Abstract
Background
The regulation of programmed cell death is critical to developmental homeostasis and normal morphogenesis of embryonic tissues. Survivin, a member of the inhibitors of apoptosis protein (IAP) family primarily expressed in embryonic cells, is both an anti-apoptosis and a pro-survival factor. Since our previous studies have demonstrated the importance of apoptosis during embryonic submandibular salivary gland (SMG) development, we postulated that survivin is a likely mediator of SMG epithelial cell survival.
Results
We investigated the developmental expression of survivin in Pseudoglandular (~ E14), Canalicular (~ E15) and Terminal Bud (~ E17) Stage SMGs. We report a significant 26% increase in transcript levels between the Canalicular and Terminal Bud Stages. Immunohistochemical studies demonstrate nuclear-localized survivin protein in epithelial cells bounding forming lumina in Canalicular and Terminal Bud Stage SMGs.
Conclusions
Survivin is known to be a pro-survival and anti-apoptotic factor. Given that survivin translocation into the nucleus is required for the induction of entry into the cell cycle and the inhibition of apoptosis, our demonstration of nuclear-localized survivin protein in presumptive ductal and proacinar lumen-bounding cells suggests that survivin may be a key mediator of embryonic SMG epithelial cell survival.
Introduction
Embryonic submandibular gland (SMG) development is best conceptualized in stages rather than gestational age [1]. Repeated branching at the distal ends of the initial epithelial SMG bud produces a bush-like structure consisting of a network of elongated epithelial branches with epithelial buds at their termini by the Pseudoglandular Stage. In the Canalicular Stage, the number of epithelial lobes has increased and the presumptive ducts begin to exhibit distinct lumina. By the Terminal Bud Stage, these branches and buds are hollowed out to form the presumptive ducts and acini. Our prior studies have indicated that lumen formation is initiated in the Canalicular Stage with the death of the central cells, with each consecutive concentric layer of cells dying until only a single layer of epithelial cells surrounds the lumina [1,2]. The demonstration of p53 concentrated to and marking the next concentric layer of epithelial cells destined for death suggests that p53/caspase3-mediated apoptosis is important to terminal bud lumen formation [2,3]. By contrast, caspase8/caspase3-mediated apoptosis is important to ductal lumen formation [3]. However, although we have identified different apoptotic pathways which mediate duct and terminal bud lumen formation, little is known about which molecule(s) mediate the apoptotic stop signal such that these lumen-bounding epithelial cells survive.
The regulation of programmed cell death is critical to developmental homeostasis and normal morphogenesis of embryonic tissues. Survivin, a member of the inhibitors of apoptosis protein (IAP) family, is unique in that it is prominently expressed in embryonic tissues, overexpressed in cancer cells, and relatively undetectable in normal adult tissues [4-7]. Gene targeting experiments indicate that survivin is both a pro-survival and an anti-apoptotic factor [5, 8]. Survivin has also been shown to be essential for mitosis during development since null embryos exhibit disrupted microtubule formation, become polyploid, and fail to survive beyond day 4.5 [9]. Survivin, expressed in a cell cycle dependent manner [9,10], has been shown in cell lines to translocate into the nucleus where it competitively binds to Cdk4/p16INK4a [11]. The resultant Cdk4/survivin complex directly or indirectly activates Cdk2/Cyclin E for S phase entry [11]. The survivin/Cdk4 complex formation also causes the release of p21 which translocates to mitochondria to form a complex with procaspase 3, which inhibits cell death [12].
Given the above, we postulated that survivin is an important pro-survival and anti-apoptotic molecule during embryonic ductal and proacinar formation. In this paper, we investigated the developmental expression of survivin transcripts and protein in embryonic SMGs. This is the first report of notable developmental changes in survivin expression and protein localization correlated with embryonic lumen formation.
Results and Discussion
Expression of Survivin Transcript
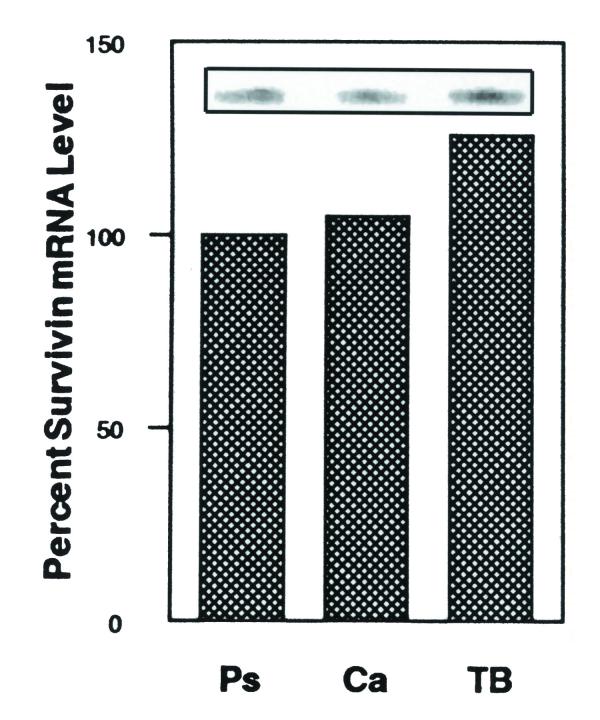
To elucidate a potential role for survivin during embryonic SMG morphogenesis, we evaluated survivin transcript levels in Pseudoglandular (~ E14), Canalicular (~ E15) and Terminal Bud (~ E17) Stage SMGs using primer extension analysis. As shown in Figure 1, survivin transcripts were detected in all stages investigated. There was no significant change in survivin mRNA levels between the Pseudoglandular and Canalicular Stages. By contrast, there was a significant ~ 26% increase (p < 0.05) in survivin transcript levels between the Canalicular and Terminal Bud Stages, this significant increase being concomitant with extensive epithelial cell apoptosis which results in lumina formation [1].
Figure 1.
Quantitative analysis of survivin trancript levels during embryonic SMG development. Inset. Primer extension analysis of Pseudoglandular (Ps), Canalicular (Ca) and Terminal Bud (TB) Stage SMGs. The relative level of survivin transcript is presented as the percent transcript level relative to that in Pseudoglandular Stage SMGs.
Determination of Antibody Specificity
Prior to determining the cell-specific distribution of survivin protein during embryonic SMG development, the specificity of the anti-survivin antibody was evaluated by determining its reactivity on Western blots of lysates of 3 cancer cell lines (MO7E, HL-60, and HELA) since cancer cell lines have been shown to overexpress survivin protein [5, 6 ]. Using the unpurified anti-survivin antiserum, survivin protein (Mr ~ 16 kDa) was detected in all cell lines but not in normal whole human blood cell lysates (Fig. 2); survivin was not detected by the control serum. After affinity purification, a single ~ 16 kDa band was detected in HL-60 human cells (Fig. 3) using this antibody; no band was seen in the affinity column eluate of the preimmune serum. Our affinity-purified antibody was then evaluated for its ability to localize survivin protein in tissue sections. Our observation of survivin immunolocalization in pulmonary epithelia (data not shown) is identical to that previously reported [4]. Based on the above results, we conclude that this anti-survivin antibody is specific for survivin protein.
Figure 2.
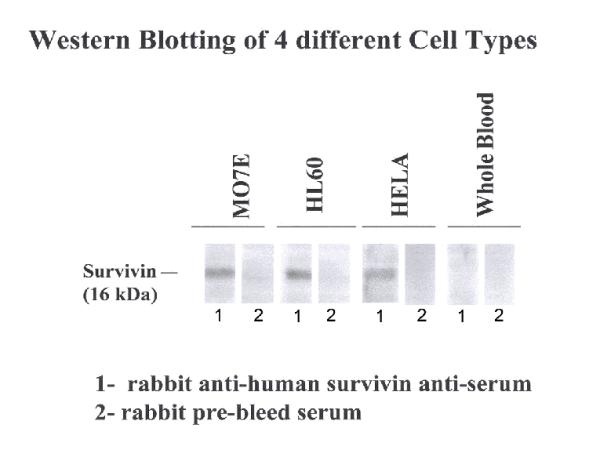
Western blot analysis using whole antiserum shows the presence of survivin protein in 3 cancer cell lines (MO7E, HL-60, and HELA). Survivin protein was not detected in whole blood lysates or control preimmune serum.
Figure 3.
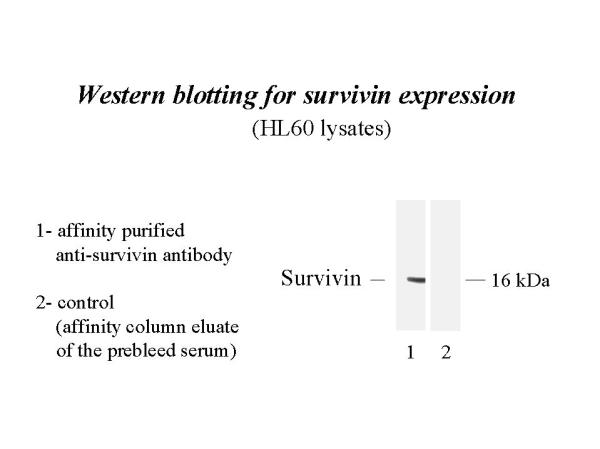
Western blot analysis for survivin expression. Using affinity-purified anti-survivin antibody, survivin protein was identified as a single ~ 16 kDa band in HL-60 lysates. No band was seen in affinity column eluate of prebleed serum.
Spatiotemporal Distribution of Survivin
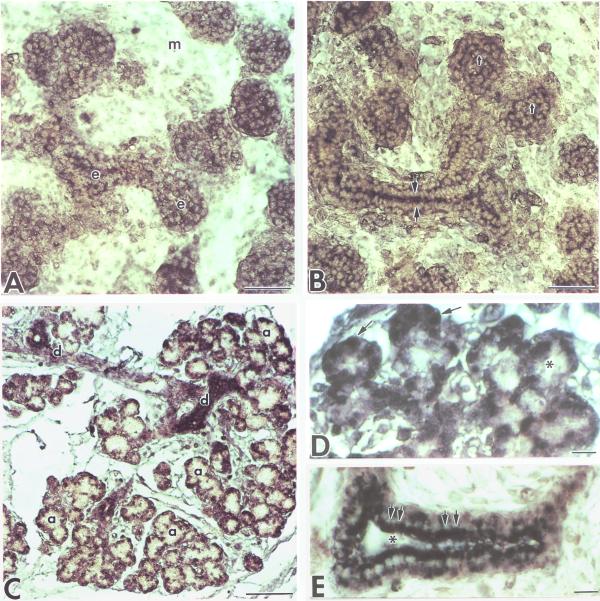
We then evaluated the pattern of survivin immunolocalization in embryonic SMGs with progressive development. In the Pseudoglandular Stage, survivin protein is diffusely distributed throughout the cytoplasm of branching epithelia and is absent from the mesenchyme (Fig. 4A). This cytoplasmic distribution of survivin protein suggests that survivin may not have translocated into the nucleus to form a survivin/Cdk4 complex at this early developmental stage. In the Canalicular Stage, epithelial cell apoptosis toward the center of presumptive ducts results in initial ductal lumen formation [1]. At the sites of lumen formation, intense survivin staining is seen in nuclei of the columnar epithelial cells bounding forming lumina (Fig. 4B). In contrast, those epithelial cells not surrounding the forming lumen do not display nuclear-localized survivin protein (Fig. 4B). As development progresses through the Terminal Bud Stage, terminal buds hollow out until only a single layer of epithelial cells surround terminal bud lumina (Fig. 4C) [1]. Nuclear-localized survivin protein is seen in these lumen-bounding terminal bud epithelial cells destined to survive (Fig. 4D), as well as in lumen-bounding ductal epithelia (Fig. 4E). Although nuclear translocation of survivin has been shown to be required for survivin-induced initiation of the cell cycle in cell lines in vitro [11], this is the first report of survivin translocation into the nuclei of embryonic cells in vivo. Cell proliferation, but not apoptosis, is seen in this single layer of terminal bud epithelial cells which is adjacent to mesenchyme and extracellular matrix [1]. This observation is consistent with the previous report that survivin is primarily found in proliferating cells [6, 8].
Figure 4.
Spatial distribution of survivin protein during embryonic SMG development. A. Survivin protein is diffusely distributed throughout the branching epithelia (e) and absent from the mesenchyme (m) in the Pseudoglandular Stage. B. Intense survivin immunostain is seen in ductal columnar epithelial cells which surround forming lumina (arrow heads) in the Canalicular Stage. By contrast, survivin protein retains its diffuse distribution in terminal bud (t) epithelia lacking lumina and in outer ductal cuboidal epithelial cells. C-E. Terminal Bud Stage SMGs exhibit distinct lumina in presumptive ducts (d) and acini (a). Note intense immunostain in the inner layer of ductal epithelia and the single layer of terminal bud epithelia which surround distinct lumina . D. Higher magnification of Terminal Bud Stage SMG exhibiting nuclear localization (arrows) of survivin protein in presumptive acini cells bounding distinct lumina (*). E. Higher magnification of a Late Terminal Bud Stage ductal epithelial bilayer which consists of an inner columnar epithelia which surround the distinct lumen and an outer cuboidal epithelia which abuts the basement membrane. Nuclear localization (double arrowheads) of survivin protein is seen in the inner layer of columnar epithelial cells surrounding the lumen (*) and not in the outer cuboidal epithelia. A-C. Bar, 50 μm. D-E, Bar, 25 μm.
Although survivin has previously been demonstrated in embryonic lung epithelia [4], this is the first report of qualitative and quantitative changes in survivin expression in any developing branching organ. Further, it is interesting to note that substantial differences in survivin protein localization exist between embryonic lungs and SMGs. Survivin protein is nuclear-localized in late embryonic SMG epithelia surrounding forming lumina (Fig. 4) whereas it remains diffusely distributed, and not nuclear-localized, in pulmonary lumen-bounding epithelia (data not shown). The likely explanation for this notable difference in these analogous branching organs is the different mechanisms of their lumina formation. The pulmonary lumen forms as an extension of the tracheal lumen into the lung bud; repeated branching of the lung bud results in an extensive epithelial tree which surrounds the pulmonary lumina [13]. Since apoptosis is not a key factor in pulmonary lumina formation, a pro-survival/anti-apoptosis signal is not needed for epithelial cell survival and, thus, the survivin pathway is not activated. By contrast, the SMG bud develops as a solid cord of epithelium, with SMG lumina being formed by the death of central epithelial cells, and subsequently each consecutive concentric layer of terminal bud epithelial cells, until a single layer of "surviving" epithelial cells surround the lumina [2]. Thus, molecule(s) mediating an apoptotic stop signal are required for these lumen-bounding epithelial cells to survive. Interestingly, Bcl-2 and NFκB, other pro-survival/anti-apoptosis factors, are absent from these Terminal Bud stage bud epithelia [3, 14]. Given that survivin translocation into the nucleus is necessary for Cdk4/survivin complex formation, induction of cell cycle entry, and inhibition of caspase 3-mediated apoptosis [11,12,15], our observation of nuclear-localized survivin protein in proacinar (Fig. 4D) and ductal epithelia (Fig. 4E) suggests that survivin may be a key factor for the survival of epithelial cells surrounding forming ductal and terminal bud lumina.
Finally, it is interesting that extensive cell proliferation, but not apoptosis, is seen in Initial Bud and Pseudoglandular Stage SMG epithelia in the absence of survivin protein. To gain insight into which molecules likely protect these early embryonic SMG cells from apoptosis, we must turn to recent gene targeting experiments [16, 17] investigating FGF-10 and its receptor, FGF-R2(IIIb). Analysis of Fgf10 null and Fgfr2(IIIb) transgenic mice indicate that FGF10/FGF-R2(IIIb) signal transduction is essential for SMG branching morphogenesis [16, 17]; SMGs are absent in E14.5 and older mice. However the presence of an initial SMG epithelial bud which subsequently undergoes cell death in the absence of FGF-R2(IIIb) signaling [16], suggests that FGF10/FGF-R2-(IIIb) signaling mediates epithelial cell survival during the Pseudoglandular Stage. What remains unclear is which factor or factors protect the initial bud epithelium from apoptosis. Further studies are needed to address this question.
Conclusions
Although apoptosis has been shown to mediate embryonic SMG ductal and proacinar lumen formation, little is known about which factor(s) mediate the anti-apoptosis/pro-survival signal. Our demonstration of a significant increase in survivin expression concomitant with SMG lumina formation, as well as survivin protein's nuclear localization in presumptive ductal and proacinar lumen-bounding cells, suggest that survivin is be a key mediator of embryonic SMG lumen-bounding epithelial cell survival.
Materials and Methods
Tissue Collection
Female B10A/SnSg mice, obtained from Jackson Laboratories (Bar Harbor, ME), were maintained and mated as previously described [1]; plug day = day 0 of gestation. Pregnant females were anesthetized on days 14-18 of gestation (E14-18) with methoxyflurane (metafane) and euthanized by cervical dislocation. This research conformed to the Animal Resource Institutional Review and Ethics Committee. Embryos were dissected in cold phosphate buffered saline (PBS) and staged according to Theiler [18]. E14-E18 SMGs and E15-E17 lungs were collected and stored at -70°C or processed for histology.
Analysis of RNA by Primer Extension
Total RNA from E14 Pseudoglandular SMGs (64 glands/3 litters); E15 Canalicular SMGs (52 glands/3 litters) and E17 Terminal Bud SMGs (48 glands/3 litters) were extracted as previously described [3]. Primer extension was conducted essentially as described in Li and Altieri [10]. A msg-PE oligonucleotide 5'-GGCTCTTTCTCTGCT CAGTTT-3' was synthesized, gel purified and 5' end-labeled; a control primer was also radiolabled. For primer extension, 40 μg total RNA per stage was hybridized with an excess of 5'-radiolabeled DNA primers. The resulting end-labeled cDNA was separated on a urea-denaturing polyacrylamide gel, visualized, and quantitated by phosphor image analysis with ImageQuant software. Relative levels of survivin mRNA were tabulated as the percent transcript level relative to that in the Pseudoglandular Stage. Subsequent analysis was by t-test for the significance of differences between percentages based on the arcsin transformation; it employs a constant representing the parametric variance of a distribution of arcsin transformations of percentages [19].
Immunohistochemistry
A rabbit polyclonal antibody was raised against a 19 aa peptide [NH2-MGAPTLPPAW OPFLKDHRI-COOH] representing amino acids 1-19 of the deduced human amino acid sequence [6]. Using anti-survivin antiserum and Western blot analysis, survivin protein (Mr ~ 16 kDa) was detected in the lysates of 3 cancer cell lines (MO7E, HL-60, and HELA), but not in normal whole human blood cell lysates; survivin was not detected by the control serum. The antibody was purified from the immune sera by affinity chromatography on a peptide column and specificity determined by Western blot analysis [3]. Extracts of the HL-60 were analyzed to demonstrate antibody specificity. No band was seen in control affinity column eluate of the prebleed serum. For immunolocalization studies, embryonic SMGs and lungs were fixed in Carnoy's fixative, processed, and immunohistochemistry conducted as previously described [3]. Immunohistochemical staining was performed using the Elite ABC kit (Vector Laboratories, Burlingham, CA).
Acknowledgments
Acknowledgements
This research was supported by NIH grant DE 11942.
Contributor Information
Tina Jaskoll, Email: tjaskoll@hsc.usc.edu.
Haiming Chen, Email: haimingc@hsc.usc.edu.
Yan Min Zhou, Email: vanminzh@hsc.usc.edu.
Dingwen Wu, Email: dingwen888@aol.com.
Michael Melnick, Email: mmelnick@hsc.usc.edu.
References
- Jaskoll T, Melnick M. Submandibular gland morphogenesis: stage-specific expression of TGF-α/EGF, IGF, TGF-β, TNF, and IL-6 signal transduction in normal embryonic mice and the phenotypic effects of TF-β2, TGF-β3 and EGF-R null mutations. Anat Rec. 1999;256:252–268. doi: 10.1002/(SICI)1097-0185(19991101)256:3<252::AID-AR5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Melnick M, Jaskoll T. Mouse submandibular gland morphogenesis: a paradigm for embryonic signal processing. Crit Rev Oral Biol Med. 2000;11:199–215. doi: 10.1177/10454411000110020401. [DOI] [PubMed] [Google Scholar]
- Melnick M, Chen H, Zhou Y, Jaskoll T. Embryonic mouse submandibular gland morphogenesis and the TNF/TNF-R1 signal transduction pathway. Anat Rec. 2001;262:318–330. doi: 10.1002/1097-0185(20010301)262:3<318::AID-AR1023>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Adida C, Crotty PL, McGrath J, Berrebi D, Altieri DC. Developmentally regulated expression of the novel cancer anti-apoptosis gene survivin in human and mouse differentiation. Am J Path. 1998;152:43–49. [PMC free article] [PubMed] [Google Scholar]
- Altieri DC, Marchisio C. Survivin Apoptosis: an interloper between cell death and cell proliferation in cancer. Lab Inv. 1999;79:1327–1333. [PubMed] [Google Scholar]
- Ambrosini G, Adida C, Altieri DC. A novel antiapoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Hatano M, Otaki M, Ogasawara T, Tokushisa T. Expression of a murine homologue of the inhibitor of apoptosis protein is related to cell proliferation. Proc Natl Acad Sci USA. 1999;96:1457–1462. doi: 10.1073/pnas.96.4.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosini G, Adida C, Siruga G, Altieri DC. Induction of apoptosis of inhibition by survivin gene targeting. J Biol Chem. 1998;273:11177–11182. doi: 10.1074/jbc.273.18.11177. [DOI] [PubMed] [Google Scholar]
- Uren AG, Wong L, Pakusch M, Fowler KJ, Burrows FJ, Vaux DL, Choo KHA. Survivin and the inner centromere protein INCENP show similar cell cycle localization and gene knockout phenotype. Current Biology. 2000;10:1319–1328. doi: 10.1016/S0960-9822(00)00769-7. [DOI] [PubMed] [Google Scholar]
- Li F, Altieri DC. The cancer antiapoptosis mouse survivin gene: characterization of locus and transcriptional requirements of basal and cell cycle-dependent expression. Canc Res. 1999;59:3143–3151. [PubMed] [Google Scholar]
- Suzuki A, Hayashida M, Ito T, Kawano H, Nakano T, Miura M, Akahane K, Shiraki K. Survivin initiates cell cycle entry by the competitive interaction with Cdk4/p16(INK4a) and Cdk2/Cyclin E complex activation. Oncogene. 2000;19:3225–3234. doi: 10.1038/sj/onc/1203665. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Ito T, Kawano H, Hayashida M, Hayasaki Y, Tsutomi Y, Akahane K, Nakano T, Miura M, Shiraki K. Survivin initiates procase3/p21 complex formation as a result of ineraction with Ckd4 to resist Fas-mediated cell death. Oncogene. 2000;19:1346–1353. doi: 10.1038/sj/onc/1203429. [DOI] [PubMed] [Google Scholar]
- Ten Have-Opbroek. Lung development in mouse embryos. Exp Lung Res. 1991;17:111–130. doi: 10.3109/01902149109064406. [DOI] [PubMed] [Google Scholar]
- Melnick M, Chen H, Zhou Y, Jaskoll T. Interleukin-6 signaling and embryonic mouse submandibular salivary gland morphogenesis. Cells, Tissues, Organs. 2001 doi: 10.1159/000047840. [DOI] [PubMed] [Google Scholar]
- Ito T, Shiraki K, Sugimoto K, Yamanaka T, Fujikawa K, Ito M, Takase K, Moriyama M, Kawamo H, Hayashida M, Nakano T, Suzuki A. Survivin promotes cell proliferation in human hepatocellular carcinoma. Hepatology. 2000;31:1080–1085. doi: 10.1053/he.2000.6496. [DOI] [PubMed] [Google Scholar]
- De Moerlooze L, Spencer-Dene B, Revest J-M, Hajihosseini M, Rosewell I, Dickson C. An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signaling during mouse organogenesis. Development. 2000;127:483–492. doi: 10.1242/dev.127.3.483. [DOI] [PubMed] [Google Scholar]
- Oheyo O, Hori Y, Yamasaki M, Harada H, Sekine K, Kato S, Itoh N. FGF10 acts as a major ligand for FGF Receptor 2 IIIb in mouse multiorgan development. Bioch Bioph Res Comm. 2000;277:643–649. doi: 10.1006/bbrc.2000.3721. [DOI] [PubMed] [Google Scholar]
- Theiler K. The House Mouse. Springer-Verlag, New York. 1989.
- Sokal RR, Rohlf FJ. Biometry. San Francisco: W H Freeman, 1969. pp. 607–608.