The movement of calcium across cell membranes regulates many important physiological processes in vertebrates, as various as the contraction of heart muscle, the firing of brain cells, the expression of genes by immune cells, and the secretion of hormones. Cells have two main types of calcium channels that enable calcium ions (Ca2+) to flow into the cell. Voltage-gated Ca2+ channels typically function only in electrically excitable cells, such as neurons, heart muscle cells, and insulin-producing cells in the pancreas. By contrast, store-operated Ca2+ channels work only in electrically inexcitable cells, such as lymphocytes and other cells of the immune system. Researchers have long puzzled over this pattern, in part because biochemical evidence suggested that both channel types are present in both excitable and inexcitable cells. On pages 101 and 105 of this issue, reports by Park et al. and Wang et al. (1, 2) help solve the puzzle by presenting a new mechanism that determines which type of Ca2+ channel predominates in a particular cell type. These two groups show that the protein STIM1, which was already known to activate store-operated Ca2+ channels, inhibits voltage-gated Ca2+ channels. Together, the reports help to illuminate a reciprocal calcium control mechanism that, if it goes wrong, can have life-threatening consequences.
STIM proteins first came to light in the Ca2+ signaling field 5 years ago; the name comes from their initial identification as a stromal-interacting molecule. Studies revealed that STIM proteins play an essential role in the function of store-operated Ca2+ channels in Drosophila (Stim) (3) and in human cells (STIM1 and STIM2) (4). Later, researchers screening the Drosophila genome identified another family of proteins involved in the function of store-operated Ca2+ channels: the Orai proteins (Orai1, Orai2, and Orai3 in humans) (5–7). By subtly altering the structure of Orai proteins, researchers showed that they play a key role in calcium “selectivity,” or a channel’s ability to recognize Ca2+ ions and allow them to pass through a pore in the membrane (8–10). Together, these studies showed that STIM and Orai proteins work closely together to mediate the activity of store-operated Ca2+ channels. In general, STIM proteins function as a signaling relay; when Ca2+ stores are depleted at the endoplasmic reticulum (ER) within the cell, for instance, they can help to activate and open Orai channels in the outer plasma membrane (PM) (see the figure). Experiments have demonstrated several functions of STIM proteins in activating store-operated Ca2+ channels (11).
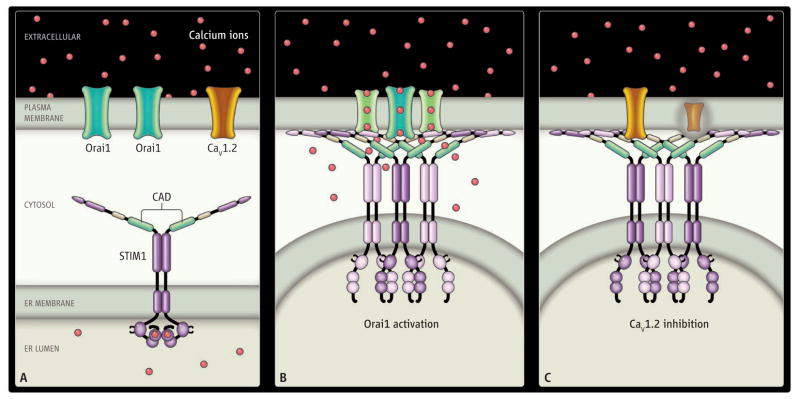
Figure. STIMulating.
STIM1 in the ER membrane activates Orai1 (green) and inhibits CaV1.2 (orange) in the plasma membrane. (A) The cell at rest. Calcium ions (red) are abundant in the extracellular space and within the ER lumen but are rare in the cytosol. Functional domains of STIM1 include the EF hand (with calcium ions bound), and the CRAC-activating domain (CAD, green). (B and C) ER-PM junctions when the cell is activated and Ca2+ is depleted from the ER lumen. Calcium ions unbinding from the EF hand of STIM1 trigger translocation of STIM1 to ER-PM junctions. The cytosolic junctional gap between ER and PM is sufficiently narrow (10 to 20 nm) to allow for direct molecular interaction of STIM1 with Orai1 and CaV1.2. The CAD of STIM1 recruits Orai1 to ER-PM junctions and opens Orai1 channels by direct binding to STIMulate Ca2+ influx (B). CaV1.2 channel proteins are also recruited to ER-PM junctions but are inhibited by the CAD interacting with the C terminus of CaV1.2 (C).
In their studies, Wang et al. and Park et al. examined the role that STIM proteins might also play in regulating voltage-gated Ca2+ channels. Specifically, they examined one medically important member of this diverse family, known as the CaV1.2 subunit (α1C). CaV1.2 forms the so-called L-type Ca2+ channels found in a wide array of cells, including those in the cortex, hippocampus, cerebellum, neuroendocrine system, heart, and arterial smooth muscle. Mutations in the CaV1.2 gene CACNA1C cause Timothy and Brugada syndromes, which are associated with life-threatening cardiac arrhythmias. A number of treatments, including the drugs verapamil, diltiazem, and various dihydropyridines, specifically target CaV1.2 channels.
The two groups found that the sequence of events involving STIM1 begins in the same way for both store-dependent and voltage-regulated channels, but the end result is very different. After Ca2+ store depletion in the ER, a structure known as the EF hand of STIM1 unbinds Ca2+, and STIM1 oligomers translocate to form clusters immediately adjacent to the plasma membrane at ER-PM junctions. If voltage-gated Ca2+ channel proteins are expressed, they, like Orai subunit proteins, accumulate adjacent to STIM1. There, STIM1 and CaV1.2 interact via specific domains that protrude into the cytosol: a CRAC-activating domain (CAD) in STIM1 and the C terminus of CaV1.2. CAD opens Orai1 channels, but—as the two new papers show—it inhibits CaV1.2 channel activity in two ways: acutely (immediately) by physical interaction, and more slowly by causing internalization, a process that inhibits the channel and results in subsequent degradation of the channel protein. The two reports differ with respect to the relative importance of the acute and internalization phases (Wang et al. find that the acute phase is strong, Park et al. less so), but the outcome is the same: STIM1 regulates Orai1 and CaV1.2 reciprocally. In inexcitable lymphocytes, where STIM1 is relatively abundant, voltage-gated Ca2+ channels are inhibited and Orai1 is activated. In neurons and presumably other excitable cells, STIM1 is not so abundant and voltage-gated Ca2+ channel activity predominates.
As the activator for one Ca2+ channel (Orai1) and an inhibitor for another (CaV1.2), STIM1 assumes a new functional importance. In excitable cells, membrane depolarization (a change in electrical charge) activates Ca2+ influx through voltage-gated Ca2+ channels, but in lymphocytes depolarization actually inhibits Ca2+ influx through Orai channels, because the electrical driving force for Ca2+ to enter is reduced. This potentially resolves a long-standing controversy regarding the type of Ca2+ channel in lymphocytes (12). Even if subunits of voltage-gated Ca2+ channels are expressed, they are not functional because of inhibition by STIM1. More important, STIM1’s reciprocal functional interaction guarantees that only one type of Ca2+ channel or the other will be active.
Many questions remain concerning the generality of the findings. Does STIM1 block other voltage-gated Ca2+ channel family members? In native tissue, CaV1.2 sub-units interact with numerous other cellular proteins that help with subcellular localization and modulation. Do they physically or functionally protect the channels from being inhibited by STIM1? When STIM1 is knocked out in mice or in rare patients with mutations of STIM1, what are the consequences for voltage-dependent Ca2+ channel function in vivo? Answers to these questions should further improve our understanding of how Ca2+ signaling is regulated.
References
- 1.Park CY, Shcheglovitov A, Dolmetsch R. Science. 2010;330:101. doi: 10.1126/science.1191027. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, et al. Science. 2010;330:105. doi: 10.1126/science.1191086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roos J, et al. J Cell Biol. 2005;169:435. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liou J, et al. Curr Biol. 2005;15:1235. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feske S, et al. Nature. 2006;441:179. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 6.Vig M, et al. Science. 2006;312:1220. doi: 10.1126/science.1127883. published online 27 April 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang SL, et al. Proc Natl Acad Sci USA. 2006;103:9357. [Google Scholar]
- 8.Prakriya M, et al. Nature. 2006;443:230. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 9.Vig M, et al. Curr Biol. 2006;16:2073. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yeromin AV, et al. Nature. 2006;443:226. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cahalan MD. Nat Cell Biol. 2009;11:669. doi: 10.1038/ncb0609-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cahalan MD, Chandy KG. Immunol Rev. 2009;231:59. doi: 10.1111/j.1600-065X.2009.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]