Abstract
Background
No high-quality study to date has shown that screening reduces lung cancer mortality, and expert groups do not recommend screening for asymptomatic individuals. Nevertheless, lung cancer screening tests are available in the U.S., and primary care physicians (PCPs) may have a role in recommending them to patients.
Purpose
This study describes U.S. PCPs’ beliefs about and recommendations for lung cancer screening, and examines characteristics of PCPs who recommend screening.
Methods
A nationally representative survey of practicing PCPs was conducted in 2006–2007. Mailed questionnaires assessed PCPs’ beliefs about lung cancer screening guidelines and the effectiveness of screening tests, and whether PCPs would recommend screening for asymptomatic patients. Data were analyzed in 2009.
Results
Nine hundred and sixty-two PCPs completed the survey (absolute response rate=70.6%; cooperation rate=76.8%). One quarter said that major guidelines support lung cancer screening. Two thirds said that low–radiation dose spiral CT (LDCT) is very or somewhat effective in reducing lung cancer mortality in current smokers; LDCT was perceived as more effective than chest × ray or sputum cytology. Responding to vignettes describing asymptomatic patients of varying smoking exposure, 67% of PCPs recommended lung cancer screening for at least one of the vignettes. Most PCPs recommending screening said they would use chest × ray; up to 26% would use LDCT. In adjusted analyses, PCPs’ beliefs and practice style were strongly associated with their lung cancer screening recommendations.
Conclusions
Many PCPs’ lung cancer screening beliefs and recommendations are inconsistent with current evidence and guidelines. Provider education regarding lung cancer screening’s evidence base and guideline content is indicated.
INTRODUCTION
More deaths in the U.S. are caused by lung cancer than by cancers of the breast, prostate, and colon and rectum combined.1,2 The 5-year survival rate for lung cancer, 16%, is substantially lower than that for breast (89%), prostate (99%), or colorectal cancer (64%).3 Because of the propensity to diagnose lung cancer at a late stage, the high mortality from the disease, and the U.S.’ large population of former and current smokers, there is periodic debate about the utility of screening patients for lung cancer.4–7 Lung cancer screening, however, is highly controversial. Chest × ray and sputum cytology were studied as screening tests in RCTs in the 1970s and 1980s, but not shown to significantly reduce lung cancer mortality.8–9 The recent introduction of low radiation–dose spiral CT (LDCT) has sparked renewed interest in lung cancer screening, although no high-quality study has yet demonstrated that this modality reduces lung cancer mortality. In the U.S., two large, RCTs of lung cancer screening, one of chest × ray versus usual care and the other of LDCT versus chest × ray, have completed accrual, but results assessing their impact on lung cancer mortality are not yet available.10–11 In the absence of strong supporting evidence, major expert groups in the U.S. do not recommend screening asymptomatic individuals, even those with heavy or long-term smoking histories, for lung cancer.12–14 Although evidence supporting lung cancer screening is lacking and practice guidelines do not recommend it, some studies have shown that many primary care physicians (PCPs) order chest × rays to screen patients for lung cancer.15–18 There also is a growing market in the U.S. for radiologic screening tests, including LDCT, which patients can obtain through self-referral.19–20 As the point of first contact with the healthcare system and/or coordinators of care for many patients, PCPs may have a role in recommending lung cancer screening tests to and/or interpreting their results for patients.
There are no recent national studies of PCPs’ knowledge, beliefs, and practices regarding lung cancer screening. To assess PCPs’ current knowledge, attitudes, recommendations, and practices regarding lung and three other types of cancer screening, the National Cancer Institute sponsored the National Survey of PCPs’ Recommendations and Practices for Breast, Cervical, Colorectal, and Lung Cancer Screening.21
In this report, data from the survey are used to address two aims. The first is to describe PCPs’ beliefs about and recommendations for lung cancer screening. The second is to examine characteristics of PCPs who recommend screening patients for lung cancer, including PCPs’ beliefs about clinical practice guidelines and test effectiveness as possible factors influencing their screening recommendations.
METHODS
Survey Methodology and Study Cohort
Between September 2006 and May 2007, a nationally representative sample of PCPs was surveyed. The American Medical Association’s Physician Masterfile was used as the sampling frame. Eligible respondents were non-Federal, office-based family physicians, general practitioners, general internists, and obstetrician/gynecologists aged ≤75 years with patient care as their major activity. PCPs listed as retired, deceased, or with an address outside the U.S. were excluded. One half of PCPs would receive a questionnaire on breast and cervical cancer screening and the other half a questionnaire on colorectal and lung cancer screening. For the colorectal/lung cancer survey, a systematic, stratified random sample of 2,576 PCPs was selected using the four specialty types as the sampling strata and after sorting the sampling frame database by PCPs’ age, gender, urban versus rural practice location, and U.S. Census region. PCPs received up to three mailings of the questionnaire. A $50 pre-paid honorarium was provided. Follow-up telephone calls were placed to the offices of PCPs who were sent the second and third mailings to encourage survey participation. Further details on the survey’s methodology have been published.22 A total of 1,266 PCPs completed the survey. The absolute response rate, calculated using a standard formula (RR3) approved by the American Association for Public Opinion Research,23 was 69.3%. The cooperation rate, which excludes physicians lacking valid contact information, was 75.0%. The present study focuses on the three primary care disciplines that see adult patients of both genders: family physicians, general practitioners, and general internists (n=962). The survey absolute response level for this group of PCPs was 70.6%; the cooperation level was 76.8%.
Questionnaire Items
Items asking about PCPs’ lung cancer screening beliefs and recommendations were specific to asymptomatic patients aged ≥50 years, and included chest × ray, sputum cytology, and LDCT. Items assessed perceived test effectiveness (“very effective”, “somewhat effective”, “not effective”, “don’t know”); each test effectiveness item was asked separately by patients’ smoking status (never, former, current smoker). Five vignettes were used to assess whether PCPs would recommend lung cancer screening for asymptomatic patients aged 50 years with varying smoking histories (including never smokers), and if so, with which tests. For each vignette, PCPs were asked to assume that the patient described had not been previously screened for lung cancer, had not expressed a preference for lung cancer screening in general or with a specific modality, and had no occupational exposure to lung carcinogens.
PCPs also were asked whether, to the best of their knowledge, five different national organizations (U.S. Preventive Services Task Force,12 American Cancer Society,25 American College of Radiology,26 American Thoracic Society,26 National Cancer Institute27) recommend the use of lung cancer screening in asymptomatic, average-risk patients. These organizations were selected based on their history of issuing guidance to healthcare professionals and/or patients regarding lung cancer screening tests, and to provide a broad representation of national expert groups. Response options were “yes, recommends”, “no, doesn’t recommend”, and “not sure”. PCPs then were asked whether, during the past 12 months, any of their patients had inquired about being screened for lung cancer. Those responding “yes” gave estimates of how many patients had so inquired.
A set of nine vignettes asking PCPs whether they would recommend colorectal cancer screening for patients of varying age and comorbidity was used to derive a measure of the intensity of PCPs’ colorectal cancer screening recommendations. This measure was constructed to assess whether PCPs who were intensive in their recommendations for another type of cancer screening would be more likely to recommend lung cancer screening. PCPs who reported that they recommended colorectal cancer screening for patients aged 50, 65, and 80 years with unresectable non-small cell lung cancer were categorized as high intensity in their colorectal cancer screening recommendations, while those who did not were categorized as low/moderate intensity.
Other items asked about physicians’ race/ethnicity, primary practice arrangement, practice size, patients’ insurance status, patient volume during a typical week, affiliation with a medical school, and type of medical record system used in the practice. Nonresponse was <3% for most survey items. The full survey instrumentation, including the wording of the vignettes, is available at: http://healthservices.cancer.gov/surveys/screening_rp/.
Items from the AMA Physician Masterfile
The AMA Physician Masterfile provided information on PCPs’ year of medical school graduation, medical school location (U.S. or international), gender, specialty, whether Board-certified in the specialty, and geographic location. The ZIP code of the PCP’s primary practice location was mapped to RUCA2 codes24 to measure the location’s urbanicity or rurality. These codes were categorized as urban, large rural city/town, and small/isolated small rural town.
Data Analysis
Descriptive statistics were used to characterize PCPs’ perceptions of the effectiveness of lung cancer screening tests, beliefs about lung cancer screening guidelines, and their recommendations for screening asymptomatic patients. A polytomous logistic regression model was estimated to assess the demographic, practice setting, patient, and practice style characteristics and physician beliefs associated with recommending lung cancer screening for asymptomatic patients. The model included a three-level dependent variable derived from the number of patient vignettes for which the PCP would recommend lung cancer screening: does not recommend screening for any vignette, recommends screening for 1–3 vignettes, and recommends screening for 4–5 vignettes. The ORs produced by this model compared the odds of recommending screening for one or more vignettes with the odds of not recommending screening for any.
Survey weights adjusting for undercoverage and survey nonresponse were applied in the analyses, which were conducted in 2009. The weighted data yield national estimates and their 95% CIs. SUDAAN version 9.0.1 was used in the analyses to account for the complex survey design.
RESULTS
Description of Respondents
Of the 962 family physicians, general practitioners, and general internists who participated in the survey, over half had graduated from medical school at least 20 years ago (Table 1). Over three quarters were board-certified in their specialty or U.S. medical school graduates. The majority were full or part owners of their practices. In all, 68% reported that one or more patients in the last 12 months had asked them about lung cancer screening. One third reported high colorectal cancer screening intensity.
Table 1.
Primary care physicians’ characteristics and practice environments, 2006–2007
| Unweighted n | Weighted % | |
|---|---|---|
|
Physician characteristics | ||
| Years since graduation from medical school: | ||
| <10 | 136 | 14.0 |
| 10–19 | 309 | 32.7 |
| 20–29 | 288 | 30.5 |
| ≥30 | 229 | 22.8 |
| Gender: | ||
| Male | 680 | 70.4 |
| Female | 282 | 29.6 |
| Race/ethnicity: | ||
| Non-Hispanic white | 698 | 71.9 |
| Non-Hispanic black | 32 | 3.6 |
| Hispanic | 46 | 5.2 |
| Asian | 149 | 15.3 |
| Othera | 37 | 4.0 |
| Specialty: | ||
| Family medicine | 481 | 50.7 |
| General practice | 66 | 4.4 |
| General internal medicine | 415 | 45.0 |
| Board certified: | ||
| Yes | 745 | 79.5 |
| No | 217 | 20.5 |
| International medical graduate: | ||
| Yes | 236 | 24.4 |
| No | 726 | 75.6 |
|
Practice setting and patient characteristics | ||
| Primary practice arrangement: | ||
| Full/part owner of physician practice | 521 | 53.8 |
| Employee of physician-owned practice | 93 | 9.5 |
| Employee of large medical group, HMO, or healthcare system | 162 | 17.6 |
| Employee of university hospital/clinic | 45 | 4.6 |
| Employee of other hospital/clinic | 110 | 11.3 |
| Other/missing | 31 | 3.2 |
| Practice size (# physicians): | ||
| 1 | 256 | 26.4 |
| 2–5 | 400 | 41.7 |
| 6–15 | 198 | 20.7 |
| ≥16 | 102 | 10.6 |
| Geographic location: | ||
| Urbanb | 762 | 80.2 |
| Large rural city/townc | 108 | 10.7 |
| Small/isolated small rural townd | 92 | 9.1 |
| Census region: | ||
| Northeast | 184 | 19.9 |
| Midwest | 253 | 23.9 |
| West | 205 | 22.9 |
| South | 320 | 33.3 |
| Type of medical record system used: | ||
| Full EMR | 177 | 18.7 |
| Partial EMR | 89 | 9.5 |
| Transitioning from paper to EMR | 144 | 15.2 |
| Paper charts | 545 | 55.9 |
| % patients uninsured: | ||
| 0–5 | 564 | 59.5 |
| 6–25 | 291 | 29.6 |
| ≥26 | 68 | 6.8 |
| Don’t know/missing | 39 | 4.0 |
| % patients with Medicaid: | ||
| 0–5 | 392 | 41.2 |
| 6–25 | 337 | 34.2 |
| 26–50 | 122 | 12.8 |
| >50 | 63 | 6.5 |
| Don’t know/missing | 48 | 5.2 |
|
Physician Practice Style | ||
| Has an affiliation with a medical school: | ||
| Yes | 316 | 33.8 |
| No | 641 | 65.7 |
| Patient volume during a typical week: | ||
| ≤75 | 316 | 32.7 |
| 76–100 | 303 | 31.7 |
| 101–125 | 202 | 21.3 |
| ≥126 | 126 | 12.8 |
| # patients who have asked about lung cancer screening in past 12 months: | ||
| 0 | 293 | 30.3 |
| 1–5 | 253 | 26.2 |
| 6–10 | 174 | 18.4 |
| 11–20 | 119 | 12.5 |
| >20 | 108 | 11.1 |
| Unknown | 15 | 1.5 |
| Colorectal cancer screening intensity: | ||
| Low/Moderate | 605 | 62.8 |
| High | 322 | 33.9 |
| Unknown | 35 | 3.4 |
Data source: National Survey of Primary Care Physicians’ Recommendations and Practices for Breast, Cervical, Colorectal, and Lung Cancer Screening
Includes American Indian/Alaska Native, Native Hawaiian/Other Pacific Islander, multiple races, other race, and unknown.
Rural Urban Commuting Area (RUCA) 2 codes 1.0, 1.1, 2.0, 2.1, 3.0, 4.1, 7.1
RUCA2 codes 4.0, 4.2, 5.0, 5.2, 6.0
RUCA2 codes 7.0, 7.2, 7.3, 7.4, 8.0, 8.2, 8.3, 9.0, 9.1, 10.0, 10.2, 10.4, 10.5, 10.6
Physicians’ Beliefs about Lung Cancer Screening
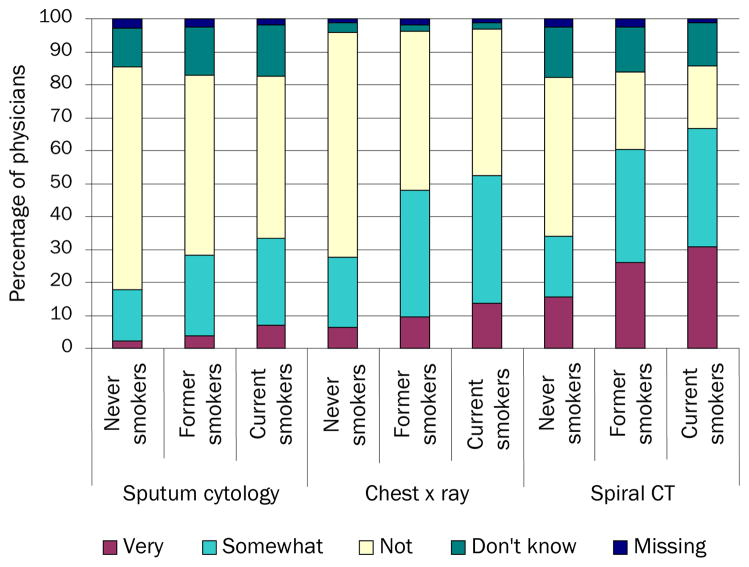
As shown in Figure 1, PCPs’ beliefs about the effectiveness of lung cancer screening tests in asymptomatic patients varied by test modality and patients’ smoking status. For all three patient types (never, former, and current smokers), lower percentages of PCPs expressed the belief that sputum cytology is very or somewhat effective in reducing lung cancer mortality compared with chest × ray and LDCT. PCPs tended to rate all three test types (sputum cytology, chest × ray, and LDCT) as more effective in current than in never smokers.
FIGURE 1.
Perceived effectiveness of tests to screen for lung cancer, by patients’ smoking status (n=962 PCPs)
Data source: National Survey of Primary Care Physicians’ Recommendations and Practices for Breast, Cervical, Colorectal, and Lung Cancer Screening
The percentages of PCPs who believed the test was very effective in reducing lung cancer mortality were highest for LDCT in current smokers (30.8%) and lowest for sputum cytology in never smokers (2.3%). Conversely, the percentages of PCPs who believed that the test was not effective in reducing lung cancer mortality were highest for chest × ray in never smokers (68.3%) and lowest for LDCT in current smokers (18.9%). Uncertainty about lung cancer screening test effectiveness was higher for LDCT and sputum cytology than for chest × ray: the percentages of PCPs reporting they did not know whether screening was effective in reducing lung cancer mortality in never, former, and current smokers was 12.9%–15.2% for LDCT, 12.0%–15.5% for sputum cytology, and 1.7%–2.8% for chest × ray.
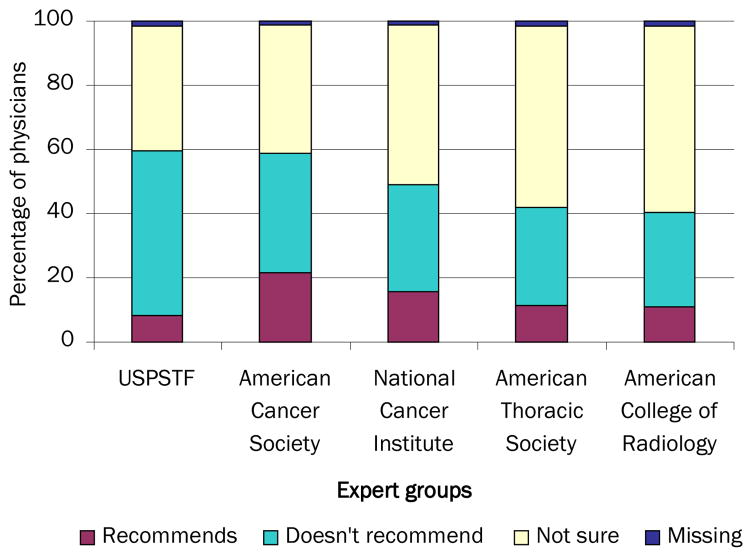
Many PCPs believed that national expert groups recommend screening asymptomatic patients for lung cancer (Figure 2). The percentages of PCPs who believed this were highest for the American Cancer Society (21.5%) and lowest for the U.S. Preventive Services Task Force (8.1%). One quarter (25.1%) believed that any expert group recommends lung cancer screening. Many PCPs also expressed uncertainty about expert groups’ lung cancer screening recommendations, with 58.2% indicating they were not sure about the recommendations of the American College of Radiology, and 38.8% reporting uncertainty about those of the U.S. Preventive Services Task Force.
FIGURE 2.
Beliefs about the lung cancer screening recommendations of expert groups (n=962 PCPs)
Data source: National Survey of Primary Care Physicians’ Recommendations and Practices for Breast, Cervical, Colorectal, and Lung Cancer Screening
USPSTF,
Physicians’ Lung Cancer Screening Recommendations
PCPs’ responses to the five vignettes presenting patients of varying smoking exposure are shown in Table 2. Most PCPs indicated they would recommend screening at least some patients for lung cancer; the propensity to recommend screening increased with patients’ smoking exposure. A minority (17.4%) recommended screening for a healthy never smoker aged 50 years; a majority (66.6%) recommended it for an otherwise healthy current smoker aged 50 years with a 20-year pack history. For all five vignettes, most PCPs who recommended screening identified chest × ray alone as the modality they would use. Although LDCT was mentioned less frequently, 13.2% indicated they would recommend this test for an otherwise healthy former smoker aged 50 years who had quit smoking 1 year ago, and 17.2% would recommend it for an otherwise healthy current smoker with a 20-year pack history. Less than 1% would recommend screening with sputum cytology alone. The combination of chest × ray and sputum cytology was mentioned by 5.3% or fewer.
Table 2.
Primary care physicians’ recommendations for lung cancer screening, by patients’ smoking status, 2006–2007 (n=962)
| Vignette A: Healthy Never Smoker Aged 50 Years | Vignette B: Healthy Never Smoker Aged 50 Years with Smoking Spouse | Vignette C: Otherwise healthy Former Smoker Aged 50 Years with 20-year pack history who quit smoking 15 years ago | Vignette D: Otherwise healthy Former Smoker Aged 50 Years with 20-year pack history who quit smoking 1 year ago | Vignette E: Otherwise healthy Current Smoker Aged 50 Years who has smoked 1 pack of cigarettes per day for 20 years | |
|---|---|---|---|---|---|
| Weighted % (95% CI) | Weighted % (95% CI) | Weighted % (95% CI) | Weighted % (95% CI) | Weighted % (95% CI) | |
| No screening | 82.1 (79.4, 84.5) | 51.3 (48.2, 54.4) | 46.9 (44.1, 49.8) | 35.9 (33.0, 38.8) | 33.2 (30.5, 36.2) |
| Screening | 17.4 (15.0, 20.0) | 48.3 (45.2, 51.4) | 52.8 (49.9, 55.6) | 63.8 (60.9, 66.7) | 66.3 (63.6, 69.4) |
| CXR only | 16.5 (14.3, 19.1) | 38.7 (35.4, 42.1) | 39.0 (35.8, 42.3) | 41.7 (38.4, 45.0) | 40.1 (37.0, 43.3) |
| SC only | 0.1 (0.0, 0.7) | 0.2 (0.0, 0.8) | 0.4 (0.1, 1.1) | 0.8 (0.4, 1.3) | 0.7 (0.4, 1.2) |
| CXR + SC | 0.2 (0.1, 0.6) | 3.2 (2.3, 4.5) | 3.3 (2.3, 4.6) | 5.3 (3.9, 7.1) | 5.0 (3.7, 6.7) |
| LDCT | 0.2 (0.1, 0.7) | 4.9 (3.6, 6.8) | 7.7 (6.1, 9.8) | 13.2 (11.1, 15.7) | 17.2 (15.0, 19.6) |
| Other | 0.2 (0.1, 0.9) | 1.0 (0.5, 1.8) | 2.1 (1.3, 3.4) | 2.4 (1.5, 3.7) | 3.3 (2.1, 5.0) |
Data source: National Survey of Primary Care Physicians’ Recommendations and Practices for Breast, Cervical, Colorectal, and Lung Cancer Screening
CXR, chest × ray; LDCT, low–radiation dose spiral CT; SC, sputum cytology
Two thirds of PCPs (66.6%) would recommend screening for at least one of the vignettes (Table 3). Of the 3.8% who recommended screening for one vignette, most (84.4%) identified the current smoker (Vignette E) as the patient they would screen. Of the 6.7% who indicated they recommended screening for two vignettes, 94.1% said they would screen the current smoker and the former smoker who quit 1 year ago (Vignettes E and D). About 12.8% responded that they recommended screening for three vignettes; of these physicians, 67.2% said they would screen the current and former smokers (Vignettes E, D, and C), and 32.8% would screen the current smoker, former smoker who quit 1 year ago, and the never smoker whose spouse smokes (Vignettes E, D, and B). Of the 26.1% who recommended screening for four vignettes, all (100%) indicated they would screen patients with any smoking exposure, active or passive (Vignettes B–E); the only patient they would not screen is the never smoker without substantial exposure to secondhand smoke (Vignette A).
Table 3.
Aggressiveness of primary care physicians’ lung cancer screening recommendations (n=962)
| Unweighted n | Weighted % (95% CI) | |
|---|---|---|
| Does not recommend for any vignette | 308 | 32.3 (29.6, 35.2) |
| Recommends for 1 vignette | 37 | 3.8 (2.7, 5.4) |
| Recommends for 2 vignettes | 64 | 6.7 (5.1, 8.7) |
| Recommends for 3 vignettes | 124 | 12.8 (10.8, 15.1) |
| Recommends for 4 vignettes | 252 | 26.1 (23.2, 29.3) |
| Recommends for all 5 vignettes | 167 | 17.2 (14.9, 19.8) |
Data source: National Survey of Primary Care Physicians’ Recommendations and Practices for Breast, Cervical, Colorectal, and Lung Cancer Screening
Characteristics of Physicians who Recommend Lung Cancer Screening
Results from the polytomous logistic regression model used to assess factors associated with PCPs who recommend screening asymptomatic patients for lung cancer are shown in Table 4. The odds of recommending screening for 1–3 and 4–5 of the patient vignettes compared with not recommending screening for any vignette were significantly greater among PCPs who are international medical graduates, believe that one or more expert groups recommend lung cancer screening, believe that one or more screening tests are very effective in reducing lung cancer mortality for former and current smokers, and whose colorectal cancer screening recommendations are intensive. In addition, the odds of recommending screening for 4–5 of the patient vignettes compared with not recommending screening for any vignette were significantly greater for PCPs who lack Board certification or who are full or part owners of their practices.
Table 4.
Polytomous logistic regression model assessing factors associated with the aggressiveness of primary care physicians’ lung cancer screening recommendationsa (n=962)
| Recommends for 1–3 Vignettes vs Does not recommend for any Vignette |
Recommends for 4–5 Vignettes vs Does not recommend for any Vignette |
|
|---|---|---|
| OR 95% CI | OR 95% CI | |
|
Physician Characteristics | ||
| Board certified | ||
| Yes | 1.0 | 1.0 |
| No | 1.0 (0.5, 2.0) | 2.4 (1.3, 4.3) |
| International medical graduate | ||
| No | 1.0 | 1.0 |
| Yes | 2.1 (1.2, 3.7) | 2.2 (1.3, 3.6) |
|
Practice Setting Characteristics | ||
| Primary practice arrangement | ||
| Employee of practice/other/missing | 1.0 | 1.0 |
| Full/part owner of practice | 1.1 (0.7, 1.9) | 1.6 (1.0, 2.5) |
|
Physician Beliefs and Practice Style | ||
| Believes any expert group recommends lung cancer screening | ||
| No | 1.0 | 1.0 |
| Yes | 2.1 (1.1, 3.8) | 5.0 (2.9, 8.6) |
| Believes any screening test2 is very effective in reducing lung cancer mortality for never smokers | ||
| No | 1.0 | 1.0 |
| Yes | 0.4 (0.1, 1.4) | 1.2 (0.4, 4.1) |
| Believes any screening test2 is very effective in reducing lung cancer mortality for former smokers | ||
| No | 1.0 | 1.0 |
| Yes | 4.5 (1.6, 12.5) | 5.5 (1.9, 15.5) |
| Believes any screening testb is very effective in reducing lung cancer mortality for current smokers | ||
| No | 1.0 | 1.0 |
| Yes | 2.6 (1.3, 5.5) | 3.7 (1.5, 8.8) |
| Intensity of colorectal cancer screening recommendations | ||
| Low/moderate | 1.0 | 1.0 |
| High | 2.2 (1.4, 3.6) | 3.0 (1.9, 4.7) |
| Unknown | 2.7 (1.0, 7.1) | 1.1 (0.3, 3.6) |
Data source: National Survey of Primary Care Physicians’ Recommendations and Practices for Breast, Cervical, Colorectal, and Lung Cancer Screening
Other model covariates included physician gender, race/ethnicity, specialty, and medical school affiliation; practice size, geographic location, Census region, type of medical record system used, % of patients who are uninsured, % of patients with Medicaid, weekly patient volume, and # patients who have asked about lung cancer screening in the past 12 months. These covariates were not significant in the multivariate model.
Chest × ray, sputum cytology, or low–radiation dose spiral CT
DISCUSSION
This study has several important findings. First, the lung cancer screening beliefs and recommendations of many U.S. PCPs are inconsistent with current evidence and guidelines. Second, many PCPs mistakenly believe that lung cancer screening’s effectiveness in reducing mortality increases with patients’ exposure to cigarette smoking; scientific evidence does not at present support this belief. Finally, factors associated with PCPs who recommend lung cancer screening include the belief that expert groups recommend it; the belief that screening is very effective in reducing lung cancer mortality in current or former smokers; and a propensity to intensively screen for cancer elsewhere.
To our knowledge, this is the first national study of PCPs’ lung cancer screening beliefs and recommendations conducted in the U.S. Although two prior national surveys of PCPs’ attitudes and practices related to early cancer detection were fielded in the 1980s, for lung cancer screening, they ascertained only whether PCPs had ever ordered chest × rays.15–16 The current study reports contemporary data and includes the primary modalities that have been considered for lung cancer early detection: chest × ray, sputum cytology, and LDCT.
A majority of U.S. PCPs believed that chest × ray and LDCT are effective in reducing lung cancer mortality among current smokers, even though RCTs have not shown this. One quarter of PCPs believed that one or more national expert groups recommend lung cancer screening, even though no major expert group currently does so.12–14 Most PCPs indicated they would recommend screening patients who were former or current smokers for lung cancer, with two thirds reporting they would screen an otherwise healthy current smoker aged 50 years with a 20-year pack history. Although most PCPs who recommended screening patients for lung cancer said they would use chest × ray, up to 26% would use LDCT. PCPs’ propensity to recommend lung cancer screening increased with patients’ smoking exposure.
Our multivariate modeling results showed PCPs’ beliefs about practice guidelines and test effectiveness and propensity to intensively screen for another type of cancer to be strongly associated with their lung cancer screening recommendations. They corroborate an earlier, much smaller study demonstrating that aggressive cancer screening behavior among PCPs is common and related to physician beliefs.28 The current study also showed that only a few physician, patient, or practice setting characteristics were associated with PCPs who recommend lung cancer screening. These included not having Board certification, being an international medical graduate, and being a practice owner. The latter finding may be related to financial benefit derived from referring patients to a service such as chest × ray in which the physician has an ownership interest; caution is needed in this interpretation, though, because the current survey did not ascertain onsite availability of lung cancer screening tests in PCPs’ practices.
Why do most PCPs believe in screening patients with smoking exposure for lung cancer when current evidence and guidelines do not support this practice? One possible explanation is the high mortality rate from lung cancer due to its generally late-stage detection and PCPs’ desire to spare patients from premature death by applying widely available technologies such as chest × ray and LDCT. Another possible explanation is the highly publicized, although controversial and later contradicted, publication in October 2006 of a case series study showing improved survival (but not lung cancer mortality reduction) for patients with smoking exposure who underwent lung cancer screening with LDCT.29–31 Even though the International Early Lung Cancer Action Project study29 is not a RCT, it has been cited to support statements that lung cancer screening saves lives.32 Some PCPs may have interpreted the study’s results as sufficiently compelling to support a clinical policy of screening patients with smoking exposure for lung cancer. More studies are needed to ascertain the specific influence of these and other factors on PCPs’ lung cancer screening beliefs and practices
Our study also suggests another reason for PCPs’ support of lung cancer screening: most lacked familiarity with clinical practice guidelines for lung cancer screening, which are consistent in stating that current evidence does not support screening any asymptomatic patient, regardless of smoking exposure. Because there are over 2000 publicly available practice guidelines covering a wide variety of clinical services,26, 33 maintaining currency with practice guidelines is a daunting challenge for physicians. The proliferation of guidelines may negatively affect physicians’ adherence to them.34 The current results indicate the need for provider education regarding lung cancer screening’s evidence base and guideline content, particularly for PCPs who are not Board-certified or are international medical graduates, since these physicians were more likely than their Board-certified or U.S. medical graduate counterparts to recommend lung cancer screening. Academic detailing,35–36 or even a “counter-detailing” approach, may have utility in offsetting supportive attitudes toward and beliefs about lung cancer screening as well as the influence of disease advocacy groups and technology availability that promote or facilitate screening. Additional research is also needed to elucidate factors influencing PCPs’ perceptions of and responses to lung cancer screening guidelines, and to develop ways to improve understanding of these guidelines.
Strengths of this study include the current survey’s large sample size and the high level of response. A limitation is that it is based on physicians’ reports of their recommendations and practices; self-reported data were not validated with other data sources such as medical records or claims. Physicians’ lung cancer screening recommendations were assessed using vignettes. Clinical vignettes, however, have been shown to be a valid and cost-effective method for assessing the quality and processes of clinical care, including cancer screening.37–39 Comparisons of physicians’ responses to clinical vignettes with their interactions with standardized patients and documentation in medical records have shown vignettes to yield data comparable to those of standardized patients, and medical records to underascertain care delivered.37–39
Lung cancer screening remains an unproven technology. Information about utilization of lung cancer screening tests in the U.S. is quite limited, and national data sources providing population estimates of cancer screening use, such as the National Health Interview Survey, to date have not included these tests. The present, nationally representative study provides new data on and a unique look at U.S. primary care physicians’ perspectives regarding lung cancer screening, as well as baseline data against which future analyses can be compared. The general public in the U.S. appears to have an overly positive view of cancer screening and limited understanding of its potential harms.40 Possible harms associated with lung cancer screening include overdiagnosis of lung cancer and other conditions, false positive results that lead to expensive and invasive diagnostic procedures, and radiation exposure.41–43 PCPs are an important information source about and access point for cancer screening. As this study’s results indicate, PCPs’ beliefs influence their lung cancer screening recommendations. Both provider education regarding lung cancer screening’s evidence base and guideline content and future research to explore additional factors that may contribute to a lack of guideline awareness and adherence among many PCPs are indicated.
Acknowledgments
Funding support for this study was provided by the National Cancer Institute (contract number N02-PC-51308), the Agency for Healthcare Research and Quality (inter-agency agreement numbers Y3-PC-5019-01 and Y3-PC-5019-02), and the CDC (inter-agency agreement number Y3-PC-6017-01).
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Cancer Institute or the CDC.
Footnotes
No financial disclosures were reported by the authors of this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Cancer Statistics Working Group. Atlanta: USDHHS, CDC, and National Cancer Institute; 2009. U.S. Cancer Statistics: 1999–2005 Incidence and Mortality Web-based Report. Available at: www.cdc.gov/uscs. [Google Scholar]
- 3.Miser WF. Cancer screening in the primary care setting: the role of the primary care physician in screening for breast, cervical, colorectal, lung, ovarian, and prostate cancers. Prim Care Clin Office Pract. 2007;34:137–167. doi: 10.1016/j.pop.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Petty TL. Screening strategies for early detection of lung cancer: the time is now. JAMA. 2000;284:1977–1980. doi: 10.1001/jama.284.15.1977. [DOI] [PubMed] [Google Scholar]
- 5.Frame PS. Routine screening for lung cancer? Maybe someday, but not yet. JAMA. 2000;284:1980–1983. doi: 10.1001/jama.284.15.1980. [DOI] [PubMed] [Google Scholar]
- 6.Carlow DR. New approaches to lung cancer: changing role for family physicians. Can Fam Phys. 2001;47:469–471. [PMC free article] [PubMed] [Google Scholar]
- 7.Lee CI, Forman HP. CT screening for lung cancer: implications on social responsibility. AJR. 2007;188:297–298. doi: 10.2214/AJR.07.5212. [DOI] [PubMed] [Google Scholar]
- 8.Berlin NI. Overview of the NCI Cooperative Early Lung Cancer Detection Program. Cancer. 2000;89:2349–2351. doi: 10.1002/1097-0142(20001201)89:11+<2349::aid-cncr6>3.3.co;2-w. [DOI] [PubMed] [Google Scholar]
- 8.Marcus PM, Bergstrahl EJ, Fagerstrom RM, et al. Lung cancer mortality in the Mayo Lung Project: Impact of extended follow-up. J Natl Cancer Inst. 2000;92:1308–1316. doi: 10.1093/jnci/92.16.1308. [DOI] [PubMed] [Google Scholar]
- 10.National Cancer Institute. About Prostate, Lung, Colorectal & Ovarian Cancer Screening Trial. Available at: http://prevention.cancer.gov/programs-resources/groups/ed/programs/plco/about.
- 11.National Cancer Institute. National Lung Screening Trial. Available at: http://www.cancer.gov.NLST.
- 12.U.S. Preventive Services Task Force. Lung cancer screening: recommendation statement. Ann Intern Med. 2004;140:738–739. doi: 10.7326/0003-4819-140-9-200405040-00014. [DOI] [PubMed] [Google Scholar]
- 13.Smith RA, Cokkinides V, Brawley OW. Cancer screening in the U.S. 2009: a review of current American Cancer Society guidelines. CA Cancer J Clin. 2009;59:27–41. doi: 10.3322/caac.20008. [DOI] [PubMed] [Google Scholar]
- 14.Bach PB, Silvestri GA, Hanger M, Jett JR. Screening for lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132:69S–77S. doi: 10.1378/chest.07-1349. [DOI] [PubMed] [Google Scholar]
- 15.American Cancer Society. Survey of physicians’ attitudes and practices in early cancer detection. CA Cancer J Clin. 1985;35:197–213. doi: 10.3322/canjclin.35.4.197. [DOI] [PubMed] [Google Scholar]
- 16.American Cancer Society. 1989 survey of physicians’ attitudes and practices in early cancer detection. CA Cancer J Clin. 1990;40:77–101. doi: 10.3322/canjclin.40.2.77. [DOI] [PubMed] [Google Scholar]
- 17.Sladden MJ, Ward JE. Do Australian family physicians screen smokers for lung cancer? Chest. 1999;115:725–728. doi: 10.1378/chest.115.3.725. [DOI] [PubMed] [Google Scholar]
- 18.Mauri D, Kamposioras K, Proiskos A, et al. Old habits die hard: chest radiography for screening purposes in primary care. Am J Manag Care. 2006;12:650–656. [PubMed] [Google Scholar]
- 19.Lee TH, Brennan TA. Direct-to-consumer marketing of high-technology screening tests. N Engl J Med. 2002;346:529–531. doi: 10.1056/NEJM200202143460715. [DOI] [PubMed] [Google Scholar]
- 20.Fenton JJ, Deyo RA. Patient self-referral for radiologic screening tests: clinical and ethical concerns. J Am Board Fam Pract. 2003;16:494–501. doi: 10.3122/jabfm.16.6.494. [DOI] [PubMed] [Google Scholar]
- 21.National Cancer Institute. National Survey of Primary Care Physicians’ Recommendations and Practices for Breast, Cervical, Lung, and Colorectal Cancer Screening. Available at: http://healthservices.cancer.gov/surveys/screening_rp/
- 22.Klabunde CN, Lanier D, Nadel MR, McLeod C, Yuan G, Vernon SW. Colorectal cancer screening by primary care physicians: recommendations and practices, 2006–2007. Am J Prev Med. 2009;37:8–16. doi: 10.1016/j.amepre.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Association for Public Opinion Research. Standard Definitions: Final Dispositions of Case Codes and Outcome Rates for Surveys Revised. 2009 Available at: http://www.aapor.org/Content/NavigationMenu/ResourcesforResearchers/StandardDefinitions/StandardDefinitions2009new.pdf.
- 24.University of Washington Rural Health Research Center. Rural Urban Commuting Area (RUCA) Codes. Available at: http://depts.washington.edu/uwruca.
- 25.Smith RA, Cokkinides V, Eyre HJ. American Cancer Society guidelines for the early detection of cancer, 2006. CA Cancer J Clin. 2006;56:11–25. doi: 10.3322/canjclin.56.1.11. [DOI] [PubMed] [Google Scholar]
- 26.Agency for Healthcare Research and Quality. National Guidelines Clearinghouse. doi: 10.1080/15360280802537332. Available at: www.guideline.gov. [DOI] [PubMed]
- 27.National Cancer Institute. Lung cancer screening (PDQ): health professional version. Available at: http://www.cancer.gov/cancertopics/pdq/screening/lung/healthprofessional.
- 28.Philips GK, Reinier K, Ashikaga T, Luebbers RA. Attitudes and beliefs of primary care physicians regarding prostate and colorectal cancer screening in a rural state. J Cancer Educ. 2005;20:167–172. doi: 10.1207/s15430154jce2003_11. [DOI] [PubMed] [Google Scholar]
- 29.International Early Lung Cancer Action Program Investigators. Henschke CI, Yankelevitz DF, Libby DM, et al. Survival of patients with stage I lung cancer detected on CT screening. New Engl J Med. 2006;355:1763–1771. doi: 10.1056/NEJMoa060476. [DOI] [PubMed] [Google Scholar]
- 30.Welch HG, Woloshin S, Schwartz LM, et al. Overstating the evidence for lung cancer screening: the International Early Lung Cancer Action Program (I-ELCAP) study. Arch Intern Med. 2007;167:2289–2295. doi: 10.1001/archinte.167.21.2289. [DOI] [PubMed] [Google Scholar]
- 31.Bach PB, Jett JR, Pastorino U, et al. Computed tomography screening and lung cancer outcome. JAMA. 2007;297:953–961. doi: 10.1001/jama.297.9.953. [DOI] [PubMed] [Google Scholar]
- 32.Kassirer JP. Stemming the craze on CT scans. Boston Globe; Feb 8, 2008. [Google Scholar]
- 33.IOM. Knowing What Works in Health Care: A Roadmap for the Nation. Washington D.C: National Academy Press; 2008. [Google Scholar]
- 34.Shaneyfelt TM, Centor RM. Reassessment of clinical practice guidelines: go gently into that good night. JAMA. 2009;301:868–869. doi: 10.1001/jama.2009.225. [DOI] [PubMed] [Google Scholar]
- 35.Jamtvedt G, Young JM, Kristofferson DT, O’Brien MA, Oxman AD. Audut and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2003;3:CD000259. doi: 10.1002/14651858.CD000259. [DOI] [PubMed] [Google Scholar]
- 36.Soumerai SB, Avorn J. Principles of educational outreach (academic detailing) to improve clinical decision making. JAMA. 1990;263:549–556. [PubMed] [Google Scholar]
- 37.Peabody JW, Luck J, Glassman P, et al. Measuring the quality of physician practice by using clinical vignettes: a prospective validation study. Ann Intern Med. 2004;141:771–780. doi: 10.7326/0003-4819-141-10-200411160-00008. [DOI] [PubMed] [Google Scholar]
- 38.Dresselhaus TR, Peabody JW, Luck J, Bertenthal D. An evaluation of vignettes for predicting variation in the quality of preventive care. J Gen Intern Med. 2004;19:1013–1018. doi: 10.1007/s11606-004-0003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peabody JW, Luck J, Glassman P, et al. Comparison of vignettes, standardized patients, and chart abstraction: a prospective validation study of 3 methods for measuring quality. JAMA. 2000;283:1715–1722. doi: 10.1001/jama.283.13.1715. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz LM, Woloshin, Fowler FJ, Welch HG. Enthusiasm for cancer screening in the U. S JAMA. 2004;291:71–78. doi: 10.1001/jama.291.1.71. [DOI] [PubMed] [Google Scholar]
- 41.Edey AJ, Hansell DM. CT lung cancer screening in the U. K Br J Radiol. 2009;82:529–531. doi: 10.1259/bjr/17503608. [DOI] [PubMed] [Google Scholar]
- 42.National Cancer Institute. Lung cancer screening leads to high rates of false positives. NCI Cancer Bulletin. 2009 June 2; [Google Scholar]
- 43.Brenner DJ, Hall EJ. Computed tomography an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]