Abstract
C-reactive protein (CRP), a marker of inflammation, has been associated with cardiovascular disease. Risk of cardiovascular disease is increased in migraineurs with aura. Results from a clinical report, case control- and a cohort study suggest that CRP is elevated in migraineurs compared to nonmigraineurs. We examined the proposed association in a case-control study nested within two large population-based studies.
The relationship between migraine and CRP (hs-CRP) was studied in 5906 men and women age 55.0 (±8.5) years in the Reykjavik Study and 1345 men and women age 27.7 (±5.5) years from the Reykjavik Study for the Young. A modified version of the International Headache Society criteria was used to categorize people into migraineurs (2 or more symptoms) or nonmigraineurs. Migraineurs with visual or sensory symptoms were further defined as having migraine with aura (MA) or without aura (MO).
Multivariable-adjusted CRP levels were similar in migraineurs and nonmigraineurs (0.83 vs. 0.79mg/l, p=0.44) for men and (0.87 vs. 0.87mg/l, p=0.90) for women. When further stratified by migraine aura and age, no differences were found between nonmigraineurs, MO and MA among men. In women MO, CRP was borderline higher than among nonmigraineurs and MA (1.01mg/l vs. 0.81 and 0.75mg/l, p=0.08 and p=0.08) in age group 19–34 years but significantly lower in age group 60–81 years (0.52mg/l vs. 1.07 and 1.01mg/l, p=0.007 and p=0.03).
CRP levels were not increased among migraine sufferers compared to nonmigraineurs. Older women migraineurs without aura had lower CRP values compared to nonmigraineurs and migraineurs with aura.
Keywords: C-reactive protein (hs-CRP), cardiovascular disease, epidemiology, inflammation, migraine
Introduction
C-reactive protein (CRP), a marker of inflammation, has been associated with risk of cardiovascular disease (1). CRP has been suggested to be abnormal among migraineurs, possibly through repeated vascular inflammation (2). Risk of stroke and coronary heart disease is increased in migraineurs compared to others, especially for those with aura (3, 4). There are limited data on the relationship between CRP and migraine.
Welch et al. performed a retrospective review of 60 migraineurs (90% female) with complex clinical features referred to secondary or tertiary clinics (2). Results indicated that 43% of the patients had elevated CRP (defined as >3 mg/l), with a suggestion of a higher proportion in the patients without aura 16/29 (55%) compared to the patients with aura 10/31 (32%).
A case-control study of 50 young adult patients with migraine and 50 controls (78% female) showed median CRP level of the migraineurs was 1.42 mg/l and 0.90 mg/l in controls (p=0.03) (5). CRP was higher in the patients without aura compared to controls, 2.11 mg/l vs. 0.90 mg/l (p=0.0002).
Compared to women with no migraine history, women with self reported history of migraine had modestly increased multivariable-adjusted odds ratio for elevated CRP in a large cohort study of female health professionals age 45 and older (1.13, 95% CI 1.05, 1.22) (6). Among current migraineurs, age-adjusted CRP was higher in the women without aura compared to women with aura (4.08 vs. 3.86 mg/l).
Thus, results from these three studies, a clinical report, a case control study and one large cohort study of female health professionals, suggest that CRP is modestly elevated in migraineurs compared to controls. Results further suggest that the elevation is more evident for migraineurs without aura compared to those with aura.
In this paper we further examine this potential relationship between CRP and migraine with and without aura in a case-control study nested within two large population-based cohorts of women and men ranging in age from 19 to 81 years.
Methods
Reykjavik Study briefly
The Reykjavik Study is a large, population-based cohort study, which started in 1967 (7). Men born 1907–1934 and women born 1908–1935, residing in the Reykjavik area and a few adjacent communities on 1 December 1966, were randomly selected for participation. A total of 19 390 persons agreed to take part, which was approximately 70% of all those invited. The study cohort, which was divided into six groups of men and women, was investigated at the Heart Preventive Clinic in Reykjavik during the period 1967–1996 (CRP was not measured in the sixth group n=478, and therefore this group was omitted from the analysis). The first examination of each person occurred between 1967 and 1993 for men and between 1968 and 1996 for women. The age range at the time of CRP measurement was 33 to 81. The average year of examination used was 1974 SD 5.7 years (1967 to 1991).
In order to study subjects younger than the participants of the Reykjavik Study, a new sample was selected in 1972, the Reykjavik Study for the young (8). This group was comprised of equal groups of men and women, 2781 in all, born 1940–1954. The subjects were invited to be examined three times in the years 1973–1974, 1983–1985 and in 2001–2003, those who participated in the third stage and had participated in either of the first two stages were sampled and the first visit was used. The age range of the Reykjavik Study for the Young at the time of CRP measurement was 19 to 45. The present analysis is based on the CRP measured at the first examination. The number of subjects that were examined at least once was 1037 men and 1109 women. The average year of examination used was 1975 SD 3.8 years.
Combining the subjects from the Reykjavik Study and the study for the young, the total number of men was 10 171 and of women 10 878, and their ages at the time of measurement were 19–81 years.
Every participant received an invitation letter that included standardized questions about health and social factors, including questions related to the presence of headache and headache features (see below).
The present analysis of the Reykjavik Study is based on a subset of participants who were selected for a nested case-control study of CRP and myocardial infarction (9). Cases were 2400 subjects without a history of myocardial infarction at entry into the study who had a major coronary event during mean (±SD) follow-up of 17.5±8.7 years, as compared with 20.6±8.2 years among controls for the Reykjavik cohort. The controls were frequency-matched to the cases with respect to year of recruitment, sex and age in five-year increments (9), in all 5906 subjects from the Reykjavik Study were used in current analysis. For the Reykjavik Study for the Young all cases (n=18) and controls (n=1327) with available CRP measurements were used, they had a mean follow-up of 20.9±6.6 years for cases and 28.2±3.8 years for controls.
Every participant received an invitation letter that included standardized questions about health and social factors, including questions related to the presence of headache and headache features (see below).
Examinations
Participants came in a fasting state to the clinic. After a 5-min rest, the supine blood pressure was measured, on two occasions, between 08.30 and 10.30 h, by a nurse, and 10–14 days later between 11.00 and 13.30 h, by a physician. Subjects were not instructed to be fasting at the second blood pressure measurement. The instruments used were mercury sphygmomanometers of the type ‘Erkameter’ wall-model (Erka, Germany). The cuffs had a rubber bladder 15–32 cm, and the total length of the cuff was 66 cm. The same types of cuffs and instruments were used throughout the study. Blood pressure was measured according to World Health Organization recommendations (10). Major coronary heart disease was defined as: death from coronary heart disease and nonfatal myocardial infarction. Deaths from coronary heart disease were ascertained from central registers on the basis of a death certificate listing an International Classification of Diseases code of 410 through 414, and the diagnosis of nonfatal myocardial infarction was based on the criteria of the Monitoring Trends and Determinants in Cardiovascular Disease study.
CRP measurements
Blood was drawn at the first visit, when subjects came in fasting. Concentrations of C-reactive protein (high sensitivity CRP, hs-CRP, referred to as CRP in this article) were measured as described (9) by latexenhanced immunoturbidimetry, with a lower limit of detection of 0.02 mg/l (Roche Diagnostics). The variation in CRP values within runs was less than 1 percent, and the between-day variability was 1 percent at a concentration of 14 mg/l and 3.7 percent at a concentration of 3.8 mg/l (9). CRP measurements of samples in the Reykjavik Study and the Reykjavik Study for the Young were identical except for the recording of the CRP values, the former recorded with two decimals and the latter with one decimal. We excluded subjects with CRP above 10 mg/l (n=313) because values above this cut-point are usually associated with acute phase stimulus (11) such as bacterial infection (12).
Definition of migraine
In this study a modified version (8) of the 1988 International Headache Society (IHS) criteria was used (13).
The questions on headache in the study questionnaire were the same for the original and younger Reykjavik cohorts. The questions are as follows.
Questions concerning symptoms during the last 12 months:
Do you get headache once or more per month?
If YES, please answer the following questions.
Is the pain usually on one side of the head?
Do you feel nauseated or vomit when you get the headache?
Do you get visual disturbances simultaneously or shortly before the pain starts?
Do you get photophobia during the headache attack?
Do you get numbness in one side of the face or numbness in either arm before the headache begins?
Subjects were considered to have migraine by “relaxed criteria”, if they answered yes to any two or more of questions 1–5 (8). Subjects were considered to have migraine “strict criteria” if they answered yes to any three or more of questions 1–5 (8). Migraineurs “relaxed criteria” with visual or sensory symptoms (questions 3 and 5) accompanied by other symptoms were further defined as having migraine with aura (MA) those without visual or sensory symptoms were defined as migraineurs without aura (MO).
The questionnaire of the present study was composed in 1967. No questions were asked about duration, intensity, phonophobia and pulsating quality of the headache which are part of the IHS criteria from 1988 (13). Also missing are questions about the less common symptoms of unilateral weakness and speech difficulty.
Analysis
We compared average CRP between subjects with migraine (with or without aura) and those without migraine using linear regression. As the distribution of CRP was log-normal, we transformed CRP in all linear regression models.
All regression analyses were performed separately for men and women and performed separately within each age category (four age categories, 19–34, 35–49, 50–59 and 60–81 years). Adjustments were made for case-control status, age, body-mass index (calculated as weight in kilograms divided by height in meters squared, BMI), cholesterol, smoking status (never smoked, former smoker, current smoker), education (elementary school or less, high school education, junior college or university education), current hormone use, current diabetes mellitus, SBP and antihypertensive therapy. Systolic blood pressure (SBP) and antihypertensive use were in three categories: a) SBP<130 mmHg, b) SBP between 130 and 160 mmHg, c) SBP≥160 mmHg and/ or antihypertiensive use. For women adjustment was made for oral contraceptive use, which has been shown to be associated with CRP levels (14). Adjustment for physical exercise (defined: 0, 0–5 and ≥6 hours per week) was made but the variables listed above were stronger predictors of CRP levels in the regression model and therefore physical exercise was left out of the final model. In addition we tested for cohort difference in association of CRP to migraine. The age group represented both in the Reykjavik Study and in the Reykjavik Study for the Young was used to estimate a possible cohort effect. A linear regression model with log transformed CRP as a function of migraine status was used. In a multivariable-adjusted model for men age 30 to 39, (n=139) in the Reykjavik Study and (n=186) in the Reykjavik Study for the Young, cohort was not a significant variable (p=0.30). Similarly for women, (n=84) for the Reykjavik Study and (n=62) for the Reykjavik Study for the Young, cohort was not a significant variable (p=0.76) in the model.
The above analysis was also performed for subjects that were not diagnosed with major coronary event during follow-up.
Significance testing was two-sided and based on a 5% probability level. Thus, results are presented with 95% confidence intervals (CI). The software package used was STATA version 9.
Results
Migraine prevalence
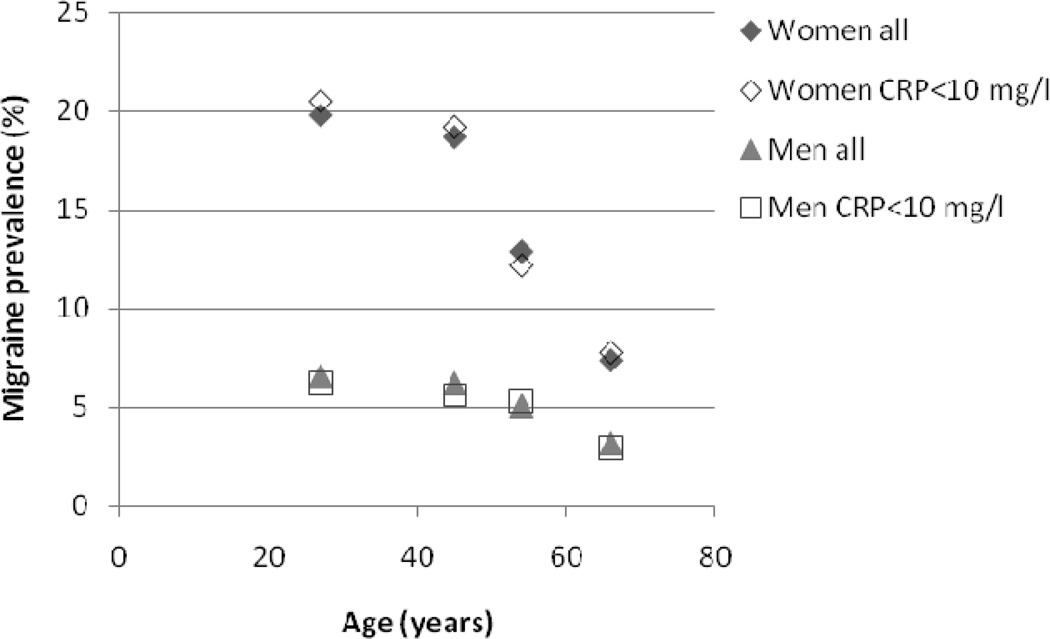
Table 1 shows characteristics of the study population, consisting of the subset of the Reykjavik Study and the Reykjavik Study for the Young participants with available CRP measurements. The crude one-year prevalence of migraine (relaxed criteria) was 5.0% among men and 14.3% among women. This prevalence was similar to the prevalence of migraine in the whole cohort (5.2% men, 14.1% women, figure 1), (8). The prevalence of MA was 3.6% for men and 8.8% for women (see table 1).
Table 1.
Characteristics for subjects with CRP measurements in the Reykjavik Study (n=5906) and the Reykjavik Study for the Young (n=1345) according to migraine† status.
| Men young cohort |
Men Reykjavik Study |
Women young cohort |
Women Reykjavik Study |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No Migraine |
MO |
MA |
No Migraine |
MO |
MA |
No Migraine |
MO |
MA |
No Migraine |
MO |
MA |
|||||||||||||
| Age, years (mean, SD) | 27.8 | 5.5 | 27.2 | 4.6 | 28.6 | 6.1 | 54.1 | 8.2 | 53.0 | 6.9 | 51.6 | 7.7 | 27.6 | 5.6 | 26.8 | 5.1 | 29.0 | 5.1 | 58.0 | 8.8 | 54.1 | 8.4 | 54.9 | 8.3 |
| BMI, kg/m2 (mean, SD) | 23.1 | 2.8 | 23.9 | 3.5 | 23.5 | 2.5 | 25.8 | 3.5 | 25.3 | 3.0 | 25.3 | 3.1 | 21.9 | 2.8 | 21.3 | 2.9 | 22.1 | 4.0 | 25.2 | 4.4 | 25.0 | 4.6 | 25.0 | 4.1 |
| Cholesterol, mmol/l (mean, SD) | 5.3 | 1.0 | 5.4 | 1.1 | 5.4 | 1.1 | 6.5 | 1.1 | 6.2 | 0.9 | 6.5 | 1.2 | 5.2 | 0.9 | 5.3 | 0.9 | 5.1 | 0.8 | 7.0 | 1.3 | 6.6 | 1.0 | 7.0 | 1.2 |
| Diabetes (n, %) | 1 | 0.2 | 0 | 0 | 0 | 0 | 185 | 4.5 | 3 | 6 | 5.0 | 3.2 | 0 | 0 | 0 | 0 | 0 | 0 | 74 | 4.8 | 0 | 0 | 8 | 5.5 |
| CRP, mg/l (geometric mean, SD)* | 0.59 | 2.77 | 0.54 | 3.38 | 0.69 | 3.01 | 1.31 | 2.65 | 0.98 | 2.40 | 1.23 | 2.81 | 0.78 | 2.91 | 0.90 | 2.94 | 0.75 | 2.78 | 1.22 | 2.77 | 0.81 | 3.09 | 1.19 | 2.79 |
| Participants, (n, %) | 574 | 100 | 16 | 100 | 25 | 100 | 3977 | 100 | 50 | 100 | 150 | 100 | 584 | 100 | 71 | 100 | 75 | 100 | 1518 | 100 | 65 | 100 | 146 | 100 |
| Headache | 21.3 | 100 | 100 | 16.7 | 100 | 100 | 35.8 | 100 | 100 | 25.4 | 100 | 100 | ||||||||||||
| Pain, unilateral | 6.3 | 87.5 | 56.0 | 4.7 | 84.0 | 63.0 | 9.2 | 77.5 | 69.3 | 6.2 | 80 | 70.5 | ||||||||||||
| Nausea/vomiting | 0.5 | 50.0 | 32.0 | 0.8 | 66.0 | 37.0 | 1.9 | 53.5 | 57.3 | 2.4 | 76.9 | 62.3 | ||||||||||||
| Photophobia | 1.9 | 87.5 | 76.0 | 0.8 | 66.0 | 53.9 | 5.1 | 87.3 | 69.3 | 1.3 | 58.5 | 60.3 | ||||||||||||
| Visual symptoms | 0.7 | 0 | 96.0 | 1.1 | 0 | 78.6 | 1.7 | 0 | 85.3 | 1.3 | 0 | 83.6 | ||||||||||||
| Sensory symptoms | 0 | 0 | 12.0 | 0.2 | 0 | 36.4 | 0.2 | 0 | 41.3 | 0.1 | 0 | 45.9 | ||||||||||||
| Elementary school or less education | 17.8 | 6.3 | 24.0 | 36.8 | 46.0 | 48.7 | 18.2 | 19.7 | 18.7 | 65.4 | 50.8 | 68.5 | ||||||||||||
| Hypertension treatment | 0.3 | 0 | 0 | 7.5 | 10.0 | 8.4 | 0.2 | 0 | 0.0 | 15.6 | 13.8 | 13.7 | ||||||||||||
| Current smoking | 57.1 | 50.0 | 68.0 | 56.1 | 50.0 | 57.8 | 46.6 | 56.3 | 46.7 | 45.7 | 41.5 | 49.3 | ||||||||||||
| Former smoking | 13.8 | 31.3 | 24.0 | 23.3 | 22.0 | 23.4 | 12.7 | 12.7 | 16.0 | 14.3 | 16.9 | 15.1 | ||||||||||||
| Medical hormone use | 0 | 0 | 0 | 0.3 | 2.0 | 1.3 | 1.0 | 0 | 0 | 4.4 | 4.6 | 11.0 | ||||||||||||
| Contraceptive use | - | - | - | - | - | - | 27.7 | 28.2 | 26.7 | 1.1 | 0 | 0.7 | ||||||||||||
Values are percentages unless otherwise indicated.
Subjects with CRP ≥10 mg/l were excluded.
Migraine, relaxed criteria defined answering yes to 2 or more out of 5 questions on migraine.
Figure 1.
Comparing the age and sex-specific 1-year prevalence of migraine between those with CRP measurements (n=7251) used in current study and the entire cohort (n=21049) of the Reykjavik Study and the Reykjavik Study for the Young
CRP values
After excluding subjects with CRP above 10 mg/l (n=313), median CRP value for all other subjects of the Reykjavik Study (n=5906) was 1.31 mg/l (25th and 75th percentile 0.63 and 2.68 mg/l) and the corresponding median CRP value for the subjects (n=1345) of the Reykjavik Study for the Young was 0.6 mg/l (25th and 75th percentile 0.3 and 1.4 mg/l).
Age-adjusted and multivariable-adjusted CRP values for nonmigraineurs, migraineurs, migraineurs without aura (MO) and migraineurs with aura (MA) are shown in Table 2. Age-adjusted values among men were borderline lower for MO subjects compared to nonmigraineurs and values for MA subjects were borderline higher than that of MO subjects. These differences were attenuated with multivariable adjustment. For women there were no statistically significant differences between the groups. Multivariate-adjusted values were similar among groups, ranging from 0.70 mg/l to 0.83 mg/l for men and 0.85 mg/l to 0.87 mg/l for women.
Table 2.
Age- and multivariable-adjusted CRP values (mg/l) with respect to migraine status relaxed criteria‡ and gender
| No Migraine |
Migraine |
P vs. No Migraine |
MO |
P vs. No Migraine |
MA |
P MA vs. MO |
|
|---|---|---|---|---|---|---|---|
| Men | n=4551 | n=241 | n=66 | n=175 | |||
| Age adjusted model* | 1.31 (1.27–1.35) | 1.24 (1.09–1.40) | 0.38 | 1.03 (0.81–1.31) | 0.05 | 1.32 (1.14–1.53) | 0.07 |
| Multivariate adjusted model† | 0.83 (0.77–0.90) | 0.79 (0.69–0.91) | 0.44 | 0.70 (0.54–0.89) | 0.14 | 0.83 (0.71–0.97) | 0.21 |
| Women | n=2102 | n=357 | n=136 | n=221 | |||
| Age adjusted model* | 1.17 (1.12–1.23) | 1.14 (1.02–1.27) | 0.63 | 1.09 (0.91–1.30) | 0.42 | 1.17 (1.02–1.34) | 0.51 |
| Multivariate adjusted model† | 0.87 (0.79–0.97) | 0.87 (0.75–0.99) | 0.90 | 0.85 (0.70–1.02) | 0.75 | 0.87 (0.75–1.03) | 0.75 |
MO: Migraine without aura, MA: Migraine with aura
Subjects in the Reykjavik Study and the Reykjavik Study for the Young with available CRP measurements. CRP values ≥10 mg/l excluded.
CRP and migraine status in a linear regression model adjusted for case-control status, age, BMI, cholesterol, smoking, education, hormone use, diabetes mellitus, SBP and antihypertensive therapy. For women adjustment was also made for birth control use. Profile used in multivariable adjusted model was: control with average values for continuous variables, non-smoker, high school education, SBP between 130 and 160 mmHg, without: diabetes, hormone use and antihypertensive therapy. Women without birth control use.
All comparisons between MA vs. No Migraine gave p-values >0.8
Migraine, relaxed criteria defined answering yes to 2 or more out of 5 questions on migraine.
Table 3 shows similar comparison as in table 2 but with stricter migraine criteria. The CRP values are almost identical for migraine defined with strict and relaxed criteria.
Table 3.
Age- and multivariable-adjusted CRP values (mg/l) with respect to migraine status and gender using strict migraine criteria‡
| No Migraine | Migraine‡ | P | |
|---|---|---|---|
| Men | n=4551 | n=92 | |
| Age adjusted model* | 1.30 (1.27–1.35) | 1.23 (1.01–1.50) | 0.58 |
| Multivariable-adjusted model† | 0.83 (0.77–0.90) | 0.79 (0.64–0.97) | 0.59 |
| Women | n=2102 | n=180 | |
| Age adjusted model* | 1.16 (1.11–1.22) | 1.09 (0.94–1.27) | 0.43 |
| Multivariable-adjusted model† | 0.87 (0.79–0.97) | 0.87 (0.73–1.03) | 0.95 |
Subjects in the Reykjavik Study and the Reykjavik Study for the Young with available CRP measurements. CRP values ≥10 mg/l excluded.
CRP and migraine status in a linear regression model adjusted for case-control status, age, BMI, cholesterol, smoking, education, hormone use, diabetes mellitus, SBP and antihypertensive therapy. For women adjustment was also made for birth control use. Profile used in multivariable adjusted model was: control with average values for continuous variables, non-smoker, high school education, SBP between 130 and 160 mmHg, without: diabetes, hormone use and antihypertensive therapy. Women without birth control use.
Migraine, strict criteria answering yes to 3 or more out of 5 questions on migraine.
Table 4 shows age and multivariate-adjusted CRP levels by age. Adjusted CRP levels increased gradually with age. In men CRP levels were consistently lower for MO subjects compared to nonmigraineurs and MA but the difference was not significant. In women nonmigraineurs and women with migraine and aura there was gradual increase in CRP levels with age. Women with migraine without aura had higher multivariable-adjusted CRP values in the young cohort, age group 19 to 34 years, (1.01 mg/l for MO vs. 0.81 mg/l for nonmigraineurs p=0.08), but with increasing age the CRP levels for women migraineurs with no aura gradually decreased and by age 60 to 81 CRP levels were significantly lower compared to migraineurs with aura and nonmigraineurs (0.52 mg/l MO vs. 1.01 mg/l MA, p=0.029; 0.52 mg/l MO vs. 1.07 mg/l control, p=0.007).
Table 4.
Age- and multivariable-adjusted CRP values and migraine status relaxed criteria for men (i) and women (ii) The Reykjavik Study and Reykjavik Study for the Young
| i) Men | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort | Average age and range |
Migraine status |
n | Age adjusted mg/l crp (95% CI) |
Migr. vs. No Migr. |
MO vs. MA |
MO vs. No Migr. |
Multivariable-adjusted mg/l crp (95% CI) |
Migr. vs. No Migr. |
MO vs. MA |
MO vs. No Migr. |
| Reykjavik | 27 | No Migr. | 513 | 0.57 (0.52, 0.63) | 0.61 (0.49, 0.76) | ||||||
| young | 19–34 | Migraine | 34 | 0.66 (0.46, 0.93) | P=0.47 | 0.68 (0.45, 1.01) | P=0.55 | ||||
| MO | 13 | 0.50 (0.28, 0.89) | P=0.24 | P=0.64 | 0.51 (0.28, 0.92) | P=0.19 | P=0.51 | ||||
| MA | 21 | 0.77 (0.49, 1.21) | 0.82 (0.50, 1.35) | ||||||||
| Reykjavik | 44 | No Migr. | 1168 | 1.14 (1.08, 1.21) | 0.71 (0.61, 0.83) | ||||||
| 35–49 | Migraine | 66 | 1.09 (0.87, 1.38) | P=0.72 | 0.67 (0.51, 0.88) | P=0.63 | |||||
| MO | 11 | 1.01 (0.56, 1.82) | P=0.77 | P=0.68 | 0.61 (0.34, 1.09) | P=0.70 | P=0.58 | ||||
| MA | 55 | 1.11 (0.86, 1.44) | 0.72 (0.56, 0.92) | ||||||||
| Reykjavik | 54 | No Migr. | 1856 | 1.30 (1.25, 1.36) | 0.74 (0.66, 0.84) | ||||||
| 50–59 | Migraine | 105 | 1.12 (0.93, 1.35) | P=0.12 | 0.69 (0.56, 0.84) | P=0.26 | |||||
| MO | 32 | 0.93 (0.66, 1.32) | P=0.20 | 0.06 | 0.62 (0.44, 0.87) | P=0.44 | P=0.26 | ||||
| MA | 73 | 1.21 (0.97, 1.52) | 0.72 (0.56, 0.92) | ||||||||
| Reykjavik | 66 | No Migr. | 924 | 1.56 (1.47, 1.66) | 1.16 (1.00, 1.36) | ||||||
| 60–79 | Migraine | 27 | 1.61 (1.14, 2.28) | P=0.86 | 1.26 (0.88, 1.82) | P=0.64 | |||||
| MO | 7 | 1.16 (0.57, 2.39) | P=0.30 | P=0.42 | 0.93 (0.45, 1.90) | P=0.26 | P=0.55 | ||||
| MA | 20 | 1.78 (1.19, 2.68) | 1.50 (0.97, 2.31) | ||||||||
| ii) Women | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort | Average age and range |
Migraine status |
n | Age adjusted mg/l crp (95% CI) |
Migr. vs. No Migr. |
MO vs. MA |
MO vs. No Migr. |
Multivariable-adjusted mg/l crp (95% CI) |
Migr. vs. No Migr. |
MO vs. MA |
MO vs. No Migr. |
| Reykjavik | 27 | No Migr. | 530 | 0.78 (0.71, 0.86) | 0.81 (0.65, 1.01) | ||||||
| young | 19–34 | Migraine | 137 | 0.80 (0.67, 0.96) | P=0.83 | 0.87 (0.67, 1.13) | P=0.44 | ||||
| MO | 69 | 0.92 (0.71, 1.19) | P=0.13 | P=0.24 | 1.01 (0.74, 1.37) | P=0.08 | P=0.08 | ||||
| MA | 68 | 0.69 (0.53, 0.90) | 0.75 (0.55, 1.02) | ||||||||
| Reykjavik | 44 | No Migr. | 236 | 0.94 (0.80, 1.09) | 0.67 (0.50, 0.90) | ||||||
| 35–49 | Migraine | 60 | 0.81 (0.61, 1.07) | P=0.35 | 0.66 (0.46, 0.94) | P=0.96 | |||||
| MO | 21 | 0.72 (0.44, 1.16) | P=0.54 | P=0.24 | 0.60 (0.36, 0.98) | P=0.56 | P=0.63 | ||||
| MA | 39 | 0.86 (0.60, 1.22) | 0.70 (0.46, 1.07) | ||||||||
| Reykjavik | 54 | No Migr. | 716 | 1.09 (1.01, 1.18) | 0.73 (0.62, 0.87) | ||||||
| 50–59 | Migraine | 101 | 1.11 (0.91, 1.36) | P=0.86 | 0.78 (0.61, 0.99) | P=0.54 | |||||
| MO | 32 | 0.86 (0.60, 1.24) | P=0.09 | P=0.21 | 0.66 (0.46, 0.96) | P=0.24 | P=0.55 | ||||
| MA | 69 | 1.25 (0.98, 1.61) | 0.85 (0.63, 1.13) | ||||||||
| Reykjavik | 66 | No Migr. | 564 | 1.52 (1.40, 1.64) | 1.07 (0.87, 1.32) | ||||||
| 60–81 | Migraine | 50 | 1.32 (1.02, 1.71) | P=0.31 | 0.86 (0.63, 1.18) | P=0.11 | |||||
| MO | 12 | 0.87 (0.51, 1.49) | P=0.07 | P=0.04 | 0.52 (0.30, 0.91) | P=0.03 | P=0.007 | ||||
| MA | 38 | 1.51 (1.12, 2.04) | 1.01 (0.71, 1.43) | ||||||||
Migraine relaxed criteria defined answering yes to 2 or more out of 5 questions on migraine. Migr.: Migraine, MO: Migraine no aura, MA: Migraine and aura.
CRP, migraine status, age and gender in the Reykjavik Study and the Reykjavik Study for the Young using linear regression adjusting for case-control status, age, BMI, cholesterol, smoking, education, hormone use, diabetes mellitus, SBP and antihypertensive use. Each age category was analysed separately and subject with CRP values≥10 mg/l were excluded. Profile used in multivariable adjusted model was: control with average values for continuous variables, non-smoker, high school education, SBP between 130 and 160 mmHg, without: diabetes, hormone use and antihypertensive therapy. Women without birth control use.
Results with and without subjects that developed coronary event during follow-up were similar. The difference in multivariable-adjusted CRP values after excluding those who later were diagnosed with MI ranged from being zero to 0.15 mg/l lower (0% to 19% decrease) for men and from being 0.14 mg/l higher to 0.05 mg/l lower (23% increase to 8% decrease) for women (data not shown).
Using 3 mg/l as a cutoff for elevated CRP levels and excluding subjects with history of myocardial infarction, oral contraceptives and other medical hormone use, the proportion of men and women with elevated CRP was similar among subjects with and without migraine, 19.3% and 20.0% for men and 14.1% and 16.6% for women. When stratifying by migraine aura, the proportion of nonmigraineurs, MO and MA with elevated CRP were 20.0%, 13.8% and 21.4% among men (p=0.43, logistic regression with age adjustment) and 16.6%, 9.7% and 16.8% for women (p=0.43).
Discussion
In this nested case-control study, age specific analysis of serum CRP levels was performed in subjects from two large cohort studies. Differences in CRP values between migraineurs and those without migraine were not significant, regardless of using linear regression with log-transformed CRP levels as a continuous variable or logistic regression with CRP as a binary variable. There was a moderate negative association between CRP levels and age among women with migraine without aura.
Inflammatory mechanisms have been involved in recent years and conceptualized in the neurogenic inflammation theory postulated by Moskowitz and co-workers (15). This theory accounts for the clinical efficacy of NSAID’s and other anti-inflammatory drugs in aborting migraine attacks. Furthermore, inflammation being an important factor in atherogenesis and atherothrombosis, the association of migraine, especially migraine with aura, with stroke could be based on vascular inflammation as a link. The inference that inflammation is an important component in subsets of migraineurs has been further supported by the recent findings that the inflammatory marker CRP may be elevated in migraineurs (2, 5, 6). However, in all of those studies, the elevation of CRP was modest at most and restricted to migraineurs without aura, a group that has not been generally found to be at increased risk of stroke (3, 16, 17). The principal finding of the present study is that CRP levels were not increased among migraine sufferers compared to nonmigraineurs. None the less, certain subtleties require further consideration.
The multivariable-adjusted values in the Reykjavik Study were fairly low because they reflect subjects with a low cardiovascular disease risk profile. Age adjusted CRP levels among men with migraine without aura were borderline lower than that of nonmigraineurs and also borderline lower than that of migraineurs with aura but after multivariate adjustment the differences between these groups were smaller and not statistically significant. CRP levels among migraineurs with aura and nonmigraineurs were similar in all age categories both for men and women.
The association between migraine status, CRP and age seemed to be more complex in women than in men. Women with migraine without aura had slightly higher multivariable-adjusted CRP values in age group 19 to 34 years. With increasing age the CRP level for women without aura fell and became significantly lower than that of nonmigraineurs in age group 60 to 81 years. Results for women in age group 19 to 34 years are similar to those published by Vanmolkot et al. (5). In their study 78% of the participants were women and the mean age was 25 years. The median CRP of those with migraine without aura was 2.11 mg/l compared to 0.90 mg/l in controls, p=0.0002. In a cohort study of women (mean age 55 years) multivariable-adjusted prevalence odds ratio, of having CRP >4.2 mg/l was 1.14 (95% CI: 1.02–1.27) for active migraine without aura and 1.10 (95% CI: 0.97–1.26) for active migraine with aura vs. 1.00 for no history of migraine as control (6). This cohort study also showed a slight positive association between attack frequency and odds of having elevated CRP (6). While it is clearly established that the prevalence of migraine is lower in older adults compared to middle-aged adults, it is not clear whether average attack frequency decreases with age. For example, Bigal et al showed that proportion of migraineurs with 10 to 14 headach days per month increased with age and the attacks were less typical in elderly individuals (18) and Prencipe et al. show similar attack frequency with increasing age among elderly migraineurs (19). In light of current knowledge on changes in attack frequency with age it is not evident what effect it could have on CRP values among different age groups of migraineurs. Headache frequency was not estimated in the Reykjavik Study so this question can not be addressed.
In the present study, although not statistically significant, multivariable-adjusted CRP levels were generally higher in migraineurs with aura compared to migraineurs without aura and contradict the results in the retrospective study of Welch and co-workers (2), the case control study of Vanmolkot et al. (5) and the cohort study of Kurth et al. (6).
The data in the current analysis from the Reykjavik Study was selected on the basis of availability of CRP measurements. The CRP measurements were part of a nested case-control study, thus raising the possibility of selection bias. However, we note that the age-specific prevalence of migraine in the sub-cohort used was almost identical to the prevalence within the entire cohort which was randomly selected. This suggests that selection bias with regard to the presence of migraine is not more prevalent in the sub-cohort than in the entire cohort of over twenty one thousand subjects (8). Due to the selection criteria there is probably an over-representation of cases with pre-existing coronary heart disease in current study. When those who did not get major coronary event during follow-up were analyzed separately CRP values were similar to the ones when cases were included. This indicates that the association between CRP values and migraine status is not different among subjects with coronary heart disease.
The questions in our study covered the most common migraine symptoms but did not include all of those identified in the 1988 IHS criteria (13). This is a cross sectional analysis and cannot account for within-individual changes in CRP values. Furthermore, cross sectional analysis cannot be used to determine any temporal relationship between CRP levels and onset of migraine. The subjects entered the study in the years 1967 to 1991 which results in a long period of time during which samples were taken and analysed. However, a study on CRP and cardiovascular disease in subjects from the Reykjavik Study showed that decade-to-decade consistency of CRP values was good (9). Alcohol use was not measured in current study. Migraineurs have been reported to be less likely to consume alcohol than nonmigraineurs (20) and alcohol is associated with decrease in CRP levels (21, 22). Therefore, alcohol use is a potential confounder that cannot be adjusted for in current study.
In the present study, contraceptive use among women resulted in two to three fold increase in CRP values (data not shown). Frölich et al. found that CRP level among 844 women, in the MONICA Augsburg survey, aged 25 to 44 years was 0.81 mg/l among non users of oral contraceptives and 2.59 mg/l among oral contraceptive users (14). Our results for women without migraine are similar when multivariable adjustment (including oral contraceptive use) was applied (tables 2 and 4).
In present study, migraine aura symptoms were more prevalent compared to migraine without aura symptoms than expected (table 4). Prior results suggest the proportion of migraineurs with aura symptoms to be 31% (20). A population based study in The Netherlands (GEM Study) of 6491 adults age 20 to 65 reported 1-year prevalence of migraine to be 25% for women and 7.5% for men (23). By combining MA and migraineurs both with and without aura in the GEM Study the prevalence of MA, MO and unspecified migraine would be 7.5%, 16% and 1.3% respectively for women and 2.3%, 4.8%, and 0.4% for men. In present study the prevalence of MA and MO for women was 8.8% and 5.5%, prevalence for men was 3.6% and 1.3%. Comparing the prevalence between the Reykjavik Study and the GEM study suggests that the prevalence of MA in the Reykjavik study is consistent with prior results, but the prevalence of MO may be underrepresented. One possible explanation could be recall bias when asked about headache symptoms, especially among milder migraine cases. This has been postulated by Liew G et al. where a higher lifetime prevalence of MA was found compared to MO among migraineurs in a population base study of older men and women (24).
There is also potential misclassification of MO subjects as MA subjects, as some of MO subjects experience non-specific visual disturbances during headache and the questions used do not distinguish between visual disturbances during and before the pain starts. The effect of this misclassification might have been to attenuate or obscure possible differences in CRP levels between migraineurs with and without aura.
The questions on migraine symptoms in the Reykjavik Study did not allow for identification of subjects with cluster headache and chronic paroxysmal hemicranias and these subjects were most likely included with the migraineurs. Two studies by Remahl et al. did not show a difference in CRP values between subjects with cluster headache and subjects without headache but these studies had few subjects (27 and 21 cases of cluster headache) thus lack power to detect small differences (25, 26). Estimated prevalence of cluster headache and chronic paroxysmal hemicranias combined is less than 0.5% (27, 28) and is therefore unlikely to affect our results to a great extent.
Information on use of aspirin and statins was not recorded in this study, and both drugs can alter CRP values. However, we note that use of these drugs was uncommon in the general population of Reykjavik between 1967 and 1991.
In conclusion, CRP levels were not increased among migraine sufferers compared to nonmigraineurs. Migraineurs without aura tended to have lower CRP values compared to nonmigraineurs and migraineurs with aura, except those of young women migraineurs without aura who had borderline higher CRP levels compared to migraineurs with aura and nonmigraineurs, respectively. The association between CRP and migraine status was similar among those who developed coronary heart disease during follow-up and those who did not.
Acknowledgements
We thank all the employees of the Icelandic Heart Preventive Clinic (Hjartavernd) for their skilful contribution to the data collection. We thank Dr. Jon Hersir Eliasson for helpful comments. This study was supported by the Icelandic Research Council and the University of Iceland Research Fund.
Footnotes
Conflict of interest: none.
References
- 1.Lowe GD, Pepys MB. C-reactive protein and cardiovascular disease: weighing the evidence. Curr Atheroscler Rep. 2006;8:421–428. doi: 10.1007/s11883-006-0040-x. [DOI] [PubMed] [Google Scholar]
- 2.Welch KM, Brandes AW, Salerno L, Brandes JL. C-reactive protein may be increased in migraine patients who present with complex clinical features. Headache. 2006;46:197–199. doi: 10.1111/j.1526-4610.2006.00330.x. [DOI] [PubMed] [Google Scholar]
- 3.Kurth T, Gaziano JM, Cook NR, Logroscino G, Diener HC, Buring JE. Migraine and risk of cardiovascular disease in women. JAMA. 2006;296:283–291. doi: 10.1001/jama.296.3.283. [DOI] [PubMed] [Google Scholar]
- 4.Bousser MG, Welch KM. Relation between migraine and stroke. Lancet Neurol. 2005;4:533–542. doi: 10.1016/S1474-4422(05)70164-2. [DOI] [PubMed] [Google Scholar]
- 5.Vanmolkot FH, de Hoon JN. Increased C-reactive protein in young adult patients with migraine. Cephalalgia. 2007;27:843–846. doi: 10.1111/j.1468-2982.2007.01324.x. [DOI] [PubMed] [Google Scholar]
- 6.Kurth T, Ridker PM, Buring JE. Migraine and biomarkers of cardiovascular disease in women. Cephalalgia. 2008;28:49–56. doi: 10.1111/j.1468-2982.2007.01467.x. [DOI] [PubMed] [Google Scholar]
- 7.Jonsdottir LS, Sigfusson N, Gudnason V, Sigvaldason H, Thorgeirsson G. Do lipids, blood pressure, diabetes, and smoking confer equal risk of myocardial infarction in women as in men? The Reykjavik Study. J Cardiovasc Risk. 2002;9:67–76. [PubMed] [Google Scholar]
- 8.Gudmundsson LS, Thorgeirsson G, Sigfusson N, Sigvaldason H, Johannsson M. Migraine patients have lower systolic but higher diastolic blood pressure compared with controls in a population-based study of 21,537 subjects. The Reykjavik Study. Cephalalgia. 2006;26:436–444. doi: 10.1111/j.1468-2982.2005.01057.x. [DOI] [PubMed] [Google Scholar]
- 9.Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 10.Rose GA, Blackburn H. Geneva: World Health Organization; 1966. Cardiovascular population studies: Methods. [Google Scholar]
- 11.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 13.Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Headache Classification Committee of the International Headache Society. Cephalalgia. 1988;8 Suppl 7:1–96. [PubMed] [Google Scholar]
- 14.Fröhlich M, Döring A, Imhof A, Hutchinson WL, Pepys MB, Koenig W. Oral contraceptive use is associated with a systemic acute phase response. Fibrinolysis and Proteolysis. 1999;13:239–244. [Google Scholar]
- 15.Waeber C, Moskowitz MA. Migraine as an inflammatory disorder. Neurology. 2005;64:S9–S15. doi: 10.1212/wnl.64.10_suppl_2.s9. [DOI] [PubMed] [Google Scholar]
- 16.Kurth T, Slomke MA, Kase CS, Cook NR, Lee IM, Gaziano JM, et al. Migraine, headache, and the risk of stroke in women: a prospective study. Neurology. 2005;64:1020–1026. doi: 10.1212/01.WNL.0000154528.21485.3A. [DOI] [PubMed] [Google Scholar]
- 17.Stang PE, Carson AP, Rose KM, Mo J, Ephross SA, Shahar E, Szklo M. Headache, cerebrovascular symptoms, and stroke: the Atherosclerosis Risk in Communities Study. Neurology. 2005;64:1573–1577. doi: 10.1212/01.WNL.0000158326.31368.04. [DOI] [PubMed] [Google Scholar]
- 18.Bigal ME, Liberman JN, Lipton RB. Age-dependent prevalence and clinical features of migraine. Neurology. 2006;67:246–251. doi: 10.1212/01.wnl.0000225186.76323.69. [DOI] [PubMed] [Google Scholar]
- 19.Prencipe M, Casini AR, Ferretti C, Santini M, Pezzella F, Scaldaferri N, Culasso F. Prevalence of headache in an elderly population: attack frequency, disability, and use of medication. J Neurol Neurosurg Psychiatry. 2001;70:377–381. doi: 10.1136/jnnp.70.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scher AI, Terwindt GM, Picavet HS, Verschuren WM, Ferrari MD, Launer LJ. Cardiovascular risk factors and migraine: the GEM population-based study. Neurology. 2005;64:614–620. doi: 10.1212/01.WNL.0000151857.43225.49. [DOI] [PubMed] [Google Scholar]
- 21.Albert MA, Glynn RJ, Ridker PM. Alcohol consumption and plasma concentration of C-reactive protein. Circulation. 2003;107:443–447. doi: 10.1161/01.cir.0000045669.16499.ec. [DOI] [PubMed] [Google Scholar]
- 22.Raum E, Gebhardt K, Buchner M, Schiltenwolf M, Brenner H. Long-term and short-term alcohol consumption and levels of C-reactive protein. Int J Cardiol. 2007;121:224–226. doi: 10.1016/j.ijcard.2006.08.104. [DOI] [PubMed] [Google Scholar]
- 23.Launer LJ, Terwindt GM, Ferrari MD. The prevalence and characteristics of migraine in a population-based cohort: the GEM study. Neurology. 1999;53:537–542. doi: 10.1212/wnl.53.3.537. [DOI] [PubMed] [Google Scholar]
- 24.Liew G, Wang JJ, Mitchell P. Migraine and coronary heart disease mortality: a prospective cohort study. Cephalalgia. 2007;27:368–371. doi: 10.1111/j.1468-2982.2007.01298.x. [DOI] [PubMed] [Google Scholar]
- 25.Remahl AI, Bratt J, Mollby H, Nordborg E, Waldenlind E. Comparison of soluble ICAM-1, VCAM-1 and E-selectin levels in patients with episodic cluster headache and giant cell arteritis. Cephalalgia. 2008;28:157–163. doi: 10.1111/j.1468-2982.2007.01487.x. [DOI] [PubMed] [Google Scholar]
- 26.Remahl IN, Waldenlind E, Bratt J, Ekbom K. Cluster headache is not associated with signs of a systemic inflammation. Headache. 2000;40:276–282. doi: 10.1046/j.1526-4610.2000.00041.x. [DOI] [PubMed] [Google Scholar]
- 27.Antonaci F, Sjaastad O. Chronic paroxysmal hemicrania (CPH): a review of the clinical manifestations. Headache. 1989;29:648–656. doi: 10.1111/j.1526-4610.1989.hed2910648.x. [DOI] [PubMed] [Google Scholar]
- 28.Torelli P, Castellini P, Cucurachi L, Devetak M, Lambru G, Manzoni GC. Cluster headache prevalence: methodological considerations. A review of the literature. Acta Biomed. 2006;77:4–9. [PubMed] [Google Scholar]