Abstract
We synthesized 2-substituted 2H-chromene derivatives from salicylaldehyde using potassium vinylic borates in the presence of secondary amines. Our goal was to generate novel compounds that might modulate transforming growth factor-β signaling, based on limited rational design. Potassium vinyl trifluoroborates react with salicylaldehydes at 80 °C in the presence of a secondary amine and produce 2-substituted 2H-chromene derivatives with a 70–90% yield. A small library of these compounds, predicted to potentially interact with transforming growth factor-β receptors, was screened for bioactivity in living zebrafish embryos. We found that the related compounds differentially affect development, and demonstrate one compound that produces severe body axis alterations in early embryogenesis and at lower doses affects specifically cardiovascular development. This compound modulates specifically a Smad-independent transforming growth factor-β-regulated mitogen-activated protein kinase pathway, namely p-SAPK/JNK. These compounds, as suggested by our biological assays, may prove useful to manipulate developmental programs and develop therapeutic tools.
Keywords: biological screening, chemical biology, chemical genetics, chemical structure, kinase/phosphatase
Forward chemical genetics involves screening a library of compounds to identify small molecules that generate a consistent phenotype in a biological assay, analogous to more traditional genetic screens. An advantage of chemical genetics over traditional genetics is the inherent conditional effect, and if it relates to human disease, a small molecule may serve as a starting point for drug discovery (1–5). It is well documented that biological pathways that govern embryonic development continue to be used in controlling adult physiology, and that deregulation of these pathways can lead to disease. Therefore, identification of small molecule modulators of gene networks active in early development can lead to a better understanding of component specificity for signaling pathways and ultimately the design of novel therapeutic and diagnostic agents for adult diseases. Currently, systematic methods for identifying small molecules that alter specific developmental processes are limited, so the number of useful, small molecule developmental probes remains small. However, using combinatorial techniques, it is possible to rapidly screen a large number of small molecules and identify those that induce a novel phenotype in a cellular or embryonic system.
We are interested in developing new compounds that interact with developmentally important receptor-mediated pathways such as the TGF-β pathway, acting as antagonist or agonist. For this purpose, we describe a chemical genetic approach by testing a library of 2-substituted 2H-chromene derivatives for their bioactivity on developing zebrafish embryos. The zebrafish embryo provides an ideal vertebrate model system for in vivo small molecule screens because of its optical transparency, accessibility during embryonic development, and permeability to small molecules (6,7).
The TGF-β signaling pathway and those controlled by related superfamily ligands (BMPs, activins, nodals) are implicated in regulating myriad developmental programs during embryogenesis and throughout life (8–14). Because of its strong association with pathways deregulated in cancer and disease, TGF-β signaling represents a prime candidate pathway for development of pharmacological modulators. One challenge is to develop specific activators and inhibitors, given the large number of ligand and receptor family members and isoforms. A clue for how to achieve this comes from mutational and structural studies. Mutagenesis studies led to the identification of amino-acid residues, including R94 of TGF-β1, implicated in binding to its receptor, TBR-II. In addition, the crystal structure of the TBR-II extracellular domain + TGF-β3 complex showed that two arginine residues, R25 and R94, of TGF-β3 are involved in the formation of hydrogen-bonded ion pairs with TBR-II residues E142 and D55, respectively (15). Mutations introduced into these sites on the TBR-II surface altered ligand binding properties and intracellular signaling (16). By screening for chemical compounds that prevent the interaction between TGF-β3 and TBR-II, it was found that 3,5,7,20,40-pentahydroxyflavone (also known as Morin) inhibits ligand binding and suppresses intracellular signaling. In a separate screen of the NCI small molecule library, flavone-containing antagonists of TGF-β/TBR-II interactions were also identified (17). Given this background, we attempted to develop new agonist/antagonists for TGF-β and TBR-II interactions and also use these molecules to investigate phenotypic changes caused by the derivatives, and to identify the pathways responsible for specific phenotypes. Because flavone molecules are a subclass of chromene derivatives, we developed new methodology to synthesize 2-substituted 2-H chromenes (18).
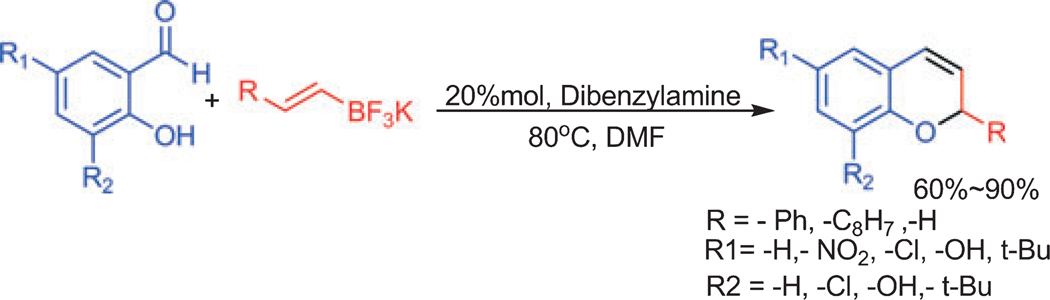
In a recent report, we demonstrated a practical and highly efficient procedure for preparing diverse chromene derivatives using potassium vinyl trifluoroborate as substrate for a petasis reaction in the presence of a catalytic amount of dibenylamine as a secondary base at 80 °C in dimethylformide, as shown in Scheme 1. While complementary to procedures developed by Wang and Finn (19) and Kalbaka et al. (20), our procedure replaces the use of organoboron derivatives with potassium organotrifluorborates. These reagents are easily prepared by addition of inexpensive KHF2 to various organoboron derivatives, and they are stable to air and moisturea. Potassium organotrifluoroborates are more stable and easier to handle than corresponding organic boronic acids; they are more reactive because of the higher nucleophilicity of the organic group on boron, and organotrifluoroborates have been used for many organic reactionsb.
Scheme 1.
An efficient procedure for generating chromene derivatives (see ref. 18).
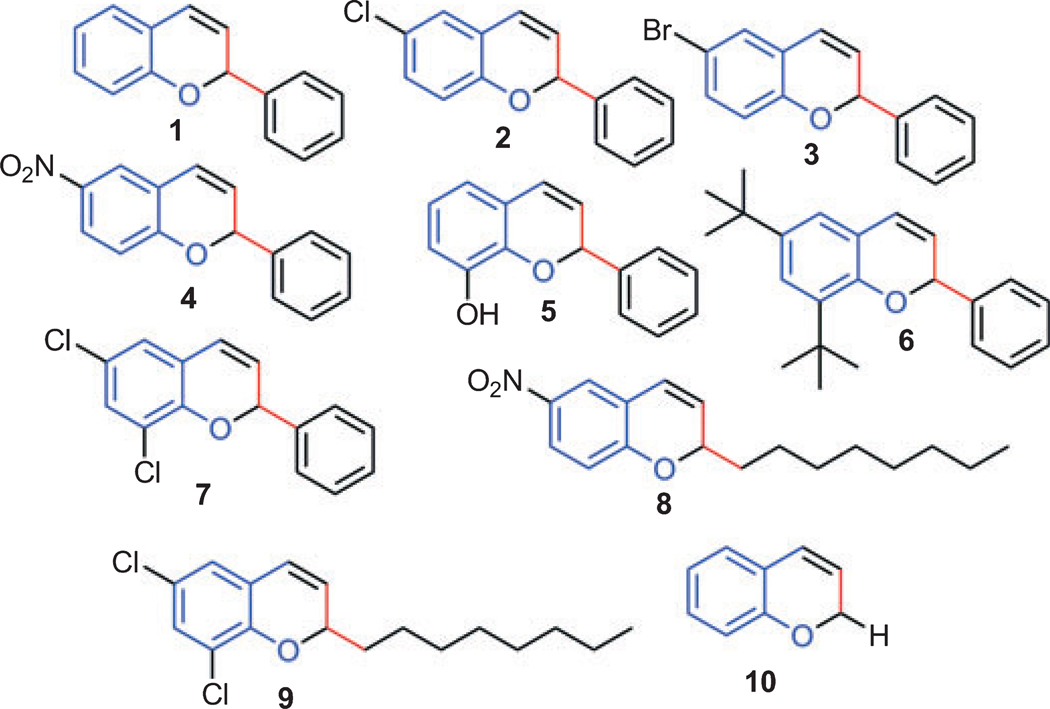
Salicylaldehyde (1 mmol), vinyl boronic acid (1 mmol), and dibenzylamine (20% mol) were added to DMF and the solution was stirred at 80 °C for 3 h. The reaction was monitored through thin layer chromatography and a new spot was identified and by NMR analysis shown to be comprised of 2-phenyl chromenesc. Using this protocol, we synthesized 10 individual compounds (Scheme 2), comprised of two sets. In the first set of compounds (1–7), the 2-position is substituted with phenyl rings and in the second set of compounds (8–10), the 2-position is substituted with hydrogen or octyl derivatives. The synthesis details for active compound 5 and 7 are givenc.
Scheme 2.
A small library of chromene derivatives was synthesized and used to screen bioactivity with zebrafish embryos. Compounds tested are shown and labelled 1–10.
As indicated above, we synthesized this small library because the literature suggests that flavone-containing compounds can modulate TGF-β/TBR-II interactions (17). As flavones are a subclass of chromene derivatives, we used this molecule as a starting point to find novel compounds that might affect TGF-β signaling pathways, and to identify possible downstream targets. After synthesis and purification of the compounds, they were screened for bioactivity using the zebrafish model.
For this purpose, zebrafish embryos were obtained at the one cell stage from paired adults, cultured in system water until 5 h postfertilization (hpf) and then grouped into individual wells of a 12 well plate, each well containing 3–10 embryos in 1 mL of 1× E3 buffer, 1% DMSO, and 1 mm Tris pH 7.5. Compounds were diluted in DMSO to 10 mm stocks, and added to the cultured embryos (1–10 µL) to a final concentration of 10 µm or 100 µm. The morphology of developing embryos was scored at 24 hpf.
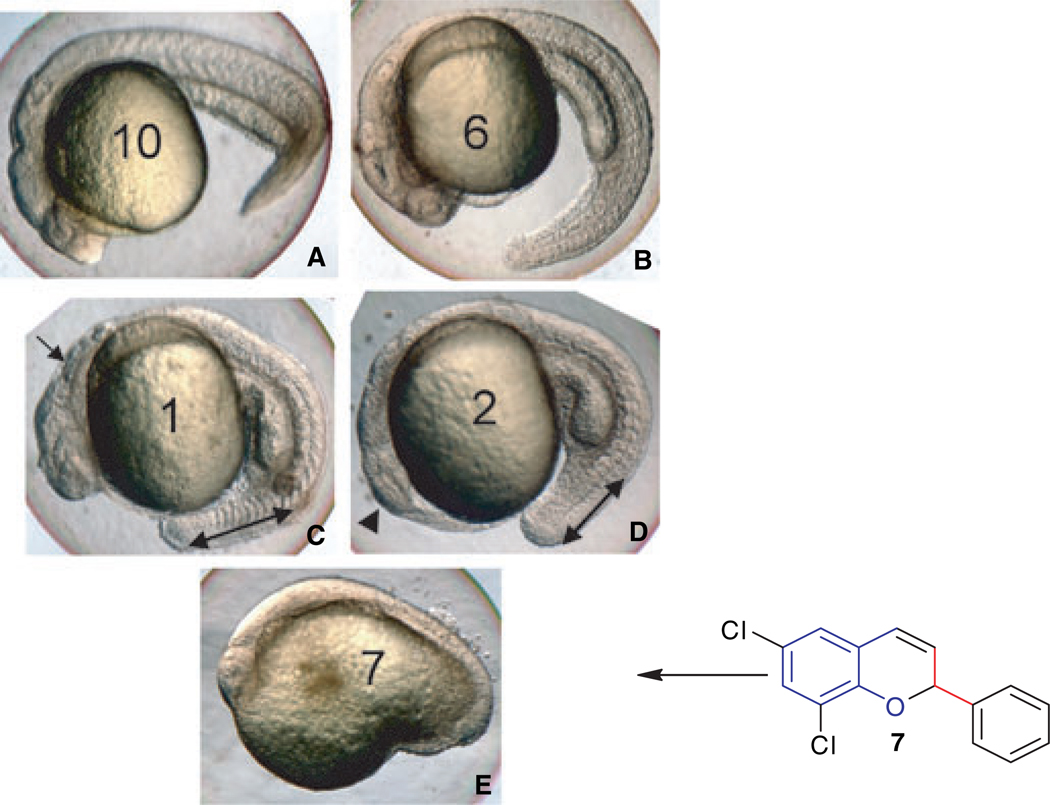
Embryos cultured in 10 µm of each compound appeared normal at this stage (24 hpf), as did embryos treated with 100 µm of compounds 6, 8, or 10. However, developmental abnormalities were obvious at 100 µm using other compounds, and these could be categorized as showing either relatively severe body axis defects (compounds 1, 2, 4 and 9), or extreme developmental delays and failure to survive beyond 24 hpf (compounds 3, 5, and 7). Examples for each class of phenotype are shown in Figure 1.
Figure 1.
Zebrafish can be used to screen bioactivity of the novel 2-chromene compounds. Zebrafish embryos were treated with compounds, as indicated, at 100 µm. Compounds were added at 5 hpf and embryos were cultured until 24 hpf. Shown are representative embryos from batches in which all embryos looked similar (n = at least 10). Embryos treated with compounds 10 (A) or 6 (B) (or 8, not shown) are essentially normal, compared to embryos treated with vehicle only (not shown). Embryos treated with compounds 1 (C) or 2 (D) (similar to 4 or 9, not shown) develop significant defects in the hindbrain (arrow in C), eye (arrowhead in D), and a shortened tail (indicated by the double-headed arrows). Embryos treated with compound 7 (E), 3 or 5 (not shown) are disrupted entirely for normal body axis development, and do not survive to 48 hpf. Views are lateral, anterior to the left.
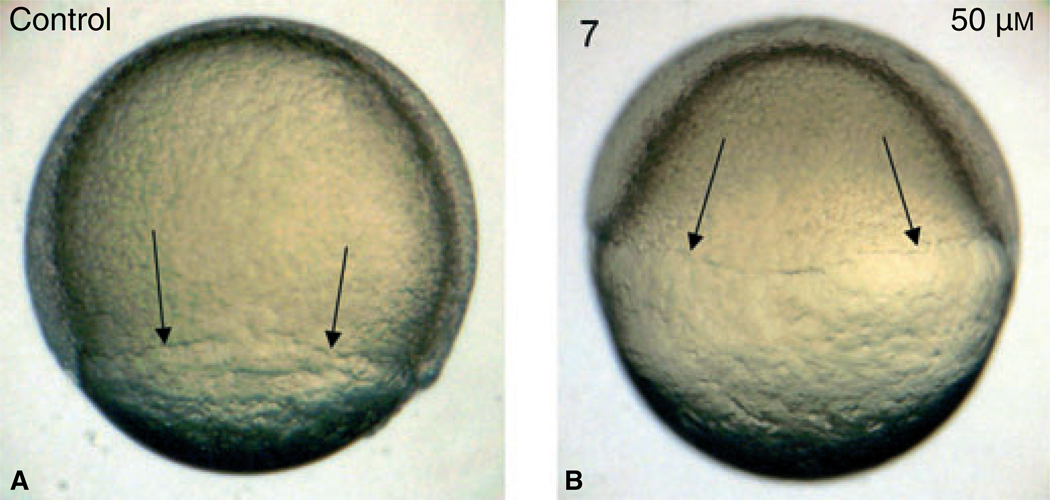
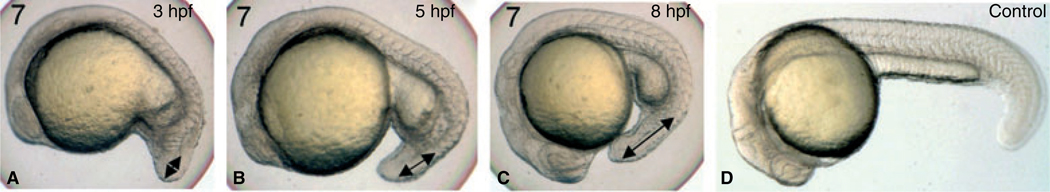
We focused on those compounds that most severely affected development, and titrated the concentration of compounds 3, 5, and 7, adding the compounds to embryos at 5 hpf in 2 mL solution, treating 15–25 embryos in each condition. At 25 µm or lower, the embryos developed normally by 24 hpf compared with control embryos treated with DMSO. At 50–100 µm, the embryos demonstrated similar and highly reproducible developmental defects, with compounds 5 and 7 being slightly more active, and with severity that reflects a dose response. We focused further analysis on compound 7. The compounds clearly function early as the embryos treated at 3 hpf with compound 7 at 50 µm show a clear defect or delay in development by 8 hpf, when epiboly (the expansion of cells around the yolk during gastrulation) has achieved only 30%, compared to 80% in control embryos (Figure 2).
Figure 2.
Compound 7 affects early embryonic processes including epiboly. Shown are representative embryos (n = 10) that were treated with vehicle (A, control) or compound 7 at 50 µm, added at 3 hpf (B), and cultured until 8 hpf. Note that epiboly in control embryos has reached by this time approximately 80% completion, while this is significantly delayed in 84-treated embryos. Views are lateral.
However, by delaying the timing for addition of the small molecules, we found that the compounds can also affect subsequent stages of development. When embryos are treated with compound 7 at 50 µm (starting at 3 hpf, 5 hpf, or 8 hpf), there is a time-dependent phenotype such that the embryos treated earliest show the greatest body axis defects, although for example tail length is still very much affected even with treatment starting at 8 hpf (Figure 3). Embryos treated at this dose at 3–5 hpf do not survive beyond 48 hpf, while those treated at 24–30 hpf develop a relatively normal body, but demonstrate a consistent cardiomyopathy (data not shown).
Figure 3.
Compound 7 affects both early and subsequent developmental processes. Shown are representative embryos (n = 25) treated with 50 µm compound 7, which was added at 3, 5, or 8 hpf, compared with embryos treated with vehicle alone (D). All embryos were cultured until 24 hpf. As indicated by the double-headed arrows, the tail effects are progressively less severe in later embryos, but still significant when added at 8 hpf compared to control. Views are lateral, anterior to the left.
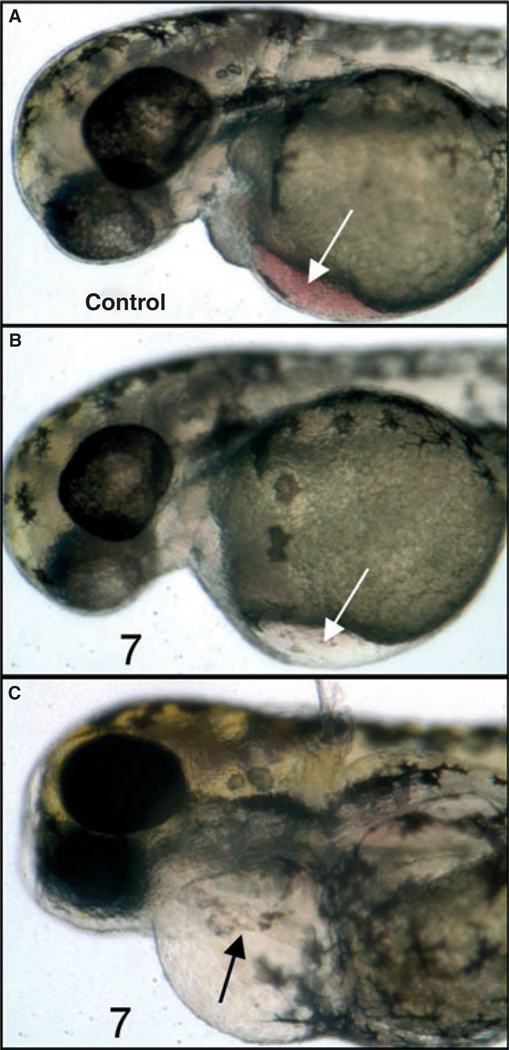
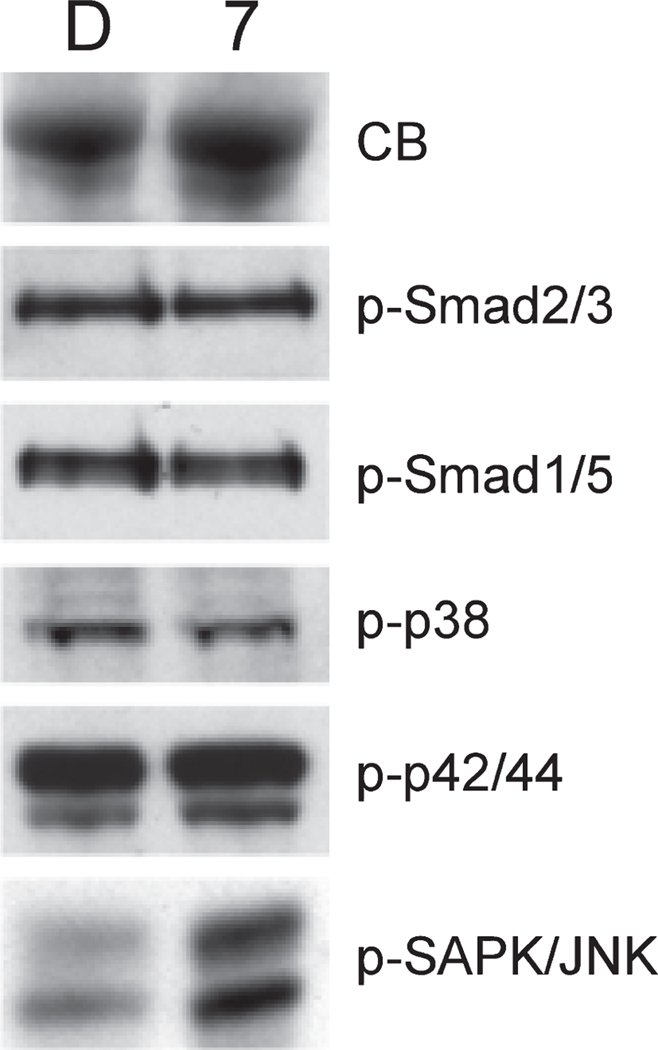
Even at 10 µm (a concentration that does not grossly affect early development), embryos treated at 3 hpf develop eventually with a notable lack of blood flow (Figures 4A, B), and those in which treatment was initiated as late as 55 hpf develop by 5 days a severe and reproducible cardiomyopathy (Figure 4C). Therefore, compound 7 strongly interferes with both early and late developmental programs and these effects can be separated in this model system depending on the time embryos are exposed to the compound. This system provides a strategy to identify the specific pathways (presumably, but not necessarily, TGF-β related) that are affected by these small molecules. For this purpose, we treated embryos at 24 hpf with compound 7 for 5 h in culture, and prepared whole cell lysates to evaluate if any changes in TGF-β regulated pathways could be documented. Our primary candidate pathways were those regulated by Smads, because these are key mediators of both TGF-β/Activin/Nodal signals (via Smad2 and Smad3) and BMP signals (via Smad1 and Smad5). Western blotting experiments were carried out using lysates prepared from embryos treated with 100 µm compound 7 or vehicle (DMSO, control). As shown in Figure 5, we found that, following this treatment, there was no change in the levels of phospho-Smad2/3 or phosph-Smad1/5 (note that antibodies are not available to distinguish p-Smad2 from p-Smad3 or p-Smad1 from p-Smad5). Although this result was somewhat surprising, there are other validated pathways that function downstream of TGF-β signaling and are independent of Smads (21), primarily through Mitogen-Activated Protein Kinases (MAPK). Therefore, we next analyzed the levels for several other candidate pathways, including phospho-p38 MAPK, phospho-p42/44 MAPK, phospho-TAK1, phospho-SAPK/JNK, and phospho-SEK1/MKK4. No significant or reproducible changes were found for p-p38 or p-p42/44 (Figure 5), while p-SEK1 and p-TAK1 levels were either not changed or not reliably detected at convincing levels (not shown). However, the analysis revealed a significant and reproducible increase in levels of p-SAPK/JNK after treatment with compound 7 (Figure 5). Therefore, compound 7 does alter a known and restricted key signaling pathway downstream of TGF-β receptor signaling, and appears to provide a means to specifically modulate Smad-independent signaling.
Figure 4.
Compound 7 alters hematopoietic and cardiac development. Shown are representative embryos (n = 25) treated with vehicle alone (A, control) 10 µm compound 7 added at 3 hpf (B), or 10 µm compound 7 added at 30 hpf (C). Embryos were cultured until 55 hpf (A, B) or 5 dpf (C). Note the relative lack of circulating erythroid cells in B, compared to A (white arrows), and the defective and linear heart (black arrow) and edema in C.
Figure 5.
Compound 7 modulates specifically levels of phospho-SAPK/JNK. Shown are representative results from western blotting experiments. Fifty embryos at 24 hpf were cultured for 5 h either in vehicle (DMSO, indicated by lanes marked D) or in 100 µm compound 7 (indicated by lanes marked 7). Embryos were then deyolked and dissociated directly into SDS sample buffer (4 µL per embryo). Each lane contains a lysate representing 5 embryo equivalents. Samples were electrophoresed in a 10% SDS-PAGE-Tris gel (Invitrogen, Carlsbad, CA, USA), transferred to nylon and probed using primary antibodies (Cell Signaling Technology, Danvers, MA, USA) specific to the phosphorylated (active) isoforms for each protein. After incubation with appropriate secondary antibodies, filters were developed using ECL (Santa Cruz, Santa Cruz, CA, USA) and exposed to film. An equivalent set of samples was stained directly from the gel using Coomasie Blue (CB), demonstrating equal protein loading. Antibodies used are indicated next to each panel. Note that only p-SAPK/JNK levels are changed, being increased substantially by exposure of embryos to compound 7. Equivalent results were reproduced with independent experiments.
In summary, we reported previously a practical synthesis of chromene derivatives using potassium organotrifluoborates, and have now used this procedure to generate a novel set of related small molecules that are modeled to potentially interact with TGF-β receptors. We showed that these compounds are differentially bioactive and can affect both early developmental processes (epiboly) and subsequent organogenesis (cardiac development). This result is encouraging with respect to specificity, as it is expected that a compound that modulates TGF-β signaling will generate different phenotypes at different times. This is because TGF-β signaling functions early in organizing embryo patterning, but subsequently continues to be used in multiple different ways to control subsequent steps of organ development and cell differentiation. This result highlights the potential advantage of using small molecules as conditional modulators, relative to genetic mutations, that are much more difficult to control conditionally. It is possible that the compounds interact with different receptor complexes at different times, or that they interact with the same component of distinct receptor complexes. We did not find altered levels of phospho-Smads in embryos treated with our primary candidate compound. However, we did find that compound 7 modulates a specific relevant pathway, namely p-SAPK/JNK, which is known to be downstream of TGF-β, and can mediate Smad-independent signaling. Additional experiments will be required to delineate how compound 7 activates p-SAPK/JNK, for example, through activation of upstream kinases, or inhibition of phosphatases. Importantly, the approach may provide a strategy to modulate TGF-β signaling with specificity through manipulation of defined downstream pathways. In the future, an understanding of the mechanism behind this specificity could expedite development of a new class of chromene derivative compounds and their application to new drug discovery, for example, relevant to cardiovascular diseases. Experiments are in progress to dissect the mechanism by which compound 7 modulates p-SAPK/JNK levels, and to identify the altered gene expression pathways behind these particular phenotypes.
Acknowledgments
BCD acknowledges Professor M. Donald Blaufox for his financial support and encouragement and AECOM for start-up funding. TE is supported by grants from the NIH (HL56182 and HL64282). Each of the authors contributed significantly to this study.
Abbreviations
- dpf
days postfertilization
- Hpf
hours postfertilization
- MAPK
mitogen-activated protein kinase
- TBR
TGF-β receptor
- TGF-β
transforming growth factor-β
Footnotes
For the synthesis of organotrifluoroborates, see: (a) Vedejs, E., Chapman, R.W., Fields, S.C., Lin, S., Schrimpf, M.R. (1995) J Org Chem;60:3020; (b) Vedejs, E., Fields, S.C., Hayashi, R., Hitchcock, S.R., Powell, D.R., Schrimpf, M.R. (1999) J Am Chem Soc;121:2460; (c) Molander, G.A., Figueroa, R. (2005) Aldrichimica Acta;38:49; (d) Darses, S., Genet, J.-P. (2003) Eur J Org Chem;4313; (e) Molander, G.A., Ellis, N. (2007) Acc Chem Res;40:275–286.
For the use of BF3K in organic synthesis, see:(a) Kabalka, G.W., Venkataiah, B., Dong, G. (2003) Org Lett;3803–3805; (b) Kabalka. G.W., Reddy.M. (2004) Organometallics;23:4519–4521; (c) Kabalka, G.W., Venkataiah, B., Dong, G. (2004) Tetrahedron Lett;729–731; (d) Kabalka, G.W., Dong, G., Venkataiah, B. (2005) Tetrahedron Lett;46:763–765; (e) Molander, G.A., Katona, B.W., Machrouhi, F.J. (2002) Org Chem;67:8416; (f) Darses, S., Michaud, G., Genet, J.-P. (1999) Eur J Org Chem;1875; (g) Vedejs, E., Chapman, R.W., Fields, S.C., Lin, S., Schrimpf, M.R. (1995) J Org Chem;60:3020; (h) Darses, S., Michaud, G., Genet, J.-P. (1998) Tetrahedron Lett;39:5045; (i) Molander, G.A., Biolatto, B. (2002) Org Lett;4:1867.(j) Molander, G.A., Petrillo, D.E. (2006) J Am Chem Soc;128:9634–9635.(k) Molander, G.A., Ribagorda, M. (2003) J Am Chem Soc;125:11148–11149.(l) Molander, G.A., Figueroa, R. (2006) cis-Org Lett;8:75–78.(m) Stefani, H.A., Cella, R., Dö rr, F.A., Pereira, C.M.P., Zeni, G., Gomes, M. Jr. (2005) Tetrahedron Lett;46:563–567.(n) Fang, G.-H., Yan, Z.-J., Deng, M.-Z. (2004) Org Lett;6:357–360.
Experimental details for synthesis: Synthesis of Compound 5: A solution of 2,3-dihydroxybenzaldehyde (276.2 mg, 2 mmol), potassium trans styryltrifluoraborate (420 mg, 2 mmol), was added with dibenzylamine (82 mg, 20 mol%) in anhydrous DMF (10 mL). The reaction mixture was heated at 80 °C for overnight. The reaction mixture was quenched with aqueous saturated NH4Cl (8 mL), extracted with CH2Cl2 (10 mL×3), and combined organic layers were washed with water (10 mL×3). The water layer was extracted with CH2Cl2 (5 mL×2). The combined organic layers were dried with Na2SO4, and evaporated. The residue was purified by flash column chromatography using hexane: ethyl acetate (98:2) as the eluent to give a yellow solid, 242.2 mg (54.0%). 1H-NMR (300MHz, CDCl3): δ 7.50–7.39 (m,5H), 6.87–6.82 (m,2H), 6.67 (dd, J = 6.9, 2.4 Hz, 1H), 6.60 (dd, J = 9.9, 2.1 Hz, 1H), 5.98 (t, J = 3 Hz, 1H), 5.85 (dd, J = 9.9, 3.3 Hz, 1H), 5.44(s, 1H). 13C-NMR CD3OD: δ 145.04, 141.31, 140.65, 128.49, 126.94, 125.20, 123.97, 122.63, 121.14, 117.80, 116.47, 77.10. ESI MS: calcd for C15H12O2 ([M+H]+) 225.088; found: 225.12. Synthesis of Compound 7: A solution of 3, 5-Dichlorosalicyladehyde (382 mg, 2 mmol), potassium trans styryltriffluoraborate (420 mg, 2mmol), was added to dibenzylamine (82 mg, 20 mol%) in anhydrous DMF (10 mL). The reaction mixture was heated at 80 °C for overnight. The reaction mixture was quenched with aqueous saturated NH4Cl (8 mL). Extracted with CH2Cl2 (15 mL×3), and combined organic layers was washed with water (10 mL×3), water layers was extracted with CH2Cl2 (10 mL×2). Combined organic layers were dried with Na2SO4, and evaporated. The residue was purified by flash column chromatography using hexane:ethyl acetate (98:2) as the eluent to give a thick liquid (460.95 mg, 83%). 1H-NMR(300 MHz, CDCl3): δ 7.61~7.36(m, 5H), 7.25 (d, J = 2.2 Hz, 1H), 6.93 (d, J = 2.4Hz, 1H), 6.51 (dd, J = 10Hz, 1.5 Hz,1H), 6.09 (dd, J = 4Hz, 1.5 Hz, 1H), and 5.98 (dd, J = 10Hz, 4 Hz 1H). 13C NMR (75MHz, CD3OD: δ 145.04,140.65, 129.25, 128.49, 127.20, 126.94, 125.20, 123.97, 120.05, 119.75, 119.25, 116.47, and 77.25. ESI MS: calcd for C15H10 Cl2O ([M+-H]+): 277.154 found 277.152.
References
- 1.Lokey RS. Forward chemical genetics: progress and obstacles on the path to a new pharmacopoeia. Curr Opin Chem Biol. 2003;7:91–96. doi: 10.1016/s1367-5931(02)00002-9. [DOI] [PubMed] [Google Scholar]
- 2.Mitchison TJ. Towards a pharmacological genetics. Chem Biol. 1994;1:3–6. doi: 10.1016/1074-5521(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 3.Schreiber SL. Chemical genetics resulting from a passion for synthetic organic chemistry. Bioorg Med Chem. 1998;6:1127–1152. doi: 10.1016/s0968-0896(98)00126-6. [DOI] [PubMed] [Google Scholar]
- 4.Specht KM, Shokat KM. The emerging power of chemical genetics. Curr Opin Cell Biol. 2002;14:155–159. doi: 10.1016/s0955-0674(02)00317-4. [DOI] [PubMed] [Google Scholar]
- 5.Tan DS. Sweet surrender to chemical genetics. Nat Biotechnol. 2002;20:561–563. doi: 10.1038/nbt0602-561. [DOI] [PubMed] [Google Scholar]
- 6.MacRae CA, Peterson RT. Zebrafish-based small molecule discovery. Chem Biol. 2003;10:901–908. doi: 10.1016/j.chembiol.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Peterson RT, Link BA, Dowling JE, Schreiber SL. Small molecule developmental screens reveal the logic and timing of vertebrate development. Proc Natl Acad Sci U S A. 2000;97:12965–12969. doi: 10.1073/pnas.97.24.12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flanders KC, Burmester JK. Medical applications of transforming growth factor-β. Clin Med Res. 2003;1:13–20. doi: 10.3121/cmr.1.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Massague J, Blain SW, Lo RS. TGF-β signaling in growth control, cancer, and heritable disorders. Cell. 2000;103:295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- 10.Massague J, Chen YG. Controlling TGF-β signaling. Genes Dev. 2000;14:627–644. [PubMed] [Google Scholar]
- 11.Massague J, Wotton D. Transcriptional control by the TGF-β/Smad signaling system. EMBO J. 2000;19:1745–1754. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts A, Sporn M. Peptide growth factors and their receptors. In: Sporn M, Roberts A, editors. Handbook of Experimental Pharmacology. Volume 1. Heidelberg, Berlin: Springer; 1995. pp. 419–472. [Google Scholar]
- 13.Ten Dijke P, Goumans MJ, Itoh F, Itoh S. Regulation of cell proliferation by Smad proteins. J Cell Physiol. 2002;191:1–16. doi: 10.1002/jcp.10066. [DOI] [PubMed] [Google Scholar]
- 14.Whitman M. Smads and early developmental signaling by the TGF-β superfamily. Genes Dev. 1998;12:2445–2462. doi: 10.1101/gad.12.16.2445. [DOI] [PubMed] [Google Scholar]
- 15.Hart PJ, Deep S, Taylor AB, Shu Z, Hinck CS, Hinck AP. Crystal structure of the human TβR2 ectodomain – TGF-β3 complex. Nat Struct Biol. 2002;9:203–208. doi: 10.1038/nsb766. [DOI] [PubMed] [Google Scholar]
- 16.Shimanuki T, Hara T, Furuya T, Imamura T, Miyazono K. Modulation of the functional binding sites for TGF-β on the type II receptor leads to suppression of TGF-β signaling. Oncogene. 2007;26:3311–3320. doi: 10.1038/sj.onc.1210123. [DOI] [PubMed] [Google Scholar]
- 17.Burmester JK, Salzman SA, Zhang KQ, Dart RA. Small molecule antagonists of the TGF-β1/TGF-β receptor binding interaction. Med Oncol. 2006;23:553–562. doi: 10.1385/MO:23:4:553. [DOI] [PubMed] [Google Scholar]
- 18.Liu F, Evans T, Das B. Synthesis of 2-substituted 2H-chromenes using potassium vinyltrifluorborates. Tetrahedron Lett. 2008;49:1578–1581. doi: 10.1016/j.tetlet.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Q, Finn MG. 2H-chromenes from salicylaldehydes by a catalytic petasis reaction. Org Lett. 2000;2:4063–4065. doi: 10.1021/ol006710r. [DOI] [PubMed] [Google Scholar]
- 20.Kalbaka G, Venkataiah B, Das B. Synthesis of 2H-chromenes in ionic liquid solvents. Synlett. 1994;12:2194–2196. [Google Scholar]
- 21.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]