Abstract
Background
Peripheral arterial disease (PAD) is common, but often not diagnosed. A biomarker index would be useful to raise suspicion of PAD, so as to trigger appropriate vascular testing and management.
Methods
The study comprised 549 subjects, 197 subjects with both coronary artery disease and peripheral arterial disease (CAD+PAD); 81 subjects with CAD only; and 262 subjects with no hemodynamically significant disease (NHSD) of the coronary or peripheral arteries. Multiple linear regression was performed to generate a biomarker panel score that could predict ABI. Logistic regression was used to investigate the relationship between disease status and the panel score as well as other risk factors (e.g. age, diabetes status, smoking status). ROC analysis was performed to test the prediction power of the biomarker panel score.
Results
Among the plasma markers tested, β2M and cystatin C had the highest correlation with ankle-brachial index, and higher than any of the conventional risk factors of age, smoking status, and diabetes status. A biomarker panel score derived from β2M, cystatin C, hsCRP, and glucose had an increased association with PAD status (OR=12.4, 95% confidence interval 6.6-23.5 for highest vs lowest quartile) which was still significant after adjusting for known risk factors (OR=7.3, 95% confidence interval 3.6-14.9 for highest vs lowest quartile).
Conclusions
After taking into account the traditional risk factors for PAD, a biomarker panel comprising β2M, cystatin C, hsCRP, and glucose adds useful information to assess the risk of disease.
Introduction
Peripheral arterial disease (PAD) affects 8 to 12 million individuals in the United States and is also prevalent in Europe and Asia (1-5). Classically, PAD causes limb fatigue or pain brought on by exertion and relieved by rest, i.e intermittent claudication, and reduces functional capacity and quality of life (6). It is frequently associated with coronary and cerebral disease (6,7). Patients with PAD are at increased risk from myocardial infarction, stroke, aortic aneurysm, and vascular death, as well as ischemic ulceration and amputation (7,8).
The high risk of vascular events in PAD is reduced by aggressive risk factor modification. In these individuals, the use of statins, angiotensin converting enzyme inhibitors, and antiplatelet therapy reduces morbidity and mortality (9). Unfortunately, PAD is underdiagnosed and undertreated. In fact, many of those affected do not manifest the classic symptomatology. Classic claudication is only noted by 10-30% of patients (6,10) and atypical leg discomfort occurs in 20-40% (11). Up to 50% of patients do not complain of leg symptoms. However, even these individuals have a reduced lifespan without aggressive treatment (6,12) (13) (14). Thus, it is important to diagnose PAD, even in the patient without classic symptomatology. Discovery of biomarkers that are highly associated with PAD would aid greatly in identifying such patients.
Biomarker index scores are increasingly used in medicine to refine diagnosis and to aid in prognostication. For example, such index scores are used to assess the risk of progressing from cirrhosis to hepatocellular carcinoma in patients infected with hepatitis virus (15,16), or to assess the likelihood that breast cancer will recur (17). Generally, these index scores perform better than individual markers. Few studies have explored combinations of markers to create a discrete index score to stratify individuals according to their risk of having PAD. None have used an agnostic proteomic profiling approach to develop a biomarker index. We conducted this study to develop an index score based on a combination of proposed biomarkers for peripheral arterial disease, including two based on proteomic discovery.
Methods
Subjects
A total of 549 subjects were investigated in the study. The subjects were randomly selected from the ongoing GenePAD study. This study of the genetic determinants of PAD comprises individuals undergoing coronary angiography at Stanford University or Mount Sinai Medical Centers. The PAD status of these individuals was not known to the investigators at the time of informed consent and recruitment into the study. Ankle-brachial index (ABI) was determined immediately after recruitment, followed by a comprehensive clinical characterization which included coronary angiography. Patients with PAD had a resting ABI of <0.90, or in those with non-compressible ankle arteries, a toe-brachial index of <0.60. Glomerular filtration rate (GFR) was estimated by the Modification of Diet in Renal Disease (MDRD) method (18).
Coronary angiograms were reviewed by an experienced angiographer blinded to the subject's ABI. A significant coronary lesion was defined as an angiographic stenosis of ≥ 60% in any vessel. The GenePAD study was funded by the National Heart, Lung and Blood Institute (NHLBI) and approved by the Stanford University and Mount Sinai School of Medicine Committees for the Protection of Human Subjects.
Measurement of markers
Venipuncture was performed on fasting subjects and serum and plasma samples were stored at -75°. Glucose, high density lipoprotein cholesterol (HDL), triglycerides and total cholesterol were all measured by standard assays using AU5400 Chemistry Immuno-Analyzer (Olympus Inc). Low density lipoprotein cholesterol (LDL) was measured by standard assay using AU640 Chemistry Immuno-Analyzer (Olympus). B2M (β2M), high sensitivity C-reactive protein (CRP) and Cystatin C assays were measured using standard nephelometry using BNII-Nephelometry system (Dade Behring Inc). All assay reagents were supplied by the relevant manufacturer with the exception of B2M nephelometric kit (The Binding Site Inc). Controls were purchased from Bio-Rad Laboratories or Cliniqa Corporation.
Data analysis and statistics
Dichotomous variables are expressed as prevalence in number and percent, and continuous data are expressed as the median (25th-75th percentiles). Univariate comparisons of risk factors and laboratory results were calculated using the Fisher exact test for dichotomous variables and using the nonparametric Mann-Whitney U test for continuous variables. Spearman coefficient of rank correlation was performed to assess the relationship of data to ABI. Calculations were performed using Prism 4.0 (Graphpad). Multiple linear regression was performed using all combinations of the markers to generate a multi-marker panel score that could predict ABI. Because the output of the linear regression analysis was positively correlated with ABI, the biomarker panel score was defined as the inverse of the linear regression output so that a higher score would indicate a higher risk. Logistic regression was used to investigate the relationship between the disease status and the biomarker panel score as well as other risk factors (e.g. age, diabetes status, smoking status). ROC analysis was performed to test the predictive power of the biomarker panel score. All subjects were assigned a score using the AHA Framingham risk score charts based on data obtained at recruitment. The odds ratio was calculated in the logistic regression analysis. R was used in the linear regression analysis. SAS was used for logistic regression analysis and odds ratio calculation. Analyze-it was used for ROC analysis.
Results
All study participants underwent coronary angiography (n = 549), and included a group with no hemodynamically significant atherosclerosis (NHSD; n=262); one with CAD + PAD (n=197); and a group with CAD alone (n=81). The NHSD group was younger than the CAD+PAD group. Also, as expected, the group with CAD + PAD had a significantly higher incidence of cardiovascular risk factors such as smoking, diabetes, and hypertension. All biochemical markers for cardiovascular risk were higher in the CAD+PAD group than in the NHSD group (Table 1).
Table 1.
Subject Demographics and Biomarkers
| NHSD (n=262) | CAD/PAD (n=197) | CAD (n=81) | NHSD vs CAD/PAD | CAD/PAD vs CAD | |
|---|---|---|---|---|---|
| Male sex (n,%) | 138 (53%) | 77 (39%) | 43 (46%) | 0.005 | 0.23 |
| Age, (years) | 63 (56-71) | 70 (64-77) | 72 (67-78) | <0.001 | 0.14 |
| Body mass index, (kg/m2) | 28.3 (24.7-33.1) | 27.9 (25.1-31.6) | 28.5 (25.9-31.8) | 0.3 | 0.32 |
| Smoking (n,%) | 119 (45%) | 135 (69%) | 45 (56%) | <0.001 | 0.05 |
| Hypertension (n,%) | 185 (71%) | 170 (86%) | 70 (86%) | <0.001 | 1.0 |
| Diabetes (n,%) | 52 (20%) | 89 (45%) | 22 (27%) | <0.001 | 0.007 |
| Resting ABI | 1.11 (1.02-1.19) | 0.79 (0.7-0.9) | 1.07 (1.0-1.14) | <0.001 | <0.001 |
| GFR | 86.3 (63.1-108) | 64.3 (46.7-84.6) | 71.4 (50.2-94.6) | <0.001 | 0.16 |
| B2M (mg/dl) | 1.67 (1.41-2.05) | 2.19 (1.7-3.4) | 1.92 (1.57-2.43) | <0.001 | 0.002 |
| Cystatin C (mg/dl) | 0.66 (0.58-0.77) | 0.83 (0.7-1.2) | 0.77 (.65-.89) | <0.001 | 0.016 |
| HsCRP (mg/dl) | 1.5 (0.6-3.7) | 2.2 (0.9-6.3) | 1.4 (0.7-3.9) | <0.001 | 0.031 |
| Triglycerides (mg/dl) | 88 (63-131) | 105 (70.2-145) | 91.1 (68.9-130) | 0.007 | 0.166 |
| Total cholesterol (mg/dl) | 150 (125-173) | 138 (110-157) | 132 (107-152) | <0.001 | 0.184 |
| LDL cholesterol (mg/dl) | 85 (66-109) | 73 (58-95) | 77 (58-94) | <0.001 | 0.808 |
| HDL cholesterol (mg/dl) | 42 (34-51) | 38 (30-45) | 37 (30–46) | 0.003 | 0.91 |
| Glucose (mg/dl) | 85.4 (79.2-96.1) | 94.6 (82.7-130) | 91.1 (80.8-102) | <0.001 | 0.085 |
Correlation analysis was performed to determine which characteristics were most highly associated with ABI. The traditional risk factors most strongly associated with PAD were diabetes status and age (Table 2). Among biochemical markers, β2M and cystatin C correlated most strongly with ABI.
Table 2.
Spearman Correlations Between Risk Factors, Biomarkers and ABI
| r | 95% CI | p | |
|---|---|---|---|
| Gender | -0.017 | -0.102, 0.069 | 0.69 |
| Age | -0.231 | -0.310, -0.148 | <0.001 |
| Body mass index, kg/m2 | 0.017 | -0.069, 0.102 | 0.694 |
| Smoking | -0.159 | -0.242, -0.075 | <0.001 |
| Hypertension | -0.148 | -0.230, -0.063 | <0.001 |
| Diabetes | -0.239 | -0.318, -0.157 | <0.001 |
| GFR | 0.238 | 0.155, 0.318 | <0.001 |
| B2M, mg/L | -0.297 | -0.373, -0.217 | <0.001 |
| Cystatin C, mg/L | -0.302 | -0.378, -0.222 | <0.001 |
| hsCRP, mg/L | -0.180 | -0.261, -0.096 | <0.001 |
| Triglycerides, mg/dL | -0.110 | -0.194, -0.025 | 0.009 |
| Total Chol, mg/dL | 0.031 | -0.055, 0.116 | 0.472 |
| LDL, mg/dL | 0.092 | 0.006, 0.176 | 0.031 |
| HDL, mg/dL | 0.001 | -0.084, 0.087 | 0.97 |
| Glucose, mg/dL | -0.200 | -0.281, -0.116 | <0.001 |
Linear regression using these variables was performed to generate indices for various combinations of two, three, and all four biomarkers. For the four marker index, each of the biomarkers is significant (p<0.001) in the model. These linear regression indices were positively correlated with ABI, i.e. a lower value of the linear regression index indicated lower ABI. The biomarker panel score was defined as the inverse of the linear regression index so that a higher biomarker panel score would indicate higher likelihood of positive PAD status. The odds ratio was calculated for each of the quartiles for each of the individual markers as well as for each of the combinations (Table 3). The panel score comprising all four markers had the highest odds ratio when comparing the highest quartile vs the lowest quartile, and its significance was still apparent even after adjusting for traditional risk factors of age, diabetes, and smoking. After adjusting for the traditional risk factors, individuals in the top quartile of the four marker index had a 7-fold greater chance of having PAD (Table 3).
Table 3.
Unadjusted and Adjusted Risks for the Diagnosis for PAD Based on Based on Biomarkers. (Data expressed as odds ratio, (95% confidence interval)
| Marker | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 |
|---|---|---|---|---|---|
| β2M | 6.1* (3.0 -12.2) | 3.2† (1.5-6.9) | 5.7* (2.8-11.6) | 5.4* (2.6-11.0) | 2.5§ (1.1-5.5) |
| Cystatin C | 5.6* (2.9-10.8) | 3.1* (1.5-6.2) | 5.3* (2.7-10.3) | 5.3* (2.7-10.3) | 2.6† (1.3-5.4) |
| hsCRP | 2.2† (1.2-3.8) | 2.8* (1.5-5.3) | 2.3† (1.3-4.0) | 1.9† (1.0-3.3) | 2.6† (1.3-4.9) |
| Glucose | 3.4* (1.9-6.0) | 1.5* (0.8-2.7) | 2.3§ (1.2-4.2) | 3.3* (1.8-5.8) | 1.8§ (0.9-3.5) |
| β2M + hsCRP | 3.2† (1.9-5.6) | 4.1† (2.3-7.5) | 3.1† (1.8-5.4) | 2.9§ (1.6-5.0) | 3.6§ (1.9-6.8) |
| β2M + Cystatin C | 5.9* (3.3-10.4) | 4.1† (2.3-7.5) | 5.6* (3.1-10.0) | 5.6* (3.1-10.0) | 3.5§ (1.9-6.7) |
| β2M + Glucose | 5.7* (3.2-10.1) | 5.7* (3.1-10.5) | 3.9* (2.1-7.2) | 5.5* (3.1-10.0) | 3.6§ (1.8-7.1) |
| hsCRP + Cystatin C | 6.9* (3.8-12.4) | 6.7* (3.6-12.6) | 6.6* (3.6-12.2) | 6.2* (3.3-11.4) | 6.1* (3.1-12.0) |
| hsCRP + Glucose | 5.9* (3.3-10.4) | 6.3* (3.4-11.5) | 4.3† (2.4-7.9) | 5.5† (3.0-9.9) | 4.1§ (2.1-7.9) |
| Cystatin C + Glucose | 6.8* (3.8-12.1) | 6.3* (3.4-11.6) | 4.9* (2.6-9.1) | 6.4* (3.5-11.6) | 3.9§ (2.0-7.8) |
| β2M + hsCRP + Cystatin C | 6.8* (3.8-12.1) | 5.2* (2.8-9.5) | 6.7* (3.7-12.2) | 6.1* (3.4-11.1) | 4.6* (2.4-8.8) |
| β2M + hsCRP + Glucose | 5.6* (3.2-9.9) | 6.4* (3.5-11.8) | 4.1† (2.3-7.5) | 5.2† (2.9-9.3) | 4.2§ (2.2-8.3) |
| hsCRP + Cystatin C + Glucose | 6.8* (3.8-12.1) | 6.9* (3.7-12.8) | 5.0* (2.7-9.3) | 6.2* (3.4-11.3) | 4.5§ (2.3-8.8) |
| β2M +Cystatin C + Glucose | 10.8* (5.9-20.1) | 8.4* (4.4-15.9) | 8.4* (4.4-16.0) | 10.0* (5.4-18.9) | 5.4* (2.7-10.8) |
| β2M + hsCRP + Cystatin C + Glucose | 12.4* (6.6-23.5) | 11.7* (6.0-22.6) | 9.4* (4.8-18.2) | 11.2* (5.9-21.4) | 7.3* (3.6-14.9) |
Model 1: Unadjusted
Model 2: Adjusted for age
Model 3: Adjusted for diabetes status
Model 4: Adjusted for smoking
Model 5: Adjusted for age, diabetes, smoking
p < 0.001
p < 0.01
p < 0.05
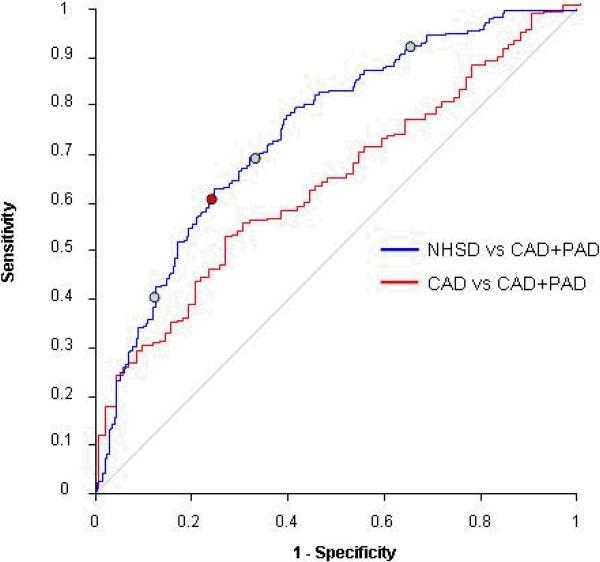
ROC analysis was performed to determine the diagnostic accuracy of the individual markers and marker combinations. The marker panel that encompassed all four markers performed the best in distinguishing the NHSD from the group subjects with both CAD and PAD. The AUC for the four marker panel was 0.747 (95% confidence interval .702-.791). At a cutoff corresponding to the 75th percentile, the index had a sensitivity of 90.4% and specificity of 36.6% (Figure 1).
Figure 1.
ROC analysis of four marker index comparing NHSD vs CAD+PAD and CAD vs CAD+PAD subjects. Blue circles indicate cutoffs at 25th%, median, and 75th % percentile. Red circle indicates point at which the sum of sensitivity and specificity is maximized.
Because these results compare patients with CAD and PAD with those with no significant hemodynamic disease, the elevation in biomarkers might be associated with the pathophysiology of CAD, of PAD, or both. Thus, the series of individuals with only CAD (n=81) was compared to the CAD + PAD group. These groups were very similar with respect to the burden of the traditional risk factors, although diabetes and tobacco use were more prevalent in the CAD + PAD group. The mean value of each of the biomarkers, cystatin C, CRP and β2M were greater in the CAD+PAD group (Table 1). The biomarker panel score was able to distinguish between the CAD group and the CAD + PAD group, although the AUC of the ROC was lower than that obtained when comparing CAD + PAD vs NHSD groups (Table 4).
Table 4.
Area Under the Curve Derived from Receiver- Operator Curves for the Diagnosis of PAD Using Combination Biomarkers
| Marker | NHSD vs CAD+PAD | CAD vs CAD+PAD |
|---|---|---|
| β2M | 0.697 (0.648, 0.746) | 0.613 (0.544, 0.681) |
| Cystatin C | 0.704 (0.655, 0.752) | 0.593 (0.524, 0.662) |
| hsCRP | 0.593 (0.54, 0.645) | 0.583 (0.511, 0.655) |
| Glucose | 0.637 (0.585, 0.69) | 0.563 (0.492, 0.633) |
| β2M + HsCRP | 0.617 (0.565, 0.668) | 0.600 (0.529, 0.671) |
| β2M + Cystatin C | 0.690 (0.641, 0.74) | 0.557 (0.486, 0.627) |
| β2M + Glucose | 0.677 (0.627, 0.726) | 0.606 (0.536, 0.675) |
| hsCRP + Cystatin C | 0.669 (0.62, 0.718) | 0.612 (0.542, 0.682) |
| hsCRP + Glucose | 0.683 (0.632, 0.733) | 0.627 (0.559, 0.696) |
| Cystatin C + Glucose | 0.709 (0.66, 0.757) | 0.623 (0.554, 0.691) |
| β2M + hsCRP + Cystatin C | 0.693 (0.644, 0.741) | 0.589 (0.52, 0.659) |
| β2M + hsCRP + Glucose | 0.691 (0.642, 0.741) | 0.639 (0.571, 0.707) |
| β2M + Cystatin C + Glucose | 0.734 (0.687, 0.781) | 0.608 (0.539, 0.676) |
| hsCRP + Cystatin C + Glucose | 0.719 (0.671, 0.766) | 0.65 (0.583, 0.717) |
| β2M + hsCRP + Cystatin C + Glucose | 0.747 (0.702, 0.791) | 0.636 (0.568, 0.703) |
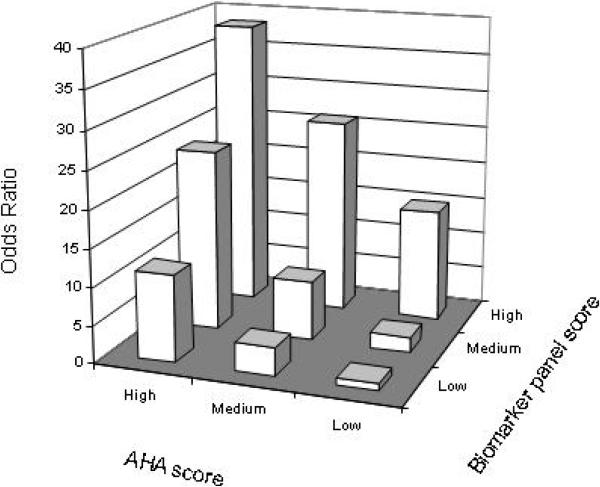
Figure 2 illustrates the odds ratio of CAD+PAD status in analyses in which study participants were stratified into nine groups according to the value of the AHA risk score and that of the four biomarker panel score. Individuals were assigned an AHA risk score using the traditional cardiovascular risk factors as described (19). AHA risk scores of <5 (low), 5 to 10 (medium), and >10 (high) were associated with increasing risk of PAD ( p=0.006 for men and <0.001 for women using the score from the linear regression by ANOVA). The odds ratio was calculated by comparing each of the 8 groups to the one with the lowest risk of disease (low AHA risk score and low biomarker panel score). As shown, there was a positive interaction between the two scores. Individuals with a low AHA risk score and a low biomarker panel score had the least risk of PAD. Individuals with a high AHA risk score and a high biomarker panel score had the greatest risk of PAD. Notably, individuals with a low AHA risk score had a considerable risk of PAD if they had a high biomarker panel score.
Figure 2.
Odds ratio of CAD+PAD status by AHA risk score and by biomarker panel score. There is a positive interaction between the two assessments of disease risk. Individuals were assigned an AHA risk score using the traditional cardiovascular risk factors as described (19). AHA risk scores of <5 (low), 5 to 10 (medium) and >10 (high) were associated with increasing risk of PAD (p=0.006 for men and p<0.001 for women using the score from the linear regression by ANOVA). The tertile cutoffs of the biomarker panel score were used to determine the risk level: low (<.991), medium (.991-1.033), and high (>1.033).
Discussion
In this case-control study, we demonstrated that a four biomarker panel comprising β2M, cystatin C, hsCRP, and glucose is associated with the presence of PAD independent of traditional risk factors. Individuals in the top quartile of the four biomarker panel score had a 7-fold greater risk of PAD. Thus, we were able to identify subjects at greatly increased risk for the presence of PAD, even after adjusting for traditional CV risk factors. Such a biomarker panel may be useful in alerting the clinician to the possibility of PAD in patients who might otherwise be undiagnosed.
These findings extend our recent observations using plasma proteomic profiling for PAD. We found that β2M and cystatin C are associated with PAD and systemic atherosclerosis (20). Several other biomarkers have been described as having increased association with PAD including CRP (21-23), pregnancy associated plasma protein A (24), lipoprotein(a) (25,26), interleukins, and fibrinogen (27). However, these markers were not studied in models that incorporated other risk factors and/or biomarkers. Cystatin C was included in our model as it was implicated as a biomarker of PAD by our initial proteomic profiling studies (20), and by a previous report (28). Several studies have shown that cystatin C is more strongly associated with all-cause and cardiovascular mortality than other measures of renal function such as serum creatinine or estimated GFR (28-31), underlining the strong link between impaired renal function and atherosclerosis. Like cystatin C, β2M is associated with renal dysfunction (32,33). In patients on dialysis, β2M levels are greatly elevated and contribute to amyloid deposition with associated cardiovascular dysfunction (34). Elevated levels of β2M have been associated with atherosclerosis (35) but not with PAD until our recent report (20). The association of cystatin C and β2M with PAD may in part reflect the association between renal insufficiency and PAD, which has been demonstrated in several studies β2M (36,37). However, we have previously shown that the relationship between β2M and PAD was largely independent of estimated creatinine clearance (20).
Another explanation for the elevation of β2M in PAD is the fact that this protein may be shed from cells with injury or inflammation. The original hypothesis driving our proteomic profiling effort was that in PAD patients, a repetitive ischemia-reperfusion injury of the limbs might cause characteristic changes in the microvasculature and tissue, including the shedding of endothelial proteins (20). Notably, β2M is not covalently attached to the major histocompatibility complex, which may explain its tendency to be released during injury.
Inflammation plays a prominent role in atherosclerotic syndromes (23). Because of its probable role in immunity and inflammation, the association of β2M with PAD or with alterations in vascular structure (38) could also be related to vascular inflammation (39). The inflammatory modulator CRP is also increased in patients with atherosclerosis and is predictive of the development of PAD (40).
We hypothesized that combining biomarkers may create a panel with higher classification accuracy than the individual biomarkers, in part because each marker may reflect different pathophysiologies contributing to PAD. CRP levels did not correlate strongly with renal function in our study (data not shown). In fact, CRP levels have been shown to be significantly related to adiposity and insulin resistance in a range of population studies, whereas renal impairment is often associated with reduced body mass, particularly in the elderly. Thus, we suggest that the use of other markers in combination with a measure of CRP may provide complementary information in this context.
The association of fasting glucose with ABI was not surprising in view of the strong relationship between diabetes and PAD. However, many patients with or at risk of atherosclerosis have elevated fasting glucose prior to the diagnosis of diabetes, largely as a manifestation of insulin resistance. Thus, the use of fasting glucose is useful in this setting and would be applicable to population based screening. Lipid levels were assessed in our study, and they did not add to the predictive ability of the model (data not shown). This finding is consistent with other studies that have found that lipids are a better marker for CAD than PAD.
After adjustment for smoking, diabetes and age, the combination marker score was able to identify a group with an odds ratio of >7 for PAD, in a population of patients referred for coronary angiography. Accordingly, this marker panel may be a useful diagnostic adjunct. Currently, clinical assessments of risk factor burden, such as the AHA risk score, incorporate “traditional” CV risk factors and are used to predict risk of future events. To the extent that the AHA risk score reflects the burden of CV risk factors, it approximates the clinician's assessment of the risk of vascular disease. Accordingly, we compared the predictive power of the biomarker panel against the accepted AHA risk score. We found a positive interaction between the biomarker panel and the AHA risk score. Subjects at highest risk were those with both a high AHA score, and a high biomarker panel score. Notably, there was a group of subjects with low AHA risk scores but high biomarker scores, that were at high risk of PAD. Thus the biomarker panel might identify a group of high risk patients that would be missed using standard CV risk factor analysis.
Currently, PAD patients are underdiagnosed, and undertreated (10,41). The primary practitioner lacks the specialized equipment and trained personnel to perform ABI measurements in the office setting. A blood test that increases the clinical index of suspicion could identify patients that merit greater scrutiny for PAD. Patients with elevated scores would be referred to vascular specialists who could provide further evaluation and appropriate management. In particular, intensive risk factor modification confers longevity in these patients and extends freedom from major adverse cardiovascular events (42).
Study limitations
Although this blood based index may be helpful in identifying patients at increased risk for systemic atherosclerosis, further studies are needed to determine its association with morbidity and mortality in larger populations, including those from lower risk populations.
References
- 1.Allison MA, Ho E, Denenberg JO, et al. Ethnic-specific prevalence of peripheral arterial disease in the United States. Am J Prev Med. 2007;32:328–33. doi: 10.1016/j.amepre.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Hasimu B, Li J, Nakayama T, et al. Ankle brachial index as a marker of atherosclerosis in Chinese patients with high cardiovascular risk. Hypertens Res. 2006;29:23–8. doi: 10.1291/hypres.29.23. [DOI] [PubMed] [Google Scholar]
- 3.Brevetti G, Schiano V, Verdoliva S, et al. Peripheral arterial disease and cardiovascular risk in Italy. Results of the Peripheral Arteriopathy and Cardiovascular Events (PACE) study. J Cardiovasc Med (Hagerstown) 2006;7:608–13. doi: 10.2459/01.JCM.0000237909.26377.9f. [DOI] [PubMed] [Google Scholar]
- 4.Hayoz D, Bounameaux H, Canova CR. Swiss Atherothrombosis Survey: a field report on the occurrence of symptomatic and asymptomatic peripheral arterial disease. J Intern Med. 2005;258:238–43. doi: 10.1111/j.1365-2796.2005.01536.x. [DOI] [PubMed] [Google Scholar]
- 5.Criqui MH, Vargas V, Denenberg JO, et al. Ethnicity and peripheral arterial disease: the San Diego Population Study. Circulation. 2005;112:2703–7. doi: 10.1161/CIRCULATIONAHA.105.546507. [DOI] [PubMed] [Google Scholar]
- 6.McDermott MM, Liu K, Greenland P, et al. Functional decline in peripheral arterial disease: associations with the ankle brachial index and leg symptoms. Jama. 2004;292:453–61. doi: 10.1001/jama.292.4.453. [DOI] [PubMed] [Google Scholar]
- 7.Steg PG, Bhatt DL, Wilson PW, et al. One-year cardiovascular event rates in outpatients with atherothrombosis. Jama. 2007;297:1197–206. doi: 10.1001/jama.297.11.1197. [DOI] [PubMed] [Google Scholar]
- 8.Newman AB, Shemanski L, Manolio TA, et al. Ankle-arm index as a predictor of cardiovascular disease and mortality in the Cardiovascular Health Study. The Cardiovascular Health Study Group. Arterioscler Thromb Vasc Biol. 1999;19:538–45. doi: 10.1161/01.atv.19.3.538. [DOI] [PubMed] [Google Scholar]
- 9.Hankey GJ, Norman PE, Eikelboom JW. Medical treatment of peripheral arterial disease. Jama. 2006;295:547–53. doi: 10.1001/jama.295.5.547. [DOI] [PubMed] [Google Scholar]
- 10.Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. Jama. 2001;286:1317–24. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 11.McDermott MM, Greenland P, Liu K, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. Jama. 2001;286:1599–606. doi: 10.1001/jama.286.13.1599. [DOI] [PubMed] [Google Scholar]
- 12.Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381–6. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 13.Vogt MT, Cauley JA, Newman AB, Kuller LH, Hulley SB. Decreased ankle/arm blood pressure index and mortality in elderly women. Jama. 1993;270:465–9. [PubMed] [Google Scholar]
- 14.Newman AB, Tyrrell KS, Kuller LH. Mortality over four years in SHEP participants with a low ankle-arm index. J Am Geriatr Soc. 1997;45:1472–8. doi: 10.1111/j.1532-5415.1997.tb03198.x. [DOI] [PubMed] [Google Scholar]
- 15.Snyder N, Nguyen A, Gajula L, et al. The APRI may be enhanced by the use of the FIBROSpect II in the estimation of fibrosis in chronic hepatitis C. Clin Chim Acta. 2007;381:119–23. doi: 10.1016/j.cca.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 16.Parkes J, Guha IN, Roderick P, Rosenberg W. Performance of serum marker panels for liver fibrosis in chronic hepatitis C. J Hepatol. 2006;44:462–74. doi: 10.1016/j.jhep.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 17.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–26. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 19.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 20.Wilson AM KE, Harada RK, Nair N, Balasubramian N, Meng X-P, Zhang F, Beck K, Olin J, Fung ET, Cooke JP. Beta 2 microglobulin as a biomarker in peripheral arterial disease : proteomic and clinical studies. Circulation. 2007 doi: 10.1161/CIRCULATIONAHA.106.683722. In Press. [DOI] [PubMed] [Google Scholar]
- 21.Vu JD, Vu JB, Pio JR, et al. Impact of C-reactive protein on the likelihood of peripheral arterial disease in United States adults with the metabolic syndrome, diabetes mellitus, and preexisting cardiovascular disease. Am J Cardiol. 2005;96:655–8. doi: 10.1016/j.amjcard.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 22.Vainas T, Stassen FR, de Graaf R, et al. C-reactive protein in peripheral arterial disease: relation to severity of the disease and to future cardiovascular events. J Vasc Surg. 2005;42:243–51. doi: 10.1016/j.jvs.2005.03.060. [DOI] [PubMed] [Google Scholar]
- 23.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–43. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 24.Mueller T, Dieplinger B, Poelz W, Haltmayer M. Increased pregnancy-associated plasma protein-A as a marker for peripheral atherosclerosis: results from the Linz Peripheral Arterial Disease Study. Clin Chem. 2006;52:1096–103. doi: 10.1373/clinchem.2005.065763. [DOI] [PubMed] [Google Scholar]
- 25.Dieplinger B, Lingenhel A, Baumgartner N, et al. Increased Serum Lipoprotein(a) Concentrations and Low Molecular Weight Phenotypes of Apolipoprotein(a) Are Associated with Symptomatic Peripheral Arterial Disease. Clin Chem. 2007;53:1298–305. doi: 10.1373/clinchem.2007.088013. [DOI] [PubMed] [Google Scholar]
- 26.Tseng CH. Lipoprotein(a) is an independent risk factor for peripheral arterial disease in Chinese type 2 diabetic patients in Taiwan. Diabetes Care. 2004;27:517–21. doi: 10.2337/diacare.27.2.517. [DOI] [PubMed] [Google Scholar]
- 27.McDermott MM, Guralnik JM, Corsi A, et al. Patterns of inflammation associated with peripheral arterial disease: the InCHIANTI study. Am Heart J. 2005;150:276–81. doi: 10.1016/j.ahj.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 28.O'Hare AM, Newman AB, Katz R, et al. Cystatin C and incident peripheral arterial disease events in the elderly: results from the Cardiovascular Health Study. Arch Intern Med. 2005;165:2666–70. doi: 10.1001/archinte.165.22.2666. [DOI] [PubMed] [Google Scholar]
- 29.Lassus J, Harjola VP, Sund R, et al. Prognostic value of cystatin C in acute heart failure in relation to other markers of renal function and NT-proBNP. Eur Heart J. 2007;28:1841–7. doi: 10.1093/eurheartj/ehl507. [DOI] [PubMed] [Google Scholar]
- 30.Ix JH, Shlipak MG, Chertow GM, Whooley MA. Association of cystatin C with mortality, cardiovascular events, and incident heart failure among persons with coronary heart disease: data from the Heart and Soul Study. Circulation. 2007;115:173–9. doi: 10.1161/CIRCULATIONAHA.106.644286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shlipak MG, Katz R, Sarnak MJ, et al. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med. 2006;145:237–46. doi: 10.7326/0003-4819-145-4-200608150-00003. [DOI] [PubMed] [Google Scholar]
- 32.Aksun SA, Ozmen D, Ozmen B, et al. Beta2-microglobulin and cystatin C in type 2 diabetes: assessment of diabetic nephropathy. Exp Clin Endocrinol Diabetes. 2004;112:195–200. doi: 10.1055/s-2004-817933. [DOI] [PubMed] [Google Scholar]
- 33.Jovanovic D, Krstivojevic P, Obradovic I, Durdevic V, Dukanovic L. Serum cystatin C and beta2-microglobulin as markers of glomerular filtration rate. Ren Fail. 2003;25:123–33. doi: 10.1081/jdi-120017475. [DOI] [PubMed] [Google Scholar]
- 34.Takayama F, Miyazaki S, Morita T, Hirasawa Y, Niwa T. Dialysis-related amyloidosis of the heart in long-term hemodialysis patients. Kidney Int Suppl. 2001;78:S172–6. doi: 10.1046/j.1523-1755.2001.59780172.x. [DOI] [PubMed] [Google Scholar]
- 35.Zumrutdal A, Demircan S, Seydaoglu G, et al. Atherosclerosis in haemodialysis patients without significant comorbidities: determinants of progression. Nephrology (Carlton) 2006;11:489–93. doi: 10.1111/j.1440-1797.2006.00694.x. [DOI] [PubMed] [Google Scholar]
- 36.de Vinuesa SG, Ortega M, Martinez P, Goicoechea M, Campdera FG, Luno J. Subclinical peripheral arterial disease in patients with chronic kidney disease: prevalence and related risk factors. Kidney Int Suppl. 2005:S44–7. doi: 10.1111/j.1523-1755.2005.09310.x. [DOI] [PubMed] [Google Scholar]
- 37.Fabsitz RR, Sidawy AN, Go O, et al. Prevalence of peripheral arterial disease and associated risk factors in American Indians: the Strong Heart Study. Am J Epidemiol. 1999;149:330–8. doi: 10.1093/oxfordjournals.aje.a009817. [DOI] [PubMed] [Google Scholar]
- 38.Saijo Y, Utsugi M, Yoshioka E, et al. Relationship of beta2-microglobulin to arterial stiffness in Japanese subjects. Hypertens Res. 2005;28:505–11. doi: 10.1291/hypres.28.505. [DOI] [PubMed] [Google Scholar]
- 39.Tzoulaki I, Murray GD, Lee AJ, Rumley A, Lowe GD, Fowkes FG. C-reactive protein, interleukin-6, and soluble adhesion molecules as predictors of progressive peripheral atherosclerosis in the general population: Edinburgh Artery Study. Circulation. 2005;112:976–83. doi: 10.1161/CIRCULATIONAHA.104.513085. [DOI] [PubMed] [Google Scholar]
- 40.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Plasma concentration of C-reactive protein and risk of developing peripheral vascular disease. Circulation. 1998;97:425–8. doi: 10.1161/01.cir.97.5.425. [DOI] [PubMed] [Google Scholar]
- 41.Okaa RK, Umoh E, Szuba A, Giacomini JC, Cooke JP. Suboptimal intensity of risk factor modification in PAD. Vasc Med. 2005;10:91–6. doi: 10.1191/1358863x05vm611oa. [DOI] [PubMed] [Google Scholar]
- 42.Beckman JA, Jaff MR, Creager MA. The United States preventive services task force recommendation statement on screening for peripheral arterial disease: more harm than benefit? Circulation. 2006;114:861–6. doi: 10.1161/CIRCULATIONAHA.105.607846. [DOI] [PubMed] [Google Scholar]