Abstract
Previous work demonstrated that cystic fibrosis (CF) cells exhibit an increase in cAMP-mediated signaling as a characteristic response to lost CFTR function. Evidence for increased cAMP-mediated signaling in CF included increased phosphorylation of the cAMP response element binding protein (CREB) and elevated β-arrestin-2 (βarr2) expression. However, subsequent studies reveal that CREB activation in CF cells is independent of protein kinase- A (PKA). The goal of this study is to test the hypothesis that elevated βarr2 expression leads to increased CREB activation in a PKA-independent mechanism. βarr2-GFP expressing tracheal epithelial cells (βarr2-GFP) exhibit an increase of pCREB content and subsequent CRE activation compared to GFP expressing control cells. βarr2 activation of the ERK cascade represents a candidate mechanism leading to CREB activation. ERK exhibits increased activation in βarr2-GFP cells compared to cont-GFP cells and ERK inhibition diminishes CRE activation in both GFP and βarr2-GFP cells. To test directly whether CREB regulation in CF is βarr2-dependent, nasal epithelium excised from wt mice (Cftr +/+; βarr2 +/+), CF mice (Cftr −/−; βarr2 +/+), and DKO mice (Cftr −/−; βarr2 −/−) were analyzed for pCREB protein content. Removal of βarr2 expression from CF mice reduces both pCREB and pERK content to wt levels. These data indicate that CF-related CREB regulation is mediated directly through βarr2 expression via the ERK pathway.
Keywords: Beta-arrestin-2, cAMP response element, mitogen activated protein kinase, cystic fibrosis
CF is caused by the loss of cystic fibrosis transmembrane conductance regulator (CFTR) function, a cAMP-activated chloride channel. Discerning the cell biological manifestations of losing CFTR function is an ongoing interest. Among the manifestations of lost CFTR function on epithelial cells are a reduction in nitric oxide synthase-2 (NOS2), increased RhoA expression and activation, and altered cholesterol homeostasis both in CF cell models and in vivo (1, 2). Recently we have demonstrated that cholesterol accumulation in CF cells is reversible upon treatment with the cAMP binding competitor Rp-cAMPS, suggesting an alteration in some aspects of the cAMP-signaling pathway (3). Evidence of alterations within the cAMP pathway include elevated phosphorylated cAMP response element binding protein (pCREB) content and increased expression of β-arrestin-2 (βarr2), with βarr2 protein expression being increased in CF cell models, Cftr −/− mouse nasal epithelia (MNE), and nasal scrapes obtained from CF patients (3).
Among other functions, arrestins can serve as a regulator of cAMP-related signaling as they work in conjunction with G-protein coupled receptor kinases (GRKs) to internalize various GPCRs such as the β2-adrenergic receptor (β2-AR) (4). There is evidence that the GRK/arrestin pathway is upregulated in CF cells independent of previous studies identifying increased βarr2 expression from this group. Barnes and colleagues have demonstrated that GRK2 and GRK5 are overexpressed in CF lung tissue and that, consistent with this finding, expression levels of the β2-AR are reduced at the cell surface in CF samples (5). Studies by Sharma and Jeffery also confirm Barnes’ findings of reduced β2-AR expression at the surface of CF lung cells (6). In addition to the regulation of GPCRs, arrestins have been linked to a number of cell signaling cascades. Consistent with signaling manifestations we have identified in CF, both βarr1 and βarr2 have been shown to mediate the activation of the small GTPase RhoA in response to angiotensin and other stimuli (7, 8). RhoA function has been implicated by our work in several regulatory functions associated with CF including being responsible for reduced NOS2 expression and contributing to increased cytokine production (9, 10). Elevated βarr2 expression has also been shown to result in increased IL-8 and IL-6 production in an NF-κB-independent manner (11). The potential impact of βarr2 expression on CF related signaling events requires further investigation.
The hypothesis tested in this study is that elevated βarr2 expression in CF cells and tissues is sufficient to stimulate CREB activation. It is demonstrated that eliminating βarr2 expression in CF mice via the breeding of a Cftr/βarr2 double knock-out mouse eliminates both elevated ERK and CREB activation observed in CF mouse models further supporting the proposed mechanism. These data identify a novel function of βarr2-mediated signaling and provide mechanistic insight into how this signaling cascade influences CF signaling phenotypes in vivo.
Materials and Methods
Cell Culture
Human epithelial 9/HTEo- cells overexpressing the CFTR regulatory domain (pCEPR) and mock-transfected 9/HTEo- cells (pCEP) were a generous gift from the lab of Dr. Pamela B. Davis (Case Western Reserve University, Cleveland, OH). Cells were cared for as previously described (12). The βarr2-GFP cells and control-GFP cells were created in house using a βarr2-pEGFP-N1 construct and a control-pEGFP-N1 construct, generous gifts from the labs of Dr. Robert Lefkowitz (Duke University, Durham, NC) and Dr. Mitchell Drumm (Case Western Reserve University, Cleveland, OH), respectively. Human epithelial 9/HTEo- cells stably transfected with βarr2-pEGFP-N1 (βarr2-GFP) and control-pEGFP-N1 (control-GFP) were grown at 37°C in 95% O3-5% CO3 on Falcon 10-cm-diameter tissue culture dishes (Biosource International, Camarillo, CA) in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Carlsbad, CA) with 10% FBS containing 0.04 mM HEPES, 2 mM L-glutamine, 1 U/mL penicillin/streptomycin, and 600 μg/mL G418 (selection drug). These cells have been subcloned twice and stably maintained. The empty vector (pUSE-positive-AMP) and constitutively active human MEK (Active MEK) cells were created in house. Human epithelial 9/HTEo- cells stably transfected with the empty and Active MEK constructs (Upstate, Lake Placid, NY) were grown at 37°C in 95% O3-5% CO3 on Falcon 10-cm-diameter tissue culture dishes (Biosource International, Camarillo, CA) in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Carlsbad, CA) with 10% FBS containing 0.04 mM HEPES, 2 mM L-glutamine, 1 U/mL penicillin/streptomycin, and 600 μg/mL G418 (selection drug). These cells were not subcloned but have been maintained in selection media. Cells treated with 50 μM Rp-cAMPS (Biomol International, Plymouth Meeting, PA), 50 μM U0126 (Calbiochem, San Diego, CA) or 50 μM PD98059 (Calbiochem, San Diego, CA) were exposed for either 72 h or 24 h.
Western Immunoblotting
Antibodies against cAMP response element binding protein (CREB) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA) and phosphorylated CREB was obtained from AbCam (Cambridge, MA); antibodies against extracellular signal-regulated kinase (ERK) and phosphorylated ERK were obtained from Santa Cruz (Santa Cruz, CA). Protein samples were prepared in 60-mm-diameter tissue culture dishes of cultured cells in ice-cold RIPA buffer (25 mM Tris, 15 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycaolate, 0.1% 0.1% sodium dodecyl sulfate) for 30 min at 4°C while shaking. Cell lysates were microcentrifuged at 4°C at 14,000 rpm for 10 min. Proteins were separated using SDS-PAGE containing 30–40 μg protein on a 7.5% or 10% acrylamide gel. The samples were transferred to an Immobilon-P membrane (Millipore, Bedford, MA) at 15 V for 30 min. The blots were blocked for 1 h at room temperature in 5% nonfat dehydrated milk in PBS containing 0.1% Tween 20 (PBS-T). Blots were incubated in primary antibody at a 1:1000 dilution in blocking solution or PBS-T overnight at 4°C. Blots were washed three times for 10 min each in PBS-T and incubated in secondary antibody conjugated to horseradish peroxidase in PBS-T for 1 h at room temperature (1:3000 dilution; Sigma-Aldrich, St. Louis, MO). Blots were washed again three times for 10 min each in PBS-T before visualization using SuperSignal chemiluminescent substrate (Pierce, Rockford, IL) and the VersaDoc Imaging System (Bio-Rad, Hercules, CA). Protein expression quantification was accomplished using densitometry software on the VersaDoc (Quality One; Bio-Rad, Hercules, CA).
CRE Luciferase
The human cAMP response element (CRE)-luciferase reporter construct (CRE-Luc) (Stratagene, Santa Clare, CA) Cells were plated at a density of 50,000 cells per well in 24-well culture dishes 48 h before transfection. For each transfection, 0.6 μl of FuGene 6 (Roche, Indianapolis, IN) was incubated for 5 minutes in 100 μl of OptiMEM (Gibco BRL, Gaithersburg, MD). Then, 0.3 μg of DNA and 0.008 μg of pRL-TK were added to the FuGene-OptiMEM mix and incubated for another 15 min. One hundred microliters of diluted transfection mix were added to each well, and cells were incubated at 37°C in 95% O2-5% CO2 for 24 h. The transfection mix was replaced with either normal growth media +/− 50 μM U0126 or 50 μM PD98059 for 24 h. Cells were then lysed in 1 ml Passive Lysis Buffer (Promega, Madison, WI) at room temperature for 30 min and assayed for luciferase activity according to manufacturer’s instructions (Promega, Madison, WI). Results are expressed in relative light units (RLU) and normalized to Renilla luciferase activity.
Mice
Mice lacking CFTR expression (Cftrtm1Unc) were obtained from Jackson Laboratories (Bar Harbor, ME). CFTR wildtype mice were siblings of Cftr −/− mice. Cftr/βarr2 double knockout (DKO) mice were created in house by crossing Cftr −/− mice and βarr2 −/− mice, which were provided by Dr. Robert Lefkowitz (Duke University, Durham, NC). All mice were used between 6 and 8 wk of age. CF mice were fed a liquid diet as described by Eckman and colleagues (13). Mice were cared for in accordance with the Case Western Reserve University IACUC guidelines by the CF Animal Core Facility. Excised mouse nasal epithelium was obtained from wildtype, CF, and DKO mice and was processed as previously described (14).
Results
βarr2 –mediated regulation of CREB phosphorylation
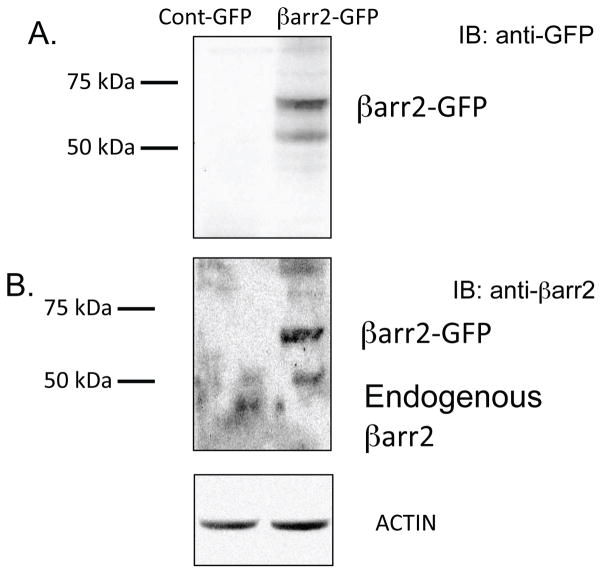
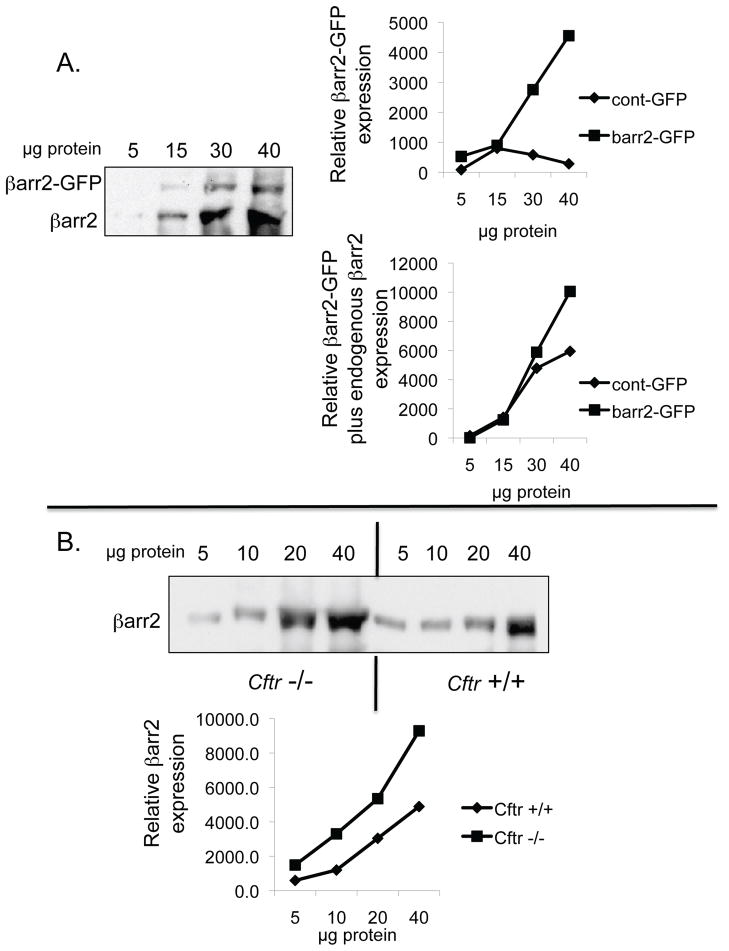
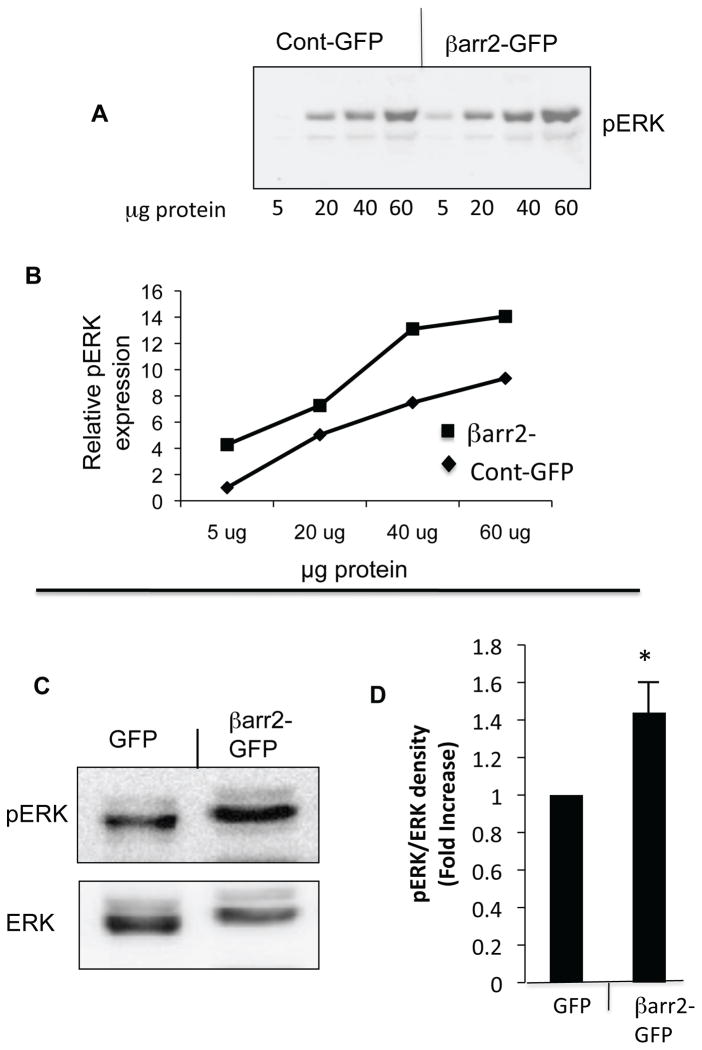
Previous work demonstrated that pCREB content and βarr2 protein expression are elevated in primary CF nasal tissue, CF cell models and Cftr −/− mouse nasal epithelium compared to respective controls (3). Evidence suggests that pCREB activation in CF-model cells is independent of PKA as neither Rp-cAMPS nor H89 impact pCREB levels (supplemental figure S1). Since βarr2 has an important regulatory role in the cAMP pathway through its regulation of β2-AR and is capable of ligand-independent signaling regulation such as signaling pathways including PI3 kinase, Src, RhoA, and ERK activation, it was hypothesized that βarr2 may influence CREB regulation in order to maintain active CREB levels during periods of β2-AR desensitization (3,7,8 15–17, 20–24). Therefore, the impact of βarr2 on pCREB content was examined in a model where βarr2-GFP (βarr2-GFP cells) was stably expressed in 9/HTEo- cells and control cells expressing GFP alone (control-GFP cells) (figure 1). Figure 1A shows GFP and βarr2-GFP expression in respective cell models probed for anti-GFP, while figure 1B examines expression as determined by anti-βarr2 immunoblot. Total expression levels of βarr2 in βarr2-GFP cells relative to endogenous βarr2 levels in control-GFP cells are comparable to βarr2 expression differences between Cftr −/− and Cftr +/+ mouse nasal epithelium (figure 2). These data suggest that the βarr2-GFP cell model exhibits a physiologically relevant level of βarr2 expression with respect to what is observed in CF models.
Figure 1.
Expression of GFP and βarr2-GFP in control-GFP and βarr2-GFP 9/HTEo- cell lines. Representative gel demonstrating GFP and βarr2-GFP expression in respective cell lines from two separate lysates.
Figure 2.
Relative expression of βarr2 in βarr2-GFP cells and in Cftr −/− mouse nasal epithelium. A) Representative gel showing both βarr2-GFP and endogenous βarr2 expression at different lysate concentrations in βarr2-GFP 9/HTEo- cells. Gel is representative of two experiments. Average of pixel density by densitometry analysis for βarr2-GFP alone and total βarr2 (βarr2-GFP + endogenous βarr2) are also shown compared to control (cont)-GFP 9/HTEo- cells. Data shown as relative expression. B) Representative gel showing endogenous βarr2 expression at different lysate concentrations in Cftr −/− and Cftr +/+ mouse nasal epithelium. Gel is representative of three experiments. Average of pixel density by densitometry analysis for βarr2- endogenous βarr2 is also shown. Data shown as relative expression.
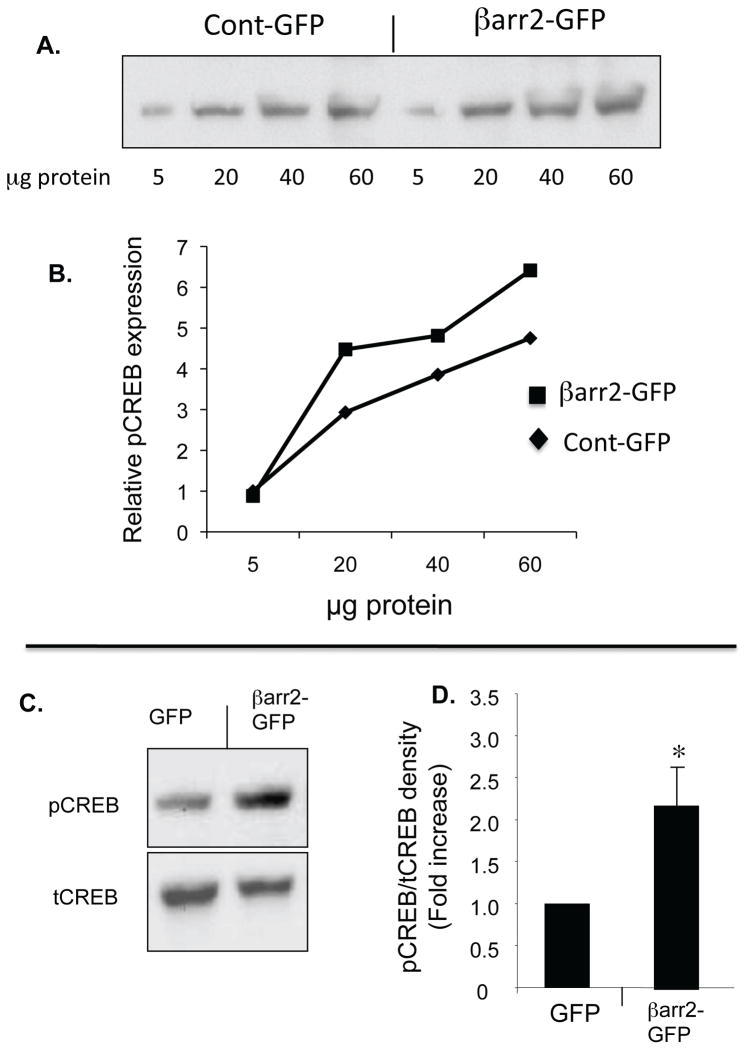
The above data demonstrate the relative extent of βarr2-GFP over expression in this cell model. Content of pCREB protein expression was determined in βarr2-GFP cells and control-GFP cells at differing lysate protein concentrations (figure 3A,B) and with respect to total CREB content (figure 3C,D). The βarr2-GFP cells exhibit a 2.2-fold increase in pCREB/total CREB ratio compared to GFP control cells (figure 3D). These data suggest that βarr2 overexpression alone is sufficient to initiate CREB activation characteristic of CF cells.
Figure 3. Expression of pCREB and tCREB in unstimulated βarr2-GFP expressing cells and control GFP expressing cells.
A) Representative gel and associated densitometry (B) showing increased pCREB content in βarr2-GFP cells compared to control-GFP cells over a range of protein concentrations. C) Representative gel showing pCREB and tCREB expression in control-GFP cells and βarr2-GFP cells. D) Densitometry analysis of pCREB/tCREB ratios using 30 μg protein. Data normalized to control-GFP cells and reported as fold increase. Error bars represent SEM with n=9 for each. Significance determined by t-test, *p<0.05.
βarr2 –mediated regulation of ERK 1/2 MAP kinase
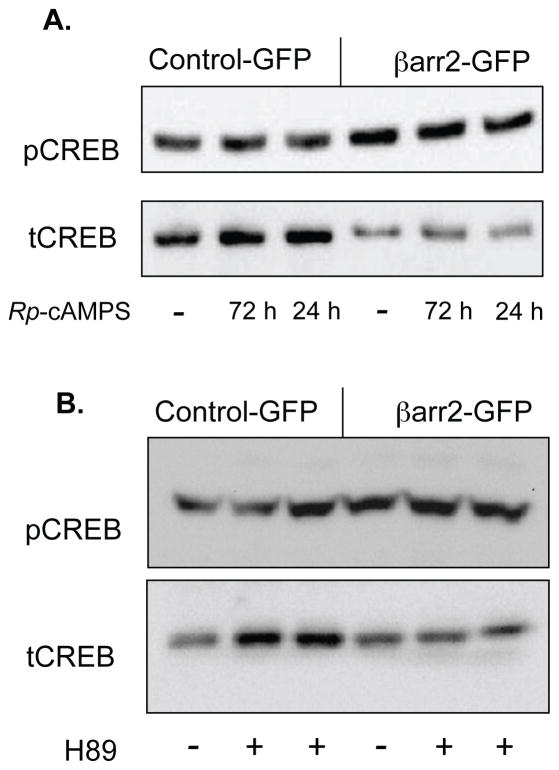
Like CF-model cells, the role of PKA was examined in CREB regulation in the βarr2-GFP cell models. Neither Rp-cAMPS nor H89 treatment significantly modulate pCREB content in control-GFP or βarr2-GFP cells suggesting a PKA-independent regulation of CREB (figure 4). Densitometry analysis reveals no significant difference in the presence of either Rp-cAMPS or H89 (not shown). Given the hypothesis that βarr2 leads to CREB activation in a PKA-independent fashion, βarr2 interactions with other pathways known to regulate CREB were explored. It has been shown that β-arrestin and ERK 1/2 can form complexes in the internalization of GPCRs and that β-arrestins are involved in the scaffolding of MAP kinase cascades, specifically for ERK 1/2 (18, 19). Because of this, we studied the effect of overexpression of βarr2 on the activation and phosphorylation of ERK 1/2. Figure 5A,B demonstrates an increase in pERK content in βarr2-GFP cells compared to control-GFP over a range of protein concentrations. Using 30 μg proteins for quantitative measurements, pCREB/tCREB ratios were found to be 1.4-fold increased in βarr2-GFP cells (figure 5C,D). Interestingly, there was inconsistency as to whether ERK1 or ERK2 exhibited more phosphorylation in βarr2-GFP cells. We have no explanation for this variance and density of both bands was used for quantification. These data suggest a strong correlation and regulatory role of βarr2 on ERK 1/2 activation.
Figure 4. Effect of PKA inhibitors on the expression of pCREB and tCREB in βarr2-GFP expressing cells and control GFP expressing cells.
A) Representative gel showing pCREB and tCREB expression in untreated and Rp-cAMPS (50 μM) treated βarr2-GFP and control-GFP cells treated for either 72 or 24 h. B) Representative gel showing pCREB and tCREB expression in untreated and H89 (75 μM) treated βarr2-GFP and control-GFP cells. Densitometry analysis reveals no significant impact of either Rp-cAMPS or H89 on pCREB content as determined by t-test.
Figure 5. Expression of pERK and tERK in unstimulated βarr2-GFP expressing cells and control GFP expressing cells.
A) Representative gel and associated densitometry (B) showing increased pERK content in βarr2-GFP cells compared to control-GFP cells over a range of protein concentrations. C) Representative gel showing pERK and tERK expression in control-GFP cells and βarr2-GFP cells. D) Densitometry analysis of pERZ/tERK ratios using 30 μg protein. Data normalized to control-GFP cells and reported as fold increase. Error bars represent SEM with n=6 for each. Significance determined by t-test, *p<0.05.
Involvement of ERK 1/2 in βarr2-mediated CREB activation
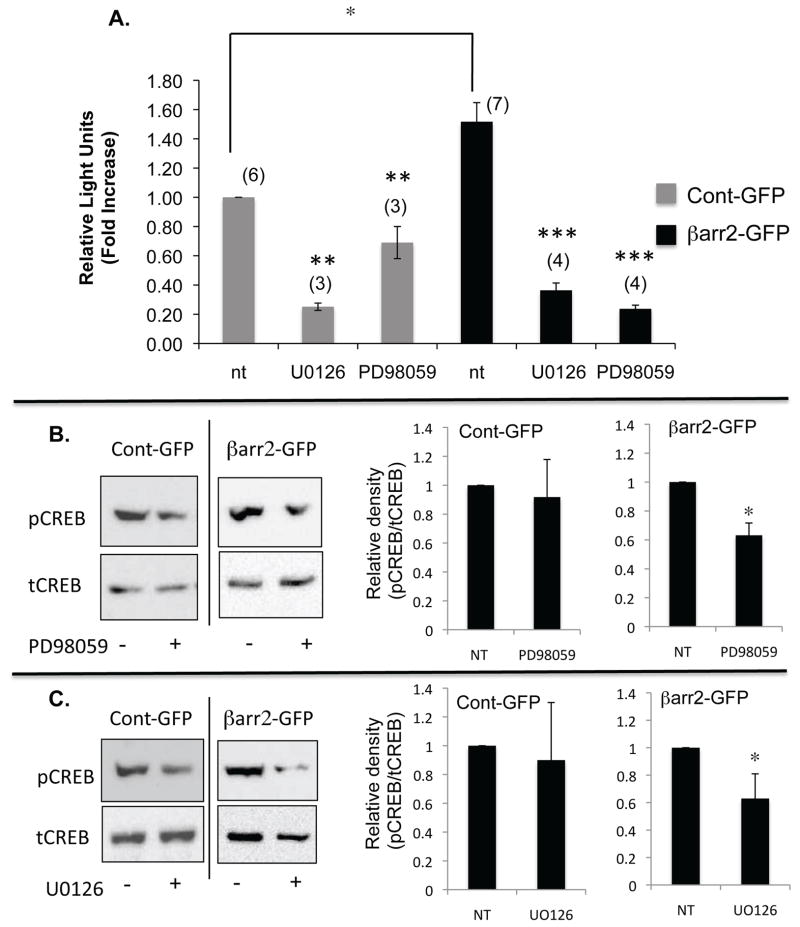
In order to further elucidate the role that ERK 1/2 may be playing in the activation and phosphorylation of CREB by βarr2, the effect of pharmacological inhibition of ERK in βarr2-GFP and cont-GFP cells was examined. Consistent with pCREB content (figure 3), CRE-luc activation is increased approximately 50% in βarr2-GFP cells compared to control-GFP cells (figure 5A). Inhibition of MEK1 with either U0126 or PD98059 results in a significant decrease in CRE activation in both control-GFP and βarr2-GFP cells (figure 6A). MEK inhibitors could interfere with several aspects of CRE stimulation, so CREB phosphorylation was examined directly. Both U0126- and PD98059-inhibitors results in a significant decrease in CREB phosphorylation compared to their untreated counterparts in βarr2-GFP cells, but no significant change is seen in control-GFP cells (figure 6B,C). These data suggest that increased expression of βarr2 may enhance a pathway utilized for basal regulation of CREB activity in epithelial cells.
Figure 6.
A) Analysis of CRE-Luc/Renilla ratios in untreated (nt) and PD98059 (50 μM) or UO126 (50 μM) treated (24 h) control-GFP and βarr2-GFP cells. Data normalized to untreated control cells and reported as fold increase. Error bars represent SEM (n shown in parentheses above each bar). Significance determined by ANOVA and significance between paired groups determined by Newman-Keuls Multiple Comparison test with *p < 0.05. B) Representative image of the effect of PD98059 on pCREB levels in Cont-GFP cells and βarr2-GFP cells. Densitometry analysis demonstrates significant reduction of pCREB/tCREB rations in βarr2-GFP cells but not in controls. Significance determined by t-test; n=6 with *p < 0.01. C) Representative image of the effect of UO126 on pCREB levels in Cont-GFP cells and βarr2-GFP cells. Densitometry analysis demonstrates significant reduction of pCREB/tCREB rations in βarr2-GFP cells but not in controls. Significance determined by t-test; n=6 with *p < 0.05.
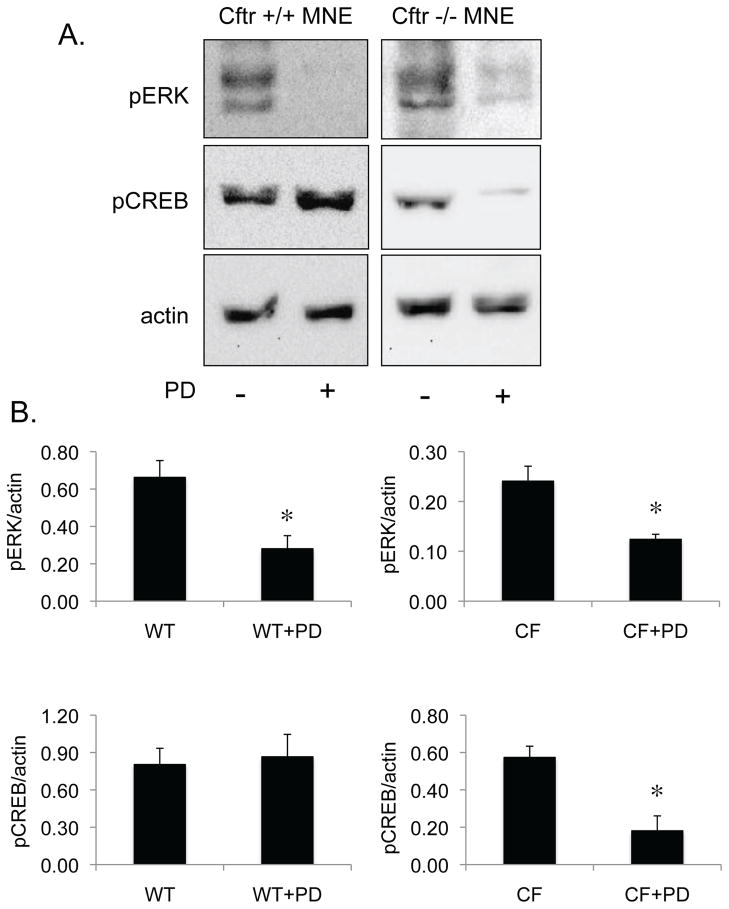
To examine the impact of MEK1/ERK regulation of CREB in primary tissue, nasal epithelial tissue from Cftr +/+ or Cftr −/− mice was excised and placed in media with our without the MEK1 inhibitor PD98059 (50 μM) overnight. Based on the above results, it was hypothesized that PD98059 would reduce pCREB content in CF but not in wt mice since CF tissue exhibits significantly increased levels of βarr2 protein expression (figure 2). Expression of pERK content was examined as a control for PD98059 effectiveness. As shown in figure 7, PD98059 reduces pERK content in both wt and CF mouse nasal tissue. However, pCREB content is only reduced in CF tissue in the presence of PD98059. These data do not demonstrate a direct role of βarr2 in the regulation of CREB, but are consistent with above data that show systems that over express βarr2 protein exhibit ERK-sensitive regulation of CREB phosphorylation.
Figure 7.
Effect of PD98059 on CREB phosphorylation in Cftr +/+ and Cftr −/− mouse nasal epithelium (MNE). A) Representative gel showing pERK, pCREB, and actin content in Cftr +/+ (WT) and Cftr −/− (CF) MNE with and without 18 h exposure to PD98059 (PD, 50 μM). B) Densitometry analysis of pERK/actin and pCREB/actin ratios in MNE of WT and CF mice. Error bars represent SEM with n=3. Significance determined by t-test with *p < 0.05.
Impact of in vivo βarr2 depletion on epithelial CREB and ERK phosphorylation
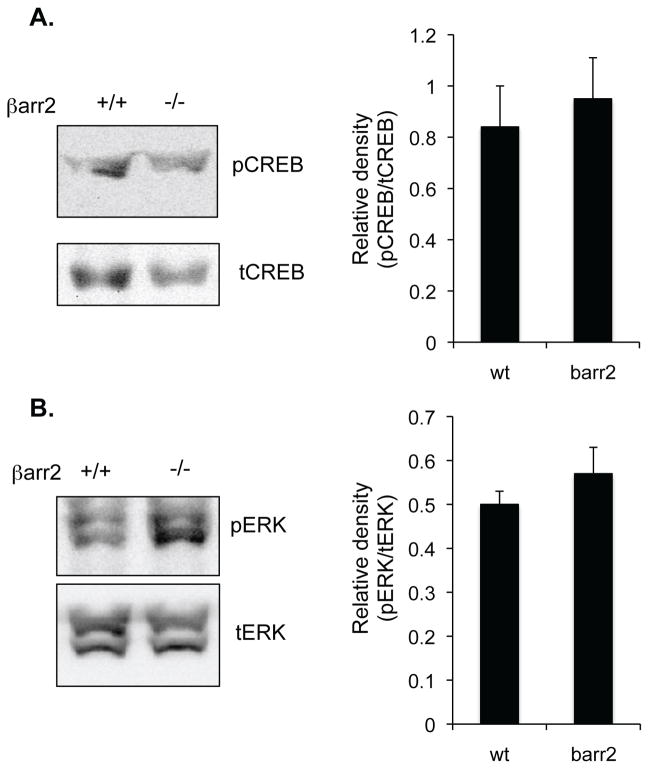
Above data demonstrate in cell models that increased expression of βarr2 can lead to increased CREB activation via the MEK/ERK pathway. Data from excised mouse nasal tissue from wt and CF mice support these data in a primary model. To more directly test the hypothesis that increased levels of βarr2 expression mediate these signaling changes in vivo, two mouse models of βarr2 depletion were examined for pCREB and pERK expression. The first model tested was βarr2 −/− mice on a C57Bl/6 background to examine the impact of losing endogenous levels of βarr2 on these outcome measures. As shown in figure 8, depletion of βarr2 expression has no effect on these expression patterns. These data suggest that endogenous levels of βarr2 expression do not influence the control of the ERK/CREB pathway in vivo.
Figure 8.
Expression of pCREB and pERK in wt and βarr2 −/− mouse nasal epithelium. A) Representative gel and densitometry analysis of pCREB/tCREB ratios in nasal epithelium of wt (βarr2 +/+) and βarr2 −/− mice.. Error bars represent SEM with n=4. Significance determined by t-test. B) Representative gel and densitometry analysis of pERK/tERK ratios in nasal epithelium of wt (βarr2 +/+) and βarr2 −/− mice.. Error bars represent SEM with n=4. Significance determined by t-test.
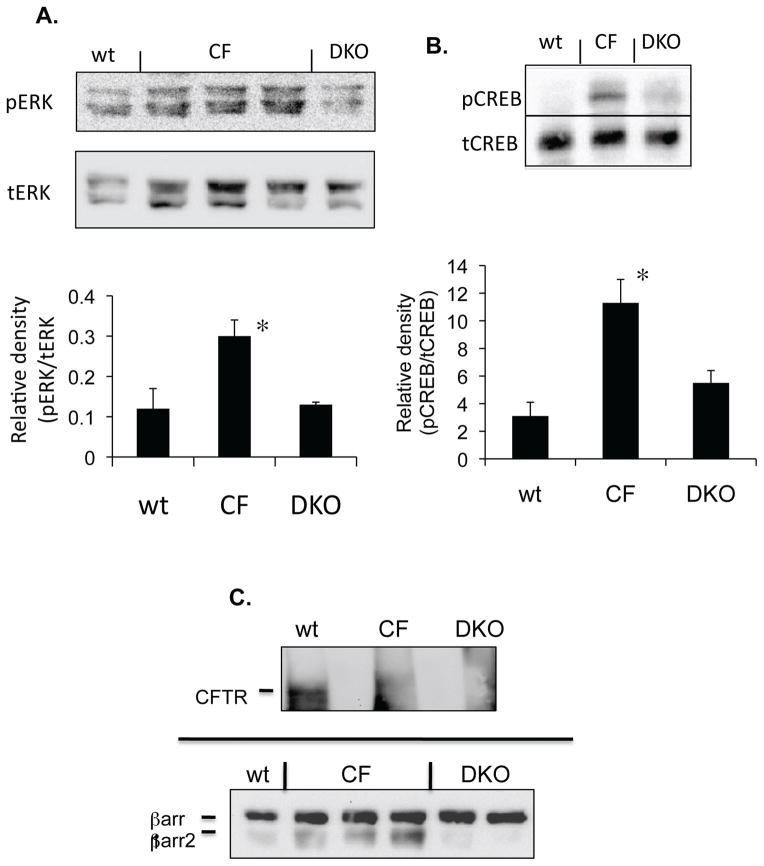
As a second model, the impact of eliminating βarr2 expression in a CF mouse model (where βarr2 protein expression is significantly elevated (3)) on pCREB and pERK content was examined. To complete this test, a Cftr/βarr2 double knock-out mouse was developed. Nasal epithelium excised from wildtype (wt), Cftr −/− (CF), βarr2 −/− (βarr2), and Cftr −/− and βarr2 −/− double knock-out (DKO) mice were analyzed for ERK and CREB phosphorylation by Western blot. CFTR and βarr2 expression in respective mouse models is shown (Figure 9C). The βarr2 blot in figure 9C is significantly over exposed so βarr2 expression in wt mouse nasal epithelium is visible. The significant increase in activation of CREB in the CF mouse (3.8-fold) is reduced to near wt levels in the Cftr/βarr2 DKO mouse (figure 9B). Similarly, pERK levels are significantly reduced to wt expression levels in DKO nasal epithelium compared to CF mice (figure 9A). These data effectively demonstrate the regulatory role of βarr2 on CF-related CREB activation and show that βarr2 expression has measurable impact on signaling regulation under in vivo conditions.
Figure 9. Effect of βarr2 deletion on CREB activation in CF mouse nasal epithelium.
A) Representative gel showing pERK and tERK expression in wt, CF, Cftr/βarr2 DKO mouse nasal epithelium. The expression of pCREB and total CREB were examined in sibling wild-type (wt) mice (n=6), Cftr −/− (CF) mice (n=4), and Cftr −/− βarr2 −/− double knock-out (DKO) mice (n=5). Densitometry analysis of pERK/tERK ratios. Significance between CF and DKO mice determined by t-test. Error bars represent SEM. Significance determined by t-test, *p < 0.01. B) Representative gel showing pCREB and tCREB expression in wt, CF, Cftr/βarr2 DKO mouse nasal epithelium. The expression of pCREB and total CREB were examined in sibling wild-type (wt) mice (n=6), Cftr −/− (CF) mice (n=4), and Cftr −/− βarr2 −/− double knock-out (DKO) mice (n=5). Densitometry analysis of pCREB/tCREB ratios. Significance determined by ANOVA and significance between paired groups determined by Newman-Keuls Multiple Comparison test with *p < 0.05. Error bars represent SEM. C) CFTR and βarr2 expression in wt, CF, and DKO mice. Representative gel demonstrating CFTR and βarr2 expression in sibling wild-type (wt) mice, Cftr −/− (CF) mice, and Cftr −/− βarr2 −/− double knock-out (DKO) mice; n=3 for each.
Discussion
The goal of this manuscript is to determine by what mechanism CREB activation is influenced by elevated βarr2 expression alone. The impetus for this study was the finding that CF cells exhibit increased expression of βarr2, a protein key to the regulation of GPCR internalization. In addition to GPCR regulation, arrestins are known to regulate a variety of signaling pathways including PI3 kinase, Src, RhoA, and ERK activation (7, 8, 17, 20–24). The hypothesis of this study is that increased βarr2 expression leads to CREB activation through a PKA-independent mechanism. It is known that CREB activation can be obtained through an ERK-dependent pathway (25), a relationship leading to the proposed mechanism of βarr2/ERK/CREB as a means to CREB activation mediated by βarr2 expression. In this manuscript we demonstrate that overexpression of βarr2 in cultured tracheal epithelial cells leads to activation of ERK, leading to ERK-dependent activation of CREB. To our knowledge, this is the first report of a regulatory relationship between βarr2 and CREB.
The physiological relevance of the βarr2/CREB regulatory relationship is evident in the examination of CF. CF is caused by the loss of function of the cystic fibrosis transmembrane conductance regulator (CFTR) (26). The loss of CFTR function results in a number of cellular changes demonstrated by gene array and proteomic studies (27–30). Among these findings is an increase in both βarr2 and pCREB protein content (3). To directly test the hypothesis that increased βarr2 expression is regulating CREB activation in CF, a Cftr/βarr2 double knock-out mouse was developed. Activated CREB and ERK 1/2 levels that are elevated in CF nasal tissue are reduced to near wt levels in the Cftr/βarr2 DKO mouse demonstrating the impact of βarr2 on CF-related signaling regulation.
Although the broader physiological impact of βarr2 expression in CF needs to be further elucidated, these findings are consistent with regulatory anomalies associated with the loss of CFTR function. Chronic activation of CREB and increased βarr2 expression would be expected to have adverse effects on cellular differentiation and promote a proliferation phenotype. A number of reports have demonstrated the role of CREB activation in the proliferation of various tumors (31, 32) and βarr2 expression has been associated with the increased proliferation of smooth muscle cells (33). Several studies have described just such a phenotype as being associated with CF cells. Leigh et al report that 17% of CF lung epithelial cells are proliferating cell nuclear antigen (PCNA) positive compared to just 0.2% of non-CF samples, an 85-fold increase (34). Similarly, one study has identified that nasal polyps isolated from CF patients proliferate at a higher rate than polyps from non-CF controls (35). Most directly, the Puchelle group has identified that CF cells are delayed in their ability to differentiate and proliferate faster than non-CF epithelial cells in a xenograph model of epithelial regeneration (36). The Puchelle group suggests that poorly differentiated/proliferating epithelium is a factor in excessive IL-8 production in CF cells (36). These data suggest that βarr2 expression may be a key intermediate in a number of CF cell regulatory events that are relevant to disease progression.
Several mechanistic questions are raised by results in this manuscript. Most studies invoke the need of Src activation in βarr2-mediated activation of ERK (37). We have examined the role of Src in this process and results are inconclusive. Although Src phosphorylation is increased in response to βarr2 expression, pharmacological examination of this pathway showed no influence of Src (not shown). Similarly, in vivo activation of Src in CF mouse models was not found (not shown).
The above data suggest that the observed cascade linking βarr2 to ERK and CREB activation is dependent on increased βarr2 expression. CF and βarr2-GFP cells models show a strong correlation between ERK function and CREB phosphorylation. The dependence on an elevated βarr2 expression for the aberrant regulation of these pathways is also seen in wt and βarr2 −/− mouse comparisons (figure 8). Depletion of βarr2 expression from an otherwise wt mouse has no significant impact on either ERK or CREB phosphorylation. Only in the presence of significant βarr2 expression increases in the context of CF tissue does depletion of βarr2 expression impact these pathways (figure 9).
Two candidate mechanisms for future study address these data and the role of excess βarr2 expression in these pathways. First is the possibility that endogenous GPCR ligands present in serum trigger βarr2-mediated activation of ERK as published (19). However, these ligand-induced signaling cascades are Src-dependent as discussed above (37), and Src does not appear to be involved in the pathway examined in this manuscript. A second mechanism being examined is the potential effects of βarr2 on microtubule function. It has been shown that non-visual arrestins interact with γ-tubulin and influence centrosome function (38). Depletion of either arrestin2 or arrestin3 leads to multinucleation and centrosome amplification (38). Chen and Xie have recently shown that treatment of cells with the microtubule disrupting agent podophyllotoxin leads to JNK, ERK, and CREB activation, although CREB activation was independent of ERK in this study (39). The effect of βarr2 over expression on microtubules may explain the lack of Src involvement. To begin exploring this potential mechanism, the impact of the microtubule disrupting agent colchicine on CREB regulation was examined. Colchicine treatment resulted in identical increases in CREB phosphorylation and activation of CRE-luc observed in CF cells and βarr2 expressing cells (figure S2). These supplemental data do not establish a direct link between βarr2 and CREB regulation through microtubules, but develop a hypothesis for future testing.
In conclusion, we have identified a novel mode of CREB regulation that is dependent on increased βarr2 expression and the subsequent activation of the MEK/ERK pathway. We also identify that βarr2 expression is directly responsible for increased CREB and ERK phosphorylation previously observed in CF cells and tissues. This mechanistic insight may clarify how increased βarr2 expression impacts cellular responses in CF.
Supplementary Material
Acknowledgments
This work is supported by a grant from the Cystic Fibrosis Foundation and by NIH/NHLBI grant HL080319. Technical support for this project was provided by core facilities of the cystic fibrosis center (P30 DK 27651).
The authors thank Dr. Pam Davis, Case Western Reserve University, for providing cell lines necessary for completion of this study and Dr. Robert Lefkowitz, HHMI, Duke University, for providing the GFP-tagged βarr2 construct and βarr2 knockout mice. The authors also thank Alyssa Harker and P. Bead for technical assistance.
Abbreviations
- βarr2
β-arrestin-2
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- CREB
cAMP response element binding protein
- DKO
double knock-out
- GPCR
G protein coupled receptor
- GRK
GPCR kinase
- NOS2
inducible nitric oxide synthase
- PKA
protein kinase-A
Footnotes
Supporting Information Available
Supporting information demonstrates insensitivity of pCREB levels in a cell model of cystic fibrosis to two separate PKA inhibitors, Rp-cAMPS and H89 (figure S1) and the effects of nocodazole on ERK and CREB activation (figure S2). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.White NM, Corey DA, Kelley TJ. Mechanistic Similarities Between Cultured Cell Models of Cystic Fibrosis and Niemann-Pick type C. American Journal of Respiratory Cell and Molecular Biology. 2004;31:538–543. doi: 10.1165/rcmb.2004-0117OC. [DOI] [PubMed] [Google Scholar]
- 2.White NM, Jiang D, Burgess JD, Bederman IR, Previs SF, Kelley TJ. Altered Cholesterol Homeostsis in Cultured and in vivo Models of Cystic Fibrosis. American Journal of Physiology Lung and Cell Molecular Physiology. 2007;292:L476–486. doi: 10.1152/ajplung.00262.2006. [DOI] [PubMed] [Google Scholar]
- 3.Manson ME, Corey DA, White NM, Kelley TJ. cAMP-Mediated Regulation of Cholesterol Accumulation in Cystic Fibrosis and Niemann-Pick Type C Cells. American Journal of Physiology Lung and Cell Molecular Physiology. 2008;295:L809–L819. doi: 10.1152/ajplung.90402.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perry SJ, Baillie GS, Kohout TA, McPhee I, Magiera MM, Ang KL, Miller WE, McLean AJ, Conti M, Houslay MD, Lefkowitz RJ. Targeting of cyclic AMP Degradation to beta 2-Adrenergic Receptors by beta-Arrestins. Science. 2002;298:834–836. doi: 10.1126/science.1074683. [DOI] [PubMed] [Google Scholar]
- 5.Mak JC, Chuang TT, Harris CA, Barnes PJ. Increased Expression of G Protein-Coupled Receptor Kinases in Cystic Fibrosis Lung. European Journal of Pharmacology. 2002;436:165–172. doi: 10.1016/s0014-2999(01)01625-9. [DOI] [PubMed] [Google Scholar]
- 6.Sharma RK, Jeffrey PK. Airway beta-Adrenoceptor Number in Cystic Fibrosis and Asthma. Clinical Science (London) 1990;78:409–417. doi: 10.1042/cs0780409. [DOI] [PubMed] [Google Scholar]
- 7.Barnes WG, Reiter E, Violin JD, Ren XR, Milligan G, Lefkowitz RJ. beta-Arrestin 1 and Galphaq/11 Coordinately Activate RhoA and Stress Fiber Formation following Receptor Stimulation. Journal of Biological Chemistry. 2005;280:8041–8050. doi: 10.1074/jbc.M412924200. [DOI] [PubMed] [Google Scholar]
- 8.Kim GH, Han JK. Essential role for beta-arrestin 2 in the regulation of Xenopus convergent extension movements. EMBO J. 2007;26:2513–2526. doi: 10.1038/sj.emboj.7601688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kreiselmeier NE, Kraynack NC, Corey DA, Kelley TJ. Statin-Mediated Correction of Stat1 Signaling and Inducible Nitric Oxide Synthase Expression in Cystic Fibrosis Epithelial Cells. American Journal of Physiology. 2003;285:L1286–L1295. doi: 10.1152/ajplung.00127.2003. [DOI] [PubMed] [Google Scholar]
- 10.Perez A, Issler AC, Cotton CU, Kelley TJ, Verkman AS, Davis PB. CFTR inhibition mimics the cystic fibrosis inflammatory profile. Am J Physiol Lung Cell Mol Physiol. 2007;292:L383–395. doi: 10.1152/ajplung.00403.2005. [DOI] [PubMed] [Google Scholar]
- 11.Fan H, Luttrell LM, Tempel GE, Senn JJ, Halushka PV, Cook JA. Beta- Arrestins 1 and 2 Differentially Regulate LPS-Induced Signaling and Pro-Inflammatory Gene Expression. Molecular Immunology. 2007;44:3092–3099. doi: 10.1016/j.molimm.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perez A, Risma KA, Eckman EA, Davis PB. Overexpression of R Domain Eliminates cAMP-Stimulated Cl− Secretion in 9/HTEo- Cells in Culture. American Journal of Physiology. 1996;271:L85–L92. doi: 10.1152/ajplung.1996.271.1.L85. [DOI] [PubMed] [Google Scholar]
- 13.Eckman EA, Cotton CU, Kube DM, Davis PB. Dietary Changes Improve Survival of CFTR S489X Homozygous Mutant Mouse. American Journal of Physiology. 1995;269:L625–L630. doi: 10.1152/ajplung.1995.269.5.L625. [DOI] [PubMed] [Google Scholar]
- 14.Kelley TK, Elmer HE. In vivo Alterations of Interferon Regulatory Factor-1 and Protein Inhibitor of Activated Stat1 Protein Levels in Cystic Fibrosis Epithelium. Journal of Clinical Investigation. 2000;106:403–410. doi: 10.1172/JCI9560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freedman NJ, Lefkowitz RJ. Desensitazation of G Protein-Coupled Receptors. Recent Progress in Hormone Research. 1996;51:319–351. [PubMed] [Google Scholar]
- 16.Reiter E, Lefkowitz RJ. GRKs and β-Arrestins: Roles in Receptor Silencing, Trafficking and Signaling. TRENDS in Endocrinology and Metabolism. 2006;17:159–165. doi: 10.1016/j.tem.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Luttrell LM, Ferguson SSG, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, Lin F-T, Kawakatsu H, Owada K, Luttrell DK, Caron MG, Lefkowitz RJ. β-Arrestin-Dependent Formation of β2 Adrenergic Receptor-Src Protein Kinase Complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- 18.DeFea KA, Vaughn ZD, O’Bryan EM, Nishijima D, Dery O, Bunnett NW. The Proliferative and Antiapoptotic Effects of Substance P are Facilitated by Formation of a β-arrestin-dependent Scaffolding Complex. Proceedings of the National Academy of Science USA. 2000;97:11086–11091. doi: 10.1073/pnas.190276697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeFea KA, Zalvesky J, Thoma MS, Dery O, Mullins RD, Bunnett NW. β-arrestin-dependent Endocytosis of Proteinase-Activated Receptor 2 is Required for Intracellular Targeting of Activated ERK 1/2. Journal of Cell Biology. 2000;148:1267–1281. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahn S, Kim J, Hara MR, Ren XR, Lefkowitz RJ. {beta}-Arrestin-2 Mediates Anti-apoptotic Signaling through Regulation of BAD Phosphorylation. J Biol Chem. 2009;284:8855–8865. doi: 10.1074/jbc.M808463200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rey A, Manen D, Rizzoli R, Caverzasio J, Ferrari SL. Proline-rich motifs in the parathyroid hormone (PTH)/PTH-related protein receptor C terminus mediate scaffolding of c-Src with beta-arrestin2 for ERK1/2 activation. J Biol Chem. 2006;281:38181–38188. doi: 10.1074/jbc.M606762200. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi H, Narita Y, Nishida M, Kurose H. Beta-arrestin2 enhances beta2-adrenergic receptor-mediated nuclear translocation of ERK. Cell Signal. 2005;17:1248–1253. doi: 10.1016/j.cellsig.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 23.Miyatake M, Rubinstein TJ, McLennan GP, Belcheva MM, Coscia CJ. Inhibition of EGF-induced ERK/MAP kinase-mediated astrocyte proliferation by mu opioids: integration of G protein and beta-arrestin 2-dependent pathways. J Neurochem. 2009;110:662–674. doi: 10.1111/j.1471-4159.2009.06156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 25.Hansen TV, Rehfeld JF, Nielsen FC. Mitogen-activated protein kinase and protein kinase A signaling pathways stimulate cholecystokinin transcription via activation of cyclic adenosine 3′,5′-monophosphate response element-binding protein. Mol Endocrinol. 1999;13:466–475. doi: 10.1210/mend.13.3.0257. [DOI] [PubMed] [Google Scholar]
- 26.Rommens JM, Iannuzzi MC, Kerem B, Drumm ML, Melmer G, Dean M, Rozmahel R, Cole JL, Kennedy D, Hidaka N. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989;245:1059–1065. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- 27.Xu Y, Liu C, Clark JC, Whitsett JA. Functional genomic responses to cystic fibrosis transmembrane conductance regulator (CFTR) and CFTR(delta508) in the lung. J Biol Chem. 2006;281:11279–11291. doi: 10.1074/jbc.M512072200. [DOI] [PubMed] [Google Scholar]
- 28.Xu Y, Clark JC, Aronow BJ, Dey CR, Liu C, Wooldridge JL, Whitsett JA. Transcriptional adaptation to cystic fibrosis transmembrane conductance regulator deficiency. J Biol Chem. 2003;278:7674–7682. doi: 10.1074/jbc.M210277200. [DOI] [PubMed] [Google Scholar]
- 29.Perez A, Davis PB. Gene profile changes after Pseudomonas aeruginosa exposure in immortalized airway epithelial cells. J Struct Funct Genomics. 2004;5:179–194. doi: 10.1023/B:JSFG.0000028982.59273.bd. [DOI] [PubMed] [Google Scholar]
- 30.Pollard HB, Eidelman O, Jozwik C, Huang W, Srivastava M, Ji XD, McGowan B, Norris CF, Todo T, Darling T, Mogayzel PJ, Zeitlin PL, Wright J, Guggino WB, Metcalf E, Driscoll WJ, Mueller G, Paweletz C, Jacobowitz DM. De novo biosynthetic profiling of high abundance proteins in cystic fibrosis lung epithelial cells. Mol Cell Proteomics. 2006;5:1628–1637. doi: 10.1074/mcp.M600091-MCP200. [DOI] [PubMed] [Google Scholar]
- 31.Linnerth NM, Baldwin M, Campbell C, Brown M, McGowan H, Moorehead RA. IGF-II induces CREB phosphorylation and cell survival in human lung cancer cells. Oncogene. 2005;24:7310–7319. doi: 10.1038/sj.onc.1208882. [DOI] [PubMed] [Google Scholar]
- 32.Siu YT, Jin DY. CREB--a real culprit in oncogenesis. FEBS J. 2007;274:3224–3232. doi: 10.1111/j.1742-4658.2007.05884.x. [DOI] [PubMed] [Google Scholar]
- 33.Kim J, Zhang L, Peppel K, Wu JH, Zidar DA, Brian L, DeWire SM, Exum ST, Lefkowitz RJ, Freedman NJ. Beta-arrestins regulate atherosclerosis and neointimal hyperplasia by controlling smooth muscle cell proliferation and migration. Circ Res. 2008;103:70–79. doi: 10.1161/CIRCRESAHA.108.172338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leigh MW, Kylander JE, Yankaskas JR, Boucher RC. Cell proliferation in bronchial epithelium and submucosal glands of cystic fibrosis patients. Am J Respir Cell Mol Biol. 1995;12:605–612. doi: 10.1165/ajrcmb.12.6.7766425. [DOI] [PubMed] [Google Scholar]
- 35.Hassid S, Degaute MP, Dawance S, Rombaut K, Nagy N, Choufani G, Decaestecker C, Danguy A, Salmon I, Kiss R. Determination of proliferative activity in nasal polyps. J Clin Pathol. 1997;50:923–928. doi: 10.1136/jcp.50.11.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hajj R, Lesimple P, Nawrocki-Raby B, Birembaut P, Puchelle E, Coraux C. Human airway surface epithelial regeneration is delayed and abnormal in cystic fibrosis. J Pathol. 2007;211:340–350. doi: 10.1002/path.2118. [DOI] [PubMed] [Google Scholar]
- 37.Pitcher JA, Tesmer JJ, Freeman JL, Capel WD, Stone WC, Lefkowitz RJ. Feedback inhibition of G protein-coupled receptor kinase 2 (GRK2) activity by extracellular signal-regulated kinases. J Biol Chem. 1999;274:34531–35434. doi: 10.1074/jbc.274.49.34531. [DOI] [PubMed] [Google Scholar]
- 38.Shankar H, Michal A, Kern RC, Kang DS, Gurevich VV, Benovic JL. Non-visual arrestins are constitutively associated with the centrosome and regulate centrosome function. J Biol Chem. 2010;285:8316–8329. doi: 10.1074/jbc.M109.062521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen YQ, Xie X. Podophyllotoxin induces CREB phosphorylation and CRE-driven gene expression via PKA but not MAPKs. Mol Cells. 2010;29:41–50. doi: 10.1007/s10059-010-0015-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.