Abstract
Background/Aim:
Helicobacter pylori is an important pathogen for gastroduodenal diseases. Infection with H. pylori can be limited by regimens of multiple antimicrobial agents. However, antibiotic resistance is a leading cause of treatment failure. The aim of this study has been to determine the resistance patterns of H. pylori strains isolated from gastric biopsies of patients with dyspepsia by agar dilution method, in Tehran, Iran
Patients and Methods:
H. pylori isolates from patients with gastrointestinal diseases were evaluated for susceptibility testing by agar dilution method. Susceptibility testing was performed to commonly used antibiotics including clarithromycin, tetracycline, amoxicillin, metronidazole and ciprofloxacin.
Results:
Among 92 patients with dyspepsia, H. pylori strains were isolated from 42 patients. Seventeen (40.5%) of the isolates were resistant to metronidazole (MICs ≥ 8 μg/l), whereas one isolate (2.4%) was resistant to amoxicillin (MICs ≤ 0. 5 μg/ml) and ciprofloxacin (MICs ≤ 1μg/ml). The resistance rates to other antibiotics in H. pylori isolates are recorded as follows: clarithromycin 6 (14.3 %), tetracycline 2 (4.8%). In 5 of 42 resistant cases, combined resistance was found.
Conclusions:
These data suggest that metronidazole should be used among Iranian patients in first-line therapy with caution, and ciprofloxacin in association with amoxicillin and a proton pump inhibitor is more recommended.
Keywords: Antibiotic susceptibility, Helicobacter pylori, Iranian patients
Helicobacter pylori is an important pathogen for gastroduodenal diseases and more than 50% of the world's population and nearly 80% of Iran's population is infected by this bacteria.[1–3] Many people with H. pylori infection never have any signs or symptoms and do not develop ulcers or other problems related to the infection, mucosa-associated lymphoid tissue (MALT) lymphoma and adenocarcinoma of the stomach.[4] So eradication therapy of symptomatic H. pylori infection by regimens of multiple antimicrobial agents plays an important role in the prevention of gastroduodenal diseases and its related complications. On the other hand, the most common causes of treatment failure can be related to antimicrobial resistance of H. pylori strain.[5,6]
The successful treatment for eradication of H. pylori infection requires combination therapy; containing a proton pump inhibitor (PPI) and prescription of two or more antibiotics simultaneously, such as amoxicillin, clarithromycin, metronidazole, and tetracycline.[6,7]
The rate of H. pylori resistance to the commonly used antibiotics varies according to different area. Also various frequencies in the same region are reported in different periods of time.[8,9] Therefore each country must provide local data to guide treatment policies. We tried to determine the resistance patterns of H. pylori strains isolated from gastric biopsies of patients with dyspepsia by agar dilution method, in Tehran, Iran.
PATIENTS AND METHODS
Population study
The study group consisted of 92 patients with dyspeptic symptoms undergoing endoscopy in Taleghani Hospitals in Tehran, Iran between April 2007 and December 2008. After endoscopic examination, the gastric biopsy specimens from the antrum were examined for the presence of H. pylori by culture and polymerase chain reaction (PCR). This study was granted Institutional Review Board approval by Shahid Beheshti University and received ethical approval from the ethics committee. None of the patients had received previous treatment for the eradication of H. pylori.
Isolation and identification
Two antral biopsy specimens from each patient were kept in transport medium consisting of Thioglycollate medium which supplemented with 1.3 g/l agar (Merck, Homburg, Germany) and 3% yeast extract (Oxoid, Basingstoke, UK). Gastric biopsies were cultured on brain heart infusion (BHI) agar supplemented with 10% (v/v) sheep blood and selective supplement (vancomycin 2.0 mg, polymyxin 0.05 mg, trimethoprim 1.0 mg) (Merck). The cultured plates were incubated at 37°C for three-five days in a microaerobic atmosphere (5% O2, 10% CO2, 85% N2) in a CO2 incubator (Innova-Co 170; New Brunswick Scientific, Edison, NJ, USA). The organisms were identified as H. pylori by Gram staining, colony morphology, and positive oxidase, catalase and urease reactions.
DNA extraction and PCR amplification analysis
The bacteria were harvested, and genomic DNA was extracted by using the QIAamp tissue DNA extraction kit (QIAGEN, Hilden, Germany) according to the manufacturer's instructions.
For molecular confirming of H. pylori colonies, the glmM gene was identified by PCR using specific primer pairs: forward 5’ GGATAAGCTTTTAGGGGTGTTAGGGG-3’ and reverse 5’ GCTTACTTTCTAACACTAACGCGC-3’ with a 296-bp size product.[10] The PCR was performed in a final volume of 25 μl containing 10 X PCR buffer, 500 nM of each primer, 2 mM MgCl2; 200 μM each deoxyribonucleotide triphosphate (dNTP), 1.5 U Taq DNA polymerase, and 200 ng DNA sample. PCR was performed in a thermocycler (AG 22331; Eppendorf, Hamburg, Germany) under the following conditions: initial denaturation for 5 min at 94°C was followed by 30 cycles of 93°C for 1 min, 58°C for 30 s and 72°C for 1 min. After a final extension at 72°C for 10 min, the PCR products were examined by electrophoresis on 1.2% agarose contained gels according to standard procedures. H. pylori strain 26695 were used as control strain. Individual cultures representing colonies from each patient were frozen at -70°C until susceptibility testing was performed.
Antibiotic susceptibility testing
The minimum inhibitory concentration (MICs) was carried out by agar dilution method using Muller-Hinton agar (Merck Co, Germany) containing 5% defibrinated sheep blood. In each plate, various concentrations of antibiotics from 0.016 to 256 μg/ml were added to media when it was cooled up to 45°C. H. pylori suspensions were prepared equivalent to a no. 2 McFarland standard and 3 μl of them were inoculated on Muller-Hinton agar plates containing different concentrations of antibiotics.[11] A plate without antibiotics was used as control. The cultured plates were incubated at 37°C for three-five days in a microaerobic atmosphere (5% O2, 10% CO2, 85% N2) in a CO2 incubator (Innova-Co 170; New Brunswick Scientific, Edison, NJ, USA). After 72 h of incubation, the MIC of each antibiotic was determined.[11] The resistance breakpoints for amoxicillin (MAST, London, United Kingdom), clarithromycin (Sandoz Ind. S.A., Spain), metronidazole (MAST, London, United Kingdom), ciprofloxacin (TEMED CO., Iran), and tetracycline (Ningxia Qiyuan Pharmaceutical Co., China) were defined as ≥ 0.5, ≥ 1.0, ≥8, > 1.0, and ≥4 μg/ml, respectively.[9,11–13]
Data analysis
Chi squared and Fisher's exact tests were used for analysis of categorical data. Analyses were done using Sigma Stat for Windows V2.03 (SPSS, Chicago, IL, USA). A P value less than 0.05 was accepted as statistically significant.
RESULTS
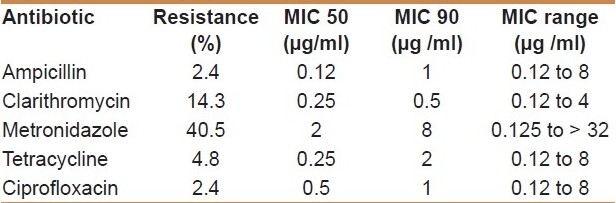
Out of 92 patients with dyspepsia, 42 patients including 24(57 %) males and 18 (43 %) females (mean age: for males: 45±18 years and females: 38±14) had endoscopic biopsy with H. pylori positive culture using specific media. The highest resistance rate was for metronidazole (40.5%), whereas one isolate (2.4%) was resistant to ampicillin and ciprofloxacin. The resistance rates to other antibiotics in H. pylori isolates are recorded as follows: clarithromycin 6 (14 %), tetracycline 2 (5%).
MIC values varied from 0.12 to 4 μg/ml for clarithromycin, and from 0.12 to 8 μg/ml for tetracycline, ampicillin and ciprofloxacin. Metronidazole had a reduced activity (MIC50 2 μg/ml); 40% of the isolates (n = 17) were resistant to this compound; and MIC values varied from 0.125 to > 32 μg/ml. The MIC50 and MIC90 of each antibiotic are shown in Table 1. Multidrug resistant H. pylori isolates were detected in 5 (12%) of 42 isolates. Among these combined resistances, the maximum resistance rate was observed in three (7 %) cases of metronidazole plus clarithromycin. Statistical analysis revealed that resistance rate to either antibiotic in H. pylori isolates was not significantly associated with the sex and age of patients (P>0.05).
Table 1.
Resistance to antimicrobial agents in H. pylori isolated from dyspeptic patients
DISCUSSION
H. pylori is an important pathogen for gastroduodenal diseases. In most cases, H. pylori infection causes an asymptomatic chronic gastric inflammation, but can also cause severe gastroduodenal diseases in some infected persons, including chronic atrophic gastritis, peptic ulcers, and gastric adenocarcinoma.[1]
In the past, H. pylori isolates were susceptible to many different antibiotics, but now the successful treatment of it is challenging.[14] Antibiotic resistance in H. pylori isolates is widespread and increasing of resistant isolates can make considerable clinical problem for antibiotic therapy. For this reason, detection of susceptibility in each area for every year can help to gain a better understanding of the effect of resistance on therapy outcome.
In this study, we report the susceptibility of Iranian H. pylori isolates to commonly used antibiotics including metronidazole, clarithromycin, ampicillin, tetracycline, and ciprofloxacin.
A high rate of resistance to metronidazole was found in Iranian isolates [Table 1]. Different range of resistance to metronidazole from 22%, 30% and 54% were reported in previous studies in Iran.[15–17] The high resistance rate of metronidazole was observed in India (77.9%) and Kuwait (70%) too.[18,19] Also there are variable susceptibility rates in developed countries; 15.8% in Bulgaria and 25% in the USA.[12,20] It was shown that H. pylori genetic drift and adapting to different situation environment can be the causes of metronidazole resistance of H. pylori isolates.[21] Because of using of metronidazole in treatment of diarrheal disease including parasitic and bacterial infections in Iran, and it is also the first line therapy in gastritis infected with H. pylori strains, high resistance to metronidazole can be expected.[22] In addition, testing conditions, including choice of medium, age of the colonies, incubation period and conditions, and inoculums size, have been shown to influence the results of susceptibility testing for metronidazole.[23] There were no significant association between rate of metronidazole resistant in H. pylori strains and age and sex in studied patients.
Among antibiotics examined, clarithromycin was the second low effective antibiotic with 14.3% resistance. In several studies conducted in Iran, different resistance rates to clarithromycin were reported; 4.16, 16 and 2.4%.[16,24,25] Albeit in developed countries approximately 10% of the H. pylori strains were clarithromycin-resistant, but in developing countries this resistance rate was higher.[26–28] Because of current usage of clarithromycin for treatment of H. pylori in Iran, this resistance may be related to cross resistance between other macrolide antibiotics such as erythromycin and clarithromycin.[15] On the other hand, using the clarithromycin may result in selection of resistant strains, but this acquired resistance to clarithromycin is stable.[29]
Resistance to amoxicillin was observed in 2.4% of the isolates. The low rate of resistance in previous studies in Iran was reported.[17,24] In Europe and USA, resistance rates are less than 1%.[23] In contrast, high resistance rates have been reported in South Korea (18.5%) and in Indonesia (19.4%).[7,30]
In some countries such as Kuwait and France, amoxicillin-resistant H. pylori has not been found.[18,30,31] Van Zwet et al. have shown that the amoxicillin-resistant phenotype can be changed in vitro condition to amoxicillin-susceptible strains.[31] Also colonization of the stomach with other b-lactam-resistant bacteria may lead to transfer of amoxicillin resistance to H. pylori by transformation or a conjugation-like mechanism.[32,33]
The low prevalence of tetracycline (5%) and ciprofloxacin-resistant (7%) H. pylori isolates in Iran was reported in other studies.[24] It has been shown that primary resistance to tetracycline is rare in H. pylori isolates.[13] The high rate of susceptibility in H. pylori strains to ciprofloxacin suggests that this antibiotic is an alternative drug that could be used for eradication of H. pylori. The low resistance rates to ciprofloxacin were 1, 2 and 5% in India, Egypt, and south-west of Nigeria, respectively.[13,34,35]
On the basis of our findings, the maximum multiple resistance rates were observed in three cases of metronidazole plus clarithromycin (7%). Statistical analysis revealed that resistance rate to either antibiotic in H. pylori isolates was not significantly associated with the gender of patients (P>0.05). Also, there was no statistical significance between antimicrobial resistance rates and different age groups.
Generally the unrestricted and unsuitable use of the antibiotics can be the causes of developing of antibiotic resistance in H. pylori strains. On the other hand, the most important causes of therapy failure are related to increased prevalence of antibiotic resistance in H. pylori; thus each country must provide local data to guide treatment policies. In conclusion considering the increasing resistance rate, ciprofloxacin and tetracycline and other probably suitable antibiotics should be recommended instead of metronidazole and clarithromycin in the eradication treatment of gastro-duodenal diseases with H. pylori infection in Iran.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175–86. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 2.Malekzadeh R, Sotoudeh M, Derakhshan MH, Mikaeli J, Yazdanbod A, Merat S. Prevalence of gastric precancerous lesions in Ardabil, a high incidence province for gastric adenocarcinoma in the North-West of Iran. J Clin Pathol. 2004;57:37–42. doi: 10.1136/jcp.57.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alborzi A, Soltani J, Pourabbas B, Oboodi B, Haghighat M, Hayati M, et al. Prevalence of Helicobacter pylori infection in children (south of Iran) Diagn Microbiol Infect Dis. 2006;54:259–61. doi: 10.1016/j.diagmicrobio.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Graham DY. Campylobacter pylori and peptic ulcer disease. Gastroenterol. 1989;96:615–25. doi: 10.1016/s0016-5085(89)80057-5. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman JS, Cave DR. Treatment of Helicobacter pylori. Curr Opin Gastroenterol. 2001;17:30–4. doi: 10.1097/00001574-200101000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Broutet N, Tchamgoue S, Pereira E, Lamouliatte H, Salamon R, Megraud F. Risk factors for failure of Helicobacter pylori therapy - results of an individual data analysis of 2751 patients. Aliment Pharmacol Ther. 2003;17:99–109. doi: 10.1046/j.1365-2036.2003.01396.x. [DOI] [PubMed] [Google Scholar]
- 7.Kim JM, Kim JS, Jung HC, Kim N, Kim YJ, Song IS. Distribution of antibiotic MICs for Helicobacter pylori strains over a 16-year period in patients from Seoul, South Korea. Antimicrob Agents Chemother. 2004;48:4843–7. doi: 10.1128/AAC.48.12.4843-4847.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharara AI, Chedid M, Araj GF, Barada KA, Mourad FH. Prevalence of Helicobacter pylori resistance to metronidazole, clarithromycin, amoxicillin and tetracycline in Lebanon. Int J Antimicrob Agents. 2002;19:155–8. doi: 10.1016/s0924-8579(01)00482-4. [DOI] [PubMed] [Google Scholar]
- 9.Glupczynski Y, Megraud F, Lopez-Brea M, Andersen LP. European multicentre survey of in vitro antimicrobial resistance in Helicobacter pylori. Eur J Clin Microbiol Infect Dis. 2001;20:820–3. doi: 10.1007/s100960100611. [DOI] [PubMed] [Google Scholar]
- 10.Kauser F, Hussain MA, Ahmed I, Ahmad N, Habeeb A, Khan AA, et al. Comparative genomics of Helicobacter pylori isolates recovered from ulcer disease patients in England. BMC Microbiol. 2005;5:32. doi: 10.1186/1471-2180-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fourth Informational Supplement M100-S14. Wayne, PA: NCCLS; 2004. National Committee for Clinical Laboratory Standards, Performance standards for antimicrobial susceptibility testing. [Google Scholar]
- 12.Boyanova L, Koumanova R, Gergova G, Popova M, Mitov I, Kovacheva Y, et al. Prevalence of resistant Helicobacter pylori isolates in Bulgarian children. J Med Microbial. 2002;51:786–90. doi: 10.1099/0022-1317-51-9-786. [DOI] [PubMed] [Google Scholar]
- 13.Megraud F, Lehn N, Lind T, Bayerdörffer E, O’Morain C, Spiller R, et al. Antimicrobial susceptibility testing of Helicobacter pylori in a large multicenter trial: the MACH 2 study. Antimicrob Agents Chemother. 1999;43:2747–52. doi: 10.1128/aac.43.11.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Realdi G, Dore MP, Piana A, Atzei A, Carta M, Cugia L. Pretreatment antibiotic resistance in Helicobacter pylori Infection: Results of three randomized controlled studies. Helicobacter. 1999;4:106–12. doi: 10.1046/j.1523-5378.1999.99002.x. [DOI] [PubMed] [Google Scholar]
- 15.Falsafi T, Mobasheri F, Nariman F, Najafi M. Susceptibilities to different antibiotics of Helicobacter pylori strains isolated from patients at the pediatric medical center of Tehran, Iran. J Clin Microbiol. 2004;42:387–9. doi: 10.1128/JCM.42.1.387-389.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fallahi GH, Maleknejad S. Helicobacter pylori culture and antimicrobial resistance in Iran. Indian J Pediatr. 2007;74:127–30. doi: 10.1007/s12098-007-0003-4. [DOI] [PubMed] [Google Scholar]
- 17.Khashei R, Shojaei H, Adibi P, Shavakhi A, Aslani MM, Naser DA. Genetic Diversity and Drug Resistance of Helicobacter pylori Strains in Isfahan, Iran. Iran J Basic Med Sci. 2008;11:174–82. [Google Scholar]
- 18.Albert JM, Al-Mekhaizeem K, Neil L, Dhar R, Dhar PM, Al-Ali M, et al. High prevalence and level of resistance to metronidazole, but lack of resistance to other antimicrobials in Helicobacter pylori, isolated from a multiracial population in Kuwait. Aliment Pharmacol Ther. 2006;24:1359–66. doi: 10.1111/j.1365-2036.2006.03144.x. [DOI] [PubMed] [Google Scholar]
- 19.Kim JM, Kim JS, Kim N, Kim SG, Jung HC, Song IS. Comparison of primary and secondary antimicrobial minimum inhibitory concentrations for Helicobacter pylori isolated from Korean patients. Int J Antimicrob Agents. 2006;28:6–13. doi: 10.1016/j.ijantimicag.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 20.Duck WM, Sobel J, Pruckler JM, Song Q, Swerdlow D, Friedman C, et al. Antimicrobial resistance incidence and risk factors among Helicobacter pylori-infected persons, United States. Emerg Infect Dis. 2004;10:1088–94. doi: 10.3201/eid1006.030744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sisson G, Jeong JY, Goodwin A, Bryden L, Rossler N, Lim-Morrison S, et al. Metronidazole activation is mutagenic and causes DNA fragmentation in Helicobacter pylori and in Escherichia coli containing a cloned H.pylori RdxA(+) (Nitroreductase) gene. J Bacteriol. 2000;182:5091–6. doi: 10.1128/jb.182.18.5091-5096.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fakheri H, Malekzadeh R, Merat S, Merat M, Khatibian A, Fazel B, et al. Clarithromycin vs.furazolidone in quadruple therapy regimens for the treatment of Helicobacter pylori in a population with a high metronidazole resistance rate. Aliment Pharmacol Ther. 2001;15:411–6. doi: 10.1046/j.1365-2036.2001.00931.x. [DOI] [PubMed] [Google Scholar]
- 23.Megraud F. H.pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut. 2004;53:1374–84. doi: 10.1136/gut.2003.022111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rafeey M, Ghotaslou R, Nikvash S, Hafez AA. Primary resistance in Helicobacter pylori isolated in children from Iran. J Infect Chemother. 2007;13:291–5. doi: 10.1007/s10156-007-0543-6. [DOI] [PubMed] [Google Scholar]
- 25.Siavoshi F, Safari F, Doratotaj D, Khatami GR, Fallahi GH, Mirnaseri MM. Antimicrobial resistance of Helicobacter pylori isolates from Iranian adults and children. Arch Iran Med. 2006;9:308–14. [PubMed] [Google Scholar]
- 26.Alarcon T, Domingo D, Lopez-Brea M. Antibiotic resistance problems with Helicobacter pylori. Int J Antimicrob Agents. 1999;12:19–26. doi: 10.1016/s0924-8579(99)00051-5. [DOI] [PubMed] [Google Scholar]
- 27.Yilmaz O, Demiray E. Clinical role and importance of fluorescence in situ hybridization method in diagnosis of Helicobacter pylori infection and determination of clarithromycin resistance in H.pylori eradication therapy. World J Gastroenterol. 2007;13:671–5. doi: 10.3748/wjg.v13.i5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bindayna KM. Antibiotic susceptibilities of Helicobacter pylori. Saudi Med J. 2001;22:53–7. [PubMed] [Google Scholar]
- 29.Megraud F. Epidemiology and mechanism of antibiotic resistance in Helicobacter pylori. Gastroenterology. 1998;115:1278–82. doi: 10.1016/s0016-5085(98)70101-5. [DOI] [PubMed] [Google Scholar]
- 30.Kumala W, Rani A. Patterns of Helicobacter pylori isolate resistance to fluoroquinolones, amoxicillin, clarithromycin and metronidazole. Southeast Asian J Trop Med Public Health. 2006;37:970–4. [PubMed] [Google Scholar]
- 31.Van Zwet A, Vandenbrouke-Grauls CM, Thijs JC, van der Wouden EJ, Gerrits MM, Kusters JG. Stable amoxicillin resistance in Helicobacter pylori. Lancet. 1998;352:595. doi: 10.1016/s0140-6736(98)00064-6. [DOI] [PubMed] [Google Scholar]
- 32.Kuipers EJ, Israel DA, Kusters JG, Blaser MJ. Evidence for a conjugation-like mechanism of DNA transfer in Helicobacter pylori. J Bacteriol. 1998;180:2901–5. doi: 10.1128/jb.180.11.2901-2905.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han SR, Bhakdi S, Maeurer MJ, Schneider T, Gehring S. Stable and unstable amoxicillin resistance in Helicobacter pylori: should antibiotic resistance testing be performed prior to eradication therapy? J Clin Microbiol. 1999;37:2740–1. doi: 10.1128/jcm.37.8.2740-2741.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thyagarajan SP, Ray P, Das BK, Ayyagari A, Khan AA, Dharmalingam S, et al. Geographical difference in antimicrobial resistance pattern of Helicobacter pylori clinical isolates from Indian patients: Multicentric study. J Gastroenterol Hepatol. 2003;18:1373–8. doi: 10.1046/j.1440-1746.2003.03174.x. [DOI] [PubMed] [Google Scholar]
- 35.Sherif M, Mohran Z, Fathy H, Rockabrand DM, Rozmajzl PJ, Frenck RW. Universal high-level primary metronidazole resistance in Helicobacter pylori isolated from children in Egypt. J Clin Microbiol. 2004;10:4832–4. doi: 10.1128/JCM.42.10.4832-4834.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


