Abstract
Background
Vigorous exertion and endurance training have been reported to increase atrial fibrillation (AF). Associations of habitual light or moderate activity with AF incidence have not been evaluated.
Methods and Results
We prospectively investigated associations of leisure-time activity, exercise intensity, and walking habits, assessed at baseline and updated during follow-up visits, with incident AF, diagnosed by annual 12-lead-electrocardiograms and hospital discharge records, from 1989–2001 among 5,446 adults ≥65 in the Cardiovascular Health Study. During 47,280 person-years follow-up, 1,061 new AF cases occurred (incidence=22.4/1,000 person-years). In multivariable-adjusted analyses, leisure-time activity was associated with lower AF incidence in a graded manner, with 25% (HR=0.75, 95%CI=0.61, 0.90), 22% (HR=0.78, 95%CI=0.65, 0.95), and 36% (HR=0.64, 95%CI=0.52, 0.79) lower risk in quintiles 3, 4, and 5, versus quintile 1 (p trend<0.001). Exercise intensity had a U-shaped relationship with AF (quadratic p=0.02): versus no exercise, AF incidence was lower with moderate (HR=0.72, 95%CI=0.58, 0.89), but not high (HR=0.87, 95%CI=0.64, 1.19), intensity exercise. Walking distance and pace were each associated with lower AF risk in a graded manner (p trend <0.001); assessing combined effects of distance/pace, individuals in quartile 2, 3, and 4 had 27% (HR=0.73, 95%CI=0.61, 0.86), 40% (HR=0.60, 95%CI=0.50, 0.73), and 48% (HR=0.52, 95%CI=0.42, 0.65) lower AF incidence, compared with quartile 1. Findings appeared unrelated to confounding by comorbidity or indication. Evaluating cutpoints of moderate leisure-time activity (~600 kcal/wk), walking distance (12 blocks/wk), and pace (2 mph), 26% of all new AF cases (95% CI=7, 43%) appeared attributable to absence of these activities.
Conclusions
Light to moderate physical activities, particularly leisure-time activity and walking, are associated with significantly lower AF incidence in older adults.
Keywords: arrhythmia, exercise, prevention, atrial fibrillation
INTRODUCTION
Atrial fibrillation (AF) is a common chronic arrhythmia and results in significant morbidity and increased health care utilization due to elevated stroke risk, reduced exercise tolerance, and potential bleeding complications from anticoagulant therapy.1–3 AF is particularly problematic in older adults (≥65), in whom risk of new-onset AF is ~2% per year;1, 2, 4 both incidence and prevalence of AF will likely grow as the population continues to age. Physical activity has been reported to increase the risk of AF.5–11 However, only vigorous exertion and endurance training have been evaluated, and largely only in retrospective case-control studies and case series of younger athletes and middle-aged adults.5–11 Hypothesized mechanisms for such potential higher risk include transient influences (i.e., during or immediately after exercise) on autonomic tone and/or long-term changes in cardiac dimensions due to chronic effects of high intensity training (e.g., left ventricular hypertrophy among distance runners). No prior studies have prospectively evaluated the relationship between habitual light to moderate physical activity and incidence of AF.
Among older adults, pathophysiology of AF may often be related to increased vascular stiffness and reduced left ventricular compliance, as reflected by risk factors for AF such as higher systolic blood pressure, treated hypertension, prior myocardial infarction (MI), congestive heart failure (CHF), valvular heart disease, and left atrial enlargement.2, 4 Based on these risk factors, in older adults habitual physical activity might be expected to reduce the incidence of AF, for example by reducing blood pressure, improving vascular compliance, or reducing risk of MI or CHF. However, the relationship between habitual physical activity and incidence of AF among older adults is unknown. The significant morbidity associated with AF, including stroke risk and potential bleeding due to anticoagulation therapy, makes prevention of paramount importance. A relationship between physical activity and risk of AF later in life would suggest that simple lifestyle behaviors could potentially affect this serious condition. Given the prospectively collected information on physical activity measures, the medical, electrocardiographic (ECG), hospital, and Holter information on AF, and the focus on older adults, we examined the association of physical activity with incidence of new-onset AF among 5,446 men and women ≥65 in the Cardiovascular Health Study (CHS), an NLHBI-sponsored prospective cohort study of determinants of cardiovascular risk among older adults. We hypothesized that greater habitual physical activity would be associated with lower incidence of AF.
METHODS
Design and Population
The CHS design and recruitment experience have been described.12, 13 Briefly, 5,201 men and women ≥65 were randomly selected and enrolled from Medicare eligibility lists in 4 U.S. communities in 1989–90; an additional 687 black participants were recruited and enrolled in 1992. Each center’s institutional review committee approved the study and all participants gave informed consent. Participants were only enrolled if they were ambulatory and noninstitutionalized. Baseline evaluation included standardized physical examination, diagnostic testing, laboratory evaluation, and questionnaires on health status, medical history, lifestyle habits, and cardiovascular risk factors.12–15 After excluding 166 individuals with incomplete information on leisure-time activity, exercise intensity, or walking habits and 276 individuals with AF at baseline, 5,446 participants were included in this analysis. To minimize possible confounding by preexisting conditions that might affect both propensity to physical activity and risk of AF, we also performed sensitivity analyses excluding individuals with potentially limiting symptoms (claudication, angina, significantly limited vision, abnormal FEV1, or any reported limitation of one or more activities of daily living; n=1,150) and stratified analyses according to presence (n=1,253) or absence (n=4,193) of preexisting cardiovascular disease, including coronary heart disease (CHD), CHF, and stroke.
Assessment of Physical Activity
Usual leisure-time activity (kcal/wk) was assessed at baseline and at the 3rd and 7th annual visits using a modified Minnesota Leisure-Time Activities questionnaire, evaluating frequency and duration of 15 different activities during the prior 2 weeks.16 Usual exercise intensity was also separately characterized at baseline and at the 3rd and 7th annual visits, with responses including no exercise or low, medium, or high intensity of exercise.17 Usual walking habits, including average pace (gait speed) and distance walked, were assessed by self-report at baseline and again annually at each follow-up visit. We assessed these measures in prespecified categories of leisure-time activity (quintiles), exercise intensity (none, low, medium, high), blocks walked (quintiles), and usual pace walked (<2 mph, 2–3 mph, >3 mph). We also evaluated a prespecified walking score combining blocks walked (ordinal score for quintiles) and pace walked (ordinal score for 3 categories), evaluated in quartiles, to account for both distance and pace of usual walking.
Identification of Atrial Fibrillation
Participants were followed by means of annual examinations including annual resting 12-lead electrocardiograms through year 10, and interim 6-month telephone contacts.18 Hospital records were obtained for all hospitalizations, with adjudication of cardiovascular events by centralized events committees.18 Cases of AF were identified by (1) annual 12-lead electrocardiograms, centrally reviewed at the CHS ECG Reading Center,19 or (2) hospital discharge diagnoses (International Classification of Diseases, 9th Revision, codes 427.3, 427.31, or 427.32). Review of medical records including hospital electrocardiograms in a subset of cases demonstrated that hospital discharge diagnoses provided an accuracy (positive predictive value) of 98.6% for diagnosing AF in CHS.4 To evaluate potential for missed outcomes, results of 24-hour Holter monitoring performed at year 5 were evaluated in a subset of 819 participants.20 Fifteen individuals demonstrated sustained AF, all of whom were identified by the above criteria, and 4 individuals demonstrated intermittent AF, 3 of whom were identified by the above criteria. Thus, as determined by 24-hour Holter monitoring, only 1 in 819 individuals (0.1%) had sustained or intermittent AF not identified by the above criteria.
Statistical Analysis
Physical activity categories were assessed as ordinal variables for evaluation of differences in baseline characteristics using linear (continuous variables) or logistic (dichotomous variables) regression and also for tests for trend. Physical activity habits were updated over time using cumulative averaging to minimize misclassification (measurement error) and assess long-term effects of habitual activity; for example, walking habits at baseline were related to incidence of AF in the first year of follow-up; the average of walking habits at the baseline and first follow-up visits were related to incidence of AF in the second year; the average of walking habits at the baseline, first, and second follow-up visits were related to incidence of AF in the third year; etc. Findings using baseline data only were generally similar; we present the cumulative updated results. Cox proportional-hazards models were used to estimate relative risk (hazard ratio) of incident AF, censoring at death or last day of follow-up through June 30, 2001. Follow-up after 2001 was censored because the last physical activity assessment, clinic examination, and annual ECG occurred at year 10 (1999–2000). To minimize potential confounding, covariates were selected based on clinical interest, previously established risk factors for incident AF in older adults,4 or associations with exposures or outcomes in the current cohort. Care was taken to evaluate separately factors that might be potential confounders (such as age, gender, race, and education) vs. factors that might be potential confounders or mediators of the effect of physical activity on incidence of AF (such as body mass index, blood pressure, antihypertensive medication use, cholesterol levels, glucose levels, and left ventricular mass). We also evaluated potential mediation by intermediary nonfatal MI or CHF using time-varying adjustment. Other covariates that did not materially alter the relations between physical activity and AF risk were excluded from the final models, including month of visit, income, preexisting stroke or transient ischemic attack, use of ACE-inhibitors and lipid-lowering medication, and (among those with dietary data) fish consumption, and (in mediator models) heart rate, LDL cholesterol, HDL cholesterol, and triglycerides. Missing covariates (all ≤6% missing) were imputed by best-subset regression using age, gender, race, education, preexisting CHD (nonfatal MI, coronary revascularization, or angina), stroke, diabetes, and chronic pulmonary disease. Potential effect modification was evaluated by subgroups of age, gender, hypertension, preexisting CHD, and preexisting cardiovascular disease (CHD, CHF, or stroke) by evaluating the significance of multiplicative interaction terms using likelihood ratio tests. Population attributable risk percent (PAR%) was calculated using the formula p(RR-1)/(1 + p(RR-1)), where p is the prevalence of the risk factor and RR is the multivariable-adjusted relative risk. All p-values were two-tailed (alpha=0.05). Analyses were performed using Stata 8.2 (College Station, Texas).
Statement of Responsibility
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
RESULTS
At baseline, average participant age was 73 years (mean±SD=72.8±5.6; range 65–100); 58% were female, and 17% were nonwhite. Participant characteristics according to leisure-time activity and exercise intensity at baseline are shown (Table 1). Greater leisure-time activity was associated with slightly younger age, male gender, white race, greater education, greater alcohol use, and generally fewer cardiovascular risk factors, including slightly less current smoking and lower prevalence of diabetes, CHD, treated hypertension, and pulmonary disease. As would be expected based on physiologic effects, greater activity was also associated with lower blood pressure, heart rate, LDL-C, triglyceride, and C-reactive protein levels. In these unadjusted analyses, exercise intensity was associated with higher HDL-C, whereas leisure-time activity was associated with lower HDL-C. Leisure-time activity was not associated with preexisting CHD or beta-blocker use. These relationships were generally similar, or even more pronounced, for exercise intensity (Table 1). Findings were also generally similar for measures of walking distance and pace (data not shown). Leisure-time activity, exercise intensity, and distance and pace of walking were only modestly positively intercorrelated (Spearman r=0.23–0.57). Associations of physical activity with incident AF were assessed with and without adjustment for each of the factors in Table 1.
Table 1.
Baseline Characteristics According to Leisure-Time Activity and Exercise Intensity Among 5,446 Older Adults
| Quintiles of Leisure-Time Activity, kcal/wk | Categories of Exercise Intensity | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| I <35 |
II 35–404 |
III 405–885 |
IV 890–1838 |
V >1840 |
None | Low | Medium | High | |
| (n=1,098) | (n=1,081) | (n=1,092) | (n=1,090) | (n=1,085) | (n=477) | (n=2,587) | (n=1,877) | (n=505) | |
| Age, years | 74±6 | 73±6 | 72±5 | 73±5 | 72±5 | 75±6 | 73±6 | 72±5 | 71±5 |
| Gender, % male | 25 | 33 | 41 | 49 | 62 | 35 | 39 | 46 | 46 |
| Race, % white | 75 | 78 | 85 | 87 | 91 | 76 | 83 | 83 | 91 |
| Education ≥ high school, % | 61 | 67 | 70 | 75 | 79 | 63 | 62 | 79 | 88 |
| Current smoking, % | 14 | 14 | 13 | 10 | 10 | 12 | 14 | 10 | 9 |
| Smoking history, pack-years | 18±28 | 16±25 | 17±27 | 18±28 | 20±27 | 20±31 | 18±26 | 17±27 | 17±24 |
| Coronary heart disease, % | 22 | 17 | 18 | 20 | 18 | 26 | 19 | 19 | 15 |
| Chronic pulmonary disease, % * | 27 | 26 | 25 | 23 | 21 | 29 | 24 | 23 | 24 |
| Diabetes mellitus, % | 21 | 15 | 14 | 14 | 14 | 22 | 16 | 15 | 9 |
| Treated hypertension, % | 55 | 48 | 46 | 44 | 39 | 58 | 49 | 43 | 34 |
| Body-mass-index, kg/m2 | 28±6 | 27±5 | 27±5 | 26±4 | 26±4 | 27±5 | 27±5 | 27±5 | 26±4 |
| Systolic blood pressure, mm Hg | 139±22 | 138±22 | 136±21 | 135±21 | 135±21 | 140±24 | 137±21 | 136±22 | 134±22 |
| Heart rate, bpm | 70+11 | 69+11 | 68+11 | 67+11 | 66+11 | 70+12 | 68+11 | 67+11 | 65+10 |
| LDL cholesterol, mg/dl | 134±38 | 130±36 | 130±35 | 130±35 | 128±34 | 135±40 | 130±36 | 130±34 | 129±34 |
| HDL cholesterol, mg/dl | 55±15 | 55±16 | 54±16 | 54±15 | 53±15 | 53±16 | 54±16 | 54±15 | 56±16 |
| Triglycerides, mg/dl | 145±85 | 141±66 | 141±80 | 135±75 | 137±76 | 150±97 | 140±72 | 139±78 | 136±71 |
| C-reactive protein, mg/dl | 4.5±6.3 | 3.7±6.2 | 3.4±5.1 | 3.4±6.9 | 3.1±6.1 | 4.5±6.1 | 3.7±6.5 | 3.4±5.9 | 2.8±5.4 |
| Beta-blocker use, % | 13 | 13 | 12 | 11 | 12 | 15 | 13 | 12 | 10 |
| Alcohol use ≥ 1/wk, % | 21 | 26 | 31 | 32 | 43 | 22 | 25 | 36 | 44 |
Values are mean±SD (continuous variables) or percent (categorical variables). P for trend<0.05 for all covariates, except for nonsignificant associations between leisure-time activity and preexisting CHD and beta-blocker use; and between exercise intensity and pulmonary disease.
Physician-diagnosed asthma, emphysema, or chronic bronchitis.
Leisure-Time Activity and Exercise Intensity
During 12 years follow-up (47,280 total person-years), 1,061 new cases of AF were documented (incidence rate=22.4 cases per 1,000 person-years). After adjustment for age and gender, both leisure-time activity and exercise intensity were associated with lower AF incidence (p<0.001) (Table 2). After multivariable-adjustment, graded lower risk was evident across quintiles of leisure-time activity: compared to the lowest quintile, individuals in quintiles 3, 4, and 5 had 25%, 22%, and 36% lower risk, respectively (p trend <0.001). Conversely, after multivariable-adjustment, a nonlinear U-shaped relationship was seen between exercise intensity and incident AF: compared with no regular exercise, individuals with moderate intensity exercise had 28% lower risk of AF, but individuals with high intensity exercise did not have significantly lower risk than those with no regular exercise (Table 2). In a post-hoc test, the addition of a U-shaped (quadratic) term to the ordinal model for exercise intensity was statistically significant (p=0.02), confirming the nonlinearity of this relationship. Further adjustments for factors which might be potential confounders or mediators of effects of physical activity on AF, including body mass index (kg/m2), treated hypertension (yes/no), systolic blood pressure (quintiles), fasting cholesterol (quintiles), fasting glucose (quintiles), C-reactive protein (quintiles), and estimated left ventricular mass from ECG (quintiles), modestly affected these associations: the extreme quintile relative risk for leisure-time activity went from 0.64 to 0.67 (95% CI=0.54, 0.83), and the relative risk for moderate and for high exercise intensity went from 0.72 to 0.73 (95% CI=0.59, 0.91) and from 0.87 to 0.93 (95% CI=0.66, 1.28), respectively.
Table 2.
Risk of New-Onset Atrial Fibrillation in 5,446 Older Adults According to Leisure-Time Activity and Exercise Intensity
| Leisure-Time Activity, quintiles (n*) | I (n=1,098) | II (n=1,081) | III (n=1,092) | IV (n=1,090) | V (n=1,085) | P for Trend |
|---|---|---|---|---|---|---|
| No. of Events | 247 | 208 | 192 | 222 | 192 | |
| Person-years | 9,131 | 9,390 | 9,432 | 9,567 | 9,762 | |
| Age and gender-adj. RR (95% CI) | 1.0 (reference) | 0.81 (0.67–0.97) | 0.70 (0.58–0.85) | 0.76 (0.63–0.92) | 0.60 (0.49–0.73) | <0.001 |
| Multivariable RR (95% CI)† | 1.0 (reference) | 0.86 (0.71–1.03) | 0.75 (0.61–0.90) | 0.78 (0.65–0.95) | 0.64 (0.52–0.79) | <0.001 |
|
| ||||||
| Exercise Intensity (n*) | None (n=477) | Low (n=2,587) | Moderate (n=1,877) | High (n=505) | P for Trend | |
|
| ||||||
| No. of Events | 127 | 545 | 324 | 65 | ||
| Person-years | 3,933 | 23,216 | 16,848 | 3,283 | ||
| Age and gender-adj. RR (95% CI) | 1.0 (reference) | 0.76 (0.63–0.93) | 0.61 (0.49–0.75) | 0.71 (0.52–0.96) | <0.001 | |
| Multivariable RR (95% CI)† | 1.0 (reference) | 0.85 (0.69–1.03) | 0.72 (0.58–0.89) | 0.87 (0.64–1.19) | 0.02 | |
Number of individuals in each category at baseline.
Adjusted for age (years), gender (male/female), race (white/non-white), enrollment site (4 sites), education (<high school, high school, >high school), smoking status (never, former, current), pack-years of smoking (4 categories), coronary heart disease (yes/no), chronic pulmonary disease (yes/no), diabetes mellitus (yes/no), alcohol use (6 categories), and beta-blocker use (yes/no). RR=relative risk (hazard ratio).
Distance and Pace of Walking
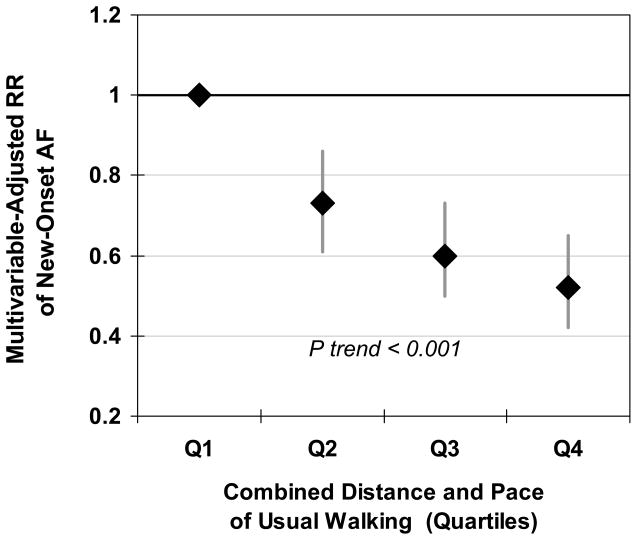
Walking habits were also associated with risk of AF (Table 3). In multivariable-adjusted analyses, compared to walking 0–4 blocks/week, individuals walking 5–11, 12–23, 24–59, and 60+ blocks/week had 22%, 24%, 33%, and 44% lower risk, respectively (p trend <0.001). Compared to pace <2 mph, individuals with pace of 2–3 mph and >3 mph had 32% and 41% lower risk, respectively (p trend <0.001). We also assessed the combined effects of walking distance and pace by means of a prespecified walking score (ordinal score for the sum of the categories of distance [0–4] and pace [0–2] of walking), evaluated in quartiles. A graded inverse relationship was evident between walking distance/pace and incidence of AF (Figure 1); individuals in the highest quartile had 48% lower risk (95% CI=35, 58%; p trend <0.001). Further adjustments for other factors that might be either confounders or mediators (as above) modestly mitigated these associations: compared to the lowest quartile of distance/pace walked, the relative risk for individuals in the highest quartile went from 0.52 to 0.56 (95% CI=0.45, 0.69).
Table 3.
Risk of New-Onset Atrial Fibrillation in 5,446 Older Adults According to Walking Habits
| Walking Distance, blocks/wk (n*) | 0–4 (n=1,145) | 5––11 (n=855) | 12–23 (n=981) | 24–59 (n=1,205) | 60+ (n=1,260) | P for Trend |
|---|---|---|---|---|---|---|
| No. of Events | 272 | 218 | 212 | 191 | 168 | |
| Person-years | 8,867 | 9,188 | 9,452 | 9,789 | 9,984 | |
| Age and gender-adj. RR (95% CI) | 1.0 (reference) | 0.78 (0.65–0.93) | 0.71 (0.59–0.86) | 0.62 (0.51–0.75) | 0.51 (0.42–0.63) | <0.001 |
| Multivariable RR (95% CI)† | 1.0 (reference) | 0.78 (0.65–0.94) | 0.76 (0.63–0.91) | 0.67 (0.55–0.81) | 0.56 (0.45–0.69) | <0.001 |
|
| ||||||
| Walking Pace (n*) | <2 mph (n=1,656) | 2–3 mph (n=2,314) | >3 mph (n=1,476) | P for Trend | ||
|
| ||||||
| No. of Events | 527 | 427 | 107 | |||
| Person-years | 16,805 | 22,494 | 7,982 | |||
| Age and gender-adj. RR (95% CI) | 1.0 (reference) | 0.62 (0.55–0.71) | 0.51 (0.41–0.63) | <0.001 | ||
| Multivariable RR (95% CI)† | 1.0 (reference) | 0.68 (0.59–0.77) | 0.59 (0.48–0.74) | <0.001 | ||
Number of individuals in each category at baseline.
Adjusted for age (years), gender (male/female), race (white/non-white), enrollment site (4 sites), education (<high school, high school, >high school), smoking status (never, former, current), pack-years of smoking (4 categories), coronary heart disease (yes/no), chronic pulmonary disease (yes/no), diabetes mellitus (yes/no), alcohol use (6 categories), and beta-blocker use (yes/no). RR=relative risk (hazard ratio).
Figure 1.
Relative risk (hazard ratio) of incident atrial fibrillation during 12 years of follow-up among 5,446 older adults according to usual walking habits (evaluated in quartiles), combining distance and pace of walking, adjusted for age, gender, race, enrollment site, education, smoking status, pack-years of smoking, coronary heart disease, chronic pulmonary disease, diabetes mellitus, alcohol use, and beta-blocker use. Diamonds represent risk estimates and bars represent 95% CIs, with the lowest category of walking habits as the reference group.
Assessment of Confounding by Comorbidity
To assess confounding by physical activity limitation, we excluded individuals with comorbidities that could significantly limit activity, including claudication, angina, significantly limited vision, abnormal FEV1, or any reported limitation of one or more activities of daily living at baseline (n=1,150). Results were not greatly altered, with lower AF risk in multivariable-adjusted analyses with greater leisure-time activity (comparing extreme quintiles, HR=0.68, 95% CI=0.54, 0.86; p trend=0.003), moderate exercise intensity (comparing moderate to none, HR=0.70, 95% CI=0.54, 0.90) and greater distance/pace walked (comparing extreme quartiles, HR=0.54, 95% CI=0.42, 0.69; p trend <0.001). In stratified analyses, there was little evidence that associations of physical activity with incident AF varied more than would be expected by chance alone according to age, gender, hypertension, preexisting CHD (p interaction>0.10 for each), or presence or absence or preexisting cardiovascular disease (Table 4).
Table 4.
Risk of New-Onset Atrial Fibrillation According to Baseline Physical Activity Habits in 5,446 Older Adults With and Without Preexisting Cardiovascular Disease.*
| Categories of Physical Activity | P Trend | P Interaction† | ||||||
|---|---|---|---|---|---|---|---|---|
| Ordinal | Categorical | |||||||
| Leisure-Time Activity, quintiles | I | II | III | IV | V | |||
| No history of CVD (n=4,193) | 1.0 (reference) | 0.86 (0.69–1.09) | 0.73 (0.58–0.93) | 0.74 (0.58–0.94) | 0.66 (0.51–0.85) | 0.001 | 0.83 | 0.86 |
| History of CVD (n=1,253) | 1.0 (reference) | 0.87 (0.63–1.20) | 0.82 (0.58–1.15) | 0.91 (0.66–1.25) | 0.63 (0.44–0.91) | 0.04 | ||
| Exercise Intensity | None | Low | Moderate | High | ||||
| No history of CVD (n=4,193) | 1.0 (reference) | 0.84 (0.65–1.09) | 0.70 (0.53–0.93) | 0.95 (0.65–1.38) | 0.11 | 0.87 | 0.30 | |
| History of CVD (n=1,253) | 1.0 (reference) | 0.90 (0.66–1.23) | 0.77 (0.54–1.10) | 0.74 (0.41–1.31) | 0.11 | |||
| Distance/Pace Walked, quartiles | I | II | III | IV | ||||
| No history of CVD (n=4,193) | 1.0 (reference) | 0.73 (0.59–0.90) | 0.58 (0.46–0.73) | 0.51 (0.39–0.67) | <0.001 | 0.39 | 0.70 | |
| History of CVD (n=1,253) | 1.0 (reference) | 0.75 (0.56–0.99) | 0.68 (0.50–0.92) | 0.56 (0.38–0.82) | 0.002 | |||
Preexisting coronary heart disease (nonfatal myocardial infarction, coronary revascularization, or angina), congestive heart failure, or stroke. In 4,193 individuals without preexisting CVD, 709 AF cases occurred; in 1,253 individuals with preexisting CVD, 352 AF cases occurred. Values represent hazard ratios (95% CI), adjusted for age (years), gender (male/female), race (white/non-white), enrollment site (4 sites), education (<high school, high school, >high school), smoking status (never, former, current), pack-years of smoking (4 categories), chronic pulmonary disease (yes/no), diabetes mellitus (yes/no), alcohol use (6 categories), and beta-blocker use (yes/no).
Evaluated using likelihood ratio testing comparing nested models with vs. without interaction terms for preexisting cardiovascular disease multiplied by the measure of physical activity, with the potential interaction modelled both ordinally and categorically.
Mediation by Preceding MI and CHF
We evaluated the extent to which observed relationships between physical activity and AF might be mediated by lower risk of preceding MI or CHF by adjusting for both preexisting and incident MI and CHF as time-varying covariates. In multivariable-adjusted analyses (covariates as in Table 4), preceding MI or CHF was a strong risk factor for incident AF (HR=4.64, 95% CI=4.08, 5.26). Adjusting for preceding MI or CHF, relationships of physical activity with incident AF were slightly attenuated: the extreme quintile relative risk for leisure-time activity went from 0.64 to 0.67 (95% CI=0.55, 0.83), the relative risk for moderate compared to no exercise intensity went from 0.72 to 0.76 (95% CI=0.61, 0.95), and the extreme quartile relative risk for distance/pace of walking went from 0.52 to 0.56 (95% CI=0.45, 0.70).
Population Attributable Risk
We calculated the proportion of cases of new-onset AF in the population attributable to lack of moderate activities, defined as leisure-time activity below the median (<616 kcal/wk), walking less than 12 blocks per week, or walking at a pace less than 2 mph, adjusting for these habits simultaneously in the multivariable model (other covariates as in Table 2). In sum, the proportion of all new cases of AF attributable to the absence of these moderate activities was 26% (95% CI=7, 43%).
DISCUSSION
Incidence of AF rises sharply with age, and most cases in the population occur after age 65. While vigorous exertion and endurance training have been reported as risk factors for AF in younger and middle-aged populations, this is the first study to focus on older adults and to evaluate prospectively the relationships of light to moderate habitual physical activity with incidence of AF. Greater leisure-time activity and walking were associated with graded lower incidence of AF, with progressively lower risk as both leisure-time activity and distances and paces of walking increased. Conversely, intensity of exercise had a U-shaped relationship with AF, with lower risk among individuals exercising with moderate, but not high, intensity.
Physical activity and exercise may have both acute (during a bout) and chronic (related to habitual activity) physiologic effects. For example, whereas the risk of sudden cardiac death may be transiently increased during vigorous exercise, habitual physical activity is associated with an overall decreased risk of sudden cardiac death.21–23 Higher risk of AF has been reported in case series and retrospective studies of younger athletes and middle-aged adults with high intensity or endurance exercise,5–10 which if causal could reflect relatively transient higher risk during/immediately following a bout of exercise, and/or more chronic left ventricular structural changes related to prolonged high intensity training.24, 25 In a retrospective study, 262 military veterans likely to have undergone long-term vigorous exercise had higher prevalence of AF compared with population volunteers (6.1% vs. 4.6%), largely due to greater lone AF (which offset lower prevalence of risk factor-related AF).5 Among 137 patients undergoing ablation of isthmus-dependent atrial flutter, the 31 patients performing semi-competitive endurance sports had more frequent post-ablation AF at 1 year (81% vs. 48%; multivariable HR=1.81, p=0.02), compared to those not undertaking such endurance sports.9 In a retrospective study, 70 middle-aged patients with lone AF seen at an arrhythmia clinic were more likely to have engaged in long-term sports training (46%) than the general population (15%)7 and more likely to report current sports practice (31%) compared with population controls (14%).10 Thus, case series and retrospective studies suggest that long-term endurance athletes have higher risk of AF, particularly lone AF, than the general population. Conversely, such analyses may be limited by selection bias and recall bias. In a Danish cohort of middle-aged adults, no significant associations were seen between bouts of strenuous activity in the workplace and risk of AF,26 but physical activity habits outside of work were not assessed.
In the present work, we examined prospectively the risk of AF associated with usual, habitual, or chronic levels of light to moderate physical activity. Thus, the observed risk estimates may in essence reflect the balance between long-term benefits associated with habitual activity and (potentially) higher acute risk “during” activity. In contrast to prior retrospective reports and case series,5–10 high intensity exercise was not associated with higher AF risk; this could relate to relatively lower maximal intensity of exercise in these older adults compared with younger adults, differences in pathoetiology of AF later in life, and/or design differences (e.g., prospective vs. retrospective investigation). However, high intensity exercise was also not associated with lower AF risk, suggesting an overall net neutral association of high intensity exercise with AF incidence in older adults. In comparison, moderate physical activities, such as greater leisure-time activity, distances and paces of walking, and moderate intensity exercise, were associated with significantly lower risk. These results suggest that long-term benefits for AF risk of light to moderate physical activities in older adults outweigh any potential higher risks of AF associated with the acute activity or exercise.
Moderate physical activity has several physiologic benefits that could reduce the incidence of AF in older adults.27–30 Physical activity induces and maintains weight loss; additional effects on maintenance of lean body mass may also be particularly relevant later in life. Physical activity lowers resting heart rate and blood pressure, improves fasting and postprandial glucose control, and improves serum lipoprotein levels and mental well-being. Physical activity may also improve endothelial function, lower systemic inflammation, and facilitate quitting smoking. Each of these factors are risks for AF. When we adjusted for differences in left ventricular mass and metabolic risk factors such as body mass index, blood pressure, glucose, cholesterol, and C-reactive protein levels, the relationships of the physical activity measures and incident AF were partly attenuated, suggesting that part of the observed lower risk may be mediated by effects of activity on these risk factors. Associations of physical activity with lower AF risk also appeared potentially mediated, in part, by lower risk of preceding MI or CHF.
In considering relationships between physical activity and incident AF, potential confounding by underlying comorbidity must be carefully assessed. Some individuals may have comorbidities that both limit their physical activity and increase risk of AF, which would cause physical activity to appear more protective than the true effect. Conversely, other individuals may increase their physical activity in response to diagnosis of a condition that also increases risk of AF (confounding by indication), which would cause physical activity to appear less protective than the true effect. Multivariable adjustments are one method to decrease such confounding. We also used restriction and stratification to assess such potential confounding. The findings did not appear to be attributable to confounding by presence of comorbid conditions, such as chronic pulmonary disease, preexisting CHD, or preexisting cardiovascular disease. Notably, the relationships of most potential confounders were similar or even more prominent for exercise intensity than for leisure-time activity (Table 1), but multivariable-adjusted analyses revealed different relationships of exercise intensity (U-shaped risk) vs. leisure-time activity (graded lower risk) with incident AF, suggesting that confounding alone would not fully account for the observed relationships.
Our analysis has several strengths. The prospective assessment of physical activity and other covariates reduces potential bias from recall differences. The cohort design minimizes selection bias (i.e., the non-cases represent the true population from which the cases arose). Standardized assessment of a wide variety of participant characteristics increases the capacity to adjust for confounding. Close follow-up, annual ECGs, and review of all hospitalizations reduce potential for missed or misclassified outcomes. The use of repeated assessments of physical activity and other risk factors over time reduces misclassification due to changes in activity and assesses long-term effects. The large number of events provides ample statistical power. The population-based recruitment strategy enhances generalizability.
Potential limitations are also evident. Physical activity was self-reported and assessed average activity in the prior two weeks at each visit, and some misclassification of the true activity of each individual is likely (although cumulative averaging over time reduces such error). Cases of asymptomatic paroxysmal AF may have been missed, reducing power to detect associations. The possibility of residual confounding due to unmeasured or imprecisely measured factors cannot be excluded. On the other hand, these findings are consistent with observational studies showing lower incidence of CHD and diabetes with greater physical activity; the latter relationship has been confirmed in randomized controlled trials.31,32 The associations were observed in older adults participating in a cohort study and may not be generalizable to younger individuals.
Overall, 1 in 5 of these older U.S. adults developed AF during 12 years of follow-up. Our findings suggest that moderate physical activity may meaningfully reduce this risk, and that up to one-quarter of new cases of AF in older adults may be attributable to absence of moderate leisure-time activity and regular walking at a moderate distance and pace. These results suggest that these easily achievable lifestyle habits should be further evaluated as potential preventive measures to reduce the incidence of AF in the particularly high-risk and growing population of older adults.
Acknowledgments
The authors express their gratitude to the CHS participants. A full list of participating CHS investigators and institutions is at http://www.chs-nhlbi.org.
Funding Sources
The National Heart, Lung, and Blood Institute, NIH, provided support for Dr. Mozaffarian (K08-HL-075628) and for the research reported in this article (contracts N01-HC-35129, N01-HC-45133, N01-HC-75150, N01-HC-85079 through N01-HC-85086, N01 HC-15103, N01 HC-55222, and U01 HL080295), with additional contribution from the National Institute of Neurological Disorders and Stroke.
Footnotes
Conflict of Interest Disclosures
None
References
- 1.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82:2N–9N. doi: 10.1016/s0002-9149(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 3.Wolf PA, Mitchell JB, Baker CS, Kannel WB, D’Agostino RB. Impact of atrial fibrillation on mortality, stroke, and medical costs. Arch Intern Med. 1998;158:229–234. doi: 10.1001/archinte.158.3.229. [DOI] [PubMed] [Google Scholar]
- 4.Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, White R, Furberg CD, Rautaharju PM. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. doi: 10.1161/01.cir.96.7.2455. [DOI] [PubMed] [Google Scholar]
- 5.Karjalainen J, Kujala UM, Kaprio J, Sarna S, Viitasalo M. Lone atrial fibrillation in vigorously exercising middle aged men: case-control study. BMJ. 1998;316:1784–1785. doi: 10.1136/bmj.316.7147.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furlanello F, Bertoldi A, Dallago M, Galassi A, Fernando F, Biffi A, Mazzone P, Pappone C, Chierchia S. Atrial fibrillation in elite athletes. J Cardiovasc Electrophysiol. 1998;9:S63–68. [PubMed] [Google Scholar]
- 7.Mont L, Sambola A, Brugada J, Vacca M, Marrugat J, Elosua R, Pare C, Azqueta M, Sanz G. Long-lasting sport practice and lone atrial fibrillation. Eur Heart J. 2002;23:477–482. doi: 10.1053/euhj.2001.2802. [DOI] [PubMed] [Google Scholar]
- 8.Hoogsteen J, Schep G, Van Hemel NM, Van Der Wall EE. Paroxysmal atrial fibrillation in male endurance athletes. A 9-year follow up. Europace. 2004;6:222–228. doi: 10.1016/j.eupc.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Heidbuchel H, Anne W, Willems R, Adriaenssens B, Van de Werf F, Ector H. Endurance sports is a risk factor for atrial fibrillation after ablation for atrial flutter. Int J Cardiol. 2006;107:67–72. doi: 10.1016/j.ijcard.2005.02.043. [DOI] [PubMed] [Google Scholar]
- 10.Elosua R, Arquer A, Mont L, Sambola A, Molina L, Garcia-Moran E, Brugada J, Marrugat J. Sport practice and the risk of lone atrial fibrillation: a case-control study. Int J Cardiol. 2006;108:332–337. doi: 10.1016/j.ijcard.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 11.Heidbuchel H, Panhuyzen-Goedkoop N, Corrado D, Hoffmann E, Biffi A, Delise P, Blomstrom-Lundqvist C, Vanhees L, Ivarhoff P, Dorwarth U, Pelliccia A. Recommendations for participation in leisure-time physical activity and competitive sports in patients with arrhythmias and potentially arrhythmogenic conditions Part I: Supraventricular arrhythmias and pacemakers. Eur J Cardiovasc Prev Rehabil. 2006;13:475–484. doi: 10.1097/01.hjr.0000216543.54066.72. [DOI] [PubMed] [Google Scholar]
- 12.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, O’Leary D, Psaty B, Rautaharju P, Tracy R. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 13.Tell GS, Fried LP, Hermanson B, Manolio TA, Newman AB, Borhani NO. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol. 1993;3:358–366. doi: 10.1016/1047-2797(93)90062-9. [DOI] [PubMed] [Google Scholar]
- 14.Psaty BM, Kuller LH, Bild D, Burke GL, Kittner SJ, Mittelmark M, Price TR, Rautaharju PM, Robbins J. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5:270–277. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 15.Kumanyika SK, Tell GS, Shemanski L, Martel J, Chinchilli VM. Dietary assessment using a picture-sort approach. Am J Clin Nutr. 1997;65:1123S–1129S. doi: 10.1093/ajcn/65.4.1123S. [DOI] [PubMed] [Google Scholar]
- 16.Geffken DF, Cushman M, Burke GL, Polak JF, Sakkinen PA, Tracy RP. Association between physical activity and markers of inflammation in a healthy elderly population. Am J Epidemiol. 2001;153:242–250. doi: 10.1093/aje/153.3.242. [DOI] [PubMed] [Google Scholar]
- 17.Siscovick DS, Fried L, Mittelmark M, Rutan G, Bild D, O’Leary DH. Exercise intensity and subclinical cardiovascular disease in the elderly. The Cardiovascular Health Study. Am J Epidemiol. 1997;145:977–986. doi: 10.1093/oxfordjournals.aje.a009066. [DOI] [PubMed] [Google Scholar]
- 18.Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, Cruise RG, Theroux S. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 19.Rautaharju PM, MacInnis PJ, Warren JW, Wolf HK, Rykers PM, Calhoun HP. Methodology of ECG interpretation in the Dalhousie program; NOVACODE ECG classification procedures for clinical trials and population health surveys. Methods Inf Med. 1990;29:362–374. [PubMed] [Google Scholar]
- 20.Mozaffarian D, Psaty BM, Rimm EB, Lemaitre RN, Burke GL, Lyles MF, Lefkowitz D, Siscovick DS. Fish intake and risk of incident atrial fibrillation. Circulation. 2004;110:368–373. doi: 10.1161/01.CIR.0000138154.00779.A5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siscovick DS, Weiss NS, Fletcher RH, Lasky T. The incidence of primary cardiac arrest during vigorous exercise. N Engl J Med. 1984;311:874–877. doi: 10.1056/NEJM198410043111402. [DOI] [PubMed] [Google Scholar]
- 22.Lemaitre RN, Siscovick DS, Raghunathan TE, Weinmann S, Arbogast P, Lin DY. Leisure-time physical activity and the risk of primary cardiac arrest. Arch Intern Med. 1999;159:686–690. doi: 10.1001/archinte.159.7.686. [DOI] [PubMed] [Google Scholar]
- 23.Siscovick DS. Triggers of clinical coronary heart disease. Epidemiology. 2006;17:495–497. doi: 10.1097/01.ede.0000231372.34197.2a. [DOI] [PubMed] [Google Scholar]
- 24.Scharhag J, Schneider G, Urhausen A, Rochette V, Kramann B, Kindermann W. Athlete’s heart: right and left ventricular mass and function in male endurance athletes and untrained individuals determined by magnetic resonance imaging. J Am Coll Cardiol. 2002;40:1856–1863. doi: 10.1016/s0735-1097(02)02478-6. [DOI] [PubMed] [Google Scholar]
- 25.Perseghin G, De Cobelli F, Esposito A, Lattuada G, Terruzzi I, La Torre A, Belloni E, Canu T, Scifo P, Del Maschio A, Luzi L, Alberti G. Effect of the sporting discipline on the right and left ventricular morphology and function of elite male track runners: a magnetic resonance imaging and phosphorus 31 spectroscopy study. Am Heart J. 2007;154:937–942. doi: 10.1016/j.ahj.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 26.Frost L, Frost P, Vestergaard P. Work related physical activity and risk of a hospital discharge diagnosis of atrial fibrillation or flutter: the Danish Diet, Cancer, and Health Study. Occup Environ Med. 2005;62:49–53. doi: 10.1136/oem.2004.014266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson PD, Buchner D, Pina IL, Balady GJ, Williams MA, Marcus BH, Berra K, Blair SN, Costa F, Franklin B, Fletcher GF, Gordon NF, Pate RR, Rodriguez BL, Yancey AK, Wenger NK. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity) Circulation. 2003;107:3109–3116. doi: 10.1161/01.CIR.0000075572.40158.77. [DOI] [PubMed] [Google Scholar]
- 28.Netz Y, Wu MJ, Becker BJ, Tenenbaum G. Physical activity and psychological well-being in advanced age: a meta-analysis of intervention studies. Psychol Aging. 2005;20:272–284. doi: 10.1037/0882-7974.20.2.272. [DOI] [PubMed] [Google Scholar]
- 29.Bassuk SS, Manson JE. Epidemiological evidence for the role of physical activity in reducing risk of type 2 diabetes and cardiovascular disease. J Appl Physiol. 2005;99:1193–1204. doi: 10.1152/japplphysiol.00160.2005. [DOI] [PubMed] [Google Scholar]
- 30.Taylor AH, Ussher MH, Faulkner G. The acute effects of exercise on cigarette cravings, withdrawal symptoms, affect and smoking behaviour: a systematic review. Addiction. 2007 doi: 10.1111/j.1360-0443.2006.01739.x. [DOI] [PubMed] [Google Scholar]
- 31.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 32.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]