Abstract
Background
Gaps exist in the modern literature that describes patterns of development in living groups of actinopterygian fishes. Relatively recent descriptions of development exist for the teleost fishes, bowfin, sturgeon, paddlefish and bichirs. Such literature dealing with the gars is to be found in older work, done approximately a century ago. The present study concerns the gars, of which the garpike, Lepisosteus osseus, is a representative example.
Results
The embryonic period of life of this fish is divided, as required for experimentation, into 34 stages, from fertilization to exhaustion of the yolk supply. Diagnostic structural characteristics are cited for each stage, and the rate of development is indicated.
Conclusions
Three features of development are especially noted that compare or contrast with other members of the Neopterygii, and with the Chondrostei. These are meroblastic cleavage, a well-defined yolk syncytial layer (ysl), and a pit at the posterodorsal edge of the blastoderm, which defines an overhanging dorsal lip. Meroblastic cleavage and the ysl in the garpike show an affinity to those character states in the teleosts, though not with Amia, the other neopterygian fish. The posterodorsal pit and dorsal lip are reminiscent of similar features in the Chondrostei. Lepisosteus is unique among the Neopterygii with respect to this character state. Such comparisons set the stage for a broader understanding of the mechanisms for development in these organisms, and of the evolutionary relationships between them.
Background
Embryological literature includes a broad array of descriptions of development in one organism or another. A monophyletic ancestry for the vertebrates, with the possible exception of cyclostomes, implies that some common inherited mechanistic themes exist for development. Differences ought to reflect either adaptations for various environmental conditions or nonadaptive (not maladaptive) traits that appeared in conjunction with genetic drift, quantum speciation, or punctuated equilibrium. Common themes and significant differences in developmental patterns should appear through the study of as many vertebrates as possible, chosen from a list of diverse types. Phylogeny and ontogeny support each other in this context [1,2]. Comparative studies also help us to identify model systems that are perhaps better suited than more common ones to answer difficult biological questions. Development in many of the approximately fifty orders of fishes remains unknown. This paper will help, in part, to fill the knowledge gaps.
The longnose gar, Lepisosteus osseus, also known as the garpike, is one of four living species of its genus within the Osteichthyian division Ginglymodi (infraclass Neopterygii). They are restricted to the Western Hemisphere. Wiley [3], using vicariance biogeography, placed a 180 million-year age on the genus, which arose before the breakup of Pangaea. Their adult anatomy and distribution have been used to construct phylogenies for actinopterygian evolution, and details of their embryonic development are likely to have similar usefulness.
Most previous work on the early embryonic development of Lepisosteus was performed before 1912, and codified by Agassiz [4], Balfour and Parker [5], Dean [6], Eycleshymer [7, 8] and Lanzi [9]. Lack of modern (by today's standards) laboratory facilities and equipment were handicaps to those studies. Much of the description of early embryonic development in the garpike is based on material that was badly distorted by harsh fixation procedures. Moreover, the embryos, themselves, might have been unhealthy; they were sometimes raised under sub-optimal conditions. Cell-marking techniques had not been devised at the time, so inferences made about gastrulation movements or blastomere fate could not be tested experimentally. Recent embryological study of the garpike either has been restricted to the development of specific structures in older embryos, e.g. [10], or has been reported in sketchy fashion ancillary to studies with another purpose, e.g. [11].
We took a fresh look at this animal to describe in familiar terms the sequence of events during its development. Relatively recent information similar to what we report here is known for four other basal fishes among the Actinopterygii, namely: paddlefish (Osteichthyes : Chondrostei: Acipenseriformes); sturgeon (Osteichthyes : Chondrostei: Acipenseriformes); bichir (Osteichthyes : Chrondrostei: Polypteriformes); and bowfin (Osteichthyes : Halecostomi: Amiiformes). Studies of those groups [12,13,14,15,16,17] illustrated the marked differences in development among them. For example, development of the sturgeon is relatively frog-like and the bowfin is much more similar to teleosts in its gastrulation pattern. We found that garpike development differs from that of the basal fish listed above, as well as teleosts.
One of us (Long) has engaged an experimental study of morphogenetic cell movements in L. osseus, which largely occupy stages 9-15 described here, see [18]. Interpretation of that and other studies will require the structural and temporal framework provided by this staging description. This normal series of developmental stages partly is a contribution to the body of science, and partly is a common reference point that will enable other investigators to use the garpike in their own laboratories.
Results
Rate of development
(Fig. 1) - The developmental rate for Lepisosteus, as is common for fish, proceeds at a pace dictated partly by the developmental program and partly by temperature. Our temperature control regime was of necessity weather-related, for specimens were raised either on a water table or in running lakewater. Developmental rates under such conditions can be measured fairly accurately over a short time period of up to several hours; but their accuracy suffers over longer periods because of environmental temperature fluctuations. We list short-term rates for early stages of development, at several temperatures, in the following paragraphs. Longer-term rates are shown in figure 1, which is based on selected developmental stages for a single batch of eggs taken in 1983. The temperatures listed each represent the average of two temperature readings taken in the running lakewater system every day, at 9 AM and 9 PM. Water temperature, at any time during a 24-hour period, fluctuated as much as a degree away from the average, i.e. warmer during the day and cooler at night. Development for this batch was not timed past six days because travel arrangements precluded it.
Figure 1.
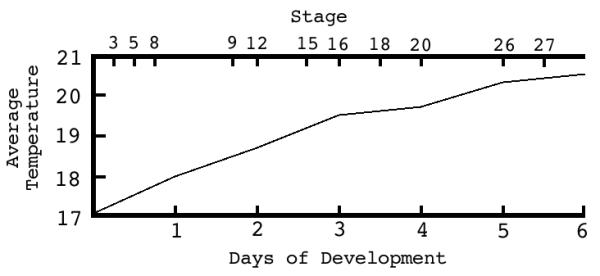
Lepisosteus osseus. Rate of Development. Average of morning and evening water temperatures over the 6 days from fertilization until hatching, related to the time and stage of development.
Stage 1, one cell
(Fig. 2A, 2B) - This stage begins at fertilization and lasts until the first cleavage furrow is readily visible, about three hours post-fertilization at 17°. The eggs are spherical, about 3 mm in diameter. As with other fish species, there is some variation in egg size from female-to-female. We measured a range of 2.8-3.1 mm for batches from various females. The eggs become quite sticky when they are placed in water, and adhere firmly to whatever substrate is available. This is true even for those that remain unfertilized.
Figure 2.
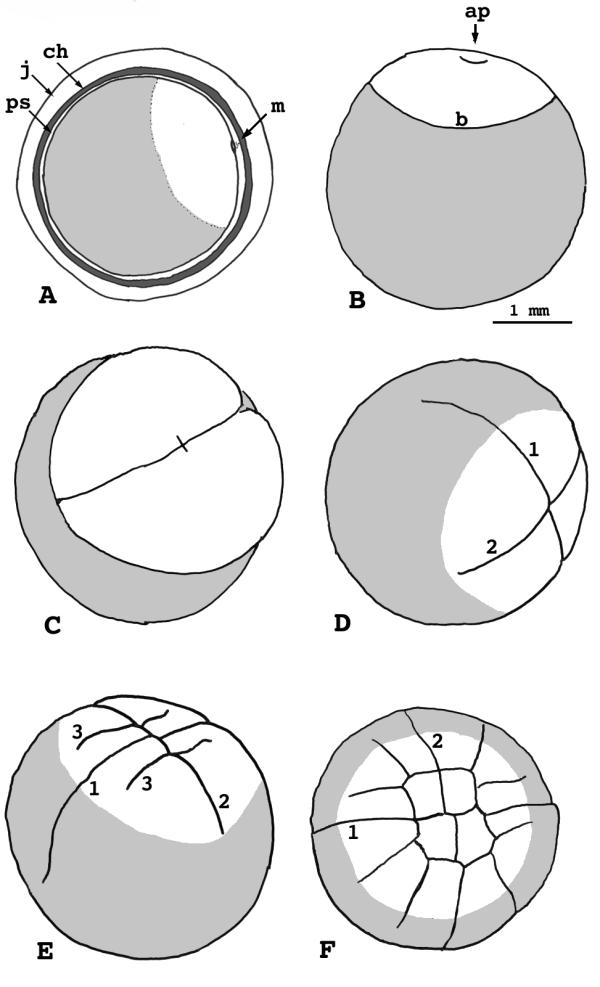
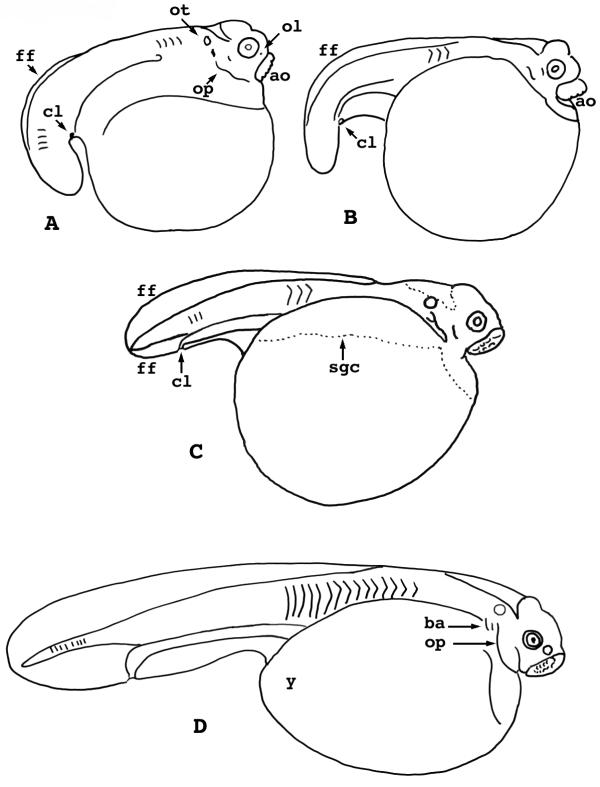
Lepisosteus osseus. Stages 1-5. A) Stage 1 showing egg envelopes. B) Stage 1 without egg envelopes, showing the dimple at the animal pole. C) Stage 2, two cells. D) Stage 3, four cells. E) Stage 4, eight cells. F) Stage 5, 16 cells. j = egg jelly ch = chorion ps = perivitelline space m = micropyle ap = animal pole b = blastodisc/blastoderm margin 1 = first cleavage furrow 2 = second cleavage furrow 3 = third cleavage furrow
The egg envelopes enlarge slowly over the next hour, revealing a narrow perivitelline space about 0.1 mm wide. A cushioning, nonadhesive jelly fills the perivitelline space. The principle envelope that surrounds the egg is the chorion, derived from the vitelline envelope of the unfertilized ovum. It is clear, allowing the egg to be viewed easily. This layer has similar optical and mechanical properties to the chorion of the medaka, Oryzias latipes, though it lacks the medaka's characteristic filamentous ornamentation. A second layer of jelly surrounds the chorion. This jelly layer is of variable thickness, up to 0.3 mm, and it provides the sticky character of the eggs.
Dean [6] described "slaty gray" eggs. This is true, except that each has a white blastodisc at the animal pole. The intersection of the white and gray regions establishes a distinctly visible border for the blastodisc. The blastodisc, itself, is about 2.3 mm in diameter and occupies about 100° of the egg's circumference. A dimple occurs at its apex, and marks the animal pole. The dimple is the point of sperm entry. It lies immediately adjacent to the micropyle, which is easily seen in the chorion and jelly layers.
Loosening of the vitelline envelope from the egg surface at fertilization allows gravity- induced egg rotation to occur, but the rotation is quite slow, perhaps impeded by the perivitelline jelly's thick consistency. It takes two hours or more to complete.
Stage 2, two cells
(Figs. 2C, 10A) - This stage begins with appearance of the first cleavage furrow, about four hours after fertilization at 17° (2 hours at 21°, 5.5 hours at 14°). The furrow first appears at the animal pole, and gradually extends to the edge of the blastodisc, which we now call the blastoderm. It will ultimately cut further, but by then subsequent furrows appear. Cleavage in this species is meroblastic; furrows divide the blastoderm similar to the teleosts. Unlike the teleostean pattern, however, the cleavage furrows continue past the blastoderm margin as grooves in the yolk cell surface. The first two grooves sometimes meet at the vegetal pole, but we found no evidence in living or fixed eggs that they extend significantly beneath the yolk cell cortex. This phenomenon led to some confusion in 19th century literature concerning whether garpike cleavage is holoblastic or meroblastic. It is meroblastic.
Figure 10.
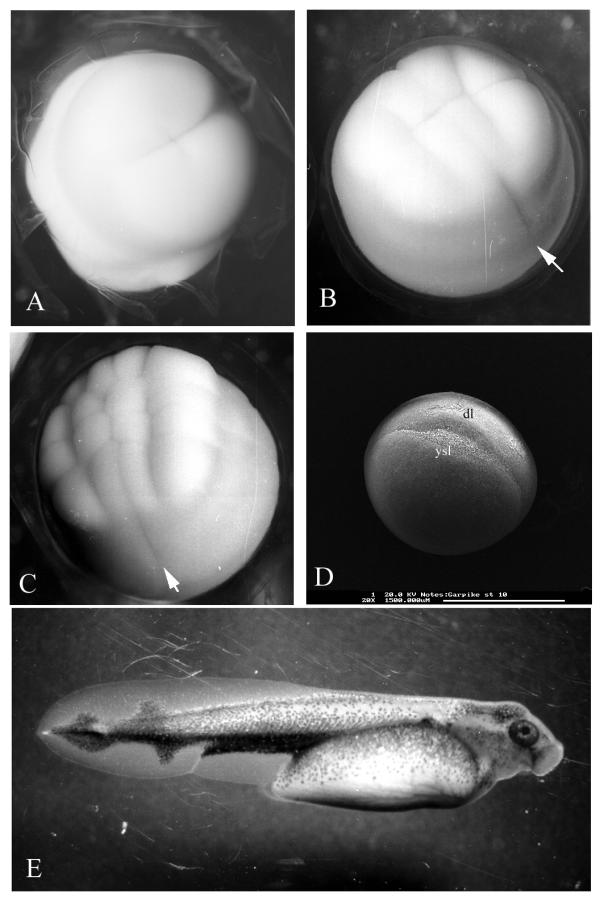
Lepisosteus osseus. A) Stage 2, live specimen treated with pronase to dissolve the egg envelopes. B) Stage 4, live specimen. C) Stage 5, live specimen. D) Stage 10, electron micrograph. E) Stage 30, live specimen. arrow = cleavage furrow extending beyond the blastoderm margin dl = dorsal lip ysl = yolk syncytial layer
Stage 3, four cells
(Fig. 2D) - The second cleavage furrow appears about 6 hours after fertilization at 17° (9 hours at 14°). Dean [6] reported its appearance at three-hours, but his specimens were subject to an uncertain temperature regimen. The second furrow proceeds like the first, dividing the blastoderm deeply and extending past its margin outward across the gray yolk cell. Like the first groove, this one will ultimately reach the vegetal pole in some specimens.
Stage 4, eight cells
(Figs. 2E, 10B) - The paired third cleavage furrows appear about 7.5 hours post-fertilization at 17° (10.5 hours at 14°). They are generally parallel to the first furrow, and produce two rows of four blastomeres each. However, a few specimens (up to 10% in some batches of eggs) show an oblique third-cleavage orientation that produces a morula with a rosette of blastomeres at its animal pole. Dean first reported this in 1895 [6].
The first cleavage groove extends to the egg's equator by now, while the second meets the blastoderm margin. The vegetal half of the morula, the future yolk cell, still exhibits a smooth surface.
Stage 5, 16-32 cells
(Figs. 2F, 10C) - The fourth round of cleavage begins about 11 hours post-fertilization at 17° (12 hours at 14°), and lasts for about two hours. By now, the first cleavage groove reaches halfway between the equator and the vegetal pole.
Stage 6, 64-512 cells
(Fig. 3A, 3B, 3C) - This stage begins the blastula phase of garpike development. Cleavage divisions carry the blastula from 64 to 512 cells. In a 64-cell embryo, about 30 complete blastomeres appear on the surface, surrounded by marginal cells that are continuous with the yolk cell. Many central blastomeres are also separated from the yolk cell by tangential cleavage divisions. Dissection shows that the deep blastoderm cells are still connected to the yolk cell, and appear to bud from it.
Figure 3.
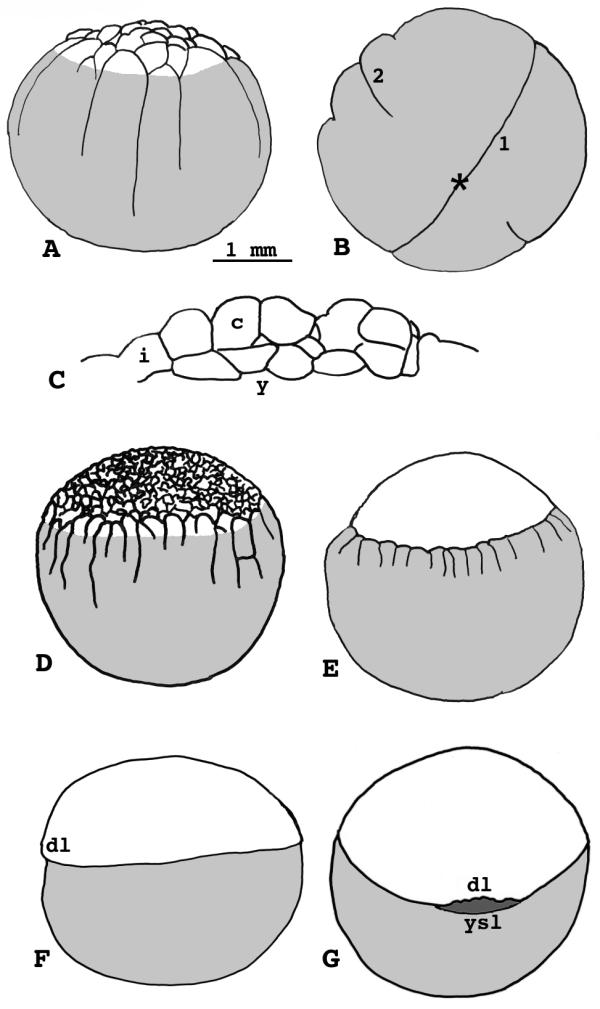
Lepisosteus osseus. Stages 6-10. A) Stage 6, side view. B) Stage 6, vegetal pole. C) Stage 6, cross-section of the blastoderm. D) Stage 7. E) Stage 8, smooth-surfaced blastula. F) Stage 9, lateral view. G) Stage 10, dorsal view. * = vegetal pole 1 = first cleavage furrow 2 = second cleavage furrow i = incomplete blastomere, continuous with the yolk cell c = complete blastomere, separated from other cells y = yolk cell dl = dorsal lip ysl = yolk syncytial layer
Stage 7, small-cell blastula
(Fig. 3D) - The surface of the blastoderm has acquired a lumpy appearance, which indicates the steady decrease in blastomere size. An enveloping cell layer can be distinguished. Many deep cells lie beneath the enveloping layer. Cleavage furrows on the yolk cell are regressing by this time; they extend only to the egg's equator, no further.
Stage 8, smooth-surfaced blastula
(Fig. 3E) - This is the final stage before epiboly. Embryos reach it by about 20 hours at 17° (16 hours at 20°). Superficial blastomeres are tiny and the blastoderm surface consists of a thin enveloping layer, similar to that in teleosts. The yolk furrows gradually regress until they are no longer visible.
A virtual, uninflated, segmentation cavity forms, floored with large yolky cells. In many specimens, the blastoderm forms a peaked mound at the animal pole. The blastoderm has an irregular rim and a flat bottom where it joins the yolk cell. Dissection reveals a continuing presence of deep central cells that appear to be budding from the yolk cell. Many of them show broad connections with the yolk cell, and many join it via a narrow stalk.
Stage 9, epiboly begins
(Fig. 3F) - The blastoderm begins epiboly by 36 hours at 18°, but has not reached the egg's equator. Some specimens show the first indication of dorsal/ventral asymmetry, as a result of a lag in epiboly of the dorsal blastoderm margin. This produces a slight bulge in the blastoderm margin at that location, as though epiboly were being retarded by its attachment to the yolk cell. The bulge is the first external expression of the embryonic shield. The germ ring appears internally at this stage as well.
Dissected specimens reveal an extensive subgerminal cavity, whose floor is paved by large yolky cells that are adherent to the yolk syncytial layer (ysl). The blastoderm can be cleanly separated (by dissection) from the ysl surface beneath the germ ring and embryonic shield, but the deep yolky cells at the animal pole adhere to the ysl. Some of them are difficult to remove, for they are still attached by stalks to the yolk cell.
Stage 10, epiboly reaches the equator
(Figs. 3G, 10D) - The hint of dorsal/ventral asymmetry seen at stage 9 is now definite. The blastoderm overhangs its margin at the dorsal midline, having a crinkled edge where it attaches to the yolk cell. The overhang appears similar to the dorsal blastopore lip in amphibian embryos, and we give it that name. The exposed yolk cell surface beneath the dorsal lip is the first external appearance of the yolk syncytial layer.
Stage 11
(Fig. 4A, 4B) - The blastoderm reaches 1/3 the distance from the equator to the vegetal pole. The germ ring is wide, approaching 1 mm; and the embryonic shield is broader still.
Figure 4.
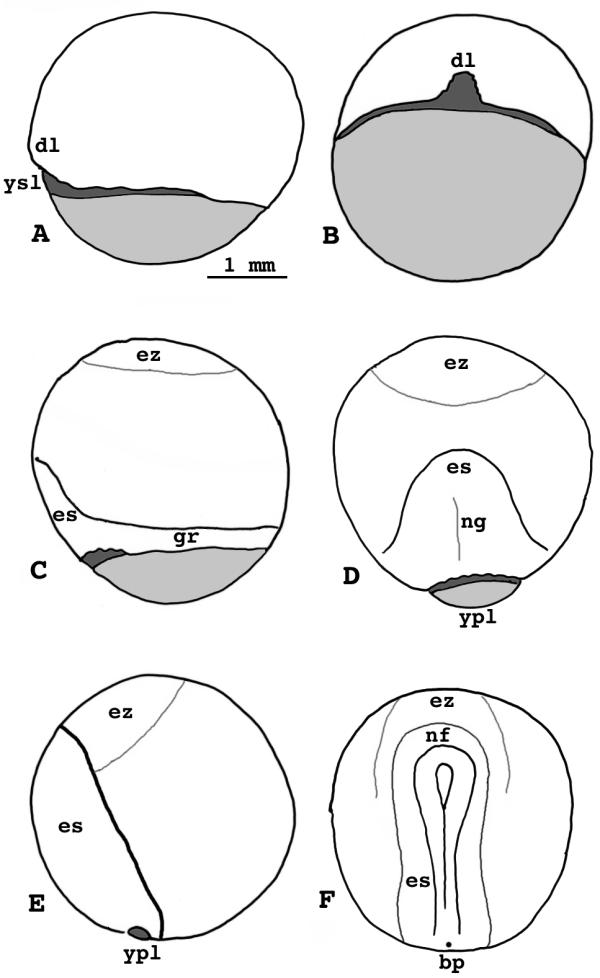
Lepisosteus osseus. Stages 11-15. A) Stage 11, lateral view. B) Stage 11, dorsal view. C) Stage 12, lateral view. D) Stage 13, dorsal view. E) Stage 14, lateral view. F) Stage 15, dorsal view. dl = dorsal lip ysl = yolk syncytial layer es = embryonic shield ez = evacuation zone at the animal pole gr = germ ring ng = neural groove ypl = yolk plug nf = neural fold bp = blastopore
The external ysl now occupies a greater portion of the blastoderm margin, and is broader at the dorsal midline than elsewhere. Viewed from the dorsal side, the blastoderm margin at the dorsal lip has the appearance of a horseshoe; its sidewalls face each other.
Deep cells anterior to the future forebrain region begin to migrate away from the animal pole, producing an evacuation zone that is devoid of cells. The evacuation zone is not visible in living specimens at this stage, and some deep cells still remain tightly attached to the ysl at the animal pole. The zone ultimately enlarges to a diameter of 1 mm at stage 14.
Stage 12
(Fig. 4C) - The blastoderm margin reaches 2/3 the distance from equator to vegetal pole. The dorsal lip overhangs a pit floored by the external ysl, a virtual gastrocoel. The embryonic shield extends above the equator. It can be dissected into two principal layers, the epiblast and the hypoblast. The roof of the evacuation zone at the animal pole is much thinner than before. The exposed surface of the yolk cell now comprises a large yolk plug.
Stage 13
(Fig. 4D) - The yolk plug is half or less the diameter of the equator. The germ ring is narrow now and the embryonic shield is longer, nearly reaching to the evacuation zone above the equator. There is a hint of a neural groove on its surface. The crinkled sidewalls of the dorsal lip flatten out as epiboly in the dorsal midline catches up to that in other areas of the blastoderm margin.
Stage 14
(Fig. 4E) - The yolk plug is 1/8 or less the equator diameter but is still visible. The embryonic shield is long, intruding anteriad on the evacuation zone, which has a jumble of floor cells. They can easily be dislodged with a hair loop. The shield extends over about 100° of the yolk cell surface. Although its neural groove has deepened somewhat, it is still quite shallow.
The subgerminal cavity, distinct from the evacuation zone, begins to inflate under the embryonic shield, but little outside it. It has a smooth floor, which is formed by the surface of the ysl.
Stage 15, yolk plug closure
(Fig. 4F) - The yolk plug is no longer visible inside the tiny blastopore. The neural folds are distinct, but feeble. They close in a posterior-anterior sequence at this stage, remaining open last at their anterior end. Internally, the posterior part of the notochord segregates from the neural keel. A crescent of cells extends forward, anterior to the neural field. Later, these cells will contribute to the adhesive organ. The subgerminal cavity inflates lateral to the neural keel. This cavity is not a blastocoel; it is a product of morphogenetic cell movements, not of cleavage.
Stage 16
(Fig. 5A, 5B) - The embryo's axis occupies from 150° to 180° of the egg circumference. The neural folds disappear and the neural keel pushes deeper, intruding on the subgerminal cavity. The prospective diencephalon enlarges slightly where the optic lobes will form.
Figure 5.
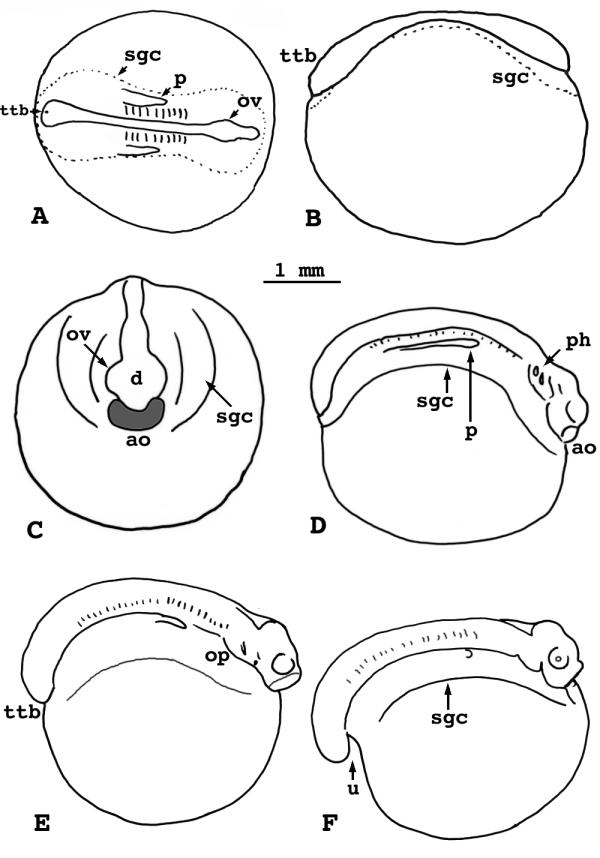
Lepisosteus osseus. Stages 16-20. A). Stage 16, dorsal view. B) Stage 16, lateral view. C) Stage 17, anterior view. D) Stage 18, lateral view. E) Stage 19, lateral view. F) Stage 20, lateral view. ttb = trunk-tail bud sgc = limit of subgerminal cavity p = pronephric duct ov = optic vesicle d = diencephalon ao = adhesive organ ph = pharyngeal pouch op = operculum u = undercut trunk-tail bud
The inflated subgerminal cavity extends the full length of the embryonic axis. The cavity extends laterally as a wide pellucid area on each side of the axis, and anterior to the brain, it extends into the evacuation zone. Somites 1-10 appear during this stage, and pronephric ducts occur from somite 4 posteriad. A slight swelling, the trunk-tail bud, lies at the posterior end.
Stage 17
(Fig. 5C) - New somites form, from the 10th to the 20th. The length of the axis exceeds 180° of the egg circumference. Expansion of the optic vesicles is obvious; they enlarge to hemispherical proportions. The telencephalon lies anterior to them. Development of the adhesive organ produces a bean-shaped swelling anterior to the brain. Thickened protrusions in the subgerminal cavity roof, lateral to the axis, mark the beginning of the pharyngeal arches.
Stage 18
(Fig. 5D) - New somites form, from the 20th to the 25th. The brain and trunk-tail bud rise prominently above the main curvature of the embryo, but neither is undercut. The single curved mass of the adhesive organ wraps tightly around the telencephalon. The optic swellings have become rounded vesicles.
The hyomandibular and hyobranchial pharyngeal pouches are visible externally, but they are best seen from below after dissection of the embryo from the yolk. The walls of the pharynx, as seen in dissection, project down laterally and begin to fold mesad to form the pharynx floor.
Stage 19
(Fig. 5E) - New somites form, from the 25th to the 30th. The trunk-tail bud projects from the embryo; it is slightly undercut, but not bent down around the yolk sac's contour. The head end is markedly raised and appears knobby because of the swollen brain vesicles, the spherical optic vesicles, and the adhesive organs. The operculum appears, extending a free edge. Ventral fusion of the pharynx sidewalls begins, signaling the appearance of the anterior intestinal portal. Lateral plate mesoderm extends about halfway out over the yolk sac. A vascular network shows on the dorsal portion of the yolk sac, but the heart is straight and not beating.
Stage 20
(Fig. 5F) - New somites form from 30th to 40th. The trunk-tail projection bends down in a curve that follows the yolk mass, and is undercut for a distance equal to that from adhesive organ to hindbrain. The posterior intestinal portal appears. The pronephros begins to bend. Lenses can be seen in the eyes. The opercular edge begins its extension. This is the stage of first motility, a slight one-sided squirm at best.
Stage 21
(Fig. 6A) - The trunk-tail outgrowth is now longer than the whole brain, but still curved along the contour of the yolk. Post-cloacal somites are now forming and the dorsal fin fold appears. The trunk is almost straight. The entire trunk is still attached to the yolk mass, but the cloacal region is beginning to lift away from it. There is a strong pronephric bend.
Figure 6.
Lepisosteus osseus. Stages 21-24. A) Stage 21. B) Stage 22. C) Stage 23. D) Stage 24. ff= fin fold cl = cloacal aperture ot = otic vesicle ol = olfactory placode op = operculum ao = adhesive organ sgc = limit of subgerminal cavity y = yolk sac ba = branchial arch
The head begins to project freely over the mouth area. The adhesive organ is larger than the eyeball, but it is not sticky. The epiphysis appears and olfactory placodes are visible. One can find pigment on the yolk sac and posterodorsal to the eye. The heart begins to beat. It is hardly curved at all. Blood vessels cover all but the midventral part of the yolk. Motility increases, and embryos move in a strong, single coil.
Stage 22
(Fig. 6B) - The free portion of the trunk is fairly straight, but the tail is bent down beyond the cloaca, which is 1/2 to 2/3 of the way out along the free ventral surface of the trunk. The dorsal finfold is obvious and the intestine is visible between the yolk mass and the cloaca. Post-cloacal somites have formed halfway out along the tail.
When viewed face on, the adhesive organ has the shape of a horseshoe. The heart begins to coil and blood flows on to the dorsal face of the yolk sac. Dissection reveals a bulging liver, although this is not visible externally. Trunk somites are arranged as chevrons halfway down the attached portion of the trunk. Motility increases to one or two coils, but there is no backlash.
Stage 23
(Fig. 6C) - The tail is finally straight. Both the dorsal and ventral fin folds of the tail are taller than the caudal somites and a ventral fin notch appears at the cloaca. Inside the chorion, the tail tip reaches the eye.
The somites become dusted with pigment. Somites are chevron-shaped only on the attached part of the trunk. The margin of the operculum is thickened but does not yet flare. Blood circulation is established over most of the yolk mass and the flow is strong in the subintestinal vein.
Stage 24
(Fig. 6D) - Tail segmentation is virtually complete; it never reaches the tail tip. The yolk mass has become ovoid. The adhesive organ becomes sticky. The free edge of the operculum begins to expand, but does not yet cover the branchial arches. Dechorionated embryos can swim clumsily when prodded.
Stage 25
(Fig. 7A) - Somites are chevron-shaped as far as the middle of the trunk. Pectoral fin buds are visible after fixation. Specimens can swim in circles when prodded.
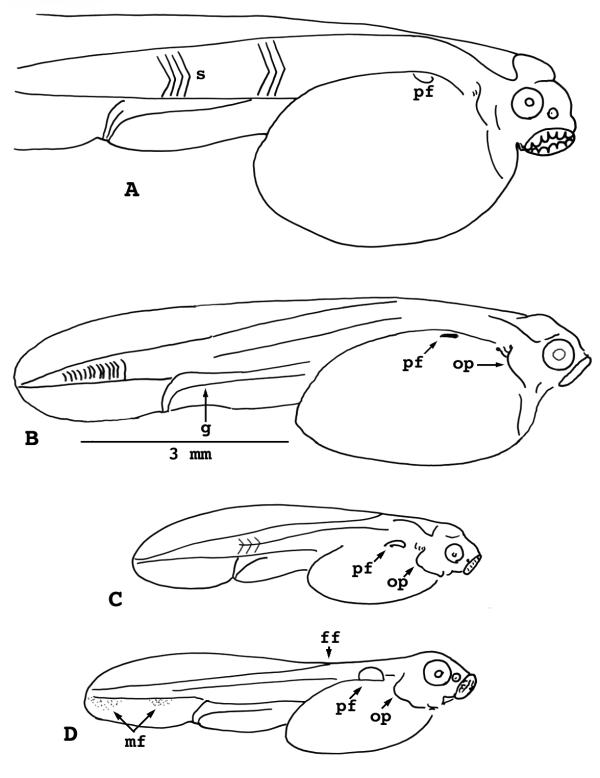
Figure 7.
Lepisosteus osseus. Stages 25-28. A) Stage 25. B) Stage 26. C) Stage 27. D) Stage 28. s = somites pf = pectoral fin bud op = operculum g = gut ff = fin fold mf = median fin anlage
Stage 26
(Fig. 7B) - Pectoral fin buds are visible in the living embryo. Somites are chevron-shaped past the cloaca. There is a prominent bulge in the operculum edge below the branchial arches.
Stage 27
(Fig. 7C) - Pigment aggregates in the ventral fin fold, indicating the location of the future anal and caudal fins. Pectoral plates now possess barely detectable ridges. The adhesive organ is equal to or larger than the eye.
Stage 28
(Fig. 7D) - Anlagen of the anal and caudal fins are clearly visible in the ventral fin fold. Erosion of the dorsal fin fold begins, back beyond the pectoral fin level. The pectoral fin is now a low disc without a membranous flange. The opercular flap, which is as wide as the eye, obscures the branchial arches in side view. Slight swellings on the gill arches presage gill filaments.
Stage 29
(Fig. 8A, 8B) - The anlage of the dorsal fin is now visible in the dorsal fin fold. Although the pectoral fin has acquired the shape of a half-moon, it still has no flange. Gill filaments are sprouting on branchial arches one and two. Most hatching occurs at this stage.
Figure 8.
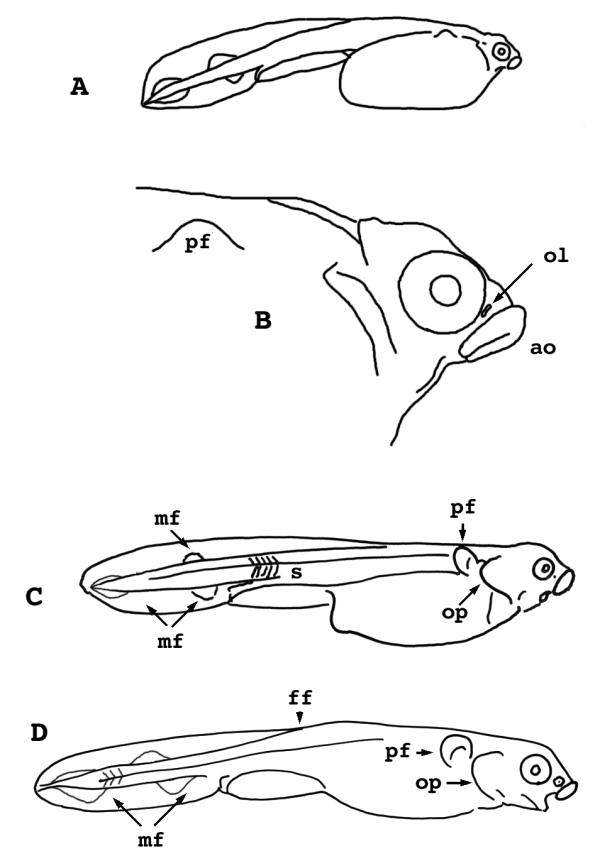
Lepisosteus osseus. Stages 29-31. A) Stage 29, whole larva. B) Stage 29, head only. C) Stage 30. D) Stage 31. ol = olfactory pit ao = adhesive organ mf = median fin anlage s = somites pf = pectoral fin op = operculum ff = fin fold
Stage 30
(Figs. 8C, 10E) - The dorsal fin fold has eroded as far as the mid-trunk. The height of the yolk mass now has been reduced nearly to the height of the tail including its fin folds. The pectoral fin is larger than a half-moon, with a flange narrower than its muscle mass. The operculum is nearly twice as wide as the eye. Gill filaments occur on branchial arch three.
Stage 31
(Fig. 8D) - The dorsal fin fold has eroded beyond the mid-trunk; and the height of the yolk mass is now less than or equal to the height of the tail including its fin folds. Somites are chevron shaped clear to the caudal fin. The pectoral fin flange is now equal to or wider than the fin's muscular disc. The edge of the operculum flap reaches the base of the pectoral fin. The diameter of the adhesive organ is now less than that of the eye. The nasal pit is still single and round, as it has been since stage 27.
Stage 32
(Fig. 9A, 9B) - Pelvic fin mats are visible as smooth bulges. The yolk mass is still perhaps twice the volume of the head. Nasal openings are still single, but elongating; and the snout begins to lengthen. The lower jaw reaches the adhesive organ. Rhythmic movements of the jaw and operculum begin. Bump-like tooth primordia are barely visible on the jaws. The pectoral fins tremble at the end of a swim.
Figure 9.
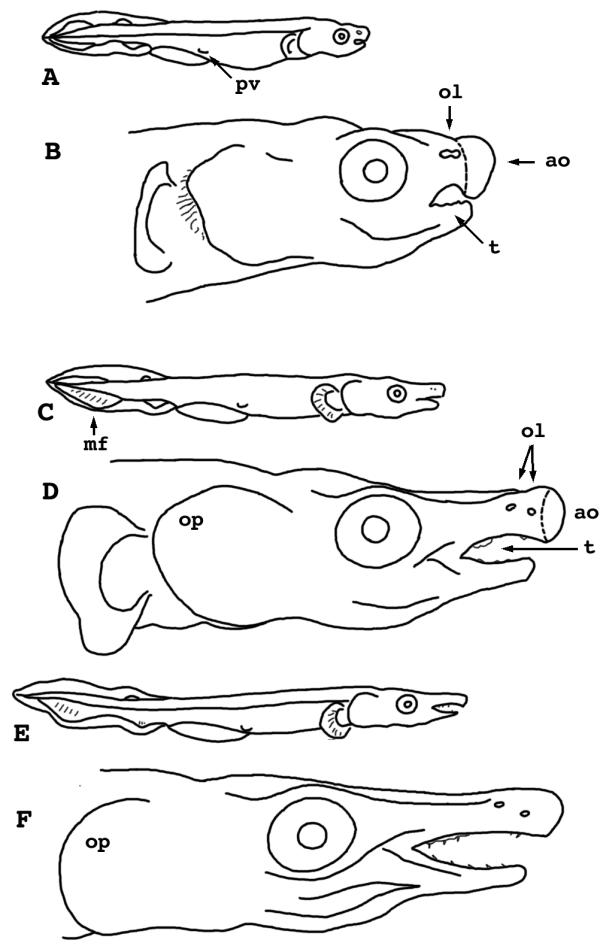
Lepisosteus osseus. Stages 32-34. A) Stage 32, whole larva. B) Stage 32, head only. C) Stage 33, whole larva. D) Stage 33, head only. E) Stage 34, whole larva. F) Stage 34, head only. pv = pelvic fin anlage ol = olfactory organ ao = adhesive organ t = tooth bud mf = median fin op = operculum
Stage 33
(Fig. 9C, 9D) - The fin fold of the tail is shrinking between the anal and caudal fins, and around the permanent dorsal fin. Rudiments of fin rays become visible in these unpaired fins. No dorsal fin fold remains anterior to the level of the cloaca. The yolk mass is now smaller than the head. The nasal apparatus finally has separated into incurrent and excurrent openings.
Stage 34
(Fig. 9E, 9F) - The yolk is finally exhausted. Pelvic fins are half-moon shaped with narrow membranous flanges. The jaws are studded with sharp teeth.
Discussion
The bowfin, Amia calva, and the garpike, Lepisosteus osseus, are frequently compared to each other in their anatomical characters, as they are both basal actinopterygians. Study of their development is an appropriate sequel to previous work on members of the Chondrostei - the paddlefish, Polyodon [12] and the sturgeons. Huso and Acipenser [13].
Garpike have three developmental characters that immediately separate them from bowfin: 1) they show meroblastic cleavage, 2) they possess a well-defined yolk syncytial layer, and 3) events of gastrulation produce a pit associated with the dorsal blastoderm margin. The pit is a formation of maneuver, a temporary structure resulting from cell migration. It has no apparent ultimate morphogenetic significance, and it eventually disappears.
Amia cleavage is clearly holoblastic, producing about a dozen large yolky macromeres, upon which the smaller cells of the blastoderm rest [14]. An initial view of Lepisosteus would lead one to draw a similar conclusion for this fish. However, only one or two garpike cleavage furrows even extend to the vegetal pole, and these are nothing more than shallow grooves in the yolk cell cortex. They eventually regress. Garpike, therefore, have a single yolk cell similar to that of teleosts. This condition sets the stage for the later appearance of the yolk syncytial layer (ysl), an otherwise uniquely teleostean feature. From a phylogenetic point of view, the garpike ysl marks its first evolutionary appearance in the actinopterygian fishes. The teleost ysl has important mechanical and communications functions in early development [19, 20, 21]. Therefore, its presence in Lepisosteus raises interesting questions about its role there. One of us (Long) is pursuing these.
Among the ray-finned fishes, bichirs, sturgeon and paddlefish display a dorsal lip around which surface cells migrate to the interior [12, 13, 16, 17]. Others, e.g. [22, 23], reported a similar condition in bowfin, a circumstance later shown to be mistaken [14, 15]. Teleost fishes lack both a blastopore and invagination; their outer enveloping layer of cells serves as a casing inside of which deep cells construct the embryo [24]. The appearance in garpike of structures appearing similar to the sturgeon's dorsal lip raises the possibility that invagination and involution of surface cells to the interior actually happens in Lepisosteus. Cell marking experiments actually show that to be the case (Long, unpublished), thus we use the terms dorsal lip, blastopore, and yolk plug as names for the appropriate embryonic structures in this report.
Their size, feeding habits, and seasonal spawning cycle make Lepisosteus embryos generally unsuitable for rapid progress through experiments, as is possible for organisms like the zebrafish, Danio rerio. Garpike embryos, however, possess combinations of features whose investigation should prove valuable in the areas of development, evolution and taxonomy. We hope that this catalogue and description of the developmental sequence in the garpike, and our notations of comparison to other fishes, will spur further investigation into the embryology of these remarkable animals.
Materials and Methods
Longnose gar spawn during the months of May and June in Michigan and Vermont, the locations at which we collected our specimens. The first two weeks of June were productive at the Michigan site. Dean [6] took eggs from a lake in New York State between May 14th and June 12th.
One of us (Ballard) collected some specimens for this study from a rocky shoreline in Vermont, on the eastern side of Lake Champlain, opposite Fort Ticonderoga. He took them from the rocks on which they were laid as soon as possible after natural spawning of the fish, which were abundant in the lake during those years (1949-1963). These eggs developed in dishes of standing water on a water table at Dartmouth College, Hanover, New Hampshire.
Later, from 1981 onward, we worked at the Gull Lake Laboratories of Michigan State University, near Hickory Corners, MI. Laboratory facilities are immediately adjacent to the lake, and only a hundred feet from the site at which we trapped garpike in spawning condition. We first used a gill net set overnight perpendicular to the shoreline, running from the shore to about a hundred feet into the lake. Later, we used a trap net set in a similar manner. Both of the devices were used under supervision of the Michigan State Department of Natural Resources, and the department owned the trap net. Adults providing gametes ranged from 75 cm to 104 cm in length; the females tended to be larger than the males. Adult females possessed a silvery body coloration, while males had a golden cast to their scales.
We dissected ripe gametes from the ovaries of gravid females and fertilized them in glass dishes by mixing them with minced testes, a procedure similar to that commonly used to fertilize frog eggs in the lab [25]. We rinsed the zygotes and incubated them in running water, pumped fresh from the lake. The first oöcytes to mature during the spawning season are located in the anterior end of the ovary. Oöcytes mature in a wave proceeding gradually posteriad as the season progresses. The ovaries are large and contain thousands of eggs. The eggs turn from white to gray as they mature.
Specimens from the 1949-1963 batches were preserved in Bouin's fluid at appropriate stages. A glutaraldehyde fixative (3% glutaraldehyde in 50% Holtfreter solution) served for the more recent work. One day in the glutaraldehyde, followed by an overnight rinse in cold (4°) 50% Holtfreter solution, prepared the specimens for transfer to 35% ethanol, and then to 70% ethanol for storage. We generally dechorionated the specimens after the Bouin's or glutaraldehyde fixation, i. e. during the first rinse. Some embryos were dechorionated with pronase, 2 mg/ml, before fixation.
Paraffin sections provided an internal view of specimens at various stages. Ballard also prepared some celloidin-imbedded hand sections more than thirty-five years ago; figure 3C was drawn from one of these. Some material was also prepared for scanning electron microscopy, and examined using the ETEC Autoscan scope at Western Maryland College. In this case, specimens were carried gradually through a series of 70% ethanol, 95% ethanol, a 50:50 mix of acetone and 95% ethanol, to acetone. They were processed through a critical point dryer and sputter coated with gold before observation. Alternately, specimens for electron microscopy were passed through a series of 95% ethanol, 97.5% ethanol, absolute ethanol, a 50:50 mix of absolute ethanol and hexamethyldisilazane (HMDS), to 100% HMDS. These were allowed to air dry in a fume hood before mounting and sputter coating. The specimen shown in figure 10D was prepared this way. Usually, we dissected fresh or recently-fixed embryos to look into their interior. Dissection easily provided the level of detail required for definitions of these stages, and the other methods confirmed the identification of internal structures. Figures 2,3,4,5,6,7,8,9 were prepared from camera lucida drawings made over the years. The design of the stages and the use of outline drawings follow the pattern established by Ballard and his associates for the normal stages of fishes [12,14,26,27,28,29]. Figure 10 shows photomicrographs of selected live specimens, and an example of one specimen prepared for electron microscopy.
Acknowledgments
Acknowledgements
We wish to thank the administration and staff of the W. K. Kellogg Biological Station and the Kellogg Bird Sanctuary for their cooperation and help over the years. W. Johnson served as our guide and resident collection expert. In each case, our collection was licensed by the Michigan State Department of Natural Resources, and generally was performed under their supervision. The trap net belonged to that department. In addition, our procedures for obtaining fish and their subsequent handling were inspected and approved by Michigan State University's animal care and use committee. We are indebted to S. Isogai for his tireless cooperation during the last phase of the project. Dartmouth College and Western Maryland College provided partial funding for the project. J. P. Wourms of Clemson University provided valuable advice during preparation of the manuscript. Finally, we wish to thank our families for their support and patience over the decades of the project.
From Bill Long: This paper culminates more than twenty years of collaboration on the embryonic development of the garpike. We pursued it partly for its scientific value, partly as a way of satisfying our curiosity about the development of ancient fishes, and partly because we enjoyed each other's company. Bill Ballard was my advisor at Dartmouth College. He was nearing retirement when we first met, and he received a special appointment from the College after his retirement, so that he could continue to foster my graduate education.
Bill began this work in 1949, after a chance observation of garpike spawning in Lake Champlain, VT. He collected eggs occasionally and studied them as time permitted. It was more of a hobby for him than another tough professional commitment, of which he had many. It was one of those things that he kept on a low burner until after his official retirement. He never "retired" in the usual sense, though his regular threats to do so were familiar to his friends and family. He did take leave from zoology at one point, caring for his beloved wife Elizabeth through her long, final illness.
But the lure of the embryos never left him, and it nurtured his own final years. It was my privilege and pleasure to share those embryos with him. I came into the garpike project twenty years ago, thirty years into its progress. We proceeded as Bill had in the past, following the garpike when time and circumstance allowed us to do so. We sometimes worked alone, but most enjoyed it when we could be together.
When it became clear that Bill's time was limited, he asked me to conclude the work for him. It was his wish, and my promise to him, that this set of Normal Embryonic Stages for the garpike would be completed and published as our last work together. And so, I offer it. He gave to me a summary of his notes; and I have extensive notes of my own, taken over our years of study. This manuscript is a mix of his words and mine. I chose to mix them freely. Give to Bill the credit for what is good about it and I'll take the blame for what's left.
Rest well, my friend
Contributor Information
Wilbur L Long, Email: wlong@wmdc.edu.
William W Ballard, Email: wlong@wmdc.edu.
References
- Collazo A, Bolker JA, Keller R. A phylogenetic perspective on teleost gastrulation. Amer Nat. 1994;144:133–152. doi: 10.1086/285665. [DOI] [Google Scholar]
- Metscher BD, Ahlberg PE. Zebrafish in context: Uses of a laboratory model in comparative studies. Devel Biol. 1999;210:1–14. doi: 10.1006/dbio.1999.9230. [DOI] [PubMed] [Google Scholar]
- Wiley EO. The phylogeny and biogeography of fossil and recent gars (Actinopterygii: Lepisosteidae). Univ Kansas Mus Nat Hist, Misc Publ No 64, 1976.
- Agassiz A. The development of Lepidosteus. Proc Am Acad Arts & Sci. 1878;14:65–76. [Google Scholar]
- Balfour FM, Parker WN. Structure and development of Lepidosteus. Phil Trans Roy Soc London. 1882;173:359–442. [Google Scholar]
- Dean B. The early development of gar-pike and sturgeon. J Morphol. 1895;11:1–62. [Google Scholar]
- Eycleshymer AC. The cleavage of the egg of Lepidosteus osseus. Anat Anz. 1899;16:529–536. [Google Scholar]
- Eycleshymer AC. The early development of Lepidosteus osseus. U Chicago Decenniel Publ. 1903;X:261–276. [Google Scholar]
- Lanzi L. Ricerche sui primi momenti di sviluppo degli Olostei (od Euganoidi) Amia Calva Bonap. e Lepidosteus osseus L. Con speciale riguardo al così detto ispessimento prostomale. Archivio Italiano Anat Embriol. 1909;8:292–306. [Google Scholar]
- Bodemer CW. The origin and development of the extrinsic ocular muscles in the gar pike (Lepisosteus osseus). J Morphol. 1957;100:83–112. [Google Scholar]
- Redmond LC. Ecology of the spotted gar (Lepisosteus oculatus Winchell) in southeastern Missouri. Master's Thesis: Univ Missouri, 1964.
- Ballard WW, Needham RG. Normal embryonic stages of Polyodon spathula (Walbaum). J Morphol. 1964;114:465–478. doi: 10.1002/jmor.1051140307. [DOI] [PubMed] [Google Scholar]
- Ballard WW, Ginsburg A. Morphogenetic movements in acipenserid embryos. J Exptl Zool. 1980;213:69–116. [Google Scholar]
- Ballard WW. Stages and rates of normal development in the holostean fish Amia calva. J Exptl Zool. 1986;238:337–354. [Google Scholar]
- Ballard WW. Morphogenetic movements and a provisional fate map of development in the holostean fish Amia calva. J Exptl Zool. 1986;238:355–372. [Google Scholar]
- Bartsch P, Gemballa S, Piotrowski T. The embryonic and larval development of Polypterus senegalus Cuvier, 1829: its staging with reference to external and skeletal features, behaviour and locomotory habits. Acta Zoologica (Stockholm) 1997;78:309–328. [Google Scholar]
- Wourms JP, Bartsch P. Early development and gastrulation of the cladistian fish Polypterus senegalus. Amer Zool. 1998;38:187A. [Google Scholar]
- Long WL, Wourms JP. Gastrulation in the gar, Lepisosteus osseus. Am Zool. 1991;31(5):81A. [Google Scholar]
- Long WL. The role of the yolk syncytial layer in determination of the plane of bilateral symmetry in the rainbow trout, Salmo gairdneri Richardson. J Exptl Zool. 1983;228:91–97. [Google Scholar]
- Long WL. Cell movements in teleost fish development. BioScience. 1984;34:84–88. [Google Scholar]
- Trinkaus JP. The yolk syncytial layer of Fundulus: its origin and history and its significance for early embryogenesis. J Exptl Zool. 1993;265:258–284. doi: 10.1002/jez.1402650308. [DOI] [PubMed] [Google Scholar]
- Dean B. The early development of Amia. Quart J Microsc Sci. 1896;38:413–451. [Google Scholar]
- Brachet A. Recherches sur la gastrulation et l'origine de l'hypoblaste du tube digestif chez Amia calva. Zool Jb Suppl 15. 1912;2:425–456. [Google Scholar]
- Ballard WW. The role of the cellular envelope in the morphogenetic movements of teleost embryos. J Exptl Zool. 1966;161:193–200. [Google Scholar]
- Rugh R. Experimental Embryolgy: Techniques and Procedures (3rd edition). Minneapolis: Burgess Publ Co, 1962.
- Ballard WW. Normal embryonic stages of Gobius niger jozo. Pubbl Staz Zool Napoli. 1969;37:1–17. [Google Scholar]
- Ballard WW. Normal embryonic stages for salmonid fishes, based on Salmo gairdneri Richardson and Salvelinus fontinalis (Mitchill). J Exptl Zool. 1973;184:7–26. [Google Scholar]
- Ballard WW, Mellinger J, Lechenault H. A series of normal stages for development of Scyliorhinus canicula, the lesser spotted dogfish (Chondrichthyes: Scyliorhinidae). J Exptl Zool. 1993;267:318–336. [Google Scholar]
- Long WL, Ballard WW. Normal embryonic stages of the white sucker, Catostomus commersoni. Copeia. 1976;1976:342–351. [Google Scholar]