Abstract
A new bifunctional ligand 3p-C-DEPA was synthesized and evaluated for use in targeted alpha radioimmunotherapy. 3p-C-DEPA was efficiently prepared via regiospecific ring opening of an aziridinium ion and conjugated with trastuzumab. The 3p-C-DEPA-trastuzumab conjugate was extremely rapid in binding 205/6Bi, and the corresponding 205/6Bi-3p-C-DEPA-trastuzumab complex was stable in human serum. Biodistribution studies were performed to evaluate in vivo stability and tumor targeting of 205/6Bi-3p-C-DEPA-trastuzumab conjugate in tumor bearing athymic mice. 205/6Bi-3p-C-DEPA-trastuzumab conjugate displayed excellent in vivo stability and targeting as evidenced by low organ uptake and high tumor uptake. The results of the in vitro and in vivo studies indicate that 3p-C-DEPA is a promising chelator for radioimmunotherapy of 212Bi and 213Bi.
Introduction
The α-emitting radioisotopes, 212Bi (t1/2 = 60.6 m) and 213Bi (t1/2 = 45.6 m) have proven to be effective for radioimmunotherapy (RIT) of cancers.1 The α-emitters with high alpha energy (5–8 MeV) and a short emission path length (50–80 µm) can be closely deposited in the target tumor cells resulting in a minimum damage to normal tissues.2 The radionuclides, 212Bi and 213Bi, with very short half-lives decay ultimately to stable bismuth nuclides and are considered to be suitable for convenient out-patient RIT.3 The therapeutic efficacy of 212Bi and 213Bi has been demonstrated in numerous pre-clinical and clinical trials involving cancer patients with leukemia, melanoma, and glioblastoma.4–7
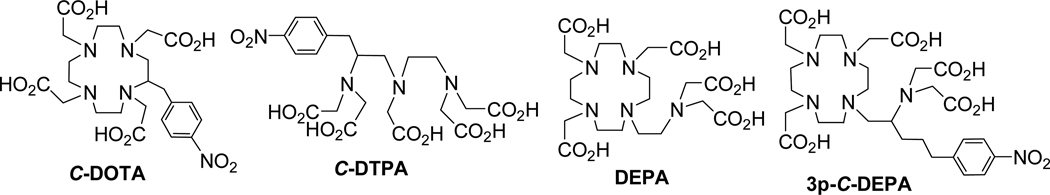
Three optimal components, a bifunctional ligand, an antibody, and a radioisotope are required for a successful RIT. An effective bifunctional ligand that can rapidly form a stable complex with the radionuclide should be employed to minimize toxic side effects related to biological deposition of the radionuclide if it becomes dissociated from the radiolabeled ligand-antibody conjugate during RIT.8 Research efforts have been directed towards improving chelation chemistry for RIT. C-DOTA and C-DTPA (Figure 1) analogues are two bifunctional ligands that are frequently explored for RIT applications.3 C-DOTA forms a kinetically inert and stable complex with Bi(III), however the slow complex formation kinetics render this chelator unacceptable for use in RIT when employing short-lived radionuclides such as 213Bi and 212Bi.9–10 The acyclic bifunctional ligand, C-DTPA, displayed rapid and high yield radiolabeling with Bi(III) radioisotopes.3 However, C-DTPA produced a less stable Bi(III)-C-DTPA complex than that of Bi(III)-C-DOTA,3,11,12
Figure 1.
Ligands in clinical and preclinical use for RIT
We recently reported the synthesis and evaluation of DEPA (Figure 1, 7-[2-(Bis-carboxymethylamino)-ethyl]-4,10-bis-carboxymethyl-1,4,7,10-tetraaza-cyclododec-1-yl-acetic acid).10 DEPA is a decadentate ligand in a hybridized form of the macrocyclic DOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetracarboxylic acid) and the acyclic DTPA (diethylenetriamine pentaacetic acid). This novel bimodal ligand DEPA was hypothesized to rapidly form a stable complex with a metal based on cooperative coordination of the macrocyclic and acyclic binding moieties. DEPA radiolabeled with 205/6Bi was indeed found to be stable in human serum without any loss of the radioactivity for two weeks and displayed excellent in vivo stability in mice.10
With the promising complexation kinetics and stability data for DEPA, we sought preparation of an effective bifunctional version of DEPA for RIT. In this paper, we report the synthesis and evaluation of a bifunctional DEPA analogue, 3p-C-DEPA (Figure 1, 2-[(carboxymethyl)][5-(4-nitrophenyl-1-[4,7,10-tris(carboxymethyl)-1,4,7,10-tetraazacyclododecan-1-yl]pentan-2-yl)amino]acetic acid) which contains the parent DEPA backbone and the isothiocyanate (NCS) group for conjugation to an antibody or a peptide. 3p-C-DEPA was synthesized, characterized, and conjugated to trastuzumab. Trastuzumab is a HER2 (human epidermal growth factor receptor 2) targeting antibody which has been reported to selectively target the HER2 protein overproduced in various tumors, including 90% of colorectal carcinomas.13–14 The corresponding 3p-C-DEPA-trastuzumab conjugate was evaluated for its radiolabeling reaction kinetics with 205/6Bi (t1/2 = 15.3 d for 205Bi; t1/2 = 6.24 d for 206Bi), a γ-emitting surrogate of 212Bi and 213Bi. In vitro analysis of the 3p-C-DEPA-trastuzumab conjugate radiolabeled with 205/6Bi included assessment of its stability in human serum and retention of reactivity with HER2 using a radioimmunoassay. Finally, the in vivo biodistribution and tumor uptake of the 205/6Bi-labeled 3p-C-DEPA-trastuzumab was assessed in mice bearing s.c. tumor (LS-174T) xenografts. For comparison and as a reference standard, the C-DOTA-trastuzumab conjugate was evaluated in the same in vitro and in vivo studies.
Experimental Procedure
Instruments and methods
1H and 13C NMR spectra were obtained using a Bruker 300 instrument and chemical shifts are reported in ppm on the δ scale relative to TMS or solvent. Electrospray iodization (ESI) high resolution mass spectra (HRMS) were obtained on JEOL double sector JMS-AX505HA mass spectrometer (University of Notre Dame, IN). Analytical HPLC was performed on an Agilent 1200 (Agilent, Santa Clara, CA) equipped with a diode array detector (λ = 254 and 280 nm), a thermostat set at 35 °C, and a Zorbax Eclipse XDB-C18 column (4.6 × 150 mm, 80Å, Agilent, Santa Clara, CA). The mobile phase of a binary gradient (0–100% B/40 min; solvent A, 0.05M AcOH/Et3N, pH 6.0; solvent B, CH3OH for method 1 or 0–50% B/30 min; solvent A, 0.05M AcOH/Et3N, pH 6.0; solvent B, CH3OH for method 2) at a flow rate of 1 mL/min was used for analytical HPLC. Semi-prep HPLC was performed on an Agilent 1200 equipped with a diode array detector (λ= 254 and 280 nm), a thermostat set at 35 °C, and a Zorbax Eclipse XDB-C18 column (9.4 × 250 mm, 80Å). The mobile phase of a binary gradient (0–100%B/160 min; solvent A = 0.05 M AcOH/Et3N, pH 6.0; solvent B = MeOH for method 3 at a flow rate of 2 mL/min was used for semi-prep HPLC. Size-exclusion HPLC (SE-HPLC) chromatograms were obtained on Agilent 1200 equipped with a diode array detector and an in-line IN/US γ-Ram Model 2 radiodetector (Tampa, FL), fitted with Bio-Silect SEC 250-5 column (Biorad, Hercules, CA) or a TSKgel G3000PW column (Tosoh Biosep, Montgomeryville, PA).
Reagents
All reagents were purchased from Sigma-Aldrich (St. Louis, MO) and used as received unless otherwise noted. Trastuzumab (Herceptin; Genetech, South San Francisco, Calif) was obtained through the Veterinary Resources Program (National Institutes of Health, Bethesda, Md). 205,6Bi was produced using a CS30 cyclotron (PET Dept, Clinical Center, NIH) and purified as described previously.15 C-DOTA was purchased from Macrocyclics (Dallas, TX).
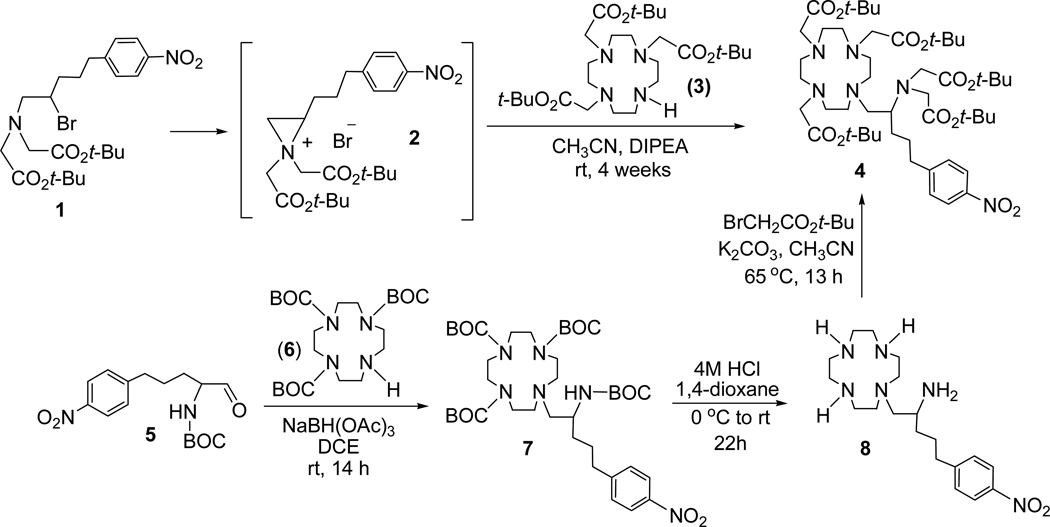
tert-Butyl 2,2',2"-(10-(2-(bis(2-tert-butoxy-2-oxoethyl)amino)-5-(4-nitrophenyl)-pentyl)-1,4,7,10-tetraazacyclododecane-1,4,7-triyl)triacetate (4)
To a solution of 116 (547.7 mg, 1.06 mmol) and DIPEA (410.9 mg, 3.18 mmol) in CH3CN (10 mL) was added tri-substituted cyclen 3 (546.6 mg, 1.06 mmol). The resulting mixture was stirred for 4 weeks at room temperature while monitoring the reaction progress using TLC. The reaction mixture containing the starting materials and the product 4 was concentrated to dryness. The residue was purified via column chromatography on silica gel (220–440 mesh) eluted with 3% CH3OH in CH2Cl2. The fractions containing the product 4 along with the starting material 3 as impurity were combined. After evaporating of the solvents, ether (20 mL) was added to the residue, and the starting material 3 formed a slurry which was removed by filtration. The filtrate containing the product 4 was washed with DI water (2 × 10 mL). The ether layer was dried over MgSO4, filtered, and concentrated in vacuo to provide pure 4 (610 mg, 61%). 1H NMR (CDCl3, 300 MHz) δ 1.37–1.45 (m, 45 H), 1.60–1.78 (m, 2 H), 1.81–1.95 (m, 1 H), 2.02–2.19 (m, 1 H), 2.39–2.50 (m, 3H), 2.56–2.83 (m, 18 H), 3.21 (s, 4H), 3.26 (s, 2 H), 3.36 (dd, J = 16.9, 21.9 Hz, 4 H), 7.36 (d, J = 8.6 Hz, 2 H), 8.09 (d, J = 8.7 Hz, 2 H); 13C NMR (CDCl3, 300MHz) δ 27.89 (t), 28.11 (q), 28.22 (q), 30.98 (t), 35.91 (t), 52.04 (t), 52.11 (t), 52.20 (t), 53.09 (t), 53.19 (t), 56.34 (t), 56.44 (t), 58.38 (t), 60.08 (d), 80.41 (s), 80.57 (s), 123.43 (d), 129.30 (d), 146.18 (s), 151.17 (s), 170.98 (s), 171.10 (s), 171.41 (s). HRMS (Positive ion FAB) Calcd for C49H85N6O12 [M + H]+ m/z 949.6225 Found: [M + H]+ m/z 949.6256.; Analytical HPLC (tR = 42 min, method 1).
Tri-tert-butyl 10-(2-(tert-butoxycarbonyl)-5-(4-nitrophenyl)pentyl)-1,4,7,10-tetraazacyclododecane-1,4,7-tricarboxylate (7)
To a mixture of 5 (1.24 g, 3.85 mmol) and 6 (1.82 g, 3.85 mmol) in 1,2-dichloroethane (30 mL) was added portionwise sodium triacetoxyborohydride (1.14 g, 5.4 mmol). The mixture was stirred at room temperature for 24 h. The reaction mixture was quenched with saturated NaHCO3 (40 mL), and the product was extracted while washing with CH2Cl2. The combined organic layers were dried over MgSO4, filtered, and concentrated in vacuo. The residue was purified by silica gel (60–230 mesh) column chromatography eluted with 25% EtOAc in hexanes to provide 7 (1.90 g, 63%). 1H NMR (CDCl3, 300 MHz) δ 1.03–1.51 (m, 36 H), 1.51–2.01 (m, 4 H), 2.50–3.99 (m, 21 H), 7.30 (d, J = 8.5 Hz, 2 H), 8.08 (d, J = 8.4 Hz, 2H); 13C NMR (CDCl3, 300 MHz) 24.69 (t), 27.16 (t), 28.57 (q), 28.69 (q), 35.13 (t), 35.68, (t), 36.64 (t), 47.49 (t), 48.46 (d), 50.54 (t), 51.20 (t), 58.18 (t), 58.64 (t), 79.18 (s), 79.28 (s), 79.57 (s), 123.56 (d), 129.19 (d), 146.27 (s), 150.39 (s), 155.14 (s), 155.73 (s), 155.95 (s), 156.72 (s). HRMS (Positive ion ESI) Calcd for C39H67N6O10 [M + H]+ m/z 779.4913 Found: [M + H]+ m/z 779.4890.
5-(4-Nitrophenyl)-1-(1,4,7,10-tetraazacyclododecan-1-yl)pentan-2-amine (8)
Compound 7 (1.84 g, 2.36 mmol) at 0–5 °C was treated dropwise with 4M HCl (g) in 1,4-dioxane (18 mL) over 20 min. The resulting mixture was warmed to room temperature and stirred for 22 h. Ether (100 mL) was added and stirred for 10 min. The resulting mixture was capped and placed in the freezer for 1 h. The slurry formed was filtered, washed with ether, and quickly dissolved in deionized (DI) water. The aqueous solution was concentrated in vacuo to provide 8 as an acidic salt (1.17 g, 89%). 1H NMR (D2O, 300 MHz) δ 1.50–1.71 (m, 4 H), 2.50–2.80 (m, 6 H), 2.82–3.40 (m, 15 H), 7.30 (d, J = 8.7 Hz, 2 H), 8.00 (d, J = 8.7 Hz, 2 H); 13C NMR (D2O, 300 MHz) δ 25.65 (t), 30.63 (t), 34.46 (t), 41.30 (t), 42.13 (t), 44.21 (t), 48.56 (t), 48.57 (d), 56.81 (t), 123.66 (d), 129.40 (d), 145.90 (s), 150.21 (s). HRMS (Positive ion ESI) Calcd for C19H35N6O2 [M + H]+ m/z 379.2816 Found: [M + H]+ m/z 379.2804.
A solution of the acidic salt 8 (670 mg, 1.7 mmol) in DI water (5 mL) was neutralized using 0.5 M NaOH. The aqueous layer was then extracted with CHCl3 (2 × 25 mL). The aqueous layer was further adjusted to pH 10 and re-extracted with CHCl3 (2 × 25 mL). The organic layers extracted from both neutral (pH 7) and basic (pH 10) solutions were combined, dried over MgSO4, filtered, and concentrated in vacuo to provide free amine 8 (452 mg, 100%). 1H NMR (CDCl3, 300 MHz) δ 1.20–1.49 (m, 2 H), 1.60–1.89 (m, 2 H), 2.15–3.00 (m, 21 H), 7.33 (d, J = 8.6 Hz, 2 H), 8.14 (d, J = 8.6 Hz, 2 H); 13C NMR (CDCl3, 300 MHz) δ 27.26 (t), 35.00 (t), 35.64 (t), 45.16 (t), 46.01 (t), 46.95 (t), 48.71 (d), 52.10 (t), 62.56 (t), 123.27 (d), 129.00 (d), 145.94 (s), 150.24 (s).
Synthesis of 4 from compound 8
To a solution of 8 (170.3 mg, 0.45 mmol) in CH3CN (5 mL) was added tert-butyl bromoacetate (438.9 mg, 2.25 mmol) and K2CO3 (311.0 mg, 2.25 mmol). The resulting mixture was heated at 65 °C and stirred for 13 h while monitoring the reaction progress by analytical HPLC. The reaction mixture was cooled to room temperature, and the solvent was evaporated. The residue was purified by semi-prep HPLC (method 3, 138–143 min) to afford 4 (37 mg, 9%).
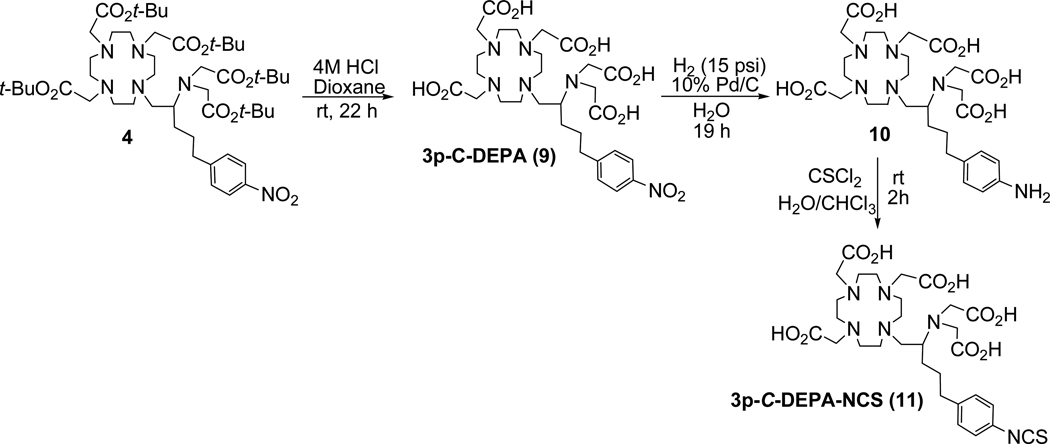
2-[(Carboxymethyl)][5-(4-nitrophenyl-1-[4,7,10-tris(carboxymethyl)-1,4,7,10-tetraazacyclododecan-1-yl]Pentan-2-yl)amino]acetic acid (9)
Compound 4 (77.0 mg, 0.08 mmol) at 0–5 °C was treated dropwise with 4M HCl (g) in 1,4-dioxane (15 mL) over 20 min. The resulting mixture was allowed to warm to room temperature for 22 h. Ether (~20 mL) was added and continuously stirred for 10 min. The resulting mixture was capped and placed in the freezer for 1 h. The solid formed was filtered, washed with ether, and quickly dissolved in DI water. Evaporation of the aqueous solution gave 9 (68.0 mg, 97%) as an off-white solid. 1H NMR (D2O, 300 MHz) δ 1.26–1.40 (m, 1H), 1.48–1.70 (m, 3H), 2.52–2.78 (m, 2H), 2.90–3.70 (m, 27H), 3.75–3.98 (m, 2H), 7.35 (d, J = 8.3 Hz, 2H), 8.06 (d, J = 8.2 Hz, 2H); 13C NMR (D2O, 300MHz) δ 27.08 (t), 34.64 (t), 48.45 (t), 48.82 (t), 50.22 (t), 51.09 (t), 52.40 (t), 53.58 (t), 54.28 (t), 55.46 (t), 59.43 (d), 123.55 (d), 129.40 (d), 145.71 (s), 150.54 (s), 169.54 (s), 172.97 (s), 173.93 (s); HRMS (Positive ion FAB) Calcd for C29H45N6O12 [M + H]+ m/z 669.3095 Found: [M + H]+ m/z 669.3086.
2,2',2"-(10-(5-(4-Aminophenyl)-2-(bis(carboxymethyl)amino)pentyl)-1,4,7,10-tetraazacyclododecane-1,4,7-triyl)triacetic acid (10)
To a solution of 9 (46.0 mg, 68.8 µmol) in DI H2O (12 mL) was added dry 10% Pd/C (14.0 mg) under Argon. The reaction mixture was subject to hydrogenation in a hydrogenation apparatus set at the constant pressure (15 psi) of H2 (g) for 19 h. The resulting mixture was filtered over a Celite bed and washed thoroughly with DI H2O. The filtrate was concentrated in vacuo to provide a light yellow solid 10 (44 mg, 100%). 1H NMR (D2O, 300 MHz) δ 1.22–1.38 (m, 1H), 1.43–1.68 (m, 3H), 2.45–2.63 (m, 2H), 2.81–3.70 (m, 27H), 3.80–3.98 (m, 2H), 7.17 (d, J = 8.5 Hz, 2H), 7.24 (d, J = 8.5 Hz, 2H); 13C NMR (D2O, 300 MHz) δ 27.04 (t), 27.46 (t), 34.18 (t), 48.50 (t), 48.98 (t), 50.28 (t), 51.12 (t), 52.50 (t), 53.69 (t), 54.50 (t), 55.49 (t), 59.40 (d), 123.01 (d), 127.55 (s), 130.10 (d), 143.28 (s), 169.50 (s), 173.13 (s), 174.22 (s); HRMS (Positive ion ESI) Calcd for C29H47N6O10 [M + H]+ m/z 639.3348 Found: [M + H]+ m/z 639.3342.
2,2',2"-(10-(2-(Bis(carboxymethyl)amino)-5-(4-isothiocyanatophenyl)pentyl)-1,4,7,10-tetraazacyclododecane-1,4,7-triyl)triacetic acid (11)
To a solution of 10 (7.0 mg, 8.2 µmol) in water (0.1 mL) was added CSCl2 (7.0 µL, 7.0 µmol) in CHCl3. The resulting mixture was stirred at room temperature for 2 h. The aqueous layer was isolated and concentrated in vacuo to give pure 3p-C-DEPA-NCS 11 as a solid (8.0 mg, 100%). 1H NMR (D2O, 300MHz) δ 1.23–1.72 (m, 4H), 2.40–2.58 (m, 2H), 2.80–3.98 (m, 29H), 7.13 (s, 4H). HRMS (Positive ion FAB) Calcd for C30H43N6O12S [M + H]+ m/z 679.2761 Found: [M + H]+ m/z 679.2747.
Conjugation of 3p-C-DEPA-NCS to trastuzumab
All absorbance measurements were obtained on an Agilent 8453 diode array spectrophotometer equipped with a 8-cell transport system (designed for 1 cm cells). Metal-free stock solutions of all buffers were prepared using Chelex®-100 resin (200–400 mesh, Bio-Rad Lab, Hercules, CA, Cat# 142–2842). Chelex resin (2.5 g) was added into the buffer solution (250 mL) and the mixture was shaken overnight in a shaker and filtered through a Corning filter system (Cat# 430513, pore size 0.2 µm). Disposable PD-10 Sephadex™ G-25M columns (GE Healthcare, #17-0851-01) were rinsed with 25 mL of the appropriate buffer prior to addition of antibody or its ligand conjugates. Amicon centricon C-50 (50,000 MWCO) centrifugal filter devices (Millipore, Cat# UFC805008) were used for purification of trastuzumab conjugate (Bedford, MA). The initial concentration of trastuzumab was determined by UV/Vis spectroscopic method. Phosphate buffered saline (PBS, 1×, 11.9 mM Phosphates, 137 mM NaCl, and 2.7 mM KCl, pH 7.4) was purchased from Fisher and used as received. Conjugation buffer (50 mM HEPES, 150 mM NaCl, pH 8.6) were prepared as 1× solutions, chelexed, and filtered through the Corning filter. Trastuzumab (6.67 mg) was diluted to 1.6 mL using conjugation buffer (1:0.6), and the resulting solution was added to a PD-10 column. Conjugation buffer (10 mL) was added to the PD-10 column to exchange the buffer solution of the antibody and collected in a sterile test tube and checked for the presence of trastuzumab via analysis of the UV/VIS spectrum at 280 nm. To a sterile test tube containing the recovered trastuzumab (6.14 mg) was added a 10-fold excess of 3p-C-DEPA-NCS (39.6 µL, 10 mM). The resulting solution was gently agitated for 16 h at room temperature and placed on a Centricon C-50 membrane and spun down to reduce volume. PBS (3 × 2 mL) was added to the remaining solution of the 3p-C-DEPA-trastuzumab conjugate, followed by centrifugation in order to remove unreacted ligand. The volume of purified conjugate antibody was brought to 1.0 mL with PBS. To measure [trastuzumab] in the 3p-C-DEPA-trastuzumab conjugate, a UV/Vis spectrometer was zeroed against a cuvette filled with 2.0 mL of PBS with a window open from 190 nm to 1100 nm. A 50 µL portion of PBS was removed and discarded, 50 µl of the 3p-C-DEPA-trastuzumab conjugate solution was added, and absorbance at 280 nm was noted. Beer’s Law was used to calculate [trastuzumab] in the conjugate with molar absorptivity of 1.42. After centrifugation, 2.98 mg (2.02 × 10−5 M, 49.0%) of the trastuzumab remained.
Spectroscopic Determination of Ligand to Protein (L/P) Ratio
A stock solution of the Cu(II)-AAIII reagent was prepared in 0.15 M NH4OAc, pH 7.0 by adding an aliquot of copper atomic absorption solution (1.55 × 10−2 M) into a 10 µM solution of AAIII to afford a 5 µM solution of Cu(II).17 This solution was stored in the dark to avoid degradation over time. A UV/Vis spectrometer was zeroed against well-dried blank 8 cuvettes with a window open from 190 nm to 1100 nm. A cuvette was filled with AAIII solution (2 mL), and the other seven cuvettes with the Cu(II)-AAIII solution (2 mL). Cu(II)-AAIII solution (50 µL) in the seven cuvettes was removed and discarded. Milli-Q water (50 µL) was added to the second cuvette, and one to five 10 µL additions of 3p-C-DEPA (0.1mM) were added to the five cuvettes to give a series of five different concentrations (2 mL total volume). The solutions in the third to the seventh cuvette were diluted to 2.0 mL by adding an aliquot of milli-Q water. 3p-C-DEPA-trastuzumab conjugate (50 µL) was added to the eighth cuvette containing Cu(II)-AAIII reagent (1950 µL). After addition of 3p-C-DEPA-trastuzumab conjugate to the Cu(II)-AAIII solution, the resulting solution was equilibrated for 10 min. The absorbance of the resulting solution at 610 nm was monitored every 30 seconds over 6 min. The average of the absorbance of each solution was calculated, and the absorbance data from the Cu(II)-AAIII solutions containing six different concentrations were used to construct a calibration plot of A610 versus [3p-C-DEPA] by the equation, Y = 0.0663−(1.5284 ×104)[3p-C-DEPA] (R2 = 0.9969), wherein Y = A610nm. The concentration of 3p-C-DEPA in the 3p-C-DEPA-trastuzumab conjugate was calculated (3.98 × 10−5 M). The L/P ratio of the 3p-C-DEPA-trastuzumab conjugate ([3.98 × 10−5 M]/[2.02 × 10−5 M]) was measured to be 1.97.
Radiolabeling of 3p-C-DEPA-trastuzumab or C-DOTA-trastuzumab conjugates with 205/6Bi
All HCl solutions were prepared from ultra-pure HCl (Fisher, Cat# A466–500). For metal-free radiolabeling, plasticware including pipette tips, tubes, and caps was soaked in 0.1M HCl overnight, washed thoroughly with Milli-Q (18.2 MΩ) water, and air-dried overnight. Ultra pure NH4OAc (Aldrich, #372331) was purchased from Aldrich and used to prepare all NH4OAc buffer solutions (0.1 M, pH 7). The buffer solutions were treated with Chelex-100 resin (Biorad, #142–2842, 1g/100 ml buffer solution), shaken overnight at room temperature, and filtered through 0.22 µm filter (Corning, #430320) prior to use. To a buffer solution (120 µL, pH 7) in a capped microcentrifuge tube (1.5 mL, Fisher Scientific #05-408-129) was sequentially added a solution of C-DOTA-trastuzumab (30 µg) in PBS (13.5 µL) and 205/6Bi (0.1 M HI, 60.5 µCi, 12.7 µL) or 3p-C-DEPA-trastuzumab (30 µg) in PBS (9.6 µL) and 205/6Bi (0.1M HI, 60.5 µCi, 12.7 µL). The final volume of the resulting solution was 146.2 µL and 142.3 µL for C-DOTA-trastuzumab and 3p-C-DEPA-trastuzumab, respectively, and the pH of the resulting reaction mixture was 5.5 for both conjugates. The reaction mixture was agitated on the thermomixer (Eppendorf, #022670549) set at 1,000 rpm at room temperature for 1 h. The labeling efficiency was determined by SE-HPLC (Biorad, Bio-Silect SEC 250-5 column, 7.8 × 30 cm, eluent: PBS, flow rate: 1 mL/min). A solution of radiolabeled mixture (10 µL for C-DOTA-trastuzumab and 7 µL for 3p-C-DEPA-trastuzumab) was withdrawn at the designated time points (1 min, 5 min, 10 min, 20 min, 30 min, and 60 min), and DTPA solution (10mM, 1 µL or 1.4 µL) was added to the mixture, and the resulting mixture was left for at least 20 min. HPLC samples were prepared and evaluated by SE-HPLC. Peaks for bound and unbound radioisotope appeared around 8.3 min and 11 min, respectively.
In vitro stability of 205/6Bi-3p-C-DEPA-trastuzumab conjugate
205/6Bi-3p-C-DEPA-trastuzumab was prepared by reaction of 3p-C-DEPA-trastuzumab with 205/6Bi at either RT or 37°C (5.0 M NH4OAc, pH 5.5). The complex formed was purified from 205/6Bi by gel filtration chromatography using PD-10 column (Disposable PD-10 Sephadex™ G-25M columns, GE Healthcare, Cat# 17-0851-01) eluted with PBS (pH 7.4, Fisher Scientific, Cat# BP2438-4). The fractions containing 205/6Bi-3p-C-DEPA-trastuzumab (700 µL, 22 µCi) were combined and added into human serum (700 µL, Gemini Bioproducts, #100110) in a microcentrifuge tube (Fisher Scientific, #05-408-129). The stability of the purified 205/6Bi-3p-C-DEPA-trastuzumab was evaluated for 4 days. The serum stability of the radiolabeled complexes was assessed by measuring the transfer of the radionuclide from each complex to serum proteins using SE-HPLC (TSKgel G3000PW column, 7.5 × 30cm, eluent: PBS, flow rate: 1 mL/min). A solution of the radiolabeled complex in serum (20–80 µL) was withdrawn at the designated time point, treated with DTPA (5 mM, 1.0 µL), incubated at room temperature for 20 min and then diluted with PBS (pH 7.4, 50–100 µL) prior to SE-HPLC.
Preparation of 205/6Bi-3p-C-DEPA-trastuzumab and 205/6Bi-C-DOTA-trastuzumab for immunoreactivity and in vivo studies
For use in the in vitro binding and in vivo biodistribution and tumor targeting studies, 205/6Bi-3p-C-DEPA-trastuzumab and 205/6Bi-C-DOTA-trastuzumab with high specific activity were prepared. In this instance, two aliquots of 205/6Bi solution (100 µL in 0.1 M HI, 1.1 mCi each) were each neutralized to pH 5.5 with NH4OAc solution (10 µL, 5 M, pH 7). An aliquot of each immunoconjugate containing 50 µg of protein in PBS was added to one or the other 205/6Bi solution. The mixtures were vortexed briefly and incubated at 37°C. After 30 min EDTA solution (5 µL, 0.1M) was added to each reaction, vortexed briefly and further incubated for 5 min. The products were purified by gel filtration chromatography using disposable PD-10 columns. The labeling efficiencies were 85.8% and 67.3% for 205/6Bi-3p-C-DEPA-trastuzumab and 205/6Bi-C-DOTA-trastuzumab, respectively, as determined by the labeled products collected from the PD-10 columns. The purity of the labeled products as determined by SE-HPLC (TSKgel G3000PW column, PBS eluate at 0.5 mL/min) were >98% for each product. The specific activities were 16 mCi/mg and 13.6 mCi/mg for205/6Bi-3p-C-DEPA-trastuzumab and 205/6Bi-C-DOTA-trastuzumab, respectively.
Radioimmunoassay
The immunoreactivity of the 205Bi-labeled trastuzumab conjugates was assessed in a radioimmunoassay using purified recombinant human Erb2/Fc chimera (rhErb2/Fc). Briefly, 50 ng of the rhErb2/Fc in PBS with Mg+2 and Ca+2(50 µL) was added to each well of a 96-well plate and allowed to adsorb to the well at 4 °C. Following an overnight incubation, the solution was removed and 100 µL of 1% BSA in PBS (BSA/PBS) was added to each well and incubated at room temperature for 30–60 min. Serial dilutions of 205/6Bi-trastuzumab (~300,000 to ~12,500 cpm in 50 µL of BSA/PBS) were added to the wells in duplicate, covered and incubated at 37 °C for 4 h. The radiolabeled antibody was then removed and the wells were washed three times with PBS. The radioactivity was removed from the wells with a solution of 0.2 M NaOH with 1 mM EDTA (100 µL), adsorbing the solution to cotton filters and transferring the filters to 12 × 75 mm polypropylene tubes. The radioactivity was measured in a γ-scintillation counter (WizardOne, Perkin Elmer, Shelton, CT), and the percentage binding calculated for each dilution. The values presented are an average of the percent bound for the serial dilutions. The specificity of the radiolabeled trastuzumab was confirmed by incubating one set of wells with radiolabeled trastuzumab and 10 µg of unlabeled trastuzumab.
In vivo biodistribution and tumor targeting studies
In vivo studies were conducted using the human colon carcinoma cell line, LS-174T, (kindly provided by Dr. J. Greiner, NCI). The LS-174T cell line was grown in Dulbecco’s minimum essential medium (DMEM) supplemented with 10 mM glutamine along with 10% FetalPlex (Gemini Bio-Products, Woodland, CA) and 1 mM non-essential amino acids. Media and supplements were obtained from Lonza (Walkersville, MD). All in vivo studies were performed using 4–6 week old female athymic (nu/nu) mice (Charles River Laboratories, Wilmington, MA). All animal protocols were approved by the National Cancer Institute Animal Care and Use Committee. Mice were injected subcutaneously (s.c.) in the right rear leg with 2×106 LS-174T cells in media (200 µL) with 20% Matrigel (BD Biosciences, San Jose, CA). Mice were utilized in studies when the tumor xenografts maximal diameter measured 0.4 – 0.6 cm. Mice (n = 4 per time point) were injected intravenously (i.v.) with the 205/6Bi-labeled trastuzumab conjugates (~7.5 µCi on 0.6 µg) and sacrificed by exsanguination at 2, 6 and 24 h. The blood, tumor, and major organs were collected, wet-weighed, and counted in a γ-scintillation counter. The percent injected dose per gram (%ID/g) and standard deviation were calculated.
Results and Discussion
The synthesis of the new bifunctional ligand 3p-C-DEPA is outlined in Scheme 1. An intramolecular substitution reaction of N,N-bisubstituted β-amino bromide 1 produced aziridinium ion 2 which was then reacted with tri-substituted cyclen 3 (1,4,7,10-tetraazacyclododecane). Regiospecific ring opening of 2 by nucleophilic attack of bulky cyclen analogue 310 occurred at the less hindered methylene carbon in 2 to provide 4 as the exclusive product (61%). The reaction was carried out at room temperature, although it proceeds extremely slowly at this mild condition (4 weeks). An intramolecular rearrangement product of 1 was obtained when the reaction was under reflux. The desired coupling product 4 was isolated via column chromatography and characterized by 1H and 13C NMR, analytical HPLC, and HRMS. To confirm the regiochemistry observed in the nucelophilic ring opening of the aziridinium ion 2 at the methylene carbon, compound 4 was separately prepared via another synthetic route starting from 5 as shown in Scheme 1. Reductive amination of 5 with 6 using sodium triacetoxyborohydride provided 7 which was subsequently treated with HCl (g) in 1,4-dioxane to afford 8. A base-promoted reaction of 8 with tert-butyl bromoacetate produced compound 4, and comparison of the spectroscopic data of 4 that was separately produced via the two synthetic routes confirmed regiochemistry in the ring opening of 2. Synthesis of the bifunctional ligands 3p-C-DEPA (9) and 3p-C-DEPA-NCS (11) is shown in Scheme 2. The tert-butyl groups in 4 was removed by the reaction of 4 with 4M HCl (g) in 1,4-dioxane to provide 3p-C-DEPA (9). The nitro group in 9 was transformed into the amino group by hydrogenolysis of 9 on Pd/C to provide 10 which was subsequently reacted with thiophosgene (g) in CHCl3 to the desired bifunctional ligand 3p-C-DEPA-NCS (11) containing the isothiocyanate group for conjugation to an antibody.
Scheme 1.
Synthesis of Precursor Molecule 4 to 3p-C-DEPA
Scheme 2.
Synthesis of 3p-C-DEPA (9) and 3p-C-DEPA-NCS (11)
3p-C-DEPA-NCS was conjugated to trastuzumab, and the concentration of trastuzumab in 3p-C-DEPA-trastuzumab conjugate was quantified by the method of Lowry.18 The Cu(II)-AAIII based UV-Vis spectrophotometric assay was used for the determination of the number of 3p-C-DEPA ligand linked to trastuzumab (L/P ratio).17 The ligand to protein (L/P) ratio for 3p-C-DEPA-trastuzumab conjugate was measured to be 1.97. For comparison, C-DOTA-NCS (Macrocyclics, TX) was conjugated to trastuzumab as reported previously.19
The purified 3p-C-DEPA trastuzumab conjugates (30~50 µg) in 0.25 M NH4OAc buffer solution at pH 5.5 was radiolabeled with 205/6Bi (0.1M HI) (60 µCi) at room temperature (RT). During the reaction time (1 h), the radiolabeling kinetics was determined by taking aliquots of the reaction mixture at 6 time points (1 min, 5 min, 10 min, 20 min, 30 min, and 60 min). The components were analyzed using SE-HPLC after challenging the reaction mixture with 10mM DTPA, and the radiolabeling efficiency (%) was determined (Table 1 and the supporting information). The data indicate that 205/6Bi labeling of 3p-C-DEPA-trastuzumab conjugate were extremely rapid (1 min, >93%) at RT. As expected, radiolabeling of C-DOTA with 205/6Bi was slow and incomplete at 24 h (60.2 ± 8.0%). The 205/6Bi-3p-C-DEPA conjugate was further evaluated for in vitro serum stability. The 205/6Bi-3p-C-DEPA conjugate was freshly prepared, purified, and incubated in human serum at 37 °C. At each time point (0 h, 1, 2, 3, 4 d), aliquots of the reaction mixture were analyzed using SE-HPLC after challenging the reaction mixture with 10mM DTPA. 205/6Bi-3p-C-DEPA-trastuzumab was found to be stable in human serum without release of the radioactivity for at least 4 days (the Supporting Information).
Table 1.
*Radiolabeling efficiency (%) of bifunctional ligands with 205/6Bi (0.25 M NH4OAc, pH 5.5, RT).
| Time (min) | 3p-C-DEPA | C-DOTA |
|---|---|---|
| 1 | 93.6 ± 0.4 | 8.4 ± 1.9 |
| 5 | 94.7 ± 0.9 | 17.2 ± 4.3 |
| 10 | 95.0 ± 0.4 | 28.7 ± 4.5 |
| 20 | 94.1 ± 0.6 | 38.0 ± 4.9 |
| 30 | 94.4 ± 1.6 | 49.7 ± 9.0 |
| 60 | 94.5 ± 0.5 | 60.2 ± 8.0 |
Radiolabeling efficiency (mean ± standard deviation) was measured in triplicate.
The 205/6Bi-3p-C-DEPA-trastuzumab and 205/6Bi-C-DOTA-trastuzumab conjugates were independently prepared at 37 °C and pH 5.5 for specific activity and immunoreactivity evaluations prior to performing comparative in vivo biodistribution studies. The respective specific activity of 16.0 mCi/mg, and 13.6 mCi/mg was measured for 205/6Bi-3p-C-DEPA and 205/6Bi-C-DOTA. The radiolabeling yields of 85.8% and 67.3% were determined for 205/6Bi-3p-C-DEPA and 205/6Bi-C-DOTA, respectively. Immunoreactivity of 205/6Bi-C-DOTA-trastuzumab and 205/6Bi-3p-C-DEPA-trastuzumab was assessed in a radioimmunoassay using recombinant human ErbB2/Fc Chimera (R&D Systems, Minneapolis, NM). The respective specific binding of 82.6% and 83.2% was measured for 205/6Bi-3p-C-DEPA-trastuzumab and 205/6Bi-C-DOTA-trastuzumab. In the presence of excess trastuzumab, 205/6Bi-3p-C-DEPA-trastuzumab and 205/6Bi-C-DOTA-trastuzumab exhibited the same non-specific binding (7.6%).
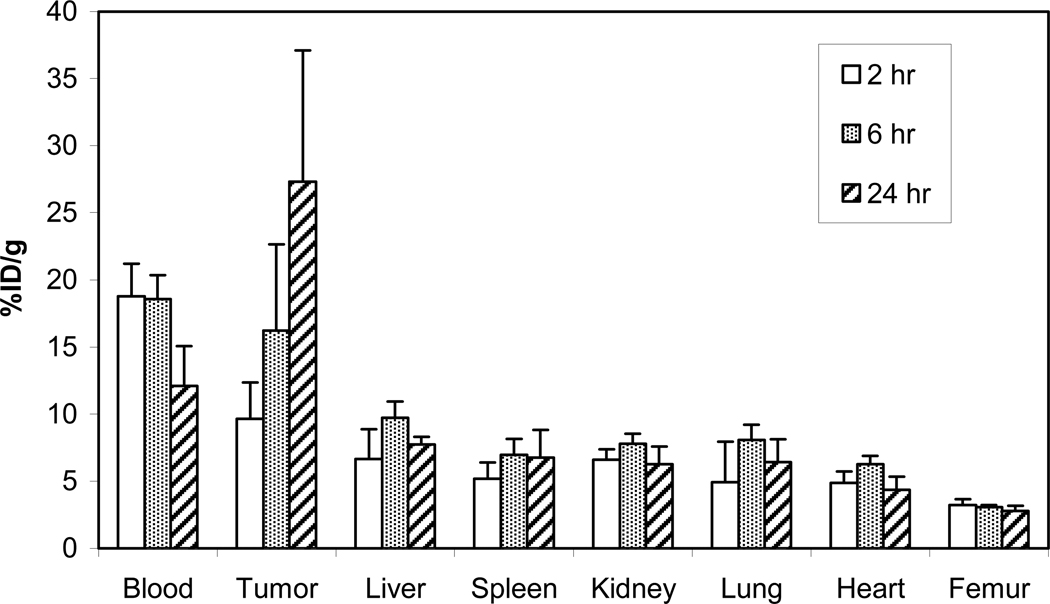
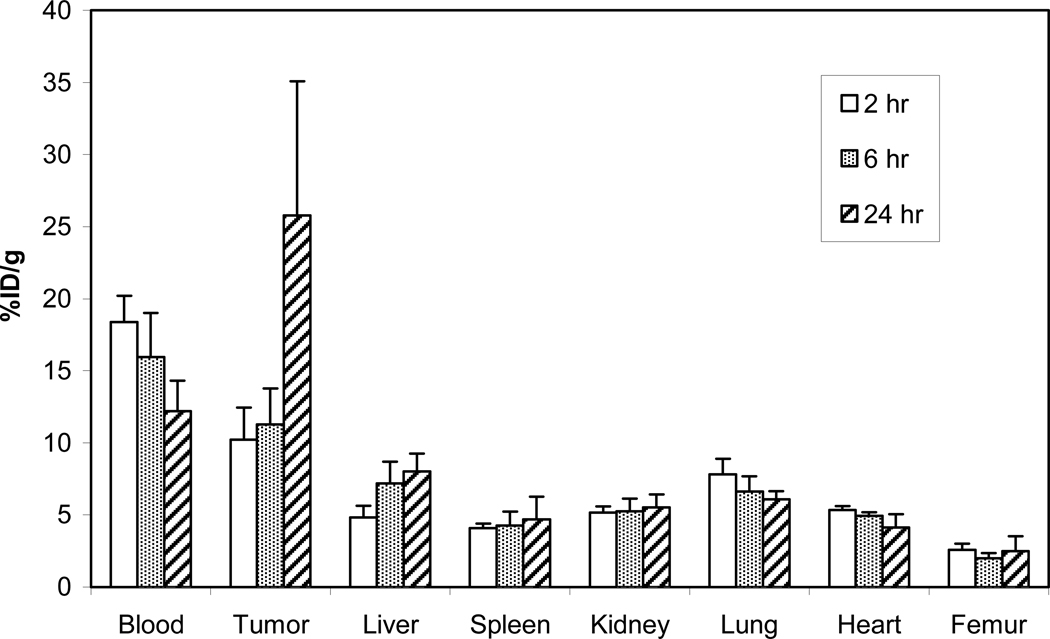
The in vivo stability and tumor targeting of 205/6Bi-3p-C-DEPA–trastuzumab was evaluated by performing biodistribution studies in LS174T-bearing athymic mice (i.v. injection, n = 4). 205/6Bi-C-DOTA-trastuzumab conjugate was evaluated for comparison. The mice were euthanized at 2 h, 6 h, and 24 h. Tumors, selected organs, and the blood were harvested, wet weighed, and the radioactivity measured in a γ-counter (Figures 2 and 3). The highest tumor uptake was observed at 24 h for 205/6Bi-3p-C-DEPA-trastuzumab and 205/6Bi-C-DOTA-trastuzumab (27.3% and 25.8%, respectively). Among the organs, the highest accretion of radioactivity was observed in the liver: 7.73% for 205/6Bi-3p-C-DEPA-trastuzumab (6 h) and 8.02% for 205/6Bi-C-DOTA-trastuzumab (24 h). Both of the 205/6Bi-labeled conjugates exhibited the highest radioactivity level in the blood at 2 h which decreased over time. Renal and liver uptake of 205/6Bi-3p-C-DEPA-trastuzumab peaked at 6 h and decreased by 24 h. 205/6Bi-3p-C-DEPA-trastuzumab and 205/6Bi-C-DOTA-trastuzumab resulted in the respective radioactivity level of 6.27% and 5.52% in the kidney at 24 h. 205/6Bi-C-DOTA-trastuzumab exhibited an upward trend in the accumulation of radioactivity in the liver over the study period. Femur uptake of 205/6Bi-3p-C-DEPA-trastuzumab peaked at 2 h (~3%) and decreased over the rest of the time points, while 205/6Bi-C-DOTA-trastuzumab displayed increased femur uptake from 2.00% at 6 h to 2.49% at 24 h. The respective tumor-to-blood ratio of 205/6Bi-3p-C-DEPA and 205/6Bi-C-DOTA at 24 h was 2.26 and 2.15. Both radioimmuno-conjugates exhibited low blood to tissue ratios (<0.7) in all tissues and higher radioactivity level in tumor as compared to the normal organs over the time points.
Figure 2.
Biodistribution of 205/6Bi-3p-C-DEPA in athymic mice bearing s.c. LS-174T tumors.
Figure 3.
Biodistribution of 205/6Bi-C-DOTA in athymic mice bearing s.c. LS-174T tumors.
In summary, the in vitro and in vivo experimental results indicate that the new ligand 3p-C-DEPA possesses great potential for RIT of 212Bi and 213Bi. Given complete and ongoing numerous preclinical and clinical studies of C-DOTA for alpha RIT, this new ligand deserves more extensive evaluations as a chelator of other potent α-emitters with a longer half-life including 212Pb (t1/2 = 10.64 h) and 225Ac (t1/2 = 10 d). 212Pb is investigated as an in vivo generator of 212Bi to abrogate the short half-life of the daughter isotope.20 225Ac with the advantage of multiple alpha emissions is a potent α emitter and known to induce apoptosis even with a single particle traversal of the cell.21 Although encouraging results on the therapeutic efficacy of C-DOTA-antibody conjugates radiolabeled with 212Pb or 225Ac as demonstrated in the preclinical studies using tumor bearing mice was reported22–25, C-DOTA-antibody conjugates radiolabeled with 203Pb or 225Ac were less stable in serum.21,26,27 The decadentate chelator 3p-C-DEPA possesses a larger macrocyclic backbone than C-DOTA and may be a suitable ligand for effectively sequestering 212Pb and 225Ac. 3p-C-DEPA will be further evaluated for various alpha RIT applications.
Conclusion
The new bifunctional ligand 3p-C-DEPA was efficiently prepared based on the synthetic route including the key reaction step, regiospecific ring opening of aziridinium ion by nucleophilic trisubstituted cyclen. 3p-C-DEPA conjugated with trastuzumab was extremely rapid in binding 205/6Bi, and the corresponding 205/6Bi-3p-C-DEPA-trastuzumab complex was stable in human serum over a 4 day study period, well beyond any concerns of stability with 212Bi and 213Bi. In vivo biodistribution data indicate that 3p-C-DEPA-trastuzumab radiolabeled with 205/6Bi displayed excellent in vivo stability as evidenced by low organ uptake and excellent tumor targeting that was favorably compared to those of 205/6Bi-C-DOTA-trastuzumab. In vitro and in vivo data demonstrate that 3p-C-DEPA is a promising ligand for RIT applications of 212Bi and 213Bi.
Supplementary Material
Acknowledgement
We acknowledge financial support from the National Institutes of Health (R01CA112503). This work was partly supported by intramural research program of NIH. Hyun A Song is the recipient of 2009 Illinois Institute of Technology Kilpatrick Fellowship.
Footnotes
Supporting material. HPLC chromatograms for assessment of radiolabeling reaction kinetics and serum stability and in vivo biodistribution data. This material is available free of charge via the Internet at http://pubs.acs.org/BC.
References
- 1.Knox SJ, Meredith RF. Clinical radioimmunotherapy. Sem. Radiat. Oncol. 2000;10:73–93. doi: 10.1016/s1053-4296(00)80045-4. [DOI] [PubMed] [Google Scholar]
- 2.Milenic DE, Brady ED, Brechbiel MW. Antibody-targeted radiation cancer therapy. Nature Rev. 2004;3:488–498. doi: 10.1038/nrd1413. [DOI] [PubMed] [Google Scholar]
- 3.Hassfjell S, Brechbiel MW. The development of the α-particle emitting radionuclides 212Bi and 213Bi, and their decay chain related radionuclides for therapeutic applications. Chem. Rev. 2001;101:2019–2036. doi: 10.1021/cr000118y. [DOI] [PubMed] [Google Scholar]
- 4.Jurcic JG, Larson SM, Sgouros G, McDevitt MR, Finn RD, Divgi CR, Ballangrud ÅM, Klaus KA, Ma D, Humm JL, Brechbiel MW, Molinet R, Scheinberg DA. Targeted alpha particle immunotherapy for myeloid leukemia. Blood. 2002;100:1233–1239. [PubMed] [Google Scholar]
- 5.Mulford DA, Pandit-Taskar N, McDevitt MR, Finn RD, Weiss MA, Apostolidis C, Morgenstern A, Divgi CR, Larson SM, Scheinberg DA, Jurcic JG. Sequential therapy with cytarabine and bismuth-213 (Bi-213)-labeled-HuM195 (anti-CD33) for acute myeloid leukemia (AML) Blood. 2004;104:496a. [Google Scholar]
- 6.Allen BJ, Raja C, Rizvi S, Li Y, Tsui W, Graham P, Thompson JF, Reisfeld RA, Kearsley J, Morgenstern A, Apostolidis C. Cancer Biol. Ther. 2005;4:1318–1324. doi: 10.4161/cbt.4.12.2251. [DOI] [PubMed] [Google Scholar]
- 7.Kneifel S, Cordier D, Good S, Ionescu MC, Ghaffari A, Hofer S, Kretzschmar M, Tolnay M, Apostolidis C, Waser B, Arnold M, Mueller-Brand J, Maecke HR, Reubi JC, Merlo A. Local targeting of malignant gliomas by the diffusible peptidic vector 1,4,7,10-tetraazacyclododecane-1-glutaric acid-4,7,10-triacetic acid-substance P. Clin. Cancer Res. 2006;12:3843–3850. doi: 10.1158/1078-0432.CCR-05-2820. [DOI] [PubMed] [Google Scholar]
- 8.Nikula TK, McDevitt MR, Finn RD, Wu C, Kozak RW, Garmasteni K, Brechbiel MW, Curcio MJ, Pippin CG, Tiffany-Jones L, Geerlings MW, Sr, Apostolidis C, Molinet R, Geerlings MW, Jr, Gansow OA, Scheinberg DA. Alpha-emitting bismuth cyclohexylbenzyl DTPA constructs of recombinant humanized anti-CD33 antibodies: Pharmacokinetics, bioactivity, toxicity, and chemistry. J. Nucl. Med. 1999;40:166–176. [PubMed] [Google Scholar]
- 9.Kumar K, Magerstadt M, Gansow OA. Lead(II) and bismuth(III) complexes of the polyazacycloalkane-N-acetic Acids nota, dota, and teta. J. Chem. Soc. Chem. Commun. 1989:145–146. [Google Scholar]
- 10.Chong HS, Lim S, Baidoo KE, Milenic DE, Ma X, Jia F, Song HA, Brechbiel MW, Lewis MR. Synthesis and biological evaluation of a novel decadentate ligand DEPA. Bioorg Med. Chem. Lett. 2008;18:5792–5795. doi: 10.1016/j.bmcl.2008.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brechbiel MW, Gansow OA. Synthesis of C-functionalized trans-cyclohexyldiethylenetri-aminepenta-acetic acids for labeling of monoclonal antibodies with the bismuth-212-α particle emitter. J. Chem. Soc. Perkin. Trans. 1992;1:1173–1178. [Google Scholar]
- 12.Milenic DE, Roselli M, Mirzadeh S, Pippin CG, Gansow OA, Colcher D, Brechbiel MW, Schlom J. In vivo evaluation of bismuth-labeled monoclonal antibody comparing DTPA-derived bifunctional chelates. Cancer Biother. Radiopharm. 2001;16:133–154. doi: 10.1089/108497801300189227. [DOI] [PubMed] [Google Scholar]
- 13.Baselga J. Phase I and II clinical trials of trastuzumab. Ann. Oncol. 2001;12:S49–S55. doi: 10.1093/annonc/12.suppl_1.s49. [DOI] [PubMed] [Google Scholar]
- 14.Agus DB, Bunn PA, Jr., Franklin W, Garcia M, Ozols RF. HER-2/neu as a therapeutic target in non-small cell lung cancer, prostate cancer, and ovarian cancer. Semin. Oncol. 2000;27:53–63. [PubMed] [Google Scholar]
- 15.Milenic DE, Garmestani K, Brady ED, Albert PS, Ma D, Abdulla A, Brechbiel MW. Targeting of HER2 antigen for the treatment of disseminated peritoneal disease. Clin. Cancer Res. 2004;10:7834–7841. doi: 10.1158/1078-0432.CCR-04-1226. [DOI] [PubMed] [Google Scholar]
- 16.Chong HS, Song HA, Kang CS, Le T, Sun X, Dadwal M, Lee HB, Lan X, Chen Y, Dai A. A highly effective bifunctional ligand for radioimmunotherapy of cancer. Chem. Commun. 2011 doi: 10.1039/c0cc05707j. In press. [DOI] [PubMed] [Google Scholar]
- 17.Brady ED, Chong HS, Milenic DE, Brechbiel MW. Development of a spectroscopy assay for bifunctional ligand-protein conjugates based on copper. Nucl. Med. Biol. 2004;31:795–802. doi: 10.1016/j.nucmedbio.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 19.Chappell LL, Ma D, Milenic DE, Garmestani K, Venditto V, Beitzel MP, Brechbiel MW. Synthesis and evaluation of novel bifunctional chelating agents based on 1,4,7,10-Tetraazacyclododecane-N,N′,N",N′″-Tetraacetic acid for radiolabeling proteins. Nucl. Med. Biol. 2003;30:581–595. doi: 10.1016/s0969-8051(03)00033-7. [DOI] [PubMed] [Google Scholar]
- 20.Atcher RW, Friedman AM, Hines JJ. An improved generator for the production of 212Pb and 212Bi from 224Ra. Int. J. Appl. Instrum. Part A. 1988;39:283–286. doi: 10.1016/0883-2889(88)90016-0. [DOI] [PubMed] [Google Scholar]
- 21.McDevitt MR, Ma D, Simon J, Frank RK, Scheinberg DA. Design and synthesis of 225Ac radioimmunotherapeuticals. Appl. Rad. Isot. 2002;57:841–847. doi: 10.1016/s0969-8043(02)00167-7. [DOI] [PubMed] [Google Scholar]
- 22.Miao Y, Hylarides M, Fisher DR, Shelton T, Moore H, Wester DW, Fritzberg AR, Winkelmann CT, Hoffman T, Quinn TP. Melanoma therapy via peptide-targeted {alpha}-radiation. Clin Cancer Res. 2005;11:5616–5621. doi: 10.1158/1078-0432.CCR-05-0619. [DOI] [PubMed] [Google Scholar]
- 23.Milenic DE, Garmestani K, Brady ED, Albert PS, Ma D, Abdulla A, Brechbiel MW. Alpha-particle radioimmunotherapy of disseminated peritoneal disease using a 212Pb-labeled radioimmunoconjugate targeting HER2. Cancer Biother Radiopharm. 2005;20:557–568. doi: 10.1089/cbr.2005.20.557. [DOI] [PubMed] [Google Scholar]
- 24.Miederer M, Henriksen G, Alke A, Mossbrugger I, Quintanilla-Martinez L, Senekowitsch-Schmidtke R, Essler M. Preclinical evaluation of the alpha-particle generator nuclide 225Ac for somatostatin receptor radiotherapy of neuroendocrine tumors. Clin Cancer Res. 2008;14:3555–3561. doi: 10.1158/1078-0432.CCR-07-4647. [DOI] [PubMed] [Google Scholar]
- 25.Ballangrud ÅM, Yang W-H, Palm S, Enmon R, Borchardt PE, Pellegrini VE, McDevitt MR, Scheinberg DA, Sgouros G. Alpha-particle emitting atomic generator (Actinium-225)-labeled trastuzumab (Herceptin) targeting of breast cancer spheroids: efficacy versus HER2/neu expression. Clin Cancer Res. 2004;10:4489–4497. doi: 10.1158/1078-0432.CCR-03-0800. [DOI] [PubMed] [Google Scholar]
- 26.Ruble G, Wu C, Squire RA, Gansow OA, Strand M. The use of 212Pb-labeled monoclonal antibody in the treatment of murine erythroleukemia. Int. J. Radiat. Oncol. Biol. Phys. 1996;34:609–616. doi: 10.1016/0360-3016(95)02119-1. [DOI] [PubMed] [Google Scholar]
- 27.Mirzadeh S, Kumar K, Gansow OA. The chemical fate of 212Bi-DOTA formed by β- decay of 212Pb-DOTA. Radiochim Acta. 1993;60:1–10. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.