Abstract
Statins may confer renal protection in a variety of glomerular diseases, including diabetic nephropathy (DN). However, various glomerular lesions have different etiologies and may have different responses to statins. This study was performed to determine the differential effects of simvastatin (SMV) on glomerular pathology including mesangial expansion and podocyte injury in a mouse model of early stage type 2 diabetes mellitus (DM). Type 2 DM was induced in male C57BL/6 mice by feeding a high fat diet (HF; 45 kcal% fat). After 22 weeks, one group of HF mice was treated with SMV (HF-SMV; 7 μg/day/g BW) and another group was treated with vehicle (HF-vehicle) for 4 weeks via osmotic mini-pump. A third group served as age-matched normal diet vehicle controls (ND-vehicle; 10 kcal% fat). At the end of treatment, glomerular morphology was evaluated in a blind manner to determine the progression of DN. Body weight, blood glucose, insulin, HDL-cholesterol and triglycerides, but not LDL-cholesterol, were increased in HF mice. Over the course of treatment, the 24-hour urinary albumin excretion (UAE) was unchanged in ND-vehicle. HF mice exhibited elevated UAE, which decreased with SMV, but was unchanged with vehicle. The absolute mesangial volume and the relative mesangial volume per glomerular volume increased in HF-vehicle and remained elevated with SMV treatment. The immuno-staining of nephrin, a protein marker of the integrity of podocyte slit diaphragms, was decreased in HF-vehicle; however, the nephrin quantity of the HF-SMV group was not different from ND-vehicle. It is concluded that SMV reverses podocyte damage, but does not affect mesangial expansion in the kidneys of early stage proteinuria of type 2 DM.
Keywords: diabetic nephropathy, mice, simvastatin, statins, albuminuria
Introduction
Diabetes mellitus (DM) can lead to a host of pathological alterations characterized by multiple organ dysfunctions, of which the diabetic nephropathies (DN) are a major cause of morbidity and mortality. A study from this laboratory 1 and others 2, 3 described glomerular lesions in animal models of type 2 diabetes mellitus. These pathologies invariably included effacement of podocyte foot processes, mesangial expansion and albuminuria. Recent studies have shown that statins may reverse the albuminuria 4–6 in type 2 DM models. However, the glomerular pathologies associated with the statin-reversal of albuminuria have not been determined.
Statins, 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors, are potent inhibitors of cholesterol synthesis and are used extensively in the treatment of hypercholesterolemia. Statins may confer renal protection in a variety of glomerular diseases including diabetic nephropathy 7. It is usually assumed that the beneficial effects of statins result from the competitive inhibition of cholesterol biosynthesis. However, statins may exert additional beneficial effects, beyond their cholesterol-lowering properties. Statins prevent the synthesis of isoprenoid intermediates, such as farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP), which interacts with a range of intracellular signaling molecules, such as Rho and Ras 8. Thus, the pleiotrophic effects of statins can have a variety of effects on the glomerular pathology of type 2 DM.
Mesangial cells are smooth muscle-like contractile cells that are interspersed among the glomerular capillaries. Mesangial expansion by means of proliferation, hypertrophy and matrix deposition is an early event in the progression of diabetic nephropathy 9, 10. The high fat diet mouse model of type 2 DM exhibits mesangial expansion with glomerular enlargement and hyperfiltration 1 after 13 weeks of diet. After 22 weeks, mesangial dedifferentiation and glomerular collagen IV deposition 1 are observed in renal sections.
Podocytes serve as a filtration barrier to albumin. The foot processes of podocytes interdigitate with a modified tight junction called a slit diaphragm, which is comprised of many signaling proteins including nephrin and podocin 11. In diabetic nephropathy, the effacement of podocyte foot processes and loss of slit diaphragm proteins result in leakage of albumin 11.
Microalbuminuria is often the first clinical sign of renal dysfunction in patients with diabetes mellitus. With increasing rates of albumin excretion, there is a progressive increase in renal and cardiovascular risk 12. Although albuminuria may partially result from defective proximal tubule reabsorption of proteins 13, the majority of albuminuria in subjects with diabetes mellitus is associated with mesangial expansion 5, 14 and effacement of podocyte foot proceses 4, 14.
Most patients with type 2 DM undergo a long quiescent period of insulin-resistance and mild hyperglycemia before overt diabetic symptoms. Statins alleviate some of the glomerular pathologies of DN. However, it is not understood whether reversal of podocyte injury or mesangial expansion is associated with the reversal of albuminuria. Because growth factors and other mediators of inflammation differentially affect mesangial expansion and podocyte damage, SMV may affect these cellular pathologies differentially. We therefore investigated whether mesangial expansion and/or podocyte injury exhibited in type 2 DM mice, were differentially affected by SMV treatment.
Methods and Materials
Animal care and sampling
Male C57BL/6 mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA) and maintained in the specific pathogen-free facility of the University of Nebraska Medical Center. All experiments were performed under the guidelines of the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center.
Four-week old weanling mice were placed on a normal or high fat diet (45 kcal% fat, Research Diets, NJ) and water ad libitum with a 12-h day/night rhythm. The high fat diet comprised fat, protein and carbohydrate as 45%, 20%, and 35% of the total calories, respectively. The normal diet comprised fat, protein and carbohydrate as 10%, 20% and 70% of the total calories, respectively. At the time of initiating the diets and two days before sacrifice, weight and blood samples were obtained the morning after overnight fasting (clean cages and bedding, no food, water ad libitum). Blood samples were withdrawn retro-orbitally under isofluorane inhalation anesthesia. Blood insulin levels were measured using an ELISA method according to the manufacturer’s instruction (CRYSTAL CHEM, IL). Blood glucose was measured with an Accu-Chek glucometer (Roche Diagnostic Corporation, IN). Blood triglyceride, total cholesterol, and HDL cholesterol were measured by an enzymatic/colorimetric method according to manufacturer’s instructions (Pointe Scientific, MI).
Implantation of osmotic pump and surgery
After placing the mice on either a normal or high fat diet for a period of 22 weeks, the mice were treated via subcutaneous implantation of an osmotic pump (DURECT Corporation, CA) with SMV (7 g/day/g body weight, gift from Merck & Co, Inc., NJ) or vehicle for 4 weeks. The dosage was based a human dosage of 20 mg for a 60 kg person per day, but increased by 10-fold because mice have a much higher GFR (10 times) and liver metabolism rate than humans. SMV was dissolved in 60% ethanol, and the latter was used as the vehicle control. After 4 weeks treatment, the GFR was determined under anesthesia and the mice were sacrificed. GFR was measured by FITC-labeled inulin method as previously described by this laboratory 15 and others 16. In brief, mice were anesthetized with Inactin (0.14 mg/g BW) and a tracheal tube inserted. Catheters were inserted in the bladder, jugular vein and carotid artery. The catheters were covered with foil to shield the FITC-inulin (Sigma-Aldrich, MO) from light. The mean arterial blood pressure (MAP) was monitored with a pressure transducer. Mice with a MAP less than 80 mm Hg were not utilized for further experimentation because GFR fails to autoregulate below this value. Mice were infused with a physiological saline solution containing (in mmol): 145 NaCl, 5 KCl, 10 HEPES, 1% FITC-inulin (pH 7.4), at a rate of 16μl/g BW/hr. Urine was collected for one hour subsequent to a one hour equilibration period. At the end of the collecting period, blood was withdrawn from the carotid artery. The urine and plasma concentrations of FITC-inulin were measured by the emitted fluorescence using a microplate reader. GFR was calculated as the urinary flow rate times the concentration of inulin in the urine divided by the concentration of inulin in the plasma.
Urine albumin excretion assay
A murine microalbuminuria ELISA kit (Exocell Inc. PA) was used to assay mouse 24-hour urine albumin excretion. Forty-eight hour urine samples were collected in metabolic cages. To complete the assay, 10 μl urine was diluted by 120 μl diluent. Diluted sample and rabbit anti-murine albumin antibody were added to albumin-coated wells. The antibody interacted and bound with the albumin immobilized to the stationary phase. A subsequent reaction with anti-rabbit -HRP conjugate labeled the probe with enzyme. After washing, the HRP bound albumin was detected using a chromogenic reaction. Color intensity was determined by a spectrometer (SpectraMAX 190, from Molecular Devices, CA) and was inversely proportional to the logarithm of albumin in the fluid phase.
Immunohistochemistry
For histological analyses, the mice were sacrificed; the complete kidneys were rapidly removed, and immediately immersed in 10% neutral buffered formalin and embedded in paraffin. Sections 5 μM thick were mounted on glass slides and previously described procedures were used for immunohistochemical analysis 1. Briefly, sections were deparaffinized, rehydrated and boiled in 10 mM citrate buffer (pH 6.0) in a pressure cooker for 2 min after steaming for antigen retrieval. Endogenous fluorescence was quenched in 0.1% osmium tetroxide for 2 minutes and then rinsed by tap water and set overnight. Sections were incubated with 10% horse serum to prevent nonspecific binding. The sections were then incubated with goat anti-nephrin (Santa Cruz, CA) overnight at 4ºC. After three rinses with PBS for 15 minutes, the sections were incubated with Alexa Fluor® 488 Donkey anti-goat antibody (from Molecular Probe, CA) for 1 hour at room temperature. Sections were rinsed again with PBS and then evaluated under fluorescence microscopy. The images were processed using Adobe Photoshop software. Positive and negative controls with their respective to primary antibodies were applied for each staining. After each staining, nephrin-stained capillaries in each glomerulus were counted. In each slide, a set of 20 consecutive glomeruli were selected for counting.
Electron microscopy
Measurements of relative mesangial per glomerular volume (Vv(mes/glom) was performed using the point grid system and Image Pro Plus image analysis software as described previously 17–19. In brief, ultra-thin renal sections (70 – 80 nm) were obtained and examined with a Philips 410LS Transmission Electron Microscope in the Dept. of Genetics, Cell Biology and Anatomy at UNMC. The sections were stained with 2% Uranyl Acetate and Reynold’s Lead Citrate. Glomeruli were photographed and printed at 1,600X magnification to produce photomontages of the entire glomerulus, defined as the circumscribed, minimal convex polygon surrounding the glomerular tuft. Using the montages, Vv(mes/glom) was calculated by superimposing a double lattice square grid with equally spaced coarse points 60 mm apart and equally spaced fine points 30 mm apart. Therefore each coarse point was equal to four fine points. The Vv(mes/glom) was estimated by counting the number of fine points (FP) falling on the mesangium in relation to the number of coarse points (CP) falling on the glomerular tuft according to the equation: Vv (mes/glom) = FPm/(CPgX4). The mesangium included the mesangial cell and extracellular matrix. Three mice from each group and 2–3 glomeruli from each animal were analyzed for a total of 8–9 values in each group (ND-veh, HF-veh, and HF-SMV). The morphology was analyzed by an electron microscopy technician who was completely unaware of the treatment groups corresponding to the numbered renal samples that were being analyzed.
Statistics
All data are presented as means ± SE. SigmaStat (version 3.00) was used for statistical analysis. Differences between means were determined by a paired t-test or ANOVA plus Student-Newman-Kuells, as appropriate.
Results
Development of diabetic mice
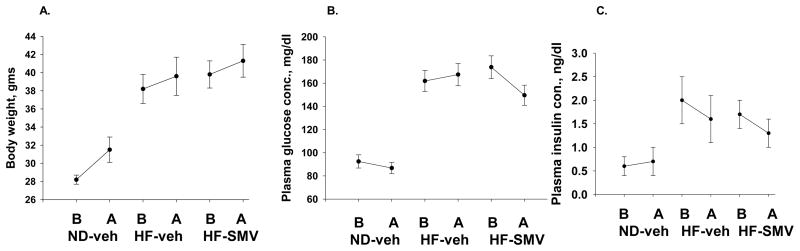
The effects of high fat diet and SMV treatment on animal weight, blood glucose and blood insulin levels are reported in figure 1. As in a previous study, male C57BL/6 mice were fed a high-fat diet to induce obesity and an early stage of type 2 DM 1. After 22 weeks on the high fat diet, the diabetic mice were treated with SMV or vehicle for 4 weeks. Age-matched controls were fed a normal diet and treated with vehicle during the same time span. Four-week treatment with SMV did not affect the weight gain of diabetic mice when compared to vehicle-treated diabetic mice (Fig. 1A). Hyperglycemia was sustained during the course of vehicle treatment. After 4 weeks of SMV treatment, the blood glucose level was not significantly different from the level before SMV treatment or the blood glucose level of the diabetic mice at the end of vehicle treatment (Fig. 1B). As previously reported, blood insulin levels increased as mice developed obesity. The blood insulin levels decreased slightly, but not significantly, in the SMV-treated diabetic mice compared to HF-vehicle mice (Fig. 1C).
Figure 1.
Effects of SMV on body weight (A), plasma glucose (B) and plasma insulin (C). A. Body weight increased in normal mice during the course of treatment from 28 to 31.5 gms. The body weight of HF-vehicle and HF-SMV did not change during the four-week treatment regimen. B. Plasma glucose concentration, significantly elevated in the HF-vehicle and HF-SMV groups compared with controls, was reduced slightly but significantly by SMV treatment. C. SMV did not affect insulin levels over the four-week treatment regimen.
Lipid profiles
Table 1 shows the results of the high fat diet and SMV treatment on lipid profiles. In comparison to the mice on a normal diet, diabetic mice (HF-vehicle) developed hypercholesterolemia. Blood cholesterol levels decreased after 4 weeks of SMV treatment, but were maintained at a constant level in HF-vehicle mice. As previously reported by our group and others 1, 20, HDL, instead of LDL, is the main composition of the blood cholesterol in mice. In contrast to human studies, SMV treatment lowered HDL in diabetic mice. Blood triglyceride (TG) levels were elevated in both vehicle and SMV treated mice and were not affected by 4 weeks of SMV treatment.
Table 1.
Lipid profiles before and after treatment
| Groups | n | Total cholesterol (mg/dl) | HDL (mg/dl) | TG (mg/dl) | |||
|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | ||
| ND-veh | 8 | 80.0 ± 1.9 | 72.0 ± 1.7 | 69.4 ± 3.1 | 66.4 ± 2.7 | 58.9 ± 3.3 | 47.6 ± 6.8 |
| HF-veh | 10 | 126.7 ± 10.0a | 126.4 ± 10.4b | 109.0 ± 7.4a | 107.7 ± 10.8b | 74.1 ± 7.0a | 97.8 ± 19.5b |
| HF-SMV | 10 | 149.8 ± 10.0a | 119.2 ± 6.2b,c | 121.3 ± 7.5a | 90.0 ± 6.2b,c | 74.9 ± 4.3a | 87.2 ± 8.1b |
p<0.05,
ANOVA analysis among groups before treatment;
ANOVA analysis among groups after treatment;
paired t-test between before and after treatment;
Renal function and morphology
Simvastatin treatment did not significantly (ANOVA) alter glomerular filtration rate (GFR). After 26 weeks of diet, the GFR (μl/min) in ND-vehicle, HF-vehicle, and HF-SMV were 245 ± 29 (n=9), 219 ± 37 (n=6), and 227 ± 35 (n=11), respectively.
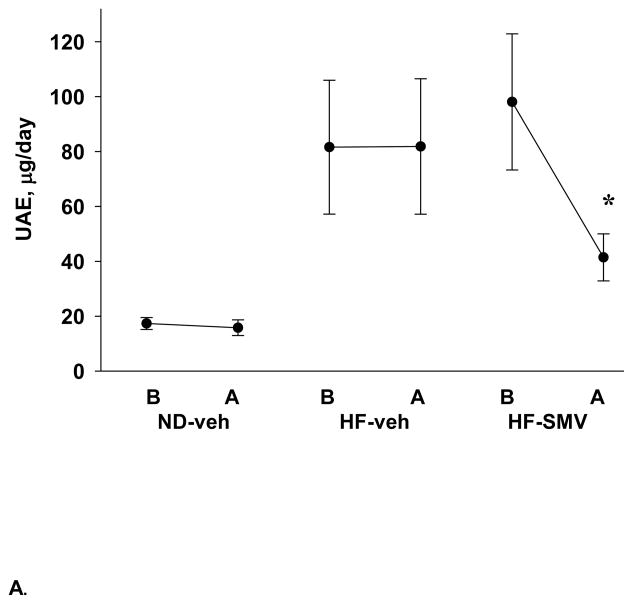
The urinary albumin excretion (UAE) was measured in all groups and the values summarized in figure 2. As shown, the UAE values were consistently low in the ND-vehicle group, with values of 17.3 and 15.8 μg/day, before and after, respectively, the four week (vehicle) treatment regimen. Mice placed on the high fat diet (HF-veh) exhibited significantly elevated UAE of 81.6 ± 24.4 μg/day, which was unchanged with vehicle treatment (81.8 ± 24.7). The mice placed in the HF-SMV group exhibited an initial UAE of 98.1 ± 24.8 μg/day, which was significantly decreased to 41.4 ± 8.6 μg/day on treatment with SMV. At termination, the kidneys of the ND-vehicle, HF-vehicle and HF-SMV mice were extracted and the glomerular pathology was compared.
Figure 2.
Summary of UAE values determined before (B) and after (A) treatment in normal diet vehicle (ND-veh; n=11), high fat diet vehicle (HF-veh; n=5) and high fat diet simvastatin (HF-SMV; n=6) groups of mice. *Denotes statistical significance (p<0.05) when comparing HF-SMV after treatment to HF-SMV before treatment.
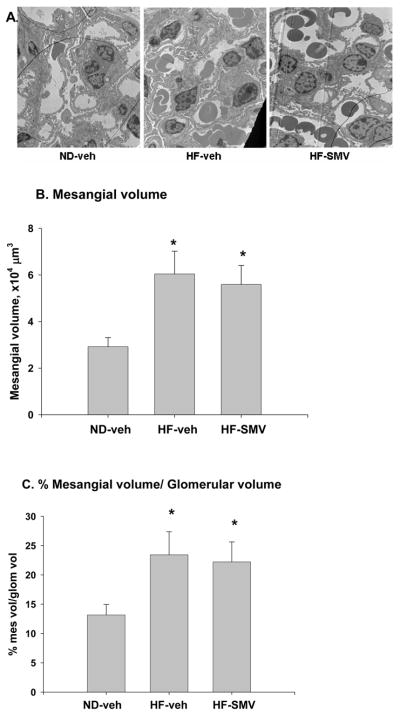
Electron microscopy was used to determine the effects of HF diet on mesangial expansion, expressed as the absolute volume and as a percentage of mesangial volume per glomerular volume [Vv(mes/glom)]. As shown by the representative micrograph in figure 3A, there was an apparent increase in Vv(mes/glom) in the HF-vehicle group compared with the ND-vehicle group. The bar plots of figure 3B show that the absolute mesangial volume was increased from 2.92 × 104 μm3 in the ND-vehicle group to 6.04 × 104 μm3 in the HF-vehicle group; however, the value for mesangial volume (5.59 × 104 μm3) in the HF-SMV group was not significantly different from that of HF-vehicle. Figure 3C summarizes the relative glomerular volume, Vv(mes/glom). in all groups. There was a significant increase in Vv(mes/glom) from 13.2% in the ND-vehicle group to 21.5% in the HF-vehicle group. However, there was no significant difference when the Vv(mes/glom) value (22.3%) for the HF-SMV group was compared with HF-vehicle. Therefore, a four-week administration of SMV does not appear to affect the relative mesangial expansion in the type 2 DM mice induced by the HF diet.
Figure 3.
Representative electron micrographs of renal sections (A) and summary bar plots for mesangial volumes (B), and mesangial volume per glomerular volumes (C) for ND-vehicle, HF-vehicle and HF-SMV. *Denotes statistical significance (p<0.05) when comparing HF-SMV and HF-vehicle with ND-vehicle using ANOVA plus SNK. Stains were 2% Uranyl Acetate and Reynold’s Lead Citrate.
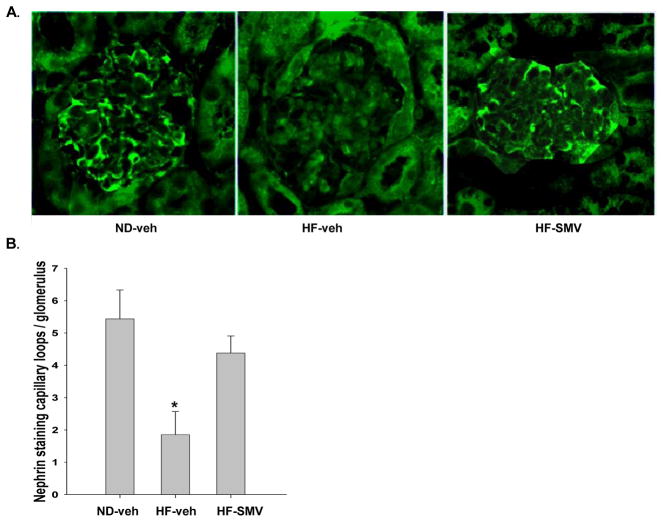
The increase in UAE in the HF mice may be elicited by podocyte damage. In order to investigate the status of the podocyte integrity in diabetic mice we studied expression of nephrin, a protein localized on slit diaphragms of the foot processes. Shown by the representative stainings of figure 4A, nephrin was clearly expressed in almost every capillary loop in renal glomeruli of the ND mice. However, nephrin staining was noted only in a few capillary loops in the glomeruli of the HF-vehicle (diabetic) mice. As shown, SMV treatment rescued nephrin expression in the HF-SMV group. To compare statistically the nephrin expression in the three groups, the positive stained capillary loops were counted in random glomeruli, the results summarized and presented in figure 4B. Nephrin staining was significantly reduced in the HF-vehicle group when compared with the ND-vehicle. The HF-SMV group returned toward the values of the ND-vehicle group and was significantly greater than the HF-vehicle group. Therefore, SMV treatment reversed the loss of nephrin in the HF mice, indicating that SMV restores the integrity of the podocytes.
Figure 4.
A. Immunohistochemical analysis of renal sections from ND-vehicle, HF-vehicle and HF-SMV. Kidneys stained with goat polyclonal anti-nephrin antibody (from Santa Cruz). Magnification × 400. B. Bar graph summarizes number of nephrin stained glomerular capillary loops in ND-vehicle (n=10), HF-vehicle (16) and HF-SMV (n=16). *Denotes statistical significance (p<0.05) when comparing HF-vehicle with HF-SMV and ND-vehicle using ANOVA plus SNK.
Discussion
A previous study in this laboratory demonstrated that C57/Bl6 mice exhibited significantly high plasma insulin and glucose levels after only 8 weeks on a high fat diet 1. Other studies have shown that a high fat diet induces type 2 DM in some strains of mice 1, 21–23. The purpose of the present study was to determine the differential effects of SMV on renal lesions noted in mice inflicted with an early stage of type 2 DM after 22–26 weeks on a high fat diet. Renal pathology included excessive albumin excretion, mesangial expansion, and diminished nephrin expression in podocytes. SMV treatment for four weeks reversed the albumin excretion and nephrin loss. Mesangial area, increased in HF mice, was unaffected by short-term treatment with SMV.
Effects of diet and SMV treatment on lipid profile
The results of the present study showed that the total cholesterol levels were significantly increased in the HF mice. However, the blood concentration of HDL, the primary component of cholesterol in mice, was increased with the high fat diet and reduced by SMV. Other studies have reported that HDL is the major component of cholesterol in mice and is reduced by SMV 1, 20.
The LDL of the mice was relatively unaffected by SMV. Thus, a reduction of LDL could not be a factor in the reversal of the renal lesions. Other studies have found that the beneficial renal effects of statins are independent of their LDL lowering effects 24, 25. In patients with type 2 DM, SMV therapy reduced albuminuria in both normotensives and hypertensives, independent of the LDL lowering effects 24. However, one study has shown that plasma cholesterol dose-dependently increased proteinuria in rats 26.
Effects of SMV on albuminuria
The finding that SMV reversed albuminuria is consistent with some recent studies showing that statins alleviate the proteinuria of diabetic nephropathy. One-year treatment of non-diabetic patients with atorvastatin decelerated the progression of kidney disease, indicated by the amelioration of proteinuria and reduction of GFR loss 27. Pitavastatin decreased albuminuria of the db/db model of type 2 diabetic mice 5 and simvastatin reduced albuminuria in an STZ-induced type 1 DM rat model 28.
Statins also reduce albuminuria in non-diabetic models of nephropathy. Cerivastatin reduced proteinuria and renal injury independently of blood pressure and cholesterol levels in stroke-prone spontaneously hypertensive rats 29. Lovastatin reduced glomerular macrophage infiltration in glomeruli and attenuated the albuminuria of rats with puromycin aminonucleoside nephrosis 30.
Although statins may reduce or prevent albuminuria in early stages of type 2 diabetes mellitus, they may not be useful for treating patients in later stages of diabetes with chronic renal disease. Fluvastatin effectively lowered LDL-cholesterol in patients with diabetic nephropathy or chronic glomerulonephritis but did not improve albuminuria or creatinine clearance 31. Importantly, treating type 2 DM patients on dialysis with atorvastatin did not reverse or slow the progression of disease 32. Therefore, it is possible that statins correct albuminuria in early stages of reversible glomerular injury but not in later stages when the cells of the filtration barrier are irreversibly damaged.
Effects of SMV on mesangial expansion
Previous studies have suggested that mesangial expansion leads to long term renal disease by extracellular matrix occlusion and restriction of capillary filtration surface area 33, 34. A variety of factors may be responsible for diabetes-associated mesangial expansion. Glucose entry into mesangial cells is not controlled by insulin. Consequently, the high plasma glucose concentration of diabetes mellitus generates in mesangial cells an abundance of diacyl glycerol (DAG), which stimulates protein kinase C 35 and increases mesangial proliferation 36. However, in comparison to glucose levels of 400 to 600 mg% in type 1 DM, glucose was only mildly elevated to 170 mg% in the present high fat model of type 2 DM. Another factor is the elevated plasma insulin levels, which could stimulate mesangial growth via insulin-like growth factor (IGF) receptors 37, which are abundantly expressed in mesangial cells. Indeed, the expression of IGF receptors is enhanced in mesangial cells isolated from the db/db type 2 DM mouse model 38.
In seeming contrast to the results of the present study, some studies have found that statins prevent mesangial proliferation in vitro 39, 40. Additionally, statins can cause apoptosis or reverse serum-stimulated mesangial proliferation 41. However, most in vivo models of diabetic nephropathy indicate that mesangial proliferation is an early event 1, 42 and early treatment may be necessary to prevent mesangial proliferation. In support of this notion, only early treatment with statins prevented mesangial proliferation in an in vivo model of proliferative nephritis 43. Administration of pitavastatin orally for 12 weeks to 12 week-old db/db mice reduced mesangial proliferation determined by PAS staining and imaging software 5. Therefore, by the time of SMV intervention in the high fat fed mouse model (22 to 26 weeks on HF diet), the mesangial cells may have already irreversibly proliferated and dedifferentiated.
Statin reversal of podocyte-associated nephrin loss and albuminuria
It is generally accepted that the numerous foot processes of podocytes interdigitate with neighboring foot processes to form a filtration slits linked by slit diaphragms, which serves as barriers to the passage of proteins 11, 44. Thus, albuminuria, one of the first signs of impending nephropathy in the early stages of type 2 diabetes, is evidence for loss of slit diaphragm integrity. The mechanism for the early loss of podocyte structure in early type 2 diabetes is unknown. However, even in nondiabetics, the central obesity, which is a component of metabolic syndrome and is modeled in the high fat fed mice of this study, is an independent risk factor for albuminuria 45.
The present study showed that SMV restores the nephrin expression that is reduced in an early stage of type 2 DM nephropathy. Nephrin is an adhesion molecule 46 localized specifically on slit diaphragms of podocytes 47, 48. Like other adhesion molecules, nephrin is phosphorylated and involved in the organization of the slit diaphragm 49, possibly by stimulating AKT-dependent signaling and remodeling of the actin cytoskeleton 50. Indeed, several cytoskeletal stimulators can induce nephrin shedding from the podocyte foot processes 51. Our results are consistent with a recent study showing that fluvastatin restored nephrin and podocyn expression in renal sections from puromycin aminonucleoside (PAN)-induced nephrosis in rats 6. Sung et al 52 also found a decrease in glomerular nephrin in a type 2 DM model (db/db) as determined by immunohistochemistry. In that study, it was interesting that Western blot showed enhanced nephrin expression in whole kidneys. However, It is possible that nephrin was shed from podocytes and remained in the kidney.
Potential mechanism for differential effects of statins on podocytes and mesangial cells
This is the first study to show that statins reversed albuminuria and podocyte injury without affecting the mesangial expansion of diabetic nephropathy. That mesangial expansion is not reversed by statins suggests a disassociation between mesangial expansion and albuminuria. The reduction of RhoA by statins may be one factor in the noted LDL-independent reversal of albuminuria by statins. The inhibition of isoprenylation by statins prevents RhoA from translocating to the plasma membrane where it is activated. RhoA signaling is over-active in PAN nephrosis and a reduction in RhoA activity, either by statins or the specific Rho-kinase inhibitor, fasudil, reversed the podocyte impairment 6. Therefore, it is possible that statins restore podocyte integrity by their action to reduce the activation of RhoA.
The differential effects of SMV on podocyte injury and mesangial expansion may be due to the property of SMV to inhibit vascular endothelial growth factor (VEGF) but not TGF-β. Inhibition of RhoA by statins reverses the effects of VEGF 53, which is associated with podocyte injury but it may not reverse the effects of TGF-β, which is more associated with mesangial expansion and collagen deposition 54, 55. Indeed, anti-TGF-β; therapy in db/db mice ameliorated matrix expansion without affecting albuminuria 56 and administration of anti-VEGF reversed albuminuria without affecting mesangial expansion 52, 57. A recent study by Whiteside and coworkers showed that high glucose leads to an increase in VEGF via PKC stimulation in mesangial cells 58. Moreover, VEGF is elevated in glomeruli from type 2 DM kidneys 57, 59, where it can cause an increase in stress fiber formation in glomerular endothelial cells as well as podocyte injury and albuminuria 52. The beneficial consequences of inhibiting RhoA activity may involve inhibiting VEGF.
Finally, evidence now indicates that podocyte injury 60, as well as mesangial expansion 5 is the result of an inflammatory response and the generation of NAD(P)H oxidase NOX4, which is a major source of oxidative stress leading to glomerular hypertrophy in the kidney 61. A recent study showed that pitavastatin ameliorated albuminuria and mesangial expansion by preventing the generation of NOX4 5. Interestingly, antioxidants also ameliorate the expression of VEGF in podocytes 60. Thus, SMV may reverse the podocyte injury in our diabetic model by preventing the NOX4 stimulation of VEGF.
Regardless of the mechanism of action of SMV, our results show that SMV applied relatively late in the progression of DN in the early stage of type 2 DM, reverses albuminuria and podocyte injury without affecting mesangial expansion. Thus, even though early treatment of statins can prevent mesangial expansion, the prevention of podocyte injury, is probably more related to the reduction of albuminuria.
Acknowledgments
This work was supported by the American Heart Association #0355405Z, the American Diabetes Association #1-05-RA-114, and the National Institutes of Diabetes and Digestive and Kidney Diseases 5RO1 DK073070 (to S.C. Sansom). P.R. Grimm was supported by a fellowship (#0610059Z) from the American Heart Association (Heartland Affiliate). We appreciate the technical assistance in electron microscopy of Thomas Bargar, Dept. of Genetics, Cell Biology and Anatomy, UNMC. Simvastatin was a gift from Merck.
Reference List
- 1.Wei P, Lane PH, Lane JT, Padanilam BJ, Sansom SC. Glomerular structural and functional changes in a high-fat diet mouse model of early-stage Type 2 diabetes. Diabetologia. 2004 August 27;47:1541–9. doi: 10.1007/s00125-004-1489-1. [DOI] [PubMed] [Google Scholar]
- 2.Erdely A, Freshour G, Maddox DA, Olson JL, Samsell L, Baylis C. Renal disease in rats with Type 2 diabetes is associated with decreased renal nitric oxide production. Diabetologia. 2004 October;47(10):1672–6. doi: 10.1007/s00125-004-1509-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coimbra TM, Janssen U, Grone HJ, et al. Early events leading to renal injury in obese Zucker (fatty) rats with type II diabetes. Kidney Int. 2000 January;57(1):167–82. doi: 10.1046/j.1523-1755.2000.00836.x. [DOI] [PubMed] [Google Scholar]
- 4.Whaley-Connell A, Demarco VG, Lastra G, et al. Insulin Resistance, Oxidative Stress, and Podocyte Injury: Role of Rosuvastatin Modulation of Filtration Barrier Injury. Am J Nephrol. 2007 October 3;28(1):67–75. doi: 10.1159/000109394. [DOI] [PubMed] [Google Scholar]
- 5.Fujii M, Inoguchi T, Maeda Y, et al. Pitavastatin ameliorates albuminuria and renal mesangial expansion by downregulating NOX4 in db/db mice. Kidney Int. 2007 August;72(4):473–80. doi: 10.1038/sj.ki.5002366. [DOI] [PubMed] [Google Scholar]
- 6.Shibata S, Nagase M, Fujita T. Fluvastatin ameliorates podocyte injury in proteinuric rats viamodulation of excessive Rho signaling. J Am Soc Nephrol. 2006 March;17(3):754–64. doi: 10.1681/ASN.2005050571. [DOI] [PubMed] [Google Scholar]
- 7.Epstein M, Campese VM. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors on renal function. Am J Kidney Dis. 2005 January;45(1):2–14. doi: 10.1053/j.ajkd.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 8.Senokuchi T, Matsumura T, Sakai M, et al. Statins suppress oxidized low density lipoprotein-induced macrophage proliferation by inactivation of the small G protein-p38 MAPK pathway. J Biol Chem. 2005 February 25;280(8):6627–33. doi: 10.1074/jbc.M412531200. [DOI] [PubMed] [Google Scholar]
- 9.Young BA, Johnson RJ, Alpers CE, et al. Cellular events in the evolution of experimental diabetic nephropathy. Kidney Int. 1995 March;47(3):935–44. doi: 10.1038/ki.1995.139. [DOI] [PubMed] [Google Scholar]
- 10.Wolf G, Sharma K, Chen Y, Ericksen M, Ziyadeh FN. High glucose-induced proliferation in mesangial cells is reversed by autocrine TGF-beta. Kidney Int. 1992 September;42(3):647–56. doi: 10.1038/ki.1992.330. [DOI] [PubMed] [Google Scholar]
- 11.Shankland SJ. The podocyte’s response to injury: role in proteinuria and glomerulosclerosis. Kidney Int. 2006 June;69(12):2131–47. doi: 10.1038/sj.ki.5000410. [DOI] [PubMed] [Google Scholar]
- 12.Lane JT. Microalbuminuria as a marker of cardiovascular and renal risk in type 2 diabetes mellitus: a temporal perspective. Am J Physiol Renal Physiol. 2004 March;286(3):F442–F450. doi: 10.1152/ajprenal.00247.2003. [DOI] [PubMed] [Google Scholar]
- 13.Tojo A, Onozato ML, Kurihara H, Sakai T, Goto A, Fujita T. Angiotensin II blockade restores albumin reabsorption in the proximal tubules of diabetic rats. Hypertens Res. 2003 May;26(5):413–9. doi: 10.1291/hypres.26.413. [DOI] [PubMed] [Google Scholar]
- 14.Menini S, Iacobini C, Oddi G, et al. Increased glomerular cell (podocyte) apoptosis in rats with streptozotocin-induced diabetes mellitus: role in the development of diabetic glomerular disease. Diabetologia. 2007 September 28; doi: 10.1007/s00125-007-0821-y. [DOI] [PubMed] [Google Scholar]
- 15.Pluznick JL, Wei P, Grimm PR, Sansom SC. BK-{beta}1 subunit: immunolocalization in the mammalian connecting tubule and its role in the kaliuretic response to volume expansion. Am J Physiol Renal Physiol. 2005 April;288(4):F846–F854. doi: 10.1152/ajprenal.00340.2004. [DOI] [PubMed] [Google Scholar]
- 16.Lorenz JN, Gruenstein E. A simple, nonradioactive method for evaluating single-nephron filtration rate using FITC-inulin. Am J Physiol. 1999 January;276(1 Pt 2):F172–F177. doi: 10.1152/ajprenal.1999.276.1.F172. [DOI] [PubMed] [Google Scholar]
- 17.Basgen JM, Rich SS, Mauer SM, Steffes MW. Measuring the volume density of the glomerular mesangium. Nephron. 1988;50(3):182–6. doi: 10.1159/000185154. [DOI] [PubMed] [Google Scholar]
- 18.Nyengaard JR. Stereologic methods and their application in kidney research. J Am Soc Nephrol. 1999 May;10(5):1100–23. doi: 10.1681/ASN.V1051100. [DOI] [PubMed] [Google Scholar]
- 19.Brito PL, Fioretto P, Drummond K, et al. Proximal tubular basement membrane width in insulin-dependent diabetes mellitus. Kidney Int. 1998 March;53(3):754–61. doi: 10.1046/j.1523-1755.1998.00809.x. [DOI] [PubMed] [Google Scholar]
- 20.Maugeais C, Tietge UJ, Broedl UC, et al. Dose-dependent acceleration of high-density lipoprotein catabolism by endothelial lipase. Circulation. 2003 October 28;108(17):2121–6. doi: 10.1161/01.CIR.0000092889.24713.DC. [DOI] [PubMed] [Google Scholar]
- 21.Park SY, Cho YR, Kim HJ, et al. Unraveling the temporal pattern of diet-induced insulin resistance in individual organs and cardiac dysfunction in C57BL/6 mice. Diabetes. 2005 December;54(12):3530–40. doi: 10.2337/diabetes.54.12.3530. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi M, Ohno T, Tsuchiya T, Horio F. Characterization of diabetes-related traits in MSM and JF1 mice on high-fat diet. J Nutr Biochem. 2004 October;15(10):614–21. doi: 10.1016/j.jnutbio.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Rossmeisl M, Rim JS, Koza RA, Kozak LP. Variation in type 2 diabetes--related traits in mouse strains susceptible to diet-induced obesity. Diabetes. 2003 August;52(8):1958–66. doi: 10.2337/diabetes.52.8.1958. [DOI] [PubMed] [Google Scholar]
- 24.Tonolo G, Ciccarese M, Brizzi P, et al. Reduction of albumin excretion rate in normotensive microalbuminuric type 2 diabetic patients during long-term simvastatin treatment. Diabetes Care. 1997 December;20(12):1891–5. doi: 10.2337/diacare.20.12.1891. [DOI] [PubMed] [Google Scholar]
- 25.Wilson SH, Chade AR, Feldstein A, et al. Lipid-lowering-independent effects of simvastatin on the kidney in experimental hypercholesterolaemia. Nephrol Dial Transplant. 2003 April;18(4):703–9. doi: 10.1093/ndt/gfg143. [DOI] [PubMed] [Google Scholar]
- 26.Attia DM, Ni ZN, Boer P, et al. Proteinuria is preceded by decreased nitric oxide synthesis and prevented by a NO donor in cholesterol-fed rats. Kidney Int. 2002 June;61(5):1776–87. doi: 10.1046/j.1523-1755.2002.00313.x. [DOI] [PubMed] [Google Scholar]
- 27.Bianchi S, Bigazzi R, Caiazza A, Campese VM. A controlled, prospective study of the effects of atorvastatin on proteinuria and progression of kidney disease. Am J Kidney Dis. 2003 March;41(3):565–70. doi: 10.1053/ajkd.2003.50140. [DOI] [PubMed] [Google Scholar]
- 28.Banes-Berceli AK, Shaw S, Ma G, et al. Effect of simvastatin on high glucose- and angiotensin II-induced activation of the JAK/STAT pathway in mesangial cells. Am J Physiol Renal Physiol. 2006 July;291(1):F116–F121. doi: 10.1152/ajprenal.00502.2005. [DOI] [PubMed] [Google Scholar]
- 29.Usui H, Shikata K, Matsuda M, et al. HMG-CoA reductase inhibitor ameliorates diabetic nephropathy by its pleiotropic effects in rats. Nephrol Dial Transplant. 2003 February;18(2):265–72. doi: 10.1093/ndt/18.2.265. [DOI] [PubMed] [Google Scholar]
- 30.Park YS, Guijarro C, Kim Y, et al. Lovastatin reduces glomerular macrophage influx and expression of monocyte chemoattractant protein-1 mRNA in nephrotic rats. Am J Kidney Dis. 1998 January;31(1):190–4. doi: 10.1053/ajkd.1998.v31.pm9428473. [DOI] [PubMed] [Google Scholar]
- 31.Yasuda G, Kuji T, Hasegawa K, et al. Safety and efficacy of fluvastatin in hyperlipidemic patients with chronic renal disease. Ren Fail. 2004 July;26(4):411–8. doi: 10.1081/jdi-120039826. [DOI] [PubMed] [Google Scholar]
- 32.Wanner C, Krane V, Marz W, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005 July 21;353(3):238–48. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 33.Ziyadeh FN. The extracellular matrix in diabetic nephropathy. Am J Kidney Dis. 1993 November;22(5):736–44. doi: 10.1016/s0272-6386(12)80440-9. [DOI] [PubMed] [Google Scholar]
- 34.Abrass CK. Diabetic nephropathy. Mechanisms of mesangial matrix expansion. West J Med. 1995 April;162(4):318–21. [PMC free article] [PubMed] [Google Scholar]
- 35.Whiteside CI, Dlugosz JA. Mesangial cell protein kinase C isozyme activation in the diabetic milieu. Am J Physiol Renal Physiol. 2002 June;282(6):F975–F980. doi: 10.1152/ajprenal.00014.2002. [DOI] [PubMed] [Google Scholar]
- 36.Ayo SH, Radnik R, Garoni JA, Troyer DA, Kreisberg JI. High glucose increases diacylglycerol mass and activates protein kinase C in mesangial cell cultures. Am J Physiol. 1991;261:F571–F577. doi: 10.1152/ajprenal.1991.261.4.F571. [DOI] [PubMed] [Google Scholar]
- 37.Conti FG, Striker LJ, Lesniak MA, Mackay K, Roth J, Striker GE. Studies on binding and mitogenic effect of insulin and insulin-like growth factor I in glomerular mesangial cells. Endocrinology. 1988 June;122(6):2788–95. doi: 10.1210/endo-122-6-2788. [DOI] [PubMed] [Google Scholar]
- 38.Oemar BS, Foellmer HG, Hodgdon-Anandant L, Rosenzweig SA. Regulation of insulin-like growth factor I receptors in diabetic mesangial cells. J Biol Chem. 1991 February 5;266(4):2369–73. [PubMed] [Google Scholar]
- 39.Nagasawa K, Muraki Y, Matsuda T, Ohnishi N, Yokoyama T. Inhibitory effect of statins on fetal bovine serum-induced proliferation of rat cultured mesangial cells and correlation between their inhibitory effect and transport characteristics. J Pharm Sci. 2000 December;89(12):1594–604. doi: 10.1002/1520-6017(200012)89:12<1594::aid-jps11>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 40.Terada Y, Inoshita S, Nakashima O, et al. Lovastatin inhibits mesangial cell proliferation via p27Kip1. J Am Soc Nephrol. 1998 December;9(12):2235–43. doi: 10.1681/ASN.V9122235. [DOI] [PubMed] [Google Scholar]
- 41.Ghosh PM, Mott GE, Ghosh-Choudhury N, et al. Lovastatin induces apoptosis by inhibiting mitotic and post-mitotic events in cultured mesangial cells. Biochim Biophys Acta. 1997 October 30;1359(1):13–24. doi: 10.1016/s0167-4889(97)00091-8. [DOI] [PubMed] [Google Scholar]
- 42.Chen LM, Li XW, Huang LW, Li Y, Duan L, Zhang XJ. [The early pathological changes of KKAy mice with type 2 diabetes] Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2002 February;24(1):71–5. [PubMed] [Google Scholar]
- 43.Yoshimura A, Nemoto T, Sugenoya Y, et al. Effect of simvastatin on proliferative nephritis and cell-cycle protein expression. Kidney Int Suppl. 1999 July;71:S84–S87. doi: 10.1046/j.1523-1755.1999.07121.x. [DOI] [PubMed] [Google Scholar]
- 44.Ronco P. Proteinuria: is it all in the foot? J Clin Invest. 2007 August;117(8):2079–82. doi: 10.1172/JCI32966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chandie Shaw PK, Berger SP, Mallat M, Frolich M, Dekker FW, Rabelink TJ. Central obesity is an independent risk factor for albuminuria in nondiabetic South Asian subjects. Diabetes Care. 2007 July;30(7):1840–4. doi: 10.2337/dc07-0028. [DOI] [PubMed] [Google Scholar]
- 46.Khoshnoodi J, Sigmundsson K, Ofverstedt LG, et al. Nephrin promotes cell-cell adhesion through homophilic interactions. Am J Pathol. 2003 December;163(6):2337–46. doi: 10.1016/S0002-9440(10)63590-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koop K, Eikmans M, Baelde HJ, et al. Expression of podocyte-associated molecules in acquired human kidney diseases. J Am Soc Nephrol. 2003 August;14(8):2063–71. doi: 10.1097/01.asn.0000078803.53165.c9. [DOI] [PubMed] [Google Scholar]
- 48.Kawachi H, Koike H, Kurihara H, Sakai T, Shimizu F. Cloning of rat homologue of podocin: expression in proteinuric states and in developing glomeruli. J Am Soc Nephrol. 2003 January;14(1):46–56. doi: 10.1097/01.asn.0000037401.02391.76. [DOI] [PubMed] [Google Scholar]
- 49.Simons M, Schwarz K, Kriz W, et al. Involvement of lipid rafts in nephrin phosphorylation and organization of the glomerular slit diaphragm. Am J Pathol. 2001 September;159(3):1069–77. doi: 10.1016/S0002-9440(10)61782-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002 May 31;296(5573):1655–7. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 51.Doublier S, Ruotsalainen V, Salvidio G, et al. Nephrin redistribution on podocytes is a potential mechanism for proteinuria in patients with primary acquired nephrotic syndrome. Am J Pathol. 2001 May;158(5):1723–31. doi: 10.1016/S0002-9440(10)64128-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sung SH, Wang A, Pyagay PE, Kanwar YS, Ziyadeh FN, Chen S. Blockade of Vascular Endothelial Growth Factor Signaling Ameliorates Diabetic Albuminuria in Mice. J Am Soc Nephrol. 2006 September 20; doi: 10.1681/ASN.2006010064. [DOI] [PubMed] [Google Scholar]
- 53.Zeng L, Xu H, Chew TL, et al. HMG CoA reductase inhibition modulates VEGF-induced endothelial cell hyperpermeability by preventing RhoA activation and myosin regulatory light chain phosphorylation. FASEB J. 2005 November;19(13):1845–7. doi: 10.1096/fj.05-4240fje. [DOI] [PubMed] [Google Scholar]
- 54.Gnudi L, Thomas SM, Viberti G. Mechanical forces in diabetic kidney disease: a trigger for impaired glucose metabolism. J Am Soc Nephrol. 2007 August;18(8):2226–32. doi: 10.1681/ASN.2006121362. [DOI] [PubMed] [Google Scholar]
- 55.Park IS, Kiyomoto H, Abboud SL, Abboud HE. Expression of transforming growth factor-β and type IV collagen in early streptozotocin-induced diabetes. Diabetes. 1997;46(3):473–80. doi: 10.2337/diab.46.3.473. [DOI] [PubMed] [Google Scholar]
- 56.Ziyadeh FN, Hoffman BB, Han DC, et al. Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-beta antibody in db/db diabetic mice. Proc Natl Acad Sci U S A. 2000 July 5;97(14):8015–20. doi: 10.1073/pnas.120055097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Flyvbjerg A, Dagnaes-Hansen F, De Vriese AS, Schrijvers BF, Tilton RG, Rasch R. Amelioration of long-term renal changes in obese type 2 diabetic mice by a neutralizing vascular endothelial growth factor antibody. Diabetes. 2002 October;51(10):3090–4. doi: 10.2337/diabetes.51.10.3090. [DOI] [PubMed] [Google Scholar]
- 58.Xia L, Wang H, Munk S, et al. Reactive Oxygen Species, PKC-{beta}1, and PKC-{zeta} Mediate High Glucose-induced Vascular Endothelial Growth Factor Expression in Mesangial Cells. Am J Physiol Endocrinol Metab. 2007 August 21; doi: 10.1152/ajpendo.00223.2007. [DOI] [PubMed] [Google Scholar]
- 59.Cha DR, Kang YS, Han SY, et al. Vascular endothelial growth factor is increased during early stage of diabetic nephropathy in type II diabetic rats. J Endocrinol. 2004 October;183(1):183–94. doi: 10.1677/joe.1.05647. [DOI] [PubMed] [Google Scholar]
- 60.Lee EY, Chung CH, Kim JH, Joung HJ, Hong SY. Antioxidants ameliorate the expression of vascular endothelial growth factor mediated by protein kinase C in diabetic podocytes. Nephrol Dial Transplant. 2006 June;21(6):1496–503. doi: 10.1093/ndt/gfl022. [DOI] [PubMed] [Google Scholar]
- 61.Gorin Y, Block K, Hernandez J, et al. Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem. 2005 November 25;280(47):39616–26. doi: 10.1074/jbc.M502412200. [DOI] [PubMed] [Google Scholar]