Abstract
A facile solid-phase synthetic approach to the synthesis of 3,4-disubstituted-2-aminothiazolium derivatives was reported. Functionalized aminomethylphenyl silica gel was used as a “volatilizable” support. The products were cleaved with 10% HF and were obtained in high yields and purities.
2-Aminotniazole is present in many chemically interesting compounds that display biological activity.1 This ring structure can be either a substituent of a bioactive scaffold or the pharmacophore of bioactive molecules. Regarding 2-aminothiazole derivatives different activities have been reported for them such as antimicrobial, antibacterial, and anti-inflammation.2 2-Aminothiazole derivatives also been tested as inhibitors of p53 to treat neurode generative disorders, as inhibitors of COX to treat pain, for the treatment of allergies, hypertension, Alzheimer's disease and tumors.3 2-Aminothiazole was also reported to form a ruthenium (III) complex that exhibited promising antitumor properties.4 Their wide range of biological activities has encouraged synthetic investigations towards facile strategies for the generation of large numbers of structurally diverse 2-aminothiazoles.
Solid-phase synthetic technology provides an ideal platform for the rapid assembly of building blocks to generate large collections of compounds having complex structures in a short time period.5 Solid-phase synthesis, combined with high throughput screening, is proven to be an efficient method for the discovery of lead compounds.6 For the conventional solid-phase synthesis, all the desired synthetic products must be cleaved from the support and then separated from the spent support. This final separation step in the synthesis of very large numbers of compounds may result in reduced yields and increased costs. To solve these problems, we first developed the concept of “volatilizable” supports, which are completely decomposed and volatile following the cleavage step leaving only the desired product in the reaction vessel.7 As part of this ongoing project, herein, we provide a facile approach to the synthesis of 3,4-disubstituted-2-aminothiazolium derivatives by the use of a “volatilizable” support.
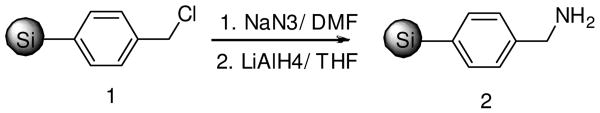
The functionalized aminomethylphenyl silica gel was used as the “volatilizable” support. The aminomethylphenyl-functionalized silica gel was synthesized by the on-resin substitution and reduction of chloromethylphenyl-functionalized silica gel 18 as shown in Scheme 1. The chloromethylphenyl-functionalized silica gel 1 (loading: 0.35 mmol/g) was treated with sodium azide in DMF at 65 °C for 24 h. After washing with DMF, the functionalized silica gel was then reduced with 0.1 M lithium aluminum hydride at room temperature overnight to yield the desired aminomethylphenyl-functionalized silica gel 2. The loading of the resin was 0.3 mmol/g, which was determined from the substitution of the Fmoc-Ala-loaded resin by coupling Fmoc-Ala-OH and then cleaving with 10% HF.
Scheme 1. Synthesis of functionalized aminomethylphenyl silica gel.
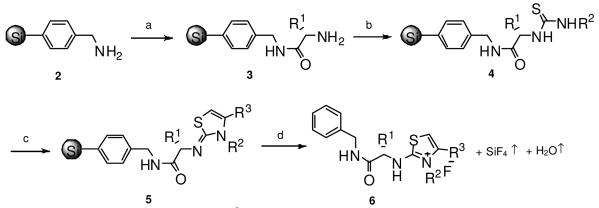
The 3,4-disubstituted-2-aminothiazolium derivatives were synthesized by on-resin cyclization of resin-bound thiourea derivatives with α-halogenoketones (Scheme 2).9 Starting from aminomethylphenyl functionalized silica gel 2, a Boc- amino acid was coupled on the resin to generate the resin bound amino acid 3. After removal of the Boc group, an isothiocyanate was coupled to yield the resin bound thiourea 4. The resin bound thiourea 4 was then reacted with an α-bromoketone, resulting in the resin bound 2-iminothiazole 5. After treatment of 10% HF at room temperate for 1 h, followed by the removal of the solvent under vacuum, the final product of 3,4-disubstituted-2-aminothiazolium 6 was obtained in high yield and purity (Table 1, 6a-k). The product was generated with the R1 position functionality protected by using side-chain protected amino acids; for example, Boc-Ser(Bzl)-OH and Boc-Lys(2-Cl-Z)-OH (6j-k).
Scheme 2. Synthesis of 2-aminothiazol-3-ium derivatives through a “volatilizable” amino-functionalized silica gel.
a. Boc-AA-OH/DIC; 55% TFA/DCM. b. R2,NCS/DCM.c. α-bromoketone, 65 °C, overnight. d. 10% HF, r.t, 1 h.
Table 1. Individual Products of 3,4-disubstituted-2-aminothiazolium derivatives.
| Entry | R1 | R2 | R3 | Purity % a | Yield% b |
|---|---|---|---|---|---|
| 6a | -CH3 | -C6H5 | -C2H5 | 92 | 84 |
| 6b | -CH3 | -C6H4(p-OCH3) | -C(CH3)3 | 91 | 88 |
| 6c | -CH2C6H5 | -C6H5 | -C2H5 | 90 | 99 |
| 6d | -CH2CH(CH3)2 | -C6H4(p-CH3) | -C6H5 | 90 | 86 |
| 6e | -CH2CH(CH3)2 | -C6H4(o-F) | -C6H4(p-F) | 80 | 80 |
| 6f | -CH(CH3)2 | -C6H4(p-NO2) | -C2H5 | 86 | 96 |
| 6g | -CH(CH3)2 | C6H4(o-Cl) | -C6H5 | 90 | 99 |
| 6h | -H | -C6H3(o,o-2CH3) | -C(CH3)3 | 90 | 93 |
| 6i | -H | -C6H3(o,p-2Cl) | -C(CH3)3 | 85 | 80 |
| 6j | -CH2O-Bzl | -C6H4(p-NO2) | -C2H5 | 70 | 73 |
| 6k | - (CH2)4NHCOOCH2C6H4(o-Cl) | -C6H4(p-CH3) | -C6H5 | 78 | 74 |
| 61 | -CH2OH | -C6H4(p-OCH3) | -C6H5 | 85 | 90 |
| 6m | -CHCH3OH | -C6H4(p-OCH3) | -C6H5 | 78 | 82 |
| 6n | -C6H4OH | -C6H4(p-OCH3) | -C6H5 | 81 | 84 |
Purity(in %) is determinate by the peak area of HPLC at 214nm.
Yields(in %) are based on the weight of crude product and are relative to the substitution of the resin.
To obtain an unprotected side-chain group at the R1 position, Fmoc-amino acids such as Fmoc-Ser(tBu)-OH, Fmoc-Thr(tBu)-OH, and Fmoc-Tyr(tBu)-OH were used instead of Boc-amino acid. After removal of the Fmoc protecting group with 20% piperidine, isothiocyanates and α-bromoketones were coupled step-wise using the same reaction conditions as shown in Scheme 1 to yield the resin bound 2-iminothiazole 5. The resin bound 2-iminothiazole 5 was then treated with a solution of TFA:water:triisopropylsilane (95:2.5:2.5 by volume) for 1.5 h at room temperature to remove the amino acid side-chain protecting groups. After treatment of 10% HF the final products of 3,4-disubstituted-2-aminothiazolium derivatives with unprotected side chain groups at the R1 position were obtained (Table 1, 61-n).
In summary, we present here a straightforward approach to the synthesis of 3,4-disubstituted-2-aminothiazolium derivatives through the use of “volatilizable” aminomethylphenyl functionalized silica gel. Boc- and Fmoc-amino acids were both used in the synthetic process, resulting in the protected and unprotected products at R1 position, respectively. The use of 10% HF in water for the cleavage of the products not only avoids the use of harsh cleavage conditions such as anhydrous HF, but also provides a facile method for isolating the desired products through the decomposition of the silica-based support. The use of functionalized aminomethylphenyl silica gel to make heterocyclic compounds is efficient and promising.
Acknowledgments
This work was supported by the State of Florida, Executive Officer of the Governor's Office of Tourism, Trade and Economic Development, National Science Foundation (R.A.H. CHE 0455072), 1P41GM079590, 1P41GM081261, and U54HG03916-MLSCN.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.(a) Latli B, D'Amour K, Casida JE. J Med Chem. 1999;42:2227. doi: 10.1021/jm980721x. [DOI] [PubMed] [Google Scholar]; (b) Das J, Chen P, Norris D, Padmanabha R, Lin J, Moquin RV, Shen Z, Cook LS, Doweyko AM, Pitt S, Pang S, Shen DR, Fang Q, de Fex HF, McIntyre KW, Shuster DJ, Gillooly KM, Behnia K, Schieven GL, Wityak J, Barrish JC. J Med Chem. 2006;49:6819. doi: 10.1021/jm060727j. [DOI] [PubMed] [Google Scholar]; (c) Rendenbach-Mueller B, Unger L, Teschendorf HJ. US Patent 5,071,864. 1991; (d) Komarov PG, Komarova EA, Kondratov RV, Christov-Tselkov K, Coon JS, Chernov MV, Gudkov AV. Science. 1999;285:1733. doi: 10.1126/science.285.5434.1733. [DOI] [PubMed] [Google Scholar]; (e) Chordia MD, Murphree LJ, Macdonald TL, Linden J, Olsson RA. Bioorg Med Chem. 2002;12:1563. doi: 10.1016/s0960-894x(02)00236-6. [DOI] [PubMed] [Google Scholar]; (f) Zhang WT, Ruan JL, Wu PF, Jiang FC, Zhang LN, Fang W, Chen XL, Wang Y, Cao BS, Chen GY, Zhu YJ, Gu J, Chen JG. J Med Chem. 2009;52:718. doi: 10.1021/jm800902t. [DOI] [PubMed] [Google Scholar]
- 2.(a) Vicini P, Geronikaki A, Anastasia K, Incertia M, Zani F. Bioorg Med Chem. 2006;14:3859. doi: 10.1016/j.bmc.2006.01.043. [DOI] [PubMed] [Google Scholar]; (b) Kane JL, Jr, Hirth BH, Liang B, Gourlie BB, Nahill S, Barsomian G. Bioorg Med Chem Lett. 2003;13:4463. doi: 10.1016/j.bmcl.2003.09.013. [DOI] [PubMed] [Google Scholar]; (c) Kim SH, Son H, Nam G, Chi DY, Kim JH. Bioorg Med Chem Lett. 2000;10:1143. doi: 10.1016/s0960-894x(00)00130-x. [DOI] [PubMed] [Google Scholar]; (d) Hashiquchi T, Yoshida T, Itoyama T, Taniquchi Y. US Patent 5,856,347. 1999; (e) Wang X, Xu F, Xu Q, Mahmud H, Houze J, Zhu L, Akerman M, Tonn G, Tang L, McMaster BE, Dairaghi DJ, Schall TJ, Collins TL, Medina JC. Bioorg Med Chem Lett. 2006;16:2800. doi: 10.1016/j.bmcl.2006.01.126. [DOI] [PubMed] [Google Scholar]
- 3.(a) Pietrancosta N, Maina F, Dono R, Moumen A, Garino C, Laras Y, Burlet S, Quéléver G, Kraus JL. Bioorg Med Chem Lett. 2005;15:1561. doi: 10.1016/j.bmcl.2005.01.075. [DOI] [PubMed] [Google Scholar]; (b) Carter JS, Kramer S, Talley JJ, Penning T, Collins P, Graneto MJ, Seibert K, Koboldt CM, Masferrer J, Zweifel B. Bioorg Med Chem Lett. 1999;9:1171. doi: 10.1016/s0960-894x(99)00157-2. [DOI] [PubMed] [Google Scholar]; (c) Hargrave KD, Hess FK, Oliver JT. J Med Chem. 1983;26:1158. doi: 10.1021/jm00362a014. [DOI] [PubMed] [Google Scholar]; (d) Patt WC, Hamilton HW, Taylor MD, Ryan MJ, Taylor DG, Connolly CJ, Jr, Doherty CAM, Klutchko SR, Sircar I, Steinbaugh BA, Batley BL, Painchaud CA, Rapundalo ST, Michniewicz BM, Olson SCJ. J Med Chem. 1992;35:2562. doi: 10.1021/jm00092a006. [DOI] [PubMed] [Google Scholar]; (e) Helal CJ, Sanner MA, Cooper CB, Gant T, Adam M, Lucas JC, Kang Z, Kupchinsky S, Ahlijanian MK, Tate B, Menniti FS, Kelly K, Peterson M. Bioorg Med Chem Lett. 2004;14:5521. doi: 10.1016/j.bmcl.2004.09.006. [DOI] [PubMed] [Google Scholar]; (f) Aberle N, Catimel J, Nice EC, Watson KG. Bioorg Med Chem Lett. 2007;17:3741. doi: 10.1016/j.bmcl.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 4.Mura P, Camalli M, Bindoli A, Sorrentino F, Casini A, Gabbiani C, Corsini M, Zanello P, Rigobello MP, Messori L. J Med Chem. 2007;50:5871. doi: 10.1021/jm0708578. [DOI] [PubMed] [Google Scholar]
- 5.(a) Geysen HM, Meloen RH, Barteling S. J Proc Natl Acad Sci USA. 1984;81:3998. doi: 10.1073/pnas.81.13.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Houghten RA. Proc Natl Acad Sci USA. 1985;82:5131. doi: 10.1073/pnas.82.15.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Houghten RA, Pinilla C, Blondelle SE, Appel JR, Dooley CT, Cuervo JH. Nature. 1991;345:8486. doi: 10.1038/354084a0. [DOI] [PubMed] [Google Scholar]; (b) An H, Cook PD. Chem Rev. 2000;100:331. [Google Scholar]
- 7.Houghten RA, Yu Y. J Am Chem Soc. 2005;127:8582. doi: 10.1021/ja0402396. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Yu Y, Giulianotti M, Houghten RA. J Comb Chem. 2008;10:613. doi: 10.1021/cc800076b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.General procedure for the synthesis of the 3, 4-disubstituted-2-aminothiazolium derivatives. 200mg functionalized aminomethylphenyl silica gel was sealed within a polypropylene mesh packet.10 Reactions were carried out in polypropylene bottles. A solution of N-Boc-amino acid (5 eqiv, 0.1M in DMF), HOBt (5 eqiv, 0.1M in DMF) and DIC (5 eqiv, 0.1M in DMF) were added to the reaction vessel. The reaction was shaken at room temperature for 2 h, followed by washing with DMF (3 times). Upon removal of the Boc- group with 55% TFA in DCM at room temperature for 30 min, the resin was washed and neutralized with 5% DIEA in DCM. The resin bound amine was reacted with isothiocyanate (5 eqiv, 0.1M in DCM) overnight. After washing with DCM (2 times), DMF (1 time), DCM (2 times) and air dried, α-bromoketone (10 eqiv, 0.1 M in DMF) was added to the reaction vessel. The reaction was performed at 65 °C for 24 h. The resin packet was then washed with DMF (3 times), DCM (3 times) and MeOH (3 times). The cleavage of the product was carried out by the treatment with 4 ml of 10% HF at room temperature for 1 h, followed by lyophilization to remove the solvent. The product of 2-aminothiazol-3-ium derivatives was obtained. The representative product 6d was characterized by electrospray LC-MS under ESI conditions and NMR. HPLC: tR = 2.8 min, CH3CN(+ 0.05% formic acid) in H2O (+ 0.05% formic acid) 5-95% in 6 min; column: luna C18, 5 μm, 50 × 4.60 mm, detection 254 nm; ESI-MS (m/z) of 6d: 469.2 [M+H]+; 1H NMR (500 MHz, DMSO-d6) δ: 0.84 (d, 6H, J = 6.5 Hz), 141-1.44 (m, 1H), 1.54-1.61 (m, 2H), 2.22 (s, 3H), 3.42-3.44 (m, 1H), 4.25 (d, 2H, J = 6.5 Hz), 6.44 (s, 1H), 6.54 (s, 1H), 7.04 (m, 4H), 7.12-7.17(m, 4H), 7.20-7.26 (m, 4H), 7.30-7.33 (m, 2H), 7.48 (t, 1H, J = 5.5 Hz); C NMR (125 MHz, DMSO-d6) δ: 22.0, 22.8, 24.5, 40.0, 41.9, 66.7, 97.0, 126.7, 127.0, 128.0, 128.2, 128.3, 128.6, 129.2, 131.3, 135.6, 136.6, 139.2, 139.4, 159.9, 172.3.
- 10.Houghten RA. Proc Natl Acad Sci USA. 1985;82:5131. doi: 10.1073/pnas.82.15.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]