5 | neg | 20 |
| Adenocarcinoma of ovary, serous | FIGO G2: moderately differentiated | IIIB | 30.5 | neg | 20 |
| Adenocarcinoma of ovary, endometrioid | FIGO G3: poorly differentiated | IIIB | 31.7 | neg | 20 |
| Adenocarcinoma of ovary, papillary serous | FIGO G2: moderately differentiated | IIIB | 31.5 | neg | 20 |
| Adenocarcinoma of ovary, papillary serous | FIGO G3: poorly differentiated | IIIB | 30.9 | neg | 20 |
| Tumour of ovary, serous, borderline | Not reported | IIIB | 31.0 | 33.3 | 20 |
| Adenocarcinoma of ovary, metastatic | Not reported | IIIB | 32.0 | 34.2 | 20 |
| Adenocarcinoma of ovary, papillary serous | FIGO G1: well differentiated | IIIC | 29.1 | neg | 20 |
| Adenocarcinoma of ovary, papillary serous | FIGO G3: poorly differentiated | IIIC | 33.2 | 31.3 | 20 |
| Adenocarcinoma of ovary, papillary serous | FIGO G3: poorly differentiated | IIIC | 32.7 | 31.1 | 20 |
| Adenocarcinoma of ovary, papillary serous | FIGO G3: poorly differentiated | IIIC | 32.5 | 32.2 | 20 |
| Adenocarcinoma of ovary, papillary serous | FIGO G3: poorly differentiated | IIIC | 31.0 | 34.6 | 20 |
| Carcinoma of ovary | FIGO G3: poorly differentiated | IIIC | 31.9 | 33.6 | 20 |
| Adenocarcinoma of ovary, papillary serous | Not reported | IIIC | 32.3 | 32.0 | 20 |
| Adenocarcinoma of ovary, serous | Not reported | IIIC | 32.4 | neg | 20 |
| Adenocarcinoma of ovary, papillary serous | FIGO G2: moderately differentiated | IV | 30.6 | 27.7 | 20 |
| Adenocarcinoma of ovary, metastatic | Not reported | IV | 32.0 | 30.9 | 20 |
| Adenocarcinoma of ovary, papillary serous | FIGO G3: poorly differentiated | IV | 32.6 | neg | 20 |
Cytoplasmic factors
There are several proteins that participate in the process of phosphorylation and degradation of β-catenin in the cytoplasm, thus preventing translocation into the nucleus. Expression of GSK3β in 23 cases of primary and metastatic serous EOC was approximately 6-fold higher on average than that in ten normal ovarian tissues. Overexpression of FRAT1 (frequently rearranged in advanced T-cell lymphomas 1), which inhibits phosphorylation of β-catenin by GSK3β, strongly correlated with nuclear β-catenin and its accumulation in the cytoplasm in human serous EOC, suggesting a potential alternative mechanism for β-catenin stabilization [60]. Cytoplasmic immunoreactivity for APC was documented in 67 out of 113 (59%) serous EOC and was comparable in primary and metastatic tumours [62], suggesting that components of the β-catenin degradation complex are in place. The same study also reported that the absence of cytoplasmic APC only rarely coincided with nuclear β-catenin [62], suggesting that β-catenin degradation did not depend on the presence of APC. Although no data are available regarding the presence of Axin or Dvl, our findings suggest the presence of a Dvl inhibitor, IDAX (inhibitor of the Dvl and Axin complex), in both normal ovary and serous EOC tissues (Table 1). On the basis of the currently available data it is tempting to speculate that the equilibrium between cytoplasmic GSK3β and FRAT1 levels could functionally regulate Wnt signalling activation in serous EOC. In addition, a possible inhibition of Dvl through IDAX could contribute to the liberation of GSK3β and Axin from the complex with Frizzled, thus allowing for β-catenin degradation.
Membranous factors
Expression of Fzd1 and Fzd5 was tested in 26 normal ovary and 38 EOC (including 22 serous) specimens [65]. Interestingly, a higher number of malignant specimens was positive for both receptors relative to normal ovary (97.1% and 14.3% of malignant EOC were FZD1- and FZD5-positive, whereas only 54.5% and 8.7% of the normal ovarian tissues were positive respectively). Moreover, patients with FZD5-positive tumours had a 6-year probability of survival of 0.2 compared with 0.55 for those with FZD5-negative tumours. In addition, our data suggest that both LRP-6 and Kremen are expressed at the RNA level in the serous ovarian carcinoma cell line DOV13 (M.V. Barbolina and M.S Stack, unpublished work). Furthermore, engagement of collagen-binding β1 integrins, a process that occurs during adhesion to submesothelial interstitial collagens in intraperitoneal EOC metastasis, up-regulates LRP6 mRNA levels in serous OVCA 433 cells [66].
Secreted factors
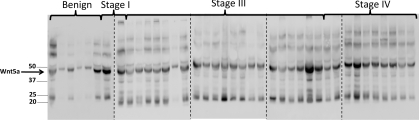
Expression of an activator of canonical Wnt signalling, Wnt-1, was found in a larger number of malignant EOC (29.4% out of a total 38, of which 22 were serous) compared with normal ovary (9.1% out of 26 total) specimens [65]. However, the same study reported that the increase in Wnt-1 expression in malignant EOC did not lead to stabilization of β-catenin [65]. Expression of Wnt-5a was also highly positive in malignant EOC specimens (80%) compared with those of the normal ovary (27.3%). Of note, both Wnt-1- and Wnt-5a-positive EOC patients had a significantly lower chance of survival compared with their Wnt-1-and Wnt-5a-negative counterparts [65]. Similar to the results summarized above for LRP-6, collagen adhesion by serous EOC cells also induces expression of Wnt-5a [66]. Furthermore, Wnt-5a is prevalent in ascites fluid obtained from women with EOC (Figure 3). Although Wnt-5a is most commonly characterized as an inhibitor of canonical Wnt signalling [67–72], emerging evidence suggests that the pathway (canonical, non-canonical or planar cell polarity) activated downstream of Wnt ligand-binding is receptor context-dependent. Using in vitro models, Wnt-5a has been shown to inhibit canonical Wnt signalling via an alternate Wnt receptor, mRor2 (mammalian receptor tyrosine kinase-like orphan receptor 2); however, Wnt-5a activates canonical Wnt signalling in the presence of Fzd4 and LRP-5. This activation potentiates nuclear β-catenin accumulation and activates Wnt reporter constructs [73]. This is consistent with data showing that Wnt-5a enhances the proliferation, migration and invasion of pancreatic cancer cell lines via β-catenin-dependent signalling pathways. In that study, Wnt5a treatment promoted nuclear β-catenin localization, which was inhibited by siRNA (small interfering RNA) targeted against Wnt-5a [74,75]. Additional studies are needed to clarify the Wnt receptor/co-receptor profiles of EOC cells of various histotypes to clarify the role of Wnt-5a in EOC pathobiology.
Figure 3. Wnt-5a in malignant EOC ascites.
Malignant ascites were collected from women with EOC and non-malignant ascites were collected from women with ovarian hyperstimulation syndrome under an institutional review board-approved protocol at Northwestern University School of Medicine. The protein concentration of each specimen was determined and samples containing equal protein (1 mg) were electrophoresed by SDS/PAGE, electroblotted on to Immobilon membranes and analysed by Western blotting using anti-Wnt-5a primary antibody (1:1000 dilution; R&D systems) and peroxidase-conjugated goat anti-(rat IgG) (1:5000 dilution; Santa Cruz Biotechnology). The blots were developed using enhanced chemiluminescence. Molecular mass in kDa is given on the left-hand side.
Expression of Wnt target genes in EOC
Indirect evidence for the existence of active Wnt signalling in serous EOC is expression of Wnt/β-catenin target genes in tumour cells or tissues. Currently over 100 target genes have been identified, the transcription of which is regulated by Wnt signalling (http://www.stanford.edu/~rnusse/pathways/targets.html). It should be noted, however, that many of these genes are also known to be regulated by other pathways, such that target gene expression alone is not sufficient evidence of activated Wnt signalling in serous EOC. Nevertheless, expression of many Wnt target genes, including CCND1 (cyclin D1) [76–79], COX2 (cyclo-oxygenase 2) [66,80], various MMPs (matrix metalloproteinases) [13,14,81–85] and MET [86–87] is observed in EOC, as summarized in Table 2, providing additional evidence in support of Wnt pathway activation.
Table 2. Expression of Wnt target genes in EOC.
Overview of published data on Wnt target gene expression in EOC. BIRC5, survivin; CLDN, claudin 1; EDN1, endothelin 1; FST, follistatin; MMP11, stromelysin 3; PLAUR, plasminogen activator, urokinase receptor; PTTG, pituitary tumour-transforming 1; RARG, retinoic acid receptor γ; SOX, sex-determining region box; JAG, jagged; SNAI, SnaiL.
| Gene | Reference(s) |
|---|---|
| CCND1 | [78,79,167,168] |
| COX2 | [111,169] |
| MMP2 | [82,83] |
| MMP9 | [83,170] |
| VEGF | [171] |
| CD44 | [172] |
| MET | [173] |
| c-Myc | [174] |
| PLAUR | [175,176] |
| MMP7 | [177] |
| CLDN | [178] |
| BIRC5 | [179] |
| EON1 | [122,123] |
| JAG | [180] |
| SOX9 | [181] |
| SOX2 | [182] |
| PTTG | [183] |
| MMP26 | [184] |
| SNAI | [185] |
| FST | [186] |
| RARG | [187] |
| MMP11 | [188] |
| LEF1 | [189] |
MICROENVIRONMENTAL ACTIVATION OF THE WNT PATHWAY
Intraperitoneal dissemination provides an unique microenvironment for ovarian carcinoma metastases. As outlined in Figure 2, progression of EOC is hallmarked by shedding of single and multi-cellular aggregates of malignant epithelial cells from the primary tumour [11,13]. Accumulation of malignant ascites in the peritoneal cavity is common, particularly in women with late-stage EOC. Thus metastasizing EOC cells exist in a milieu rich in inflammatory cells [88] and growth/signalling factors, including VEGF [89–91], EGF [11,92], TGFα, TGFβ [93,94] and LPA [23,95], providing ample opportunity for cross-talk between signalling networks. Ascites accumulation also modifies peritoneal mechanobiology, altering the force environment of both metastatic tumour cells and peritoneal mesothelium [96,97]. Formation of secondary tumours at peritoneal organs (colon, omentum, uterus and liver) is achieved by β1 integrin-mediated anchoring to the mesothelium and submesothelial matrix [14–16,98], representing a significant transition from a free-floating cell or aggregate to a three-dimensional matrix-anchored structure. Within this unique metastatic niche, current evidence suggests multiple opportunities for regulation of Wnt signalling via molecular, mechanical and adhesion-dependent microenvironmental cues.
LPA and LPA GPCRs
LPA, a bioactive lipid signalling molecule, plays a role in numerous cell processes, including proliferation, migration, adhesion and cell survival [23–26,99,100], by acting at a family of GPCRs known as LPARs (LPA receptors) [101,102]. LPA has wide-ranging influence on cell physiology and pathophysiology, including increases in cytokine and growth factor expression, alteration of surface-protein trafficking and modulation of transcription [103,104]. LPA, which is produced by both normal and malignant cells, is present in high concentration (2–80 μM) in ascites and serum from 98% of ovarian cancer patients, including those with early-stage disease [23,26,104–108]. Furthermore, increasing LPA expression is correlated with poor prognosis, suggesting its potential role as a therapeutic biomarker [109]. Results from our laboratory and others demonstrate LPA regulation of proteases [MMP2, MMP9, MT1-MMP (membrane type 1 MMP) and uPA (urokinase-type plasminogen activator)], inflammatory signalling molecules (COX2), IL-8 and adhesion molecules including E-cadherin and β1 integrin [24,100,104,110–111]. Notably, E-cadherin-based adherens junctions are stabilized by β-catenin [112], and loss of junctional stability may increase the cytoplasmic and/or nuclear pool of β-catenin [66].
LPA induces diverse cellular functions by activating one of five known receptors (LPA1–5), which modulate various signalling pathway proteins, including Rho/ROCK (Rho-associated kinase), IP3 (inositol 1,4,5-triphosphate)/Ca2+, PKC (protein kinase C), PI3K, Ras/MAPK (mitogen-activated protein kinase) and cAMP [101–117]. LPA1 (Gα12/13), LPA3 (Gαq/11) and LPA4 (Gα12/13) receptor subtypes are expressed in the ovary, with LPA4 being the most abundant. LPA2 and LPA3 are aberrantly overexpressed in several ovarian carcinoma cell lines [103,104]. This observation has been confirmed in vivo by detection of overexpressed LPAR2 and LPAR3 mRNA in human ovarian tumour tissues, compared with normal and benign tissues [105–107]. Furthermore overexpression of LPA2 and/or LPA3 potentiates a more proliferative and invasive phenotype in ovarian tumour cells by modulating IL-6, IL-8 and VEGF expression [113]. Interestingly, heterotrimeric G-proteins containing Gα12/13 interact with the cytoplasmic domain of E-cadherin, rescuing breast carcinoma cells from E-cadherinmediated migration suppression, preventing E-cadherin-based cell aggregation and displacing β-catenin from the adherens junction complex [114,115]. In colon cancer cell lines, LPA treatment (1 μM) leads to robust inactivation of GSK3β and nuclear localization of β-catenin [116]. These results correlate with a previous study demonstrating that LPA treatment (0.1–20 μM) or LPA2/LPA3 expression induced a deactivating phosphorylation of GSK3β in HEK (human embryonic kidney)-293 cells [117]. Recently, it has been shown that the Gβγ subunit of the G-protein heterotrimer can also mediate Wnt signalling, inhibiting β-catenin degradation and allowing β-catenin-mediated transcriptional activity [118]. In support of these findings, our unpublished work demonstrates that the LPA–LPAR interaction disrupts junctional localization of E-cadherin and induces nuclear translocation of β-catenin, suggesting a mechanism for Wnt ligand-independent activation of Wnt/β-catenin signalling in EOC (R. J. Burkhalter, Y. Liu and M.S. Stack, unpublished work).
Endothelins
The endothelins (ET-1, ET-2 and ET-3) are a family of peptide signalling molecules that play a diverse role in physiology and pathology. Biological function is achieved via autocrine and paracrine signalling through two GPCRs, ETAR and ETBR (ET type A and B receptors respectively) [119,120]. ET-1 and ETAR are overexpressed in ovarian cancer, driving epithelial-to-mesenchymal transition by stimulating tumour cell proliferation, invasive propensity (migration and adhesion), tumour cell escape from apoptosis and angiogenesis through multiple signalling pathways [120–123]. The ET-1/ETAR axis has been shown to promote the invasive phenotype via inhibition of GSK3β in an ovarian carcinoma cell line [123]. Studies have shown a ETAR/β-arrestin-1/Src signalling complex activates ILK, leading to GSK3β inhibition [124,125]. Subsequently, this activity leads to down-regulation of E-cadherin and increased Snail and β-catenin levels, promoting epithelial-to-mesenchymal transition [126,127]. Interestingly, studies in colon cancer and prostate cancer suggest reciprocal β-catenin regulation of ET-1 transcription through interaction with Tcf-4 [128,129]. These results suggest a potential signalling feed-forward loop for co-ordinate regulation of β-catenin and ET-1 to sustain an aggressive invasive phenotype necessary for tumour progression to metastasis.
Integrin-mediated matrix engagement activates β-catenin signalling
During the ovarian carcinoma metastatic cascade, disseminating cells anchor to the mesothelium and submesothelial matrix of peritoneal organs [11,13]. Although mechanisms regulating mesothelial receptivity have not been fully investigated, mechanical deformation of the mesothelium caused by accumulated ascites fluid may expose submesothelial matrix and/or modulate surface-expressed proteins on mesothelial cells themselves [130]. Additionally, a fibronectin-rich adhesion-promoting stroma is deposited on the otherwise non-adhesive mesothelium [131,132] Several studies have recently demonstrated a link between integrin signalling and the Wnt/β-catenin pathway activation in physiological and pathological cell signalling processes [66,133–138]. Our laboratory has modelled submesothelial anchoring of metastasizing EOC cells using three-dimensional collagen matrices or microsphere-immobilized anti-(β1 integrin) antibodies to mimic matrix-induced integrin aggregation. Integrin clustering in the serous EOC cell lines OVCA 429 and OVCA 433 results in rapid loss of junctional E-cadherin, dissolution of adherens junctions and loss of surface-localized β-catenin [66]. β-Catenin is translocated to the nucleus wherein it activates transcription of Tcf/Lef target genes, including LRP6 and Wnt5a [66]. Similarly, culturing the serous EOC cell line DOV13 in three-dimensional collagen gels leads to down-regulation of two secreted antagonists of Wnt signalling, CTGF (connective tissue growth factor) [139] and DKK1 [16]. Interestingly, down-regulation of DKK1 and CTGF also occurs in DOV13 cells cultured as spheroids relative to two-dimensional tissue culture (Table 3). On the basis of these results, it is interesting to speculate that activation of Wnt signalling in free-floating MCAs may contribute to the acquired chemoresistance of this cell population.
Table 3. Changes in expression of genes related to Wnt signalling in ovarian carcinoma MCAs compared with monolayers.
Cells were cultured as monolayers or MCAs. To create MCAs, cells were released from the monolayers using trypsin/EDTA solution, resuspended in minimal essential medium containing 2% fetal bovine serum, plated over solidified 0.5% agarose, and allowed to form spheroids overnight at 37 °C and 5% CO2. Total RNA for cDNA microarray experiments was extracted using TRIzol® (Invitrogen), according to the manufacturer's instructions. All DNA microarray gene expression studies used human oligonucleotide arrays custom printed by a dedicated core facility within the Eppley Institute for Research in Cancer and Allied Diseases, University of Nebraska Medical Center (Omaha, N.E., U.S.A.) as described previously [16]. Microarray slides were scanned with a ScanArray 4000 confocal laser system (PerkinElmer). Analysis of microarray gene expression data, accumulated from three independent experiments, was performed as described [16]. n/a, not applicable. VCAN, versican.
| Gene | Up-regulation (fold) | Down-regulation (fold) | Cell line |
|---|---|---|---|
| DKK1 | n/a | 3.3 | DOV13, ES2 |
| CTGF | n/a | 3.4 | DOV13 |
| VCAN | 2.2 | n/a | DOV13 |
| CCND1 | 2.3 | n/a | ES2 |
The mechanical microenvironment may modulate Wnt signalling through β-catenin
More than two-thirds of all cases of EOC simultaneously present with malignant ascites. Ascites development in progressive or recurrent disease is a poor prognostic indicator and is correlated with a lack of response to treatment [140–142]. In comparison with the peritoneal cavity of disease-free women that contains approximately 20 ml of peritoneal fluid, ascitic volumes average 4.9 litres in EOC patients, with a range of 0.8 to 15 litres [143–145]. Although biological components of ascites are increasingly shown to influence progression of ovarian cancer via diverse signalling pathways [74,146,147], potential biomechanical signals activated by increasing intraperitoneal fluid pressure have not been investigated. Tumour cells sense alterations in the force environment via mechanosensing cell surface-expressed proteins, including integrins, and subsequent ‘inside-out’ integrin signalling induces cytoskeletal modifications that effect cell behaviour [148–151]. Force modulation in the context of three-dimensional matrix rigidity is under investigation in several other tumour types [140,149,152–154]; however, the potential effects of the complex mix of strain, compression and shear forces conferred by ascites fluid are unknown. Our preliminary results show that static strain results in loss of surface-associated E-cadherin in serous EOC cells (J. Symowicz and M.S. Stack, unpublished work). In colon cancer, laminar shear stress increased DKK1 expression and decreased activated β-catenin [155]. Conversely, transient compression in APC-deficient cell lines facilitated phosphorylation of β-catenin, leading to destabilization of adherens junctions, nuclear translocation of β-catenin and transcriptional activation of the β-catenin target genes Myc and Twist1 [156]. These results highlight the need to consider multiple distinct mechanical stimuli when modelling the force microenvironment of tumour tissues.
FUTURE PERSPECTIVES
As summarized above and in Figure 4, the unique microenvironmental niche inhabited by ovarian tumours provides a number of diverse mechanisms for transcriptional activation of Wnt/β-catenin target gene expression, even in histotypes devoid of activating mutations in Wnt pathway components. As canonical Wnt signalling is known to regulate proliferation, differentiation and ‘stemness’ [157], clearly additional studies are needed to provide a more detailed understanding of the role of Wnt target gene expression in ovarian tumour progression and metastasis. In particular, the contribution of microenvironmentally regulated Wnt/β-catenin signalling to maintenance of stemness properties in the self-renewing population of ovarian-cancer-initiating cells [158] remains to be explored. As many extracellular proteinases are β-catenin target genes, analysis of the involvement of this pathway in proteinase-mediated intraperitoneal anchoring of metastatic EOC cells may also prove informative.
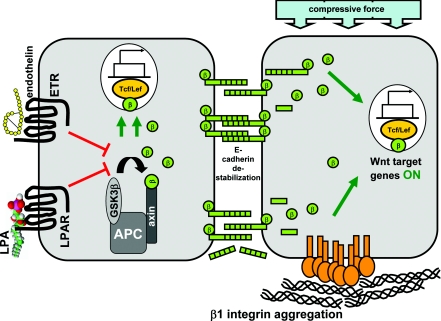
Figure 4. Potential mechanisms for activation of Wnt/β-catenin signalling in the ovarian tumour microenvironment.
In the absence of activating mutations in key components of the Wnt signalling pathway, microenvironmental factors may contribute to transcriptional activation of Wnt/β-catenin target genes. For example, interaction of LPA with LPAR or ET with ET receptors (ETR) have been shown to block the activity of GSK3β, thereby resulting in increased nuclear translocation of β-catenin and enhanced β-catenin-mediated transcriptional activity (left-hand panel). Lateral aggregation of collagen-binding β1 integrins leads to dissolution of adherens junctions and enhanced nuclear translocation of β-catenin. In addition, integrin aggregation enhances the expression of LRP-6 and Wnt-5a while down-regulating the Wnt pathway inhibitors DKK and CTGF. Mechanical compression, exerted on MCAs of metastasizing EOC cells in the form of increased intraperitoneal fluid pressure due to accumulated ascitic fluid, may also destabilize adherens junctions and promote nuclear translocation of β-catenin (right-hand panel).
A number of compounds have been identified that target various components of the Wnt signalling pathway [1,159–161]. For example, non-steroidal anti-inflammatory drugs do not block Wnt signalling themselves, but can induce degradation of Tcf or inhibit Wnt targets such as COX2 [162]. Various small-molecule inhibitors of β-catenin–Tcf interaction have been identified that disrupt β-catenin–Tcf binding, decrease reporter gene transcription, inhibit cell proliferation in vitro and block tumour growth in murine models [163–165]. An alternative approach has been to utilize compounds that stabilize Axin, thereby stimulating β-catenin degradation [166]. As summarized above, with the exception of endometrioid ovarian tumours, progression of EOC has not been commonly associated with activated Wnt signalling. Consequently, there is no available literature reporting the efficacy of Wnt signalling inhibitors in serous EOC, and a search of the clinical trials database (www.clinicaltrials.gov) for trials involving EOC patients and Wnt signalling inhibitors returned no hits. Nevertheless, in light of the accumulating evidence in support of activated Wnt signalling in EOC and increasing evidence for ligand-independent activation of the pathway through microenvironment-regulated cross-talk, preclinical studies evaluating the potential efficacy of small- molecule inhibitors of this signalling pathway are warranted. Following successful preclinical trials, future human clinical trials should be designed to identify the subset of patients most likely to benefit from this biologically targeted therapy (for example, showing nuclear β-catenin staining) and to use validated biomarkers of response.
FUNDING
This work was supported by National Institutes of Health/National Cancer Institute [grant numbers RO1 CA109545, RO1 CA086984 (to M.S.S) and CA086984-11S1 (to R.J.B)], the Illinois Department of Public Health Penny Severns Breast, Ovarian, and Cervical Cancer Fund (to M.V.B), and an Ovarian Cancer Research Foundation Program of Excellence Award (to M.V.B.)
References
- 1.Wend P., Holland J. D., Ziebold U., Birchmeier W. Wnt signalling in stem and cancer stem cells. Seminars Cell Dev. Biol. 2010;21:855–863. doi: 10.1016/j.semcdb.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 2.van Amerongen R., Nusse R. Towards an integrated view of Wnt signalling in development. Development. 2009;136:3205–3214. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- 3.Barker N. The canonical Wnt/β-catenin signalling pathway. Methods Mol. Biol. 2008;468:5–15. doi: 10.1007/978-1-59745-249-6_1. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D., Weinberg R. A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 5.Camilli T. C., Weeraratna A. T. Striking the target in Wnt-y conditions: intervening in Wnt signalling during cancer progression. Biochem. Pharmacol. 2010;80:702–711. doi: 10.1016/j.bcp.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu R., Hendrix-Lucas N., Kuick R., Zhai Y., Schwartz D. R., Akyol A., Hanash S., Misek D. E., Katabuchi H., Williams B. O., et al. Mouse model of human ovarian endometrioid adenocarcinoma based on somatic defects in the Wnt/β-catenin and PI3K/PTEN signalling pathways. Cancer Cell. 2007;11:321–333. doi: 10.1016/j.ccr.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Zhai Y., Wu R., Schwartz D. R., Darrah D., Reed H, Kolligs F. T., Nieman M. T., Fearon E. R., Cho K. R. Role of β-catenin-T-cell factor-regulated genes in ovarian endometrioid adenocarcinomas. Am. J. Path. 2002;160:1229–1238. doi: 10.1016/s0002-9440(10)62550-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gatcliffe T. A., Monk B. J., Planutis K., Holcombe R. F. Wnt signalling in ovarian tumorigenesis. Int. J. Gynecol. Cancer. 2008;18:954–962. doi: 10.1111/j.1525-1438.2007.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rask K., Nilsson A., Brannstrom M., Carlsson P., Hellberg P., Janson P. O., Hedin L., Sundfeldt K. Wnt signalling pathway in ovarian epithelial tumors: increased expression of beta-catenin and GSK3β. Br. J. Caner. 2003;89:1298–1304. doi: 10.1038/sj.bjc.6601265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee C. M., Shvartsman H., Deavers M. T., Wang S. C., Xia W., Schmandt R., Bodurka D. C., Atkinson E. N., Malpica A., Gershenson D. M., et al. β-Catenin nuclear localization is associated with grade in ovarian serous carcinoma. Gynecol. Oncol. 2003;88:363–368. doi: 10.1016/s0090-8258(02)00015-x. [DOI] [PubMed] [Google Scholar]
- 11.Hudson L. G., Zeineldin R., Stack M. S. Phenotypic plasticity of neoplastic ovarian epithelium: unique cadherin profiles in tumor progression. Clin. Exp. Metastasis. 2008;25:643–655. doi: 10.1007/s10585-008-9171-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scully R. E., Young R. H., Clement P. B. Atlas of Tumor Pathology. Washington D.C.: Armed Forces Institute of Pathology; 1998. Tumors of the ovary, maldeveloped gonads, fallopian tube and broad ligament; pp. 51–168. [Google Scholar]
- 13.Barbolina M. V., Moss N. M., Westfall S. D., Liu Y., Burkhalter R. J., Marga F., Forgacs G., Hudson L. G., Stack M. S. Microenvironmental regulation of ovarian cancer metastasis. Cancer Treat. Res. 2009;149:319–334. doi: 10.1007/978-0-387-98094-2_15. [DOI] [PubMed] [Google Scholar]
- 14.Ellerbroek S. M., Fishman D. A., Kearns A. S., Bafetti L. M., Stack M. S. Ovarian carcinoma regulation of matrix metalloproteinase-2 and membrane type 1 matrix metalloproteinase through β1 integrin. Cancer Res. 1999;59:1635–1641. [PubMed] [Google Scholar]
- 15.Ellerbroek S. M., Wu Y. I., Overall C. M., Stack M. S. Functional interplay between type I collagen and cell surface matrix metalloproteinase activity. J. Biol. Chem. 2001;276:24833–24842. doi: 10.1074/jbc.M005631200. [DOI] [PubMed] [Google Scholar]
- 16.Barbolina M. V., Adley B. P., Ariztia E. V., Liu Y., Stack M. S. Microenvironmental regulation of membrane type 1 matrix metalloproteinase activity in ovarian carcinoma cells via collagen-induced EGR1 expression. J. Biol. Chem. 2007;282:4924–4931. doi: 10.1074/jbc.M608428200. [DOI] [PubMed] [Google Scholar]
- 17.Hudson L. G., Zeineldin R., Silberberg M., Stack M. S. Activated epidermal growth factor receptor in ovarian cancer. Cancer Treat. Res. 2009;149:203–226. doi: 10.1007/978-0-387-98094-2_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siwak D. R., Carey M., Hennessy B. T., Nguyen C. T., McGahren Murray M. J., Nolden L., Mills G. B. Targeting the epidermal growth factor receptor in epithelial ovarian cancer: current knowledge and future challenges. J. Oncol. 2010 doi: 10.1155/2010/568938. doi:10.1155/2010/568938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farley J., Birrer M. J. Novel therapeutic targets. Cancer Treat. Res. 2009;149:63–84. doi: 10.1007/978-0-387-98094-2_3. [DOI] [PubMed] [Google Scholar]
- 20.Bast R. C., Jr, Hennessy B., Mills G. B. The biology of ovarian cancer: new opportunities for translation. Nat. Rev. Cancer. 2009;9:415–428. doi: 10.1038/nrc2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosen D. G., Mercado-Uribe I., Yang G., Bast R. C., Jr, Amin H. M., Lai R., Liu J. The role of constitutively active signal transducer and activator of transcription 3 in ovarian tumorigenesis and prognosis. Cancer. 2006;107:2730–2740. doi: 10.1002/cncr.22293. [DOI] [PubMed] [Google Scholar]
- 22.Karin M. Nuclear factor κB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 23.Xu Y., Wang D., Wang Z. Lipid generation and signalling in ovarian cancer. Cancer Treat. Res. 2009;149:241–268. doi: 10.1007/978-0-387-98094-2_12. [DOI] [PubMed] [Google Scholar]
- 24.Do T. V., Symowicz J. C., Berman D. M., Liotta L. A., Petricoin E. F., Stack M. S., Fishman D. A. Lysophosphatidic acid down-regulates stress fibers and up-regulates pro-matrix metalloproteinase-2 activation in ovarian cancer cells. Mol. Cancer Res. 2007;5:121–131. doi: 10.1158/1541-7786.MCR-06-0319. [DOI] [PubMed] [Google Scholar]
- 25.Wang F., Fishman D. A. Lysophosphatidic acid and invasion. Cancer Treat. Res. 2009;149:269–296. doi: 10.1007/978-0-387-98094-2_13. [DOI] [PubMed] [Google Scholar]
- 26.Mills G. B., Eder A., Fang X., Hasegawa Y., Mao M., Lu Y., Tanyi J., Tabassam F. H., Wiener J., Lapushin R., et al. Critical role of lysophospholipids in the pathophysiology, diagnosis, and management of ovarian cancer. Cancer Treat. Res. 2002;107:259–284. doi: 10.1007/978-1-4757-3587-1_12. [DOI] [PubMed] [Google Scholar]
- 27.Bienz M. β-Catenin: a pivot between cell adhesion and Wnt signalling. Curr. Biol. 2005;15:R64–R67. doi: 10.1016/j.cub.2004.12.058. [DOI] [PubMed] [Google Scholar]
- 28.Kastritis E., Murray S., Kyriakou F., Horti M., Tamvakis N., Kavantzas N., Patsouris E. S., Noni A., Legaki S., Dimopoulos M. A., Bamias A. Somatic mutations of adenomatous polyposis coli gene and nuclear β-catenin accumulation have prognostic significance in invasive urothelial carcinomas: evidence for Wnt pathway implication. Int. J. Cancer. 2009;124:103–108. doi: 10.1002/ijc.23917. [DOI] [PubMed] [Google Scholar]
- 29.Oving I. M., Clevers H. C. Molecular causes of colon cancer. Eur. J. Clin. Invest. 2002;32:448–457. doi: 10.1046/j.1365-2362.2002.01004.x. [DOI] [PubMed] [Google Scholar]
- 30.Schlosshauer P. W., Pirog E. C., Levine R. L., Ellenson L. H. Mutational analysis of the CTNNB1 and APC genes in uterine endometrioid carcinoma. Mod. Pathol. 2000;13:1066–1071. doi: 10.1038/modpathol.3880196. [DOI] [PubMed] [Google Scholar]
- 31.Shinohara A., Yokoyama Y., Wan X., Takahashi Y., Mori Y., Takami T., Shimokawa K., Tamaya T. Cytoplasmic/nuclear expression without mutation of exon 3 of the β-catenin gene is frequent in the development of the neoplasm of the uterine cervix. Gynecol. Oncol. 2001;82:450–455. doi: 10.1006/gyno.2001.6298. [DOI] [PubMed] [Google Scholar]
- 32.Howe L. R., Brown A. M. Wnt signalling and breast cancer. Cancer Biol. Ther. 2004;3:36–41. doi: 10.4161/cbt.3.1.561. [DOI] [PubMed] [Google Scholar]
- 33.Ishigaki K., Namba H., Nakashima M., Nakayama T., Mitsutake N., Hayashi T., Maeda S., Ichinose M., Kanematsu T., Yamashita S. Aberrant localization of β-catenin correlates with overexpression of its target gene in human papillary thyroid cancer. J. Clin. Endocrinol. Metab. 2002;87:3433–3440. doi: 10.1210/jcem.87.7.8648. [DOI] [PubMed] [Google Scholar]
- 34.Mazieres J., He B., You L., Xu Z., Jablons D. M. Wnt signalling in lung cancer. Cancer Lett. 2005;222:1–10. doi: 10.1016/j.canlet.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 35.Miyake N., Maeta H., Horie S., Kitamura Y., Nanba E., Kobayashi K., Terada T. Absence of mutations in the β-catenin and adenomatous polyposis coli genes in papillary and follicular thyroid carcinomas. Pathol. Int. 2001;51:680–685. doi: 10.1046/j.1440-1827.2001.01269.x. [DOI] [PubMed] [Google Scholar]
- 36.Verras M., Sun Z. Roles and regulation of Wnt signalling and β-catenin in prostate cancer. Cancer Lett. 2006;237:22–32. doi: 10.1016/j.canlet.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Zeng G., Germinaro M., Micsenyi A., Monga N. K., Bell A., Sood A., Malhotra V., Sood N., Midda V., Monga D. K., et al. Aberrant Wnt/β-catenin signalling in pancreatic adenocarcinoma. Neoplasia. 2006;8:279–289. doi: 10.1593/neo.05607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin S. Y., Xia W., Wang J. C., Kwong K. Y., Spohn B., Wen Y., Pestell R. G., Hung M. C. β-catenin, a novel prognostic marker for breast cancer: its roles in cyclin D1 expression and cancer progression. Proc. Natl. Acad. Sci. U.S.A. 2000;97:4262–4266. doi: 10.1073/pnas.060025397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanai Y., Ochiai A., Shibata T., Oyama T., Ushijima S., Akimoto S., Hirohashi S. c-erbB-2 gene product directly associates with β-catenin and plakoglobin. Biochem. Biophys. Res. Commun. 1995;208:1067–1072. doi: 10.1006/bbrc.1995.1443. [DOI] [PubMed] [Google Scholar]
- 40.Playford M. P., Bicknell D., Bodmer W. F., Macaulay V. M. Insulin-like growth factor 1 regulates the location, stability, and transcriptional activity of β-catenin. Proc. Natl. Acad. Sci. U.S.A. 2000;97:12103–12108. doi: 10.1073/pnas.210394297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Novak A., Hsu S. C., Leung-Hagesteijn C., Radeva G., Papkoff J., Montesano R., Roskelley C., Grosschedl R., Dedhar S. Cell adhesion and the integrin-linked kinase regulate the LEF-1 and β-catenin signalling pathways. Proc. Natl. Acad. Sci. U.S.A. 1998;95:4374–4379. doi: 10.1073/pnas.95.8.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharma M., Chuang W. W., Sun Z. Phosphatidylinositol 3-kinase/Akt stimulates androgen pathway through GSK3β inhibition and nuclear β-catenin accumulation. J Biol. Chem. 2002;277:30935–30941. doi: 10.1074/jbc.M201919200. [DOI] [PubMed] [Google Scholar]
- 43.Persad S., Troussard A. A., McPhee T. R., Mulholland D. J., Dedhar S. Tumor suppressor PTEN inhibits nuclear accumulation of β-catenin and T cell/lymphoid enhancer factor 1-mediated transcriptional activation. J. Cell Biol. 2001;153:1161–1174. doi: 10.1083/jcb.153.6.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu J., Stevens J., Rote C. A., Yost H. J., Hu Y., Neufeld K. L., White R. L., Matsunami N. Siah-1 mediates a novel β-catenin degradation pathway linking p53 to the adenomatous polyposis coli protein. Mol. Cell. 2001;7:927–936. doi: 10.1016/s1097-2765(01)00241-6. [DOI] [PubMed] [Google Scholar]
- 45.Matsuzawa S. I., Reed J. C. Siah-1, SIP, and Ebi collaborate in a novel pathway for β-catenin degradation linked to p53 responses. Mol. Cell. 2001;7:915–926. doi: 10.1016/s1097-2765(01)00242-8. [DOI] [PubMed] [Google Scholar]
- 46.Verras M., Sun Z. β-Catenin is involved in insulin-like growth factor 1-mediated transactivation of the androgen receptor. Mol. Endocrinol. 2005;19:391–398. doi: 10.1210/me.2004-0208. [DOI] [PubMed] [Google Scholar]
- 47.Truica C. I., Byers S., Gelmann E. P. β-Catenin affects androgen receptor transcriptional activity and ligand specificity. Cancer Res. 2000;60:4709–4713. [PubMed] [Google Scholar]
- 48.Uematsu K., He B., You L., Xu Z., McCormick F., Jablons D. M. Activation of the Wnt pathway in non small cell lung cancer: evidence of dishevelled overexpression. Oncogene. 2003;22:7218–7221. doi: 10.1038/sj.onc.1206817. [DOI] [PubMed] [Google Scholar]
- 49.Mazieres J., He B., You L., Xu Z., Lee A. Y., Mikami I., Reguart N., Rosell R., McCormick F., Jablons D. M. Wnt inhibitory factor-1 is silenced by promoter hypermethylation in human lung cancer. Cancer Res. 2004;64:4717–4720. doi: 10.1158/0008-5472.CAN-04-1389. [DOI] [PubMed] [Google Scholar]
- 50.Nozaki I., Tsuji T., Iijima O., Ohmura Y., Andou A., Miyazaki M., Shimizu N., Namba M. Reduced expression of REIC/Dkk-3 gene in non-small cell lung cancer. Int. J. Oncol. 2001;19:117–121. doi: 10.3892/ijo.19.1.117. [DOI] [PubMed] [Google Scholar]
- 51.Gamallo C., Palacios J., Moreno G., Calvo de Mora J., Suarez A., Armas A. β-catenin expression pattern in stage I and II ovarian carcinomas: relationship with β-catenin gene mutations, clinicopathological features, and clinical outcome. Am. J. Pathol. 1999;155:527–536. doi: 10.1016/s0002-9440(10)65148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saegusa M., Okayasu I. Frequent nuclear β-catenin accumulation and associated mutations in endometrioid-type endometrial and ovarian carcinomas with squamous differentiation. J. Pathol. 2001;194:59–67. doi: 10.1002/path.856. [DOI] [PubMed] [Google Scholar]
- 53.Schlosshauer P. W., Ellenson L. H., Soslow R. A. β-Catenin and E-cadherin expression patterns in high-grade endometrial carcinoma are associated with histological subtype. Mod. Pathol. 2002;15:1032–1037. doi: 10.1097/01.MP.0000028573.34289.04. [DOI] [PubMed] [Google Scholar]
- 54.Wu R., Zhai Y., Fearon ER., Cho K. R. Diverse mechanisms of β-catenin deregulation in ovarian endometrioid adenocarcinomas. Cancer Res. 2001;61:8247–8255. [PubMed] [Google Scholar]
- 55.Hendrix N. D., Wu R., Kuick R., Schwartz D. R., Fearon ER., Cho K. R. Fibroblast growth factor 9 has oncogenic activity and is a downstream target of Wnt signalling in ovarian endometrioid adenocarcinomas. Cancer Res. 2006;66:1354–1362. doi: 10.1158/0008-5472.CAN-05-3694. [DOI] [PubMed] [Google Scholar]
- 56.Schwartz D. R., Wu R., Kardia S. L., Levin A. M., Huang C. C., Shedden K. A., Kuick R., Misek D. E., Hanash S. M., Taylor J. M., et al. Novel candidate targets of β-catenin/T-cell factor signalling identified by gene expression profiling of ovarian endometrioid adenocarcinomas. Cancer Res. 2003;63:2913–2922. [PubMed] [Google Scholar]
- 57.Zhai Y., Wu R., Schwartz D. R., Darrah D., Reed H., Kolligs F. T., Nieman M. T., Fearon E. R., Cho K. R. Role of β-catenin/T-cell factor-regulated genes in ovarian endometrioid adenocarcinomas. Am. J. Pathol. 2002;160:1229–1238. doi: 10.1016/s0002-9440(10)62550-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sarrio D., Moreno-Bueno G., Sanchez-Estevez C., Banon-Rodriguez I., Hernandez-Cortes G., Hardisson D., Palacios J. Expression of cadherins and catenins correlates with distinct histologic types of ovarian carcinomas. Hum. Pathol. 2006;37:1042–1049. doi: 10.1016/j.humpath.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 59.Bell D. A. Origins and molecular pathology of ovarian cancer. Mod. Pathol. 2005;18:S19–S32. doi: 10.1038/modpathol.3800306. [DOI] [PubMed] [Google Scholar]
- 60.Wang Y., Hewitt S. M., Liu S., Zhou X., Zhu H., Zhou C., Zhang G., Quan L., Bai J., Xu N. Tissue microarray analysis of human FRAT1 expression and its correlation with the subcellular localisation of β-catenin in ovarian tumours. Br. J. Cancer. 2006;94:686–691. doi: 10.1038/sj.bjc.6602988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sagae S., Kobayashi K., Nishioka Y., Sugimura M., Ishioka S., Nagata M., Terasawa K., Tokino T., Kudo R. Mutational analysis of β-catenin gene in Japanese ovarian carcinomas: frequent mutations in endometrioid carcinomas. Jpn. J. Cancer. Res. 1999;90:510–515. doi: 10.1111/j.1349-7006.1999.tb00777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Karbova E., Davidson B., Metodiev K., Trope C. G., Nesland J. M. Adenomatous polyposis coli (APC) protein expression in primary and metastatic serous ovarian carcinoma. Int. J. Surg. Pathol. 2002;10:175–180. doi: 10.1177/106689690201000302. [DOI] [PubMed] [Google Scholar]
- 63.Lee C. M., Shvartsman H., Deavers M. T., Wang S. C., Xia W., Schmandt R., Bodurka D. C., Atkinson E. N., Malpica A., Gershenson D. M., et al. β-Catenin nuclear localization is associated with grade in ovarian serous carcinoma. Gynecol. Oncol. 2003;88:363–368. doi: 10.1016/s0090-8258(02)00015-x. [DOI] [PubMed] [Google Scholar]
- 64.Popadiuk C. M., Xiong J., Wells M. G., Andrews P. G., Dankwa K., Hirasawa K., Lake B. B., Kao K. R. Antisense suppression of pygopus2 results in growth arrest of epithelial ovarian cancer. Clin. Cancer Res. 2006;12:2216–2223. doi: 10.1158/1078-0432.CCR-05-2433. [DOI] [PubMed] [Google Scholar]
- 65.Badiglian Filho L., Oshima C. T., De Oliveira Lima F., De Oliveira Costa H., De Sousa Damiao R., Gomes T. S., Goncalves W. J. Canonical and noncanonical Wnt pathway: a comparison among normal ovary, benign ovarian tumor and ovarian cancer. Oncol. Rep. 2009;21:313–320. [PubMed] [Google Scholar]
- 66.Burkhalter R. J., Symowicz J. S., Hudson L. G., Gottardi C. J., Stack M. S. Integrin regulation of β-catenin signalling in ovarian carcinoma. J. Biol. Chem. 2011 doi: 10.1074/jbc.M110.199539. doi:10.1074/jbc.M110.199539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Danielson K. G., Pillarisetti J., Cohen I. R., Sholehvar B., Huebner K., Ng L.-J., Nicholls J. M., Cheah K. S. E., Iozzo R. V. Characterization of the complete genomic structure of the human Wnt-5A gene, functional analysis of its promoter, chromosomal mapping, and cxpression in early human embryogenesis. J. Biol. Chem. 1995;270:31225–31234. doi: 10.1074/jbc.270.52.31225. [DOI] [PubMed] [Google Scholar]
- 68.Topol L., Juaing X., Choi H., Garett-Beal L., Carolan P. J., Yang Y. Wnt5a inhibits the canonical Wnt pathway by promoting GSK-3-independent β-catenin. J. Cell Biol. 2003;162:899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ishtani T., Kishida S., Hyodo-Miura J., Ueno N., Yasuda J., Waterman M., Shibuya H., Moon R. T., Ninomiya-Tsuji J., Matsumoto K. The TAK1-NLK mitogen-activated protein kinase cascade functions in the Wnt-5a/Ca(2+) pathway to antagonize Wnt/ β-catenin signalling, Mol. Cell Biol. 2003;23:131–139. doi: 10.1128/MCB.23.1.131-139.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Holmen S. L., Salic A., Zylstra C. R., Kirschner M. W., Williams B. O. A novel set of Wnt–Frizzled fusion proteins identifies receptor components that activate β-catenin-dependent signalling. J. Biol. Chem. 2002;277:34727–34735. doi: 10.1074/jbc.M204989200. [DOI] [PubMed] [Google Scholar]
- 71.Olson D. J., Gibo D. M. Antisense Wnt-5a mimics Wnt-1-medaited C57MG mammary epithelial cell transformation. Exp. Cell Res. 1998;241:134–141. doi: 10.1006/excr.1998.4030. [DOI] [PubMed] [Google Scholar]
- 72.Torres M. A., Yang-Snyder J. A., Purcell S. M., DeMarais A. A., McGrew L. L., Moon R. T. Activities of the Wnt-1 class of secreted signalling factors are antagonized by the Wnt-5a class and by a dominant negative cadherin in early Xenopus development. J. Cell Biol. 1996;133:1123–1137. doi: 10.1083/jcb.133.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McDonald S. L., Silver A. The opposing roles of Wnt-5a in cancer. Br. J. Cancer. 2009;101:209–214. doi: 10.1038/sj.bjc.6605174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mikels A. J., Nusse N. Purified Wnt5a protein activates or inhibits β-catenin-TCF signalling depending on receptor context. PLoS Biol. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ripka S., Konig A., Buchholz M., Wagner M., Sipos B., Kloppel G., Downward J., Gress T. M., Muchl P. Wnt5a-target of CUTL1 and potent modulator of tumor cell migration and invasion in pancreatic cancer. Carcinogenesis. 2007;28:1178–1187. doi: 10.1093/carcin/bgl255. [DOI] [PubMed] [Google Scholar]
- 76.Tetsu O., McCormick F. β-Catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 77.Rahmani M., Read J. T., Carthy J. M., McDonald P. C., Wong B. W., Esfandiarei M., Si X., Luo Z., Luo H., Rennie P. S., McManus B. M. Regulation of the versican promoter by the β-catenin-T-cell factor complex in vascular smooth muscle cells. J. Biol. Chem. 2005;280:13019–13028. doi: 10.1074/jbc.M411766200. [DOI] [PubMed] [Google Scholar]
- 78.Hung W. C., Chai C. Y., Huang J. S., Chuang L. Y. Expression of cyclin D1 and c-Ki-ras gene product in human epithelial ovarian tumors. Hum. Pathol. 1996;27:1324–1328. doi: 10.1016/s0046-8177(96)90345-7. [DOI] [PubMed] [Google Scholar]
- 79.Barbieri F., Cagnoli M., Ragni N., Pedulla F., Foglia G., Alama A. Expression of cyclin D1 correlates with malignancy in human ovarian tumours. Br. J. Cancer. 1997;75:1263–1268. doi: 10.1038/bjc.1997.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Howe L. R., Subbaramaiah K., Chung W. J., Dannenberg A. J., Brown A. M. Transcriptional activation of cyclooxygenase-2 in Wnt-1-transformed mouse mammary epithelial cells. Cancer Res. 1999;59:1572–1577. [PubMed] [Google Scholar]
- 81.Wu B., Crampton S. P., Hughes C. C. W. Wnt signalling induces matrix metalloproteinase expression and regulates T cell transmigration. Immunity. 2007;26:227–239. doi: 10.1016/j.immuni.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fishman D. A., Bafetti L. M., Banionis S., Kearns A. S., Chilukuri K., Stack M. S. Production of extracellular matrix-degrading proteinases by primary cultures of human epithelial ovarian carcinoma cells. Cancer. 1997;80:1457–1463. doi: 10.1002/(sici)1097-0142(19971015)80:8<1457::aid-cncr13>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 83.Moser T. L., Pizzo S. V., Bafetti L. M., Fishman D. A., Stack M. S. Evidence for preferential adhesion of ovarian epithelial carcinoma cells to type I collagen mediated by the alpha2beta1 integrin. Int. J. Cancer. 1996;67:695–701. doi: 10.1002/(SICI)1097-0215(19960904)67:5<695::AID-IJC18>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 84.Ellerbroek S. M., Hudson L. G., Stack M. S. Proteinase requirements of epidermal growth factor-induced ovarian cancer cell invasion. Int. J. Cancer. 1998;78:331–337. doi: 10.1002/(SICI)1097-0215(19981029)78:3<331::AID-IJC13>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 85.Ghosh S., Wu Y., Stack M. S. Ovarian cancer-associated proteinases. Cancer Treat. Res. 2002;107:331–351. doi: 10.1007/978-1-4757-3587-1_16. [DOI] [PubMed] [Google Scholar]
- 86.Boon E. M., van der Neut R., van de Wetering M., Clevers H., Pals S. T. Wnt signalling regulates expression of the receptor tyrosine kinase met in colorectal cancer. Cancer Res. 2002;62:5126–5128. [PubMed] [Google Scholar]
- 87.Di Renzo M. F., Olivero M., Katsaros D., Crepaldi T., Gaglia P., Zola P., Sismondi P., Comoglio P. M. Overexpression of the Met/HGF receptor in ovarian cancer. Int. J. Cancer. 1994;58:658–662. doi: 10.1002/ijc.2910580507. [DOI] [PubMed] [Google Scholar]
- 88.Freedman R. S., Deavers M., Liu J., Wang E. Peritoneal inflammation: a microenvironment for epithelial ovarian cancer (EOC) J. Transl. Med. 2004;2:23–32. doi: 10.1186/1479-5876-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zebrowski B. K., Liu W., Ramirez K., Akgi Y., Mills G. B. Markedly elevated levels of vascular endothelial growth factor in malignant ascites. Ann. Surg. Oncol. 1999;6:373–378. doi: 10.1007/s10434-999-0373-0. [DOI] [PubMed] [Google Scholar]
- 90.Kraft A., Weindel K., Ochs A., Marth C., Zmija J., Schumacher P., Unger C., Marme D., Gastl G. Vascular endothelial growth factor in the sera and effusions of patients with malignant and nonmalignant disease. Cancer. 1999;85:178–187. [PubMed] [Google Scholar]
- 91.Santin A. D., Hermonat P. L., Ravaggi A, Cannon M. J., Pecorelli S., Parham G. P. Secretion of vascular endothelial growth factor in ovarian cancer. Eur. J. Gynaecol. Oncol. 1999;20:177–181. [PubMed] [Google Scholar]
- 92.Miyamoto S., Hirata M., Yamazaki A., Kageyama T., Hasuwa H., Mizushima H., Tanaka Y., Yagi H., Sonoda K., Kai M., et al. Heparin-binding EGF-like growth factor is a promising target for ovarian cancer therapy. Cancer Res. 2004;64:5720–5727. doi: 10.1158/0008-5472.CAN-04-0811. [DOI] [PubMed] [Google Scholar]
- 93.Saltzman A. K., Hartenbach E. M., Carter J. R., Contreras D. N., Twiggs L. B., Carson L. F., Ramakrishnan S. Transforming growth factor-alpha levels in the serum and ascites of patients with advanced epithelial ovarian cancer. Gynecol. Obstet. Invest. 1999;47:200–204. doi: 10.1159/000010095. [DOI] [PubMed] [Google Scholar]
- 94.Abendstein B., Stadlmann S., Knabbe C., Buck M., Muller-Holzner E., Zeimet A. G., Marth C., Obrist P., Krugmann J., Offner F. A. Regulation of transforming growth factor-beta secretion by human peritoneal mesothelial and ovarian carcinoma cells. Cytokine. 2000;12:1115–1119. doi: 10.1006/cyto.1999.0632. [DOI] [PubMed] [Google Scholar]
- 95.Puiffe M. L., Le Page C., Filali-Mouhim A., Zietarska M., Ouellet V., Tonin P. N., Chevrette M., Provencherm D. M., Mes-Masson A. M. Characterization of ovarian cancer ascites on cell invasion, proliferation, spheroid formation, and gene expression in an in vitro model of ovarian cancer. Neoplasia. 2007;9:820–829. doi: 10.1593/neo.07472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Esquis P., Consolo D., Magnin G., Pointaire P., Moretto P., Ynsa M. D., Beltrame J. L., Drogoul C., Simonet M., Benoit, et al. High intra-abdominal pressure enhances the penetration and antitumor effect of intraperitoneal cisplatin on experimental peritoneal carcinomatosis. Ann. Surg. 2006;244:106–112. doi: 10.1097/01.sla.0000218089.61635.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Henriksen J. H., Stage J. G., Schlichting P., Winkler K. Intraperitoneal pressure: ascetic fluid and splanchnic vascular pressures, and their role in prevention and formation of ascites. Scand. J. Clin. Lab. Invest. 1980;40:493–502. doi: 10.3109/00365518009091956. [DOI] [PubMed] [Google Scholar]
- 98.Lessan K., Aguiar D. J., Oegema T., Siebenson L., Skubitz A. P. N. CD44 and fl1 integrin mediate ovarian carcinoma cell adhesion to peritoneal mesothelial cells. Am. J. Pathol. 1999;154:1525–1537. doi: 10.1016/s0002-9440(10)65406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Moolenaar W., Jalink K., Van Corven E. Lysophosphatidic acid: a bioactive phospholipid with growth factor-like properties. Rev. Physiol. Biochem. Pharmacol. 1992;119:47–65. doi: 10.1007/3540551921_3. [DOI] [PubMed] [Google Scholar]
- 100.Fishman D. A., Liu Y., Ellerbroek S. M., Stack M. S. Lysophosphatidic acid promotes matrix metalloproteinase (MMP) activation and MMP-dependent invasion in ovarian cancer cells. Cancer Res. 2001;61:3194–3199. [PubMed] [Google Scholar]
- 101.Choi J. W., Herr D. R., Noguchi K., Yung Y. C., Lee C.-W., Mutoh T., Lin M.-E., Teo S. T., Park K. E., et al. LPA receptors: subtypes and biological actions. Annu. Rev. Pharmacol. Toxicol. 2010;50:157–186. doi: 10.1146/annurev.pharmtox.010909.105753. [DOI] [PubMed] [Google Scholar]
- 102.Fukushima N., Chun J. The LPA receptors. Prostaglandins Other Lipid Mediators. 2001;64:21–32. doi: 10.1016/s0090-6980(01)00105-8. [DOI] [PubMed] [Google Scholar]
- 103.Fang X., Yu S., Bast R. C., Liu S., Xu H.-J., Hu S.-X., LaPushin R., Claret F. X., Aggarwal B. B., Lu Y., Mills G. B. Mechanisms for lysophosphatidic acid-induced cytokine production in ovarian cancer cells. J. Biol. Chem. 2004;279:9653–9661. doi: 10.1074/jbc.M306662200. [DOI] [PubMed] [Google Scholar]
- 104.Fang X., Gaudette D., Furui T., Mao M., Estrella V., Eder A., Pustilnik T., Sasagawa T., Lapushin R., Yu S., et al. Lysophospholipid growth factors in the initiation, progression, metastases, and management of ovarian cancer. Ann. N. Y. Acad. Sci. 2000;905:188–208. doi: 10.1111/j.1749-6632.2000.tb06550.x. [DOI] [PubMed] [Google Scholar]
- 105.Xu Y., Gaudette D. C., Boyton J. D. Characterization of an ovarian cancer activating factor in ascites from ovarian cancer patients. Clin. Cancer Res. 1995;1:1223–1232. [PubMed] [Google Scholar]
- 106.Westermann A. M., Havik E., Postma F. R., Neijnen J. H., Dalesio O., Moolenaar W. H., Rodenhuis S. Malignant effusions contain lysophosphatidic acid (LPA)-like activity. Ann. Oncol. 1998;9:437–442. doi: 10.1023/a:1008217129273. [DOI] [PubMed] [Google Scholar]
- 107.Fang X., Schummer M., Mao M., Yu S., Tabassam F. H., Swaby R., Hasegawa Y., Tanyi J. L., LaPushin R., Eder A., et al. Lysophosphatidic acid is a bioactive mMediator in ovarian cancer. Biochim. Biophys. Acta. 2002;1582:257–264. doi: 10.1016/s1388-1981(02)00179-8. [DOI] [PubMed] [Google Scholar]
- 108.Xiao Y. J., Schwartz B., Washington M., Kennedy A., Webster K., Belinson J., Xu Y. Electrospray ionization mass spectrometry analysis of lysophospholipids in human ascitic fluids: comparison of the lysophospholipid contents in malignant vs. nonmalignant ascitic fluids. Anal. Biochem. 2001;290:302–313. doi: 10.1006/abio.2001.5000. [DOI] [PubMed] [Google Scholar]
- 109.Xu Y., Shen Z., Wiper D. W., Wu M., Morton R. E., Elson P., Kennedy A. W., Belinson J., Markman J., Casey G. Lysophosphatidic acid as a potential biomarker for ovarian and other gynecologic cancers. JAMA, J. Am. Med. Assoc. 1998;280:719–723. doi: 10.1001/jama.280.8.719. [DOI] [PubMed] [Google Scholar]
- 110.Gil O. D., Lee C., Ariztia E. V., Wang F.-Q., Smith P. J., Hope J. M., Fishman D. A. Lysophosphatidic acid (LPA) promotes E-cadherin ectodomain shedding and OVCA429 cell invasion in an uPA-dependent manner. Gynecol. Oncol. 2008;108:361–369. doi: 10.1016/j.ygyno.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 111.Symowicz J., Adley B. P., Woo M. M., Auersperg N., Hudson L. G., Stack M. S. Cyclooxygenase-2 functions as a downstream mediator of lysophosphatidic acid to promote aggressive behavior in ovarian carcinoma cells. Cancer Res. 2005;65:2234–2242. doi: 10.1158/0008.5472.CAN-04-2781. [DOI] [PubMed] [Google Scholar]
- 112.Nelson W. J., Nusse R. Convergence of Wnt, β-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yu S., Murph M. M., Lu Y., Liu S., Hall H. S., Liu J., Stephens C., Fang X., Mills G. B. Lysophosphatidic acid receptors determine tumorigenicity and aggressiveness of ovarian cancer cells. J. Natl. Cancer Inst. 2008;20:1630–1642. doi: 10.1093/jnci/djn378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Meigs T. E., Fields T. A., McKee D. D., Casey P. J. Interaction of Galpha12 and Galpha13 with the cytoplasmic domain of cadherin provides a mechanism for β-catenin release. Proc. Natl. Acad. Sci. U.S.A. 2001;98:519–524. doi: 10.1073/pnas.021350998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Meigs T. E., Fedor-Chaiken M., Kaplan D. D., Brackenbury R., Casey P. J. Galpha12 and Galpha13 negatively regulate the adhesive functions of cadherin. J. Biol. Chem. 2002;277:24594–24600. doi: 10.1074/jbc.M201984200. [DOI] [PubMed] [Google Scholar]
- 116.Yang M., Zhong W. W., Srivastava N., Slavin A., Yang J., Hoey T., An S. G protein-coupled lysophosphatidic acid receptors stimulate proliferation of colon cancer cells through the β-catenin pathway. Proc. Natl. Acad. Sci. U.S.A. 2005;102:6027–6032. doi: 10.1073/pnas.0501535102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fang X., Yu S., Tanyi J. L., Lu Y., Woodgett J. R., Mills G. B. Convergence of multiple signalling cascades at glycogen synthase kinase 3: edg receptor-mediated phosphorylation and inactivation by lysophosphatidic acid through a protein kinase C-dependent intracellular pathway. Mol. Cell. Biol. 2002;22:2099–2110. doi: 10.1128/MCB.22.7.2099-2110.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jernigan K. K., Cselenyi C. S., Thorne C. A., Hanson A. J., Tahinci E., Hajicek N., Oldham W. M., Lee L. A., Hamm H. E., Hepler J. R., et al. Gbetagamma activates GSK3 to promote LRP6-mediated β-catenin transcriptional activity. Sci. Signaling. 2010;3:ra37. doi: 10.1126/scisignal.2000647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Levin E. R. Endothelins. N. Engl. J. Med. 1995;333:356–363. doi: 10.1056/NEJM199508103330607. [DOI] [PubMed] [Google Scholar]
- 120.Bagnato A., Spinella F., Rosano L. The endothelin axis in cancer: the promise and the challenges of molecularly targeted therapy. Can. J. Physiol. Pharmacol. 2008;86:473–484. doi: 10.1139/Y08-058. [DOI] [PubMed] [Google Scholar]
- 121.Nelson J., Bagnato A., Battistini B., Nisen P. The endothelin axis: emerging role in cancer. Nat. Rev. Cancer. 2003;3:110–116. doi: 10.1038/nrc990. [DOI] [PubMed] [Google Scholar]
- 122.Bagnato A., Tecce R., Moretti C., Di Castro V., Spergel D., Catt K. J. Autocrine actions of endothelin-1 as a growth factor in human ovarian carcinoma cells. Clin. Cancer Res. 1995;1:1059–1066. [PubMed] [Google Scholar]
- 123.Bagnato A., Salani D., Di Castro V., Wu-Wong J. R., Tecce R., Nicotra M. R., Venuti A., Natali P. G. Expression of endothelin 1 and endothelin A receptor in ovarian carcinoma: evidence for an autocrine role in tumor growth. Cancer Res. 1999;59:720–727. [PubMed] [Google Scholar]
- 124.Rosano L., Cianfrocca R., Masi S., Spinella F., Di Castro V., Biroccio A., Salvati E., Nicotra M. R., Natali P. G., Bagnato A. β-Arrestin link endothelin A receptor to β-catenin signalling to induce ovarian cancer cell invasion and metastasis. Proc. Natl. Acad. Sci. U.S.A. 2009;106:2806–2811. doi: 10.1073/pnas.0807158106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cianfracca R., Rosano L., Spinella F., Di Castro V., Natali P. G., Bagnato A. β-arrestin-1 mediates the endothelin-1-induced activation of Akt and integrin-linked kinase. Can. J. Physiol. Pharmacol. 2010;88:796–801. doi: 10.1139/Y10-052. [DOI] [PubMed] [Google Scholar]
- 126.Rosano L., Spinella F., Di Castro V., Nicotra M. R., Dedhar S., de Herreros A. G., Natali P. G., Bagnato A. Endothelin-1 promotes epithelial-to-mesenchymal transition in human ovarian cancer cells. Cancer Res. 2005;65:11649–11657. doi: 10.1158/0008-5472.CAN-05-2123. [DOI] [PubMed] [Google Scholar]
- 127.Bagnato A., Rosano L. Epithelial–mesenchymal transition in ovarian cancer progression: a crucial role for the endothelin axis. Cells Tissues Organs. 2007;185:85–94. doi: 10.1159/000101307. [DOI] [PubMed] [Google Scholar]
- 128.Kim T. H., Xiong H., Zhang Z., Ren B. β-Catenin activates the growth factor endothelin-1 in colon cancer cells. Oncogene. 2005;24:597–604. doi: 10.1038/sj.onc.1208237. [DOI] [PubMed] [Google Scholar]
- 129.Sun P., Xiong H., Kim T. H., Ren B., Zhang Z. Positive inter-regulation between β-catenin/T Cell factor-4 signalling and endothelin-1 signalling potentiates proliferation and survival of prostate cancer cells. Mol. Pharmacol. 2006;69:520–531. doi: 10.1124/mol.105.019620. [DOI] [PubMed] [Google Scholar]
- 130.Zareie M., Keuning E. D., ter Wee P. M., Beelen R. H. J., van den Born J. Peritoneal dialysis fluid-induced changes of the peritoneal membrane are reversible after peritoneal rest in rats. Nephrol. Dial. Transplant. 2005;20:189–193. doi: 10.1093/ndt/gfh559. [DOI] [PubMed] [Google Scholar]
- 131.Nagy J. A., Morgan E. S., Herzberg K. T., Manseau E. J., Dvorak A. M., Dvorak H. F. Pathogenesis of ascites tumor growth: angiogenesis, vascular remodeling, and stroma formation in the peritoneal lining. Cancer Res. 1995;55:376–385. [PubMed] [Google Scholar]
- 132.Kenny H. A., Dogan S., Zillhardt M., Mitra A. K., Yamada S. D., Krausz T., Lengyel E. Organotypic models of metastasis: a three-dimensional culture mimicking the human peritoneum and omentum for the study of the early steps of ovarian cancer metastasis. Cancer Treat. Res. 2009;149:335–351. doi: 10.1007/978-0-387-98094-2_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Crampton S. P., Beibei W., Park E. J., Kim J. H., Solomon C., Waterman M. L., Hughes C. C. W. Integration of the β-catenin dependent Wnt pathway with integrin signalling through the adaptor molecule Grb2. PLoS ONE. 2009;4:e7841. doi: 10.1371/journal.pone.0007841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Maher M. T., Flozak A. S., Stocker A. M., Chenn A., Gottardi C. J. Activity of the β-catenin phosphodestruction complex at cell–cell contacts is enhanced by cadherin-based adhesion. J. Cell Biol. 2009;186:219–228. doi: 10.1083/jcb.200811108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kim Y., Kugler M. C., Wei Y., Kim K. K., Li X., Brumwell A. N., Chapman H. A. Integrin alpha3beta1-dependent β-catenin phophorylation links epithelial Smad signalling to cell contacts. J. Cell Biol. 2009;184:309–322. doi: 10.1083/jcb.200806067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chandramouly G., Abad P. C., Knowles D. W., Lelievre S. A. The control of tissue architecture over nuclear organization is crucial for epithelial cell fate. J. Cell Sci. 2007;120:1596–1606. doi: 10.1242/jcs.03439. [DOI] [PubMed] [Google Scholar]
- 137.Kam Y., Quaranta V. Cadherin-bound β-catenin feeds into the Wnt pathway upon adherens junctions dissociation: evidence for an intersection between β-catenin pools. PLoS ONE. 2009;4:e4580. doi: 10.1371/journal.pone.0004580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Koenig A., Mueller C., Hasel C., Adler G., Menke A. Collagen type I induces disruption of E-cadherin-mediated cell–cell contacts and promotes proliferation of pancreatic carcinoma cells. Cancer Res. 2006;66:4662–4671. doi: 10.1158/0008-5472.CAN-05-2804. [DOI] [PubMed] [Google Scholar]
- 139.Barbolina M. V., Adley B. P., Kelly D L., Shepard J., Fought A. J., Scholtens D., Penzes P., Shea L. D., Stack M. S. Downregulation of connective tissue growth factor by three-dimensional matrix enhances ovarian carcinoma cell invasion. Int. J. Cancer. 2009;125:816–825. doi: 10.1002/ijc.24347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gotlieb W. H., Feldman B., Feldman-Moran O., Zmira N., Kreizer D., Segal Y., Elran E., Ben-Baruch G. Intraperitoneal pressures and clinical parameters of total paracentesis for palliation of symptomatic ascites in ovarian cancer. Gynecol. Oncol. 2002;71:381–385. doi: 10.1006/gyno.1998.5215. [DOI] [PubMed] [Google Scholar]
- 141.Dembo A. J., Davy M., Stenwig A. E., Berle E. J., Bush R. S., Kjorstad K. Prognostic factors in patients with stage I epithelial ovarian cancer. Obstet. Gynecol. 1990;75:263–273. [PubMed] [Google Scholar]
- 142.Stanojevic Z., Rancic G., Radic S., Potic-Zecevic N., Dordevic B., Markovic M., Todorovska I. Pathogenesis of malignant ascites in ovarian cancer patients. Arch. Oncol. 2004;12:115–118. [Google Scholar]
- 143.Holm A., Halpern N. B., Aldnek J. S. Peritoneovenous shunt for intractable ascites of hepatic nephrogenic and malignant causes. Am. J. Surg. 1989;158:162–166. doi: 10.1016/0002-9610(89)90368-1. [DOI] [PubMed] [Google Scholar]
- 144.Yazdi G. P., Miedema B. W., Humphrey L. J. High mortality after abdominal operation in patients with large-volume malignant ascites. J. Surg. Oncol. 1996;62:93–96. doi: 10.1002/(SICI)1096-9098(199606)62:2<93::AID-JSO4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 145.McNamara P. Paracentesis: an effective method of symptom control in the palliative care setting? Palliative Med. 2000;14:62–66. doi: 10.1191/026921600676345896. [DOI] [PubMed] [Google Scholar]
- 146.Cowden-Dahl K. D., Symowicz J., Ning Y., Gutierrez E., Fishman D. A., Adley B. P., Stack M. S., Hudson L. G. Matrix metalloproteinase 9 is a mediator of epidermal growth factor-dependent E-cadherin loss in ovarian carcinoma cells. Cancer Res. 2008;68:4606–4613. doi: 10.1158/0008-5472.CAN-07-5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Said N. A., Najwer I., Socha M. J., Fulton D. J., Mok S. C., Motamed K. SPARC inhibits LPA-mediated mesothelial–ovarian cancer cell crosstalk. Neoplasia. 2007;9:23–35. doi: 10.1593/neo.06658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ingber D. E. Tensegrity-based mechanosensing from macro to micro. Prog. Biophys. Mol. Bio. 2008;97:163–179. doi: 10.1016/j.pbiomolbio.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Paszek M. J., Zahir N., Johnson K. R., Lakins J. N., Rozenberg G. I., Gefen A., Reinhart-King C. A., Margulies S. S., Dembo M., Boettiger D., et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 150.Thamilselvan V., Basson M. D. Pressure activates colon cancer cell adhesion by inside-out focal adhesion complex and actin cytoskeletal signalling. Gastroenterology. 2004;126:8–18. doi: 10.1053/j.gastro.2003.10.078. [DOI] [PubMed] [Google Scholar]
- 151.Ingber D. E. Cell tension, matriz mechanics, and cancer development. Cancer Cell. 2005;8:175–176. doi: 10.1016/j.ccr.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 152.Butcher D. T., Alliston T., Weaver V. M. A tense situation: forcing tumor progression. Nat. Rev. Cancer. 2009;9:108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Condeelis J., Segall J. E. Intravital imaging of cell movement in tumours. Nat. Rev. Cancer. 2003;3:921–930. doi: 10.1038/nrc1231. [DOI] [PubMed] [Google Scholar]
- 154.Wyckoff J. B., Pinner S. E., Gschmeissner S., Condeelis J. S., Sahai E. ROCK- and myosin-dependent matrix deformation enables protease-independent tumor-cell invasion in vivo. Curr. Biol. 2006;16:1515–1523. doi: 10.1016/j.cub.2006.05.065. [DOI] [PubMed] [Google Scholar]
- 155.Avvisato C. L., Yang X., Shah S., Hoxter B., Li W., Gaynor R., Pestell R., Tozeren A., Byers S. W. Mechanical force modulates global gene expression and β-catenin signalling in colon cancer cells. J. Cell Sci. 2007;120:2672–2682. doi: 10.1242/jcs.03476. [DOI] [PubMed] [Google Scholar]
- 156.Whitehead J., Vignjevic D., Futterer C., Beaurepaire E., Robine S., Farge E. Mechanical factors activates β-catenin-dependent oncogene expression in APC1638N/+mouse colon. HFSP J. 2008;2:286–294. doi: 10.2976/1.2955566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fodde R., Brabletz T. Wnt/β-catenin signalling in cancer stemness and malignant behavior. Curr. Opin. Cell Biol. 2007;19:150–158. doi: 10.1016/j.ceb.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 158.Zhang S., Balch C., Chan M. W., Lai H. C, Matei D., Schilder J. M, Yan P. S., Huang T. H., Nephew K. P. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311–4320. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Barker N., Clevers H. Mining the Wnt pathway for cancer therapeutics. Nat. Rev. Drug Discovery. 2006;5:997–1014. doi: 10.1038/nrd2154. [DOI] [PubMed] [Google Scholar]
- 160.Cho N. L., Lin C. I., Whang E. E., Carothers A. M., Moore F. D., Jr, Ruan D. T. Sulindac reverses aberrant expression and localization of beta-catenin in papillary thyroid cancer cells with the BRAFV600E mutation. Thyroid. 2010;20:615–622. doi: 10.1089/thy.2009.0415. [DOI] [PubMed] [Google Scholar]
- 161.Luo J., Chen J., Deng Z. L., Luo X., Song W. X., Sharff K. A., Tang N., Haydon R. C., Luu H. H., He T. C. Wnt signalling and human diseases: what are the therapeutic implications? Lab. Invest. 2007;87:97–103. doi: 10.1038/labinvest.3700509. [DOI] [PubMed] [Google Scholar]
- 162.Takahashi-Yanaga F., Kahn M. Targeting Wnt signalling: can we safely eradicate cancer stem cells? Clin. Cancer Res. 2010;16:3153–3162. doi: 10.1158/1078-0432.CCR-09-2943. [DOI] [PubMed] [Google Scholar]
- 163.Lepourcelet M., Chen Y. N. P., France D. S., Wang H., Crews P., Petersen F., Bruseo C., Wood A. W., Shivdasani R. A. Small molecule antagonists of the oncogenic Tcf/β-catenin protein comples. Cancer Cell. 2004;5:91–102. doi: 10.1016/s1535-6108(03)00334-9. [DOI] [PubMed] [Google Scholar]
- 164.Chen Z., Venkatesan A. M., Dehnhardt C. M., Dos Santos O., Delos Santos E., Ayral-Kaloustian S., Chen L., Geng Y., Arndt K. T., Lucas J., et al. 2,4-Diamino-quinazolines as inhibitors of β-catenin/Tcf-4 pathway: potential treatment for colorectal cancer. Bioorg. Med. Chem. Lett. 2009;19:4980–4983. doi: 10.1016/j.bmcl.2009.07.070. [DOI] [PubMed] [Google Scholar]
- 165.Dehnhardt C. M., Venkatesan A. M., Chen Z., Ayral-Kaloustian S., Dos Santos O., Delos Santos E., Curran K., Follettie M. T., Diesl V., Lucas J., Geng Y., et al. Design and synthesis of novel diaminoquinazolines with in vivo efficacy for β-catenin/T-cell transcriptional factor 4 pathway inhibition. J. Med. Chem. 2010;53:897–910. doi: 10.1021/jm901370m. [DOI] [PubMed] [Google Scholar]
- 166.Huang S. M., Mishina Y. M., Liu S., Cheung A., Stegmeier F., Michaud G. A., Charlat O., Wiellette E., Zhang Y., et al. Tankyrase inhibition stabilizes Axin and antagonizes Wnt signalling. Nature. 2009;461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 167.Barbieri F., Lorenzi P., Ragni N., Schettini G., Bruzzo C., Pedulla F., Alama A. Overexpression of cyclin D1 is associated with poor survival in epithelial ovarian cancer. Oncology. 2004;66:310–315. doi: 10.1159/000078332. [DOI] [PubMed] [Google Scholar]
- 168.Dhar K. K., Branigan K., Parkes J., Howells R. E., Hand P., Musgrove C., Strange R. C., Fryer A. A., Redman C. W., Hoban P. R. Expression and subcellular localization of cyclin D1 protein in epithelial ovarian tumour cells. Br. J. Cancer. 1999;81:1174–1181. doi: 10.1038/sj.bjc.6690826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Denkert C., Kobel M., Pest S., Koch I., Berger S., Schwabe M., Siegert A., Reles A., Klosterhalfen B., Hauptmann S. Expression of cyclooxygenase 2 is an independent prognostic factor in human ovarian carcinoma. Am. J. Pathol. 2002;160:893–903. doi: 10.1016/S0002-9440(10)64912-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cowden-Dahl K. D., Symowicz J., Ning Y., Gutierrez E., Fishman D. A., Adley B. P., Stack M. S., Hudson L. G. Matrix metalloproteinase 9 is a mediator of epidermal growth factor-dependent E-cadherin loss in ovarian carcinoma cells. Cancer Res. 2008;68:4606–4613. doi: 10.1158/0008-5472.CAN-07-5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Boocock C. A., Charnock-Jones D. S., Sharkey A. M., McLaren J., Barker P. J., Wright K., Twentyman P. R., Smith S. K. Expression of vascular endothelial growth factor and its receptors flt and KDR in ovarian carcinoma. J. Natl. Cancer Inst. 1995;87:506–516. doi: 10.1093/jnci/87.7.506. [DOI] [PubMed] [Google Scholar]
- 172.Lee J. H., Kang Y. S., Kim B. G., Park S. Y., Lee E. D., Lee K. H., Park K. B. Expression of the CD44 adhesion molecule in primary and metastatic gynecologic malignancies and their cell lines. Int. J. Gynecol. Cancer. 1995;5:193–199. doi: 10.1046/j.1525-1438.1995.05030193.x. [DOI] [PubMed] [Google Scholar]
- 173.Di Renzo M. F., Olivero M., Katsaros D., Crepaldi T., Gaglia P., Zola P., Sismondi P., Comoglio P. M. Overexpression of the Met/HGF receptor in ovarian cancer. Int. J. Cancer. 1994;58:658–662. doi: 10.1002/ijc.2910580507. [DOI] [PubMed] [Google Scholar]
- 174.Watson J. V., Curling O. M., Munn C. F., Hudson C. N. Oncogene expression in ovarian cancer: a pilot study of c-myc oncoprotein in serous papillary ovarian cancer. Gynecol. Oncol. 1987;28:137–150. doi: 10.1016/0090-8258(87)90207-1. [DOI] [PubMed] [Google Scholar]
- 175.Chambers S. K., Gertz R. E., Jr, Ivins C. M., Kacinski B. M. The significance of urokinase-type plasminogen activator, its inhibitors, and its receptor in ascites of patients with epithelial ovarian cancer. Cancer. 1995;75:1627–1633. doi: 10.1002/1097-0142(19950401)75:7<1627::aid-cncr2820750712>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 176.Sier C. F., Stephens R., Bizik J., Mariani A., Bassan M., Pedersen N., Frigerio L., Ferrari A., Dano K., Brunner N., Blasi F. The level of urokinase-type plasminogen activator receptor is increased in serum of ovarian cancer patients. Cancer Res. 1998;58:1843–1849. [PubMed] [Google Scholar]
- 177.Tanimoto H., Underwood L. J., Shigemasa K., Parmley T. H., Wang Y., Yan Y., Clarke J., O'Brien T. J. The matrix metalloprotease pump-1 (MMP-7, matrilysin): a candidate marker/target for ovarian cancer detection and treatment. Tumour Biol. 1999;20:88–98. doi: 10.1159/000030051. [DOI] [PubMed] [Google Scholar]
- 178.Soini Y., Talvensaari-Mattila A. Expression of claudins 1, 4, 5, and 7 in ovarian tumors of diverse types. Int. J. Gynecol. Pathol. 2006;25:330–335. doi: 10.1097/01.pgp.0000215298.38114.cc. [DOI] [PubMed] [Google Scholar]
- 179.Yoshida H., Ishiko O., Sumi T., Matsumoto Y., Ogita S. Survivin, bcl-2 and matrix metalloproteinase-2 enhance progression of clear cell- and serous-type ovarian carcinomas. Int. J. Oncol. 2001;19:537–542. doi: 10.3892/ijo.19.3.537. [DOI] [PubMed] [Google Scholar]
- 180.Choi J. H., Park J. T., Davidson B., Morin P. J., Shih Ie M., Wang T. L. Jagged-1 and Notch3 juxtacrine loop regulates ovarian tumor growth and adhesion. Cancer Res. 2008;68:5716–5723. doi: 10.1158/0008-5472.CAN-08-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Malki S., Bibeau F., Notarnicola C., Roques S., Berta P., Poulat F., Boizet-Bonhoure B. Expression and biological role of the prostaglandin D synthase/SOX9 pathway in human ovarian cancer cells. Cancer Lett. 2007;255:182–193. doi: 10.1016/j.canlet.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 182.Ye F., Li Y., Hu Y., Zhou C., Hu Y., Chen H. Expression of Sox2 in human ovarian epithelial carcinoma. J. Cancer Res. Clin. Oncol. 137:131–136. doi: 10.1007/s00432-010-0867-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Puri R., Tousson A., Chen L., Kakar S. S. Molecular cloning of pituitary tumor transforming gene 1 from ovarian tumors and its expression in tumors. Cancer Lett. 2001;163:131–139. doi: 10.1016/s0304-3835(00)00688-1. [DOI] [PubMed] [Google Scholar]
- 184.Ripley D., Tunuguntla R., Susi L., Chegini N. Expression of matrix metalloproteinase-26 and tissue inhibitors of metalloproteinase-3 and -4 in normal ovary and ovarian carcinoma. Int. J. Gynecol. Cancer. 2006;16:1794–1800. doi: 10.1111/j.1525-1438.2006.00714.x. [DOI] [PubMed] [Google Scholar]
- 185.Blechschmidt K., Sassen S., Schmalfeldt B., Schuster T., Hofler H., Becker K. F. The E-cadherin repressor Snail is associated with lower overall survival of ovarian cancer patients. Br. J. Cancer. 2008;98:489–495. doi: 10.1038/sj.bjc.6604115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Welt C. K., Lambert-Messerlian G., Zheng W., Crowley W. F., Jr, Schneyer A. L. Presence of activin, inhibin, and follistatin in epithelial ovarian carcinoma. J. Clin. Endocrinol. Metab. 1997;82:3720–3727. doi: 10.1210/jcem.82.11.4345. [DOI] [PubMed] [Google Scholar]
- 187.Kaiser P. C., Korner M., Kappeler A., Aebi S. Retinoid receptors in ovarian cancer: expression and prognosis. Ann. Oncol. 2005;16:1477–1487. doi: 10.1093/annonc/mdi265. [DOI] [PubMed] [Google Scholar]
- 188.Mueller J., Brebeck B., Schmalfeldt B., Kuhn W., Graeff H., Hofler H. Stromelysin-3 expression in invasive ovarian carcinomas and tumours of low malignant potential. Virchows Arch. 2000;437:618–624. doi: 10.1007/s004280000261. [DOI] [PubMed] [Google Scholar]
- 189.Rask K., Nilsson A., Brannstrom M., Carlsson P., Hellberg P., Janson P. O., Hedin L., Sundfeldt K. Wnt-signalling pathway in ovarian epithelial tumours: increased expression of β-catenin and GSK3β. Br. J. Cancer. 2003;89:1298–1304. doi: 10.1038/sj.bjc.6601265. [DOI] [PMC free article] [PubMed] [Google Scholar]