Abstract
MicroRNAs (miRNAs) are short, non-coding sequences that control gene expression via translational regulation. Through interactions with the 3'-untranslated region of messenger RNA, miRNAs trigger translational repression and play a key role in developmental timing. Furthermore, many miRNA groups have now been shown to regulate various processes in tumorigenesis, including angiogenesis and metastasis. These links highlight the importance of microRNA research in further understanding cancer development. This review article summarizes the current state of microRNA research, with a focus on the roles of microRNAs in various cancer types. Up to date knowledge of the structure and biogenesis pathway of microRNA are also reviewed.
Keywords: microRNA, biogenesis, cancer, tumorigenesis, angiogenesis
Overview
The Human Genome Project revealed approximately 25,000 protein encoding genes in 2004 [1]. While biological functions are mediated mostly by these proteins, activating mechanisms are often required for proper cellular function. Alongside physical and chemical modifications, there are various regulatory barriers to gene transcription and translation. It is well established that transcription factors play a major role in gene activation through interactions with the 5'-untranslated region (5'UTR) of messenger RNA (mRNA). Interestingly and more recently, the 3'-untranslated region (3'UTR) of mRNA has also been found to be important in translational control. Sequences known as microRNAs (miRNAs) have been uncovered as regulators of translational efficiency. MiRNAs often exert their effect by interacting with the 3'UTR of mRNAs.
MicroRNAs are non-coding RNA sequences that are approximately 22 nucleotides whose major role appears to be in gene regulation. Initially viewed as cellular detritus, these sequences are now known to be evolutionarily conserved across species [2]. As of 2010, 721 miRNAs were found and validated in the human genome [3]. Computer models have predicted the number of miRNAs to be at least 1000, encompassing 2.5% of the total number of genes [4]. These miRNAs were expected to regulate approximately one third of human genes [5]. This number may increase significantly with new tool to analyze the matching/targeting [6].
Despite advancements in the study of miRNA, little is known about the regulatory systems in play. When mapped, many miRNA genes were found to be located in genomic fragile sites and commonly in regions associated with cancer [7]. Large scale investigations using clinical samples further indicated that groups of miRNAs are differentially expressed in cancerous cells [8]. miRNA expression profiles are therefore potentially useful as biomarkers for diagnostic purposes. However, the ability of miRNAs to target multiple genes suggests both direct and far-reaching effects in oncogenesis. Nevertheless, the common trend appears to be that up-regulation of oncogenic miRNAs lowers expression of tumor suppressor genes, while down-regulation of certain miRNAs increases oncogene translation. Better understanding miRNA functionality can therefore aid in developing novel strategies to halt or disrupt cancer formation.
miRNA biogenesis pathway
Transcription
According to genomic screening of over two hundred miRNAs, more than half were transcriptionally linked to protein-coding or non-coding RNAs (ncRNA) [9]. More specifically, 40% of analyzed miRNAs were located in the introns of protein-coding RNA transcripts, while 10% of them were transcribed in the introns of ncRNA transcripts. In addition, 10% of miRNA overlap with exons of ncRNA, suggesting further complexity in the maturation of miRNAs.
Besides intronic and exonic miRNA, some miRNAs were also found located between genes. Many of these intergenic miRNAs are clustered in the genome. Multiple miRNAs separated by up to 10 kilobases are transcribed together in one transcript [10]. For the intergenic miRNAs, there has been reports of TATA box binding motifs upstream of miRNA genes [11]. Having their own transcription elements suggests that miRNAs can be independently transcribed as well, which requires the recruitment of transcription factors. Recent studies have reported the involvement of several transcription factor, such as MYC and P53, in regulating expression of miRNAs [12].
Upon p53 activation, several independent studies all identified miR-34a as being strongly up-regulated by p53 in cultured cell lines and in primary mouse embryonic fibroblasts [13]. More interestingly, expression of two other members of the miR-34 family, miR-34b and miR-34c, are also induced by p53. These two family members are transcribed from chromosomes 11 and 1, respectively, different chromosomes than the one miR-34a is transcribed from. In addition, miR-34a and miR-34c have identical seed regions and are thus predicted to target similar mRNAs. Further functional analysis demonstrated that both miR-34b and miR34c work cooperatively with p53 in suppressing cell proliferation [14]. MiR-34a induces growth arrest through regulating pathways of the transcription factor E2F [15]. This transcription factor activates miRNA transcription in order to function synergistically.
Processing in nucleus
RNA polymerase II is the first enzyme that is thought to be responsible in transcribing primary miRNA (pri-miRNA) [16]. Some human miRNAs, which are surrounded by Alu repeat have been found to be transcribed by RNA transcriptase III instead of II [17]. These transcripts containing pri-miRNA can be several kilobases long, and also have a 5' 7-methylguanosine gap and a 3' polyadenylated tail similar to conventional messenger RNA (mRNA) [18].
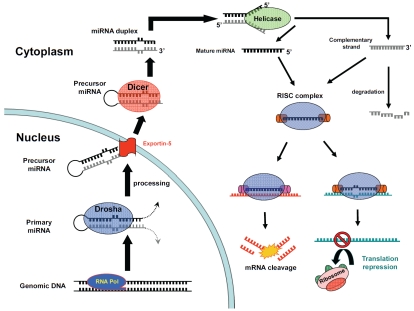
Pri-miRNA transcripts must be cleaved and spliced during miRNA maturation in order to form mature miRNA. It has a hairpin stem of approximately 33 base-pairs, a terminal loop and two single-stranded flanking regions upstream and downstream of the hairpin. Pri-miRNA is first processed into a precursor miRNA of about 70 nucleotides long. Figure 1 shows each step of the miRNA biogenesis.
Figure 1.
MicroRNA Biogenesis. Genes of miRNA are first transcribed by RNA Polymerase along with coding genes or independently. Nascently transcribed primary miRNA is then cleaved by the microprocessor composed of Drosha and Pasha in the nucleus. Resulting precursor miRNA is then exported from nucleus by nuclear pore protein Exportin-5. In the cytoplasm, Dicer removes the loop from precursor miRNA. After helicase opens the miRNA duplex, guide strand of the mature miRNA is loaded with Argonaute (Ago2) within the RISC complex, where it guides RISC to silence target mRNAs. Target mRNAs are translated ineffectively due to mRNA degradation or translation inhibition. The complementary strand is either degraded or functions as a mature miRNA.
The cleavage of pri-miRNA proceeds concurrently with the transcription of the gene or ncRNA [19]. Inside the nucleus, primary miRNA is first cleaved by a miRNA microprocessor composed of Drosha and DGCR8 (Pasha). Drosha is a RNase III endonuclease that performs the cleavage through its RNase III domain [20]. It cleaves 11 base-pairs away from the bottom of the hairpin stem where the 5' and 3' flanking regions separate [21]. In order for the microprocessor to recognize pri-miRNA, conservation of the pri-miRNA stem structure is essential. Single Nucleotide Polymorphisms (SNP) in the stem have been reported to block proper processing by Drosha [22]. The flanking single-stranded RNA below the stem loop is also mandatory for Drosha cleavage, but the sequence of terminal loop is not important in most cases [23]. These conserved aspects allow processing of pri-miRNA to occur even during transcription of the entire transcript.
While Drosha acts as the catalytic subunit of the microprocessor, DGCR8 recognizes the pri-miRNA through its double-stranded RNA binding domain, and also stabilizes its interaction with Drosha through its conserved C-terminal domain [24]. DGCR8 functions as a molecular ruler that allows Drosha to cut at an accurate position. The precursor miRNA (pre-miRNA) that is cleaved by Drosha always contain 2 nucleotide overhangs, characteristic of RNase III [25]. The 2 nucleotide overhang later becomes an important property for recognition by other proteins in downstream processes.
The expression level of the microprocessor complex and its activity is tightly maintained. The working Drosha-DGCR8 complex also cleaves stem loops present in the 5'UTR and the coding region of DGCR8 mRNA, resulting in its instability [26]. This acts as a feedback mechanism to ensure no additional complex will be assembled. The microprocessor complex can also be customized to enhance processing of a specific miRNA. A heterogenous nuclear ribonucleoprotein A1 (hnRNP A1) has been reported to participate as a subunit in the Drosha-DGCR8 complex. This RNA-binding protein binds specifically to pri-miR-18a but not to any other miRNAs in the same cluster, and the knock-down of hnRNP A1 greatly reduces expression of pre-miR-18a [27]. In this case, hnRNP A1 acts as a transacting factor by binding to the conserved loop of pri-miR-18a. This binding changes the conformation of pri-miR-18a and favors its processing by Drosha-DGCR8 complex [28]. However, this mechanism is not applicable to all the miRNAs. MicroRNA comparison across species displays low conservation in the loop sequences.
After nuclear processing, precursor miRNA is transported by Exportin-5 to the cytoplasm in a Ran guanosine triphosphate-dependent manner [29]. Exportin-5 recognizes precursor miRNA not by its sequence but by its characteristic hairpin stem and 3' protruding overhang [30]. These recognition requirements allow the transport of correctly processed precursor miRNAs only. High affinity binding of Exportin-5 to precursor miRNA protects it as soon as it is processed by Drosha [31].
Processing in cytoplasm
Once inside the cytoplasm, pre-miRNA is further processed into a shorter double stranded RNA duplex, composing of a mature strand and a complementary strand. This processing is carried out by another RNase III endonuclease, Dicer [32]. Dicer has a PAZ (Piwi-Argonaute-Zwille) domain, which preferentially binds to the 3' overhang of single-stranded RNA [33]. The distance between its PAZ domain and two RNase III domains is 65 angstroms, which is about 25 nucleotides in length according to crystal structure analysis. Therefore, after excision of pre-miRNA terminal loop, double-stranded RNA is about 22-25 nucleotide in length with 2 nucleotides overhanging on each 3' side [33,34]. Knocking down the expression of Dicer greatly reduces miRNA maturation [35], and deletion of Dicer in mice leads to lethality in early development [36]. This result demonstrates the importance of miRNAs in maintaining embryonic stem cell population, and has led to other studies on miRNA's function in embryonic cells [37].
Additional mechanisms that regulate Dicer processing have been reported in the literature. Dicer is shown to be negatively regulated by its product, let-7 miRNA, by targeting the coding region of Dicer mRNA [38]. Its targeting of Dicer mRNA leads to the negative feedback of Dicer expression and thus its activity. In one study, precursor miR-138-2 was observed abundantly in the cytoplasm of many cell types, but mature miR-138 was found to only exist in certain cell types. This observation points out the possibility that miRNA expression can be regulated post-transcriptionally and that miRNA expression may be restricted to certain cell types [39]. Although the specific mechanism has not yet been found, it is possible that a tissue-specific RNA binding protein is required to recruit Dicer in processing precursor miR-138-2. A ternary complex has been reported where TAR RNA Binding Protein (TRBP) and Protein Activator of PKR (PACT) assembles with Dicer. Dicer alone is sufficient for precursor miRNA processing. Like DGCR8 to Drosha, TRBP acts to stabilize Dicer [40]. Both TRBP and PACT were shown to enhance binding of RNA to Dicer and greatly facilitates the processing efficiency of Dicer [41]. By default, the helicase domain of Dicer inhibits its own cleaving activity [42]. TRBP interacts directly to the helicase domain of Dicer and changes it conformation to improve its processing efficiency. In addition, TRBP also functions by recruiting the excised RNA duplex to its final stop, the Argonaute proteins [43].
Guide strand loading into RISC
Following excision by Dicer, a short miRNA duplex is formed. Dicer and its cleavage-associated proteins TRBP or PACT dissociate from the miRNA duplex. However, only one strand of the miRNA duplex will act as the guide strand, which guides the functional unit. The other strand of the duplex is referred to as the complementary strand and is degraded subsequently. The mechanism for this asymmetry in strand selection still remains unclear [44], but it is suspected that strand selection is determined by a ribonucleoprotein complex called the RNA-induced Silencing Complex (RISC). RISC is composed of Dicer, TRBP for RNA binding, and Argonaute2 (Ago2) for catalytic activity [45]. Ago2 seems to pertain the ability to remove complementary strands by making an endonucleolytic cut at the passenger strand [46]. Degradation of the passenger strand facilitates the loading of the guide strand and activation of RISC. In vitro reconstitution experiments has shown that no chaperons or co-factors other than the basic machineries are required for RISC formation [47]. Dicer, TRBP, and Ago2 are the basic components needed for miRNA processing and functioning. However, particular helicases may be involved in facilitating unwinding of specific miRNAs. For example, P68 RNA helicase has been shown to facilitate selection of let-7 by RISC, and knock-down of P68 RNA helicase has been shown to inhibit let-7 functioning [48].
Theoretically, either strand of the miRNA duplex can be the guide strand. A study has attempted to predict strand selection by comparing outcomes of selection in validated precursor miRNA pairs with random RNA duplexes [49]. Random RNA duplexes do not form good base-pairing stems. It was found that miRNA pairs generally exhibit lower free energy than random duplexes. Base-pairing that includes U nucleotides, such as A:U, U:A, G:U, are not as stable as C:G pairs, which allow its incorporation into RISC. The ease in unwinding of the RNA duplex might explain in part why many miRNAs that start with a U nucleotide are selected.
Gene regulation by microRNAs
miRNAs Function as Gene Modulators
MicroRNAs are currently well recognized for their critical role in many biological processes [50]. MicroRNAs are single-stranded RNAs consisting of 18 to 24 nucleotides. They are generated from endogenous transcripts each consisting of a hairpin structure [43,51]. The accepted notion is that miRNAs function as a guide molecule in post-transcriptional gene silencing by partially base pairing with the 3'UTR of target mRNAs, leading to translational repression [52,53]. By silencing various target mRNAs, miRs play important modulatory roles in diverse regulatory pathways, including tissue development [54,55], cell proliferation [56,57], cell division [58,59], cell differentiation [60], apoptosis [61], protein secretion [62], and viral infection [63]. The majority of miRNA loci are found in intronic regions of non-coding transcription units, whereas few of them are found in exonic regions [64]. Computational analysis demonstrates that one miRNA has the ability to regulate the expression of many genes. Thus, miRNAs have been increasingly recognized as key regulators of gene expression, playing critical roles in many biological processes, such as aging, vascular diseases, angiogenesis, and tumorigenesis. However, the function of most miRNAs remains largely unknown.
Repression by mRNA degradation
Messenger RNA degradation can be initiated by either decapping or deadenylation [65], although sequence-specific endoneucleolytic cleavage by polysomal ribonuclease 1 (PMR-1) can also occur [66]. In general, mRNA degradation usually begins with deadenylation from the 3' end of the mRNA. Following deadenylation, decapping enzymes, such as DCP1 and DCP2, remove the 5' cap of mRNA, exposing itself for exonucleolytic degradation from 5'-to-3' by the enzyme Xrn1p. Alternatively, deadenylated mRNAs can be degraded from the 3'-to-5' direction by cytoplasmic exonucleases.
In a study of miRNA-mediated translational repression, some miRNA-targeted mRNAs were found to be destabilized [67]. In a study carried out in D. melanogaster, the Argonaute protein, Ago1, was found to interact with GW182, which is known as a marker for processing bodies where mRNAs are degraded or stored [68]. This interaction is also found in human cells [69]. GW182 was shown to function downstream of Argonaute proteins by silencing mRNA expression and promoting mRNA degradation. Another study also supports this finding based on similar experiments of depleting decapping enzymes or activators [70]. This then lead to the accumulation of deadenylated mRNAs in addition to the suppression of miRNA-mediated gene silencing. However, these studies also show that translational repression and mRNA degradation can occur as a combination. The subunits that are recruited during target site recognition by miRNA-incorporated RISC may determine the degree of translational inhibition.
Translational inhibition
Mature miRNAs are eventually transferred to Argonaute proteins and guide RISC activity in RNA silencing. In order to regulate translation, miRNAs base-pair with their target sites in the 3'UTR of mRNAs [71]. Translational repression has also been reported to occur even when target sites are located in 5'UTR or coding regions [72]. However, the mechanistic details of how microRNAs function in translational repression are still poorly understood. Results from different studies are sometimes contradictory because they are conducted in different systems and species.
MicroRNAs in tumorigenesis and angiogenesis
Malignant transformation is a multi-step process whereby normal cells acquire genetic and epigenetic alterations [73]. During this process, cells may acquire alterations that enhance growth and survival (i.e. oncogenic attributes) or ones that impair cancer progression (tumor suppressor-like). As a result of these transformation processes, transformed cells may become growth-independent, apoptosis-resistant, tissue-invasive and/or metastatic. Oncogenes and tumor suppressor genes are particularly powerful in transforming cells because they often strongly affect proliferation, apoptosis, or even both. For example, p53 is a well-known tumor suppressor that monitors cell cycle and induces apoptosis, but is missing or mutated in 50% of all human cancers [74].
Recent studies have indicated that expression of some miRNAs is closely correlated with cancer development and some miRNAs can function as oncogenes or tumor suppressors. Cancer cells develop different signatures of miRNA expression that distinguish them from normal cells. For example, a general decrease in miRNA expression was reported in cancers [75] perhaps due to the involvement of some of them in modulating cell differentiation. Decrease in miRNA expression may contribute to the generation and maintenance of cancer cells and tumor stem cells. Furthermore, some of these miRNAs may function as tumor suppressors. The most well-characterized examples are miR-15a and miR-16, which are down-regulated in 68% of chronic lymphocytic leukemia [76]. Their suppressor activities are further supported by the observation that they negatively regulate the expression of the anti-apoptotic factor BCL2 [77]. Down-regulation of miR-15a and miR-16 expression can increase levels of BCL2 contributng to higher anti-apoptotic activities. Another well known tumor suppressor is the let-7 family. Let-7 expression has been found to be lower in lung tumor tissue than in normal lung tissue [78]. Let-7 contains eight members and computational analysis suggests that most of the members target the expression of the RAS proto-oncogene. Negative regulation of RAS expression has been confirmed experimentally. Two miRNAs, miR-143 and miR-145, were found to be expressed at lower levels in colorectal cancer than in normal tissues, and are predicted to target several mRNAs that encode several components of signal transduction pathways associated with cancer development and these components include Raf, Rho, GTPase activating protein, G-protein γ, NF-κB, and HGK [79]. The first demonstration that a miRNA could have oncogenic potential is miR-155, encoded by BIC [80]. It was later found that, transgenic mice carrying a miR-155 transgene developed preleukemic pre-B-cell proliferation [81]. Furthermore, some miRNAs can function cooperatively with oncogenes rather than acting directly as an oncogene. MiR-17-19b is a good example of this as its expression in transgenic mice expressing c-Myc was found to accelerate lymphomagenesis [64].
MicroRNAs in hepatocellular carcinoma (HCC)
Many studies have revealed that the expression of miRNAs is deregulated in human HCC [82-89]. Among the aberrantly expressed miRNAs, some are hepato-specific. For example, miR-122a, which accounts for about 70% of the total population in adult liver, is down-regulated in HCC [90]. In fact, miR-122a acts as a key regulator of cholesterol and fatty-acid metabolism. Down-regulation of miR-122a was detected in more than 70% of HCC [89,90]. During the development of the mouse liver, the level of miR-122a expression reaches its maximal level just before birth. The loss of miR-122a expression in HCC may represent a less differentiated state of liver cells, a common phenotype of tumorigenesis. This group in fact showed that down-regulation of miR-122a expression may have a direct role in liver tumourigenesis through cyclin G1 activation [89,90]. In addition, some of the deregulated miRNAs found in HCC are not hepato-specific, and are deregulated in other neoplasm's as well. For example, up-regulation of miR-221/222 was not only reported in HCC but was also found in the colon, pancreas, stomach, bladder carcinomas and glioblastomas. Likewise, down-regulation of miR-199a, miR-200b and miR-214 was not only reported in HCC but also reported in ovarian cancer and lung cancer [82-84,88]. The oncogenic miRNA miR-373 was also found to be up-regulated in HCC as well as in testicular cancer and in breast cancer metastasis [91,92]. Interestingly, some miRNAs that are up-regulated in HCC including miR-21, miR-210, miR-213 and miR-181b, were also found to be up-regulated in hypoxic conditions.
The discovery that miRNAs play an important role in hepato-carcinogenesis has shone light on the possibility of exploiting them for molecular therapy. The approach of applying siRNA to molecular therapy has been reported to be an advantageous one as it does not appear to induce significant harmful side effects in treating liver injury [33,93]. The high hepatic uptake of siRNA also allows systemic administration. Recently, anti-miRNA oligonucleotides (AMOs) have been developed. Since miR-21 is up-regulated in HCC, transfection of anti-miR-21 into cancer cells was found to suppress cell growth associated with increased apoptosis [61,94]. In fact, the liver seems to be the organ that is most efficiently targeted by intravenous injection of anti-miRNA oligonucleotides. Since aberrantly expressed miRNAs in HCC may be associated with bio-pathological and clinical features, applying microRNA tools into the development of new therapeutic targets will be an exciting endeavor.
microRNAs and breast cancer
The possibility of measuring miRNA expresson levels in tumors to classify cancers even those of ambiguous origin is a very promising one [64,75,78,95]. The aberrant expression of microRNA levels in human breast cancer tissue compared with in normal breast tissue has been suggested to have important implications [96]. The most significantly deregulated miRNAs in breast cancer tissue confirmed to date include miR-125b, miR-145, miR-10b, and miR-204 [96]. These results were confirmed by microarray and Northern blot analyses. The expression of these miRs correlated well with specific breast cancer biopathologic features, such as estrogen and progesterone receptor expression, tumor stage, vascular invasion, and proliferation index. In fact, direct evidence that miRNAs is involved in breast cancer development is apparent from the studies of miR-21. While being overexpressed in breast carcinomas, miR-21 promotes tumor cell growth by inhibiting the putative tumor suppressor gene tropomyosin-1 [97]. Invasion and metastasis of breast cancer has also been found to be initiated by overex-pression of miR-10b [98]. Furthermore, some miRNAs such as let-7, can regulate self renewal and tumorigenesis of breast cancer cells [99]. For the miRNAs that have been lost as human breast cancer develops into its metastatic stage, restoration of the miRNAs, miR-126 and miR-335, was shown to reduce tumor cell growth and inhibit metastasis [100].
miRNAs in cancer diagnosis
Today, miRNAs are gaining increasing importance as gene expression regulators. Its ability to affect cellular activities by regulating gene expression makes it potentially powerful in regulating malignant transformations. Analysis of a number of human tissue and cell lines shows that precursor miRNA processing is reduced in cancer cell lines [101]. Many of these precursor miRNAs are retained in the nucleus, and thus they are not processed into mature miRNAs. This result is consistent with the observation of global reduction in mature miRNA expression in cancerous tissues compared with its expression in normal tissues [75]. Loss of Dicer expression has also been reported to occur in some tumors [102]. Knockdown of major miRNA biogenesis machineries- Drosha, DCGR8, and Dicer, substantially decreases production of miRNAs and promotes a more transformed phenotype of cells [103].
Expression of miRNAs is different in tumors compared with its expression in normal tissues. For example, miR-21, miR-125b, miR-145, and miR-155 expression are clearly deregulated in breast cancer as confirmed by microarray and northern blot [96]. Deregulation of miRNA expression may be a result of genetic alteration. It was found that 53% miRNAs genes were located at fragile sites, where they are much more likely to be susceptible to amplification, deletion, or breakpoint [7]. It suggests that miRNAs may have a crucial role in cancer development. Over-expression of oncogenic miRNAs can result in down-regulation of tumor suppressor genes, whereas under-expression of tumor suppressor-like miRNAs can lead to up-regulation of oncogenes. Therefore, studies linking the targets of these miRNAs with their functions will unveil the underlying role of miRNAs in cancer development.
miRNAs Function in Angiogenesis
Angiogenesis is the sprouting of new blood vessels from preexisting ones. It is a phenomenon associated with several human pathologies, including various potentially blinding ocular disorders, rheumatoid arthritis, and cancer. The regulation of angiogenesis by miRs is not a new finding [104-107]. miR-221 and miR-222 are known to modulate the angiogenic properties of human umbilical vein endothelial cells [108,109]. In fact, a number of miRNAs have been found to be abundantly expressed in angiogenesis-associated endothelial cells, and some of them are even predicted to target angiogenic receptors. One example is the targeting of c-kit, a receptor tyrosine kinase that binds stem cell factor and mediates VEGF expression, by miR-221 and miR-222 [109]. Transfection of miR-221/222 represses c-kit expression without changing mRNA level. It also inhibits endothelial cell-facilitated tube formation. Endothelial cells express VEGF receptors, and thus miRNAs could modulate angiogenesis by regulating VEGF receptor activity, thereby affecting endothelial cell migration and invasion [108,110,111]. Other points to keep in mind are that ,miRNAs have been shown to regulate angiogenesis in vascular smooth cells [112], and that Dicer and Drosha, two enzymes that control processing of miRNAs, have also been shown to be important in angiogenesis [110].
The roles of miRNAs in tumor angiogenesis have been extensively studied. Initially, it was established that the oncogene c-myc directly activates miRNA cluster miR-17-92 in human lymphocytes [113]. It was later found that miR-18 and miR-19 are the cleaved products of the miR -17-92 cluster, and thus expression of miR-18 and miR-19 are up-regulated by c-myc [114]. Another member of this family, miR-93, also plays important roles in tumorigenesis and angiogenesis [115]. Studies also indicate that expression of miR-378 promoted tumorigenesis and angiogenesis [116], and inhibit cell differentiation [117], since miR-378 can induces VEGF expression [118]. However, tumor growth is not always accompanied by promoted angiogenesis. For example, expression of the 3'untranslated region of CD44 can inhibit tumor growth but promote angiogenesis [119]. The 3'UTR can modulate endogenous miRNA activities. Expression of versican 3'UTR induces organ adhesion and inhibit tumor growth [120,121]. This appears to be due to up-regulation of versican expression. It have been known that vesicant plays important role in mediating cell activities [122-128], tumorigenesis [129,130], and angiogenesis [131,132]. These results demonstrated that not only miRNAs can regulate mRNA expression, but also mRNAs can modulate miRNA functions. By identifying the signature miRNAs in angiogenesis, investigators may be able to identify previously unrecognized targets within a disease pathway or identify binding sites for disease-specific miRNAs.This approach could ultimately lead to identification of new disease targets and innovative therapeutic approaches associated with angiogenesis.
Some examples of microRNAs in tumorigenesis and angiogenesis
miR-17∼92 is the most extensively studied miRNA cluster. This cluster contains six pairs of mature miRNAs including miR-17-5p/miR-17-3p, miR-18a/miR-18a*, miR-19a/miR-19a*, miR-20a/miR-20a*, miR-19b-1/miR-19b-1*, and miR-92a-1/miR-92a-1*. It is located in a non-protein-coding gene C13orf25 at 13q31 [133], and it transcribes a polycystronic transcript. Two paralogs are present in Chromosome 7 (7q22.1 producing cluster miR-106b∼25 for three pairs of mature miRNAs) and Chromosome X (Xq26.2 producing cluster miR-106a∼363 for six pairs of mature miRNAs). The miR-17∼92 cluster appears to play important roles in regulating diverse cell activities and even broader processes such as angiogenesis [134-136] and cancer development [133,137,138]. It has been found that miR-17∼92 can function as an oncogene in the development of B-cell lymphoma [64], consistent with the finding that transcription of this cluster is trans-activated by the oncogene c-Myc [113,114]. Indeed, miR-17∼92 and related miRNAs are overexpressed in different human cancers [139-141] because of their ability to repress expression of many tumor-associated proteins [142-144]. Comprehensive microarray analysis detected deletion of miR-17∼92 in 16-22% of ovarian cancers, breast cancers and melanomas [145]. Overexpression of miR-17∼92 has been found to promote cell proliferation and reduce apoptosis [141,143,146,147]. While inhibition of miR-17 and miR-20a induces apoptosis, inhibition of miR-18a and miR-19a has no effect, suggesting that different miRNAs in the miR-17∼92 cluster have distinct functions [148]. Indeed, overexpression of a single miRNA (miR-17) has been shown to inhibit breast cancer cell proliferation by targeting the oncogene AIB1 [149] and this finding is in contrast to the results obtained by over-expressing the entire miR-17∼92 cluster.
Nevertheless, the functions reported for the miR -17∼92 cluster and it paralogs are very different. Comprehensive microarray analysis has revealed that miR-17∼92 is deleted in some ovarian cancers, breast cancers and melanomas [145]. Overexpression of miR-17∼92 promotes cell proliferation and reduces apoptosis by regulating cell cycle progression [141,143,146,147]. Inhibition of miR-17 and miR-20a induces apoptosis, while inhibition of miR-18a and miR-19a in the same cluster has no apparent effect. These studies indicate functional distinctions among miRNAs in the miR-17∼92 cluster [148]. Recent studies show that the miR-17∼92 cluster plays an important role in tissue development. Loss-of-function of miR-17∼92 or its paralogs results in smaller embryos and enhanced postnatal death due to hypoplastic lungs and ventricular septal defects, reminiscent of B cell development disruption [150]. On the other hand, gain-of-function of the miR-17∼92 cluster causes lymphoproliferative diseases and premature death [151]. Overexpression of miR-17 and miR-20a promotes monocytic proliferation and inhibits monocytic differentiation [152]. However, overexpression of miR-19a and miR-92a has no effect, further indicating distinctive functions among the miRNAs of the cluster. These diverse and opposite functions reported for miR-17∼92 and its paralogs may be due to the specific function of miRNAs in different tissues and cells. In addition, the miR-17∼92 and its paralogs express 15 miRNA precursors. The functions of each miRNA could be different in the cluster, but the precise function of each is unclear.
The tumor suppressor-like properties of miR-15a and miR-16-1 are well-studied. Chronic Lymphocytic Leukemia (CLL) is the most common type of leukemia and is characterized by a deletion at chromosome 13q14 [153]. Frequent deletions and loss of heterozygosity in chromosome 13q14 have suggested inactivation of tumor suppressor genes at this location [154]. Deletion analysis narrowed the possibilities down to a fragment containing three non-protein coding genes. One of them is named Leukemia-associated Gene 2 (LEU2), which is flanked by Alu sequences and long interspersed element (LINE) [155]. Later miR-15a and miR-16-1 were found located in the first intron of LEU2, and reduction of both miRNAs was found in 68% of CLL cases. Further studies showed that anti-apoptotic B Cell Lymphoma 2 (BCL2) is targeted by miR-15a and miR16-1 [77]. Deletion or inactivity of miR-15a and miR-16-1 was found to relieve translational repression on BCL2. The finding that miR-15a and miR-16-1 plays a causal role in inducing CLL links miRNAs with cancer [76]. Measuring expression levels of miR15a and miR16-1 can be used to determine if patients have CLL. Mapping of miRNAs reveals that their genomic location is situated in fragile regions of the genome [7], and hence can substantially contribute to their deregulated expression in tumor tissues. Expression profiling of miRNA has been used extensively in defining the miRNA signature of different cancers [156].
Let-7 has many closely related and highly conserved family members [157]. It is lost in many human malignancies [7], and is the earliest miR found to regulate several oncogenes, including RAS, HMGA2, and MYC [78,158,159]. Mutations in the RAS family of proto-oncogenes are found in 20% to 30% of all human tumors. In fact, activating the RAS gene results in cellular transformation [160]. The 3'UTR of human RAS genes contains multiple target sites for members of the Let-7 family. Introducing let-7 into lung cancer cells reduces cell growth shown in a colony formation assay [161]. In a later study, over-expression of let-7 in several human lung cancer cell lines resulted in decreased levels of Ras, and the depletion of let-7 led to a marked increase in Ras protein expression. Thus, Let-7 miRNAs can function as tumor suppressors by maintaining the expression level of oncogenic RAS proteins. Studies also showed that expression of Let-7 is greatly reduced in breast tumor-initiating cells compared with non-breast tumor-initiating cells [162]. Targeting of many different genes by Let-7 miRNAs gives rise to characteristics of tumor-initiating cells. In addition, increased expression of let-7 in self-renewing tumor-initiating cells decreases their proliferation and metastatic capacity.
Conclusion remark
Our understanding of the roles of miRNAs in cancer is still incomplete and only a handful of tumor-associated miRNAs are shown to act as oncogenes or tumor suppressors. More studies are required to move from miRNA expression profiling to their biological activities in order to understand their involvement in the etiology of malignant transformation.
References
- 1.Finishing the euchromatic sequence of the human genome. Nature. 2004;431:931–945. doi: 10.1038/nature03001. [DOI] [PubMed] [Google Scholar]
- 2.Ibanez-Ventoso C, Vora M, Driscoll M. Sequence relationships among C. elegans, D. melanogaster and human microRNAs highlight the extensive conservation of microRNAs in biology. PLoS ONE. 2008;3:e2818. doi: 10.1371/journal.pone.0002818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griffiths-Jones S. The microRNA Registry. Nucleic Acids Res. 2004;32:D109–111. doi: 10.1093/nar/gkh023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zamore PD, Haley B. Ribo-gnome: the big world of small RNAs. Science. 2005;309:1519–1524. doi: 10.1126/science.1111444. [DOI] [PubMed] [Google Scholar]
- 5.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 6.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M, Croce CM. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci U S A. 2004;101:2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J, Banfic H, Straforini F, Tosi L, Volinia S, Rittenhouse SE. A type II phosphoinositide 3-kinase is stimulated via activated integrin in platelets. A source of phosphatidylinositol 3-phosphate. J Biol Chem. 1998;273:14081–14084. doi: 10.1074/jbc.273.23.14081. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez A, Griffiths-Jones S, Ashurst JL, Bradley A. Identification of mammalian microRNA host genes and transcription units. Genome Res. 2004;14:1902–1910. doi: 10.1101/gr.2722704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saini A, Seller Z, Davies D, Marshall JF, Hart IR. Activation status and function of the VLA-4 (alpha4beta1) integrin expressed on human melanoma cell lines. Int J Cancer. 1997;73:264–270. doi: 10.1002/(sici)1097-0215(19971009)73:2<264::aid-ijc17>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 11.Houbaviy HB, Dennis L, Jaenisch R, Sharp PA. Characterization of a highly variable eutherian microRNA gene. Rna. 2005;11:1245–1257. doi: 10.1261/rna.2890305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, Yagita H, Ishii N, Evans R, Honda K, Takeda K. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 13.Chang TC, Wentzel EA, Kent OA, Ramachandran K, Mullendore M, Lee KH, Feldmann G, Yamakuchi M, Ferlito M, Lowenstein CJ, Arking DE, Beer MA, Maitra A, Mendell JT. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell. 2007;26:745–752. doi: 10.1016/j.molcel.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corney DC, Flesken-Nikitin A, Godwin AK, Wang W, Nikitin AY. MicroRNA-34b and MicroRNA-34c are targets of p53 and cooperate in control of cell proliferation and adhesion-independent growth. Cancer Res. 2007;67:8433–8438. doi: 10.1158/0008-5472.CAN-07-1585. [DOI] [PubMed] [Google Scholar]
- 15.Tazawa H, Tsuchiya N, Izumiya M, Nakagama H. Tumor-suppressive miR-34a induces senescence-like growth arrest through modulation of the E2F pathway in human colon cancer cells. Proc Natl Acad Sci U S A. 2007;104:15472–15477. doi: 10.1073/pnas.0707351104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chowdhury TT, Salter DM, Bader DL, Lee DA. Integrin-mediated mechanotransduction processes in TGFbeta-stimulated monolayer-expanded chondrocytes. Biochem Biophys Res Commun. 2004;318:873–881. doi: 10.1016/j.bbrc.2004.04.107. [DOI] [PubMed] [Google Scholar]
- 17.Borchert GM, Lanier W, Davidson BL. RNA polymerase III transcribes human microRNAs. Nat Struct Mol Biol. 2006;13:1097–1101. doi: 10.1038/nsmb1167. [DOI] [PubMed] [Google Scholar]
- 18.Cai X, Hagedorn CH, Cullen BR. Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. Rna. 2004;10:1957–1966. doi: 10.1261/rna.7135204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morlando M, Ballarino M, Gromak N, Pagano F, Bozzoni I, Proudfoot NJ. Primary microRNA transcripts are processed co-transcriptionally. Nat Struct Mol Biol. 2008;15:902–909. doi: 10.1038/nsmb.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee MH, Yang HY. Regulators of G1 cyclin-dependent kinases and cancers. Cancer Metastasis Rev. 2003;22:435–449. doi: 10.1023/a:1023785332315. [DOI] [PubMed] [Google Scholar]
- 21.Han J, Lee Y, Yeom KH, Kim YK, Jin H, Kim VN. The Drosha-DGCR8 complex in primary microRNA processing. Genes Dev. 2004;18:3016–3027. doi: 10.1101/gad.1262504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duan R, Pak C, Jin P. Single nucleotide polymorphism associated with mature miR-125a alters the processing of pri-miRNA. Hum Mol Genet. 2007;16:1124–1131. doi: 10.1093/hmg/ddm062. [DOI] [PubMed] [Google Scholar]
- 23.Han J, Lee Y, Yeom KH, Nam JW, Heo I, Rhee JK, Sohn SY, Cho Y, Zhang BT, Kim VN. Molecular basis for the recognition of primary microRNAs by the Drosha-DGCR8 complex. Cell. 2006;125:887–901. doi: 10.1016/j.cell.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 24.Yeom KH, Lee Y, Han J, Suh MR, Kim VN. Characterization of DGCR8/Pasha, the essential cofactor for Drosha in primary miRNA processing. Nucleic Acids Res. 2006;34:4622–4629. doi: 10.1093/nar/gkl458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takeshita A, Murakami Y, Yamashita Y, Ishida M, Fujisawa S, Kitano S, Hanazawa S. Porphyromonas gingivalis fimbriae use beta2 integrin (CD11/CD18) on mouse peritoneal macrophages as a cellular receptor, and the CD18 beta chain plays a functional role in fimbrial signaling. Infect Immun. 1998;66:4056–4060. doi: 10.1128/iai.66.9.4056-4060.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han J, Pedersen JS, Kwon SC, Belair CD, Kim YK, Yeom KH, Yang WY, Haussler D, Blelloch R, Kim VN. Posttranscriptional crossregulation between Drosha and DGCR8. Cell. 2009;136:75–84. doi: 10.1016/j.cell.2008.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guil S, Caceres JF. The multifunctional RNA-binding protein hnRNP A1 is required for processing of miR-18a. Nat Struct Mol Biol. 2007;14:591–596. doi: 10.1038/nsmb1250. [DOI] [PubMed] [Google Scholar]
- 28.Michlewski G, Guil S, Semple CA, Caceres JF. Posttranscriptional regulation of miRNAs harboring conserved terminal loops. Mol Cell. 2008;32:383–393. doi: 10.1016/j.molcel.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lund E, Guttinger S, Calado A, Dahlberg JE, Kutay U. Nuclear export of microRNA precursors. Science. 2004;303:95–98. doi: 10.1126/science.1090599. [DOI] [PubMed] [Google Scholar]
- 30.Gwizdek C, Ossareh-Nazari B, Brownawell AM, Doglio A, Bertrand E, Macara IG, Dargemont C. Exportin-5 mediates nuclear export of minihelix-containing RNAs. J Biol Chem. 2003;278:5505–5508. doi: 10.1074/jbc.C200668200. [DOI] [PubMed] [Google Scholar]
- 31.Bohnsack MT, Czaplinski K, Gorlich D. Exportin 5 is a RanGTP-dependent dsRNA-binding protein that mediates nuclear export of pre-miRNAs. Rna. 2004;10:185–191. doi: 10.1261/rna.5167604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–366. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- 33.Song E, Lee SK, Wang J, Ince N, Ouyang N, Min J, Chen J, Shankar P, Lieberman J. RNA interference targeting Fas protects mice from fulminant hepatitis. Nat Med. 2003;9:347–351. doi: 10.1038/nm828. [DOI] [PubMed] [Google Scholar]
- 34.Carmell MA, Xuan Z, Zhang MQ, Hannon GJ. The Argonaute family: tentacles that reach into RNAi, developmental control, stem cell maintenance, and tumorigenesis. Genes Dev. 2002;16:2733–2742. doi: 10.1101/gad.1026102. [DOI] [PubMed] [Google Scholar]
- 35.Hutvagner G, McLachlan J, Pasquinelli AE, Balint E, Tuschl T, Zamore PD. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science. 2001;293:834–838. doi: 10.1126/science.1062961. [DOI] [PubMed] [Google Scholar]
- 36.Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, Li MZ, Mills AA, Elledge SJ, Anderson KV, Hannon GJ. Dicer is essential for mouse development. Nat Genet. 2003;35:215–217. doi: 10.1038/ng1253. [DOI] [PubMed] [Google Scholar]
- 37.Suh MR, Lee Y, Kim JY, Kim SK, Moon SH, Lee JY, Cha KY, Chung HM, Yoon HS, Moon SY, Kim VN, Kim KS. Human embryonic stem cells express a unique set of microRNAs. Dev Biol. 2004;270:488–498. doi: 10.1016/j.ydbio.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 38.Forman JJ, Legesse-Miller A, Coller HA. A search for conserved sequences in coding regions reveals that the let-7 microRNA targets Dicer within its coding sequence. Proc Natl Acad Sci USA. 2008;105:14879–14884. doi: 10.1073/pnas.0803230105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Obernosterer G, Leuschner PJ, Alenius M, Martinez J. Post-transcriptional regulation of microRNA expression. Rna. 2006;12:1161–1167. doi: 10.1261/rna.2322506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haase AD, Jaskiewicz L, Zhang H, Laine S, Sack R, Gatignol A, Filipowicz W. TRBP, a regulator of cellular PKR and HIV-1 virus expression, interacts with Dicer and functions in RNA silencing. EMBO Rep. 2005;6:961–967. doi: 10.1038/sj.embor.7400509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee Y, Hur I, Park SY, Kim YK, Suh MR, Kim VN. The role of PACT in the RNA silencing pathway. Embo J. 2006;25:522–532. doi: 10.1038/sj.emboj.7600942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma E, MacRae IJ, Kirsch JF, Doudna JA. Autoinhibition of human dicer by its internal helicase domain. J Mol Biol. 2008;380:237–243. doi: 10.1016/j.jmb.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chendrimada TP, Gregory RI, Kumaraswamy E, Norman J, Cooch N, Nishikura K, Shiekhattar R. TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature. 2005;436:740–744. doi: 10.1038/nature03868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwarz DS, Hutvagner G, Du T, Xu Z, Aronin N, Zamore PD. Asymmetry in the assembly of the RNAi enzyme complex. Cell. 2003;115:199–208. doi: 10.1016/s0092-8674(03)00759-1. [DOI] [PubMed] [Google Scholar]
- 45.Zervas CG, Gregory SL, Brown NH. Drosophila integrin-linked kinase is required at sites of integrin adhesion to link the cytoskeleton to the plasma membrane. J Cell Biol. 2001;152:1007–1018. doi: 10.1083/jcb.152.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rand TA, Petersen S, Du F, Wang X. Argonaute2 cleaves the anti-guide strand of siRNA during RISC activation. Cell. 2005;123:621–629. doi: 10.1016/j.cell.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 47.Macrae IJ, Ma E, Zhou M, Robinson CV, Doudna JA. In vitro reconstitution of the human RISC-loading complex. Proc Natl Acad Sci U S A. 2008 doi: 10.1073/pnas.0710869105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salzman DW, Shubert-Coleman J, Furneaux H. P68 RNA helicase unwinds the human let-7 microRNA precursor duplex and is required for let-7-directed silencing of gene expression. J Biol Chem. 2007;282:32773–32779. doi: 10.1074/jbc.M705054200. [DOI] [PubMed] [Google Scholar]
- 49.Khvorova A, Reynolds A, Jayasena SD. Functional siRNAs and miRNAs exhibit strand bias. Cell. 2003;115:209–216. doi: 10.1016/s0092-8674(03)00801-8. [DOI] [PubMed] [Google Scholar]
- 50.Ambros V. MicroRNA pathways in flies and worms: growth, death, fat, stress, and timing. Cell. 2003;113:673–676. doi: 10.1016/s0092-8674(03)00428-8. [DOI] [PubMed] [Google Scholar]
- 51.Hutvagner G, Zamore PD. A microRNA in a multiple-turnover RNAi enzyme complex. Science. 2002;297:2056–2060. doi: 10.1126/science.1073827. [DOI] [PubMed] [Google Scholar]
- 52.He J, Zhang JF, YiC, Lv Q, Xie WD, Li JN, Wan G, Cui K, Kung HF, Yang J, Yang BB, Zhang Y. miRNA-mediated functional changes through co-regulating function related genes. PLoS One. 5:e13558. doi: 10.1371/journal.pone.0013558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang CH, Lee DY, Deng Z, Jeyapalan Z, Lee SC, Kahai S, Lu WY, Zhang Y, Yang BB. MicroRNA miR-328 regulates zonation morphogenesis by targeting CD44 expression. PLoS One. 2008;3:e2420. doi: 10.1371/journal.pone.0002420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnston RJ, Hobert O. A microRNA controlling left/right neuronal asymmetry in Caenorhabditis elegans. Nature. 2003;426:845–849. doi: 10.1038/nature02255. [DOI] [PubMed] [Google Scholar]
- 55.Zhao Y, Samal E, Srivastava D. Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature. 2005;436:214–220. doi: 10.1038/nature03817. [DOI] [PubMed] [Google Scholar]
- 56.Cheng AM, Byrom MW, Shelton J, Ford LP. Antisense inhibition of human miRNAs and indications for an involvement of miRNA in cell growth and apoptosis. Nucleic Acids Res. 2005;33:1290–1297. doi: 10.1093/nar/gki200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen JF, Mandel EM, Thomson JM, Wu Q, Callis TE, Hammond SM, Conlon FL, Wang DZ. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet. 2006;38:228–233. doi: 10.1038/ng1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hatfield SD, Shcherbata HR, Fischer KA, Nakahara K, Carthew RW, Ruohola-Baker H. Stem cell division is regulated by the microRNA pathway. Nature. 2005;435:974–978. doi: 10.1038/nature03816. [DOI] [PubMed] [Google Scholar]
- 59.Croce CM, Calin GA. miRNAs, cancer, and stem cell division. Cell. 2005;122:6–7. doi: 10.1016/j.cell.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 60.Naguibneva I, Ameyar-Zazoua M, Polesskaya A, Ait-Si-Ali S, Groisman R, Souidi M, Cuvellier S, Harel-Bellan A. The microRNA miR-181 targets the homeobox protein Hox-A11 during mammalian myoblast differentiation. Nat Cell Biol. 2006;8:278–284. doi: 10.1038/ncb1373. [DOI] [PubMed] [Google Scholar]
- 61.Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65:6029–6033. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- 62.Poy MN, Eliasson L, Krutzfeldt J, Kuwajima S, Ma X, Macdonald PE, Pfeffer S, Tuschl T, Rajewsky N, Rorsman P, Stoffel M. A pancreatic islet-specific microRNA regulates insulin secretion. Nature. 2004;432:226–230. doi: 10.1038/nature03076. [DOI] [PubMed] [Google Scholar]
- 63.Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science. 2005;309:1577–1581. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- 64.He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, Powers S, Cordon-Cardo C, Lowe SW, Hannon GJ, Hammond SM. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828–833. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thomson JM, Parker J, Perou CM, Hammond SM. A custom microarray platform for analysis of microRNA gene expression. Nat Methods. 2004;1:47–53. doi: 10.1038/nmeth704. [DOI] [PubMed] [Google Scholar]
- 66.Jin W, Dodson MV, Moore SS, Basarab JA, Guan LL. Characterization of microRNA expression in bovine adipose tissues: a potential regulatory mechanism of subcutaneous adipose tissue development. BMC Mol Biol. 11:29. doi: 10.1186/1471-2199-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.BaggaS, Bracht J, Hunter S, Massirer K, Holtz J, Eachus R, Pasquinelli AE. Regulation by let-7 and lin-4 miRNAs results in target mRNA degradation. Cell. 2005;122:553–563. doi: 10.1016/j.cell.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 68.Behm-Ansmant I, Rehwinkel J, Doerks T, Stark A, Bork P, Izaurralde E. mRNA degradation by miRNAs and GW182 requires both CCR4:NOT deadenylase and DCP1:DCP2 decapping complexes. Genes Dev. 2006;20:1885–1898. doi: 10.1101/gad.1424106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Meister G, Landthaler M, Peters L, Chen PY, Urlaub H, Luhrmann R, Tuschl T. Identification of novel argonaute-associated proteins. Curr Biol. 2005;15:2149–2155. doi: 10.1016/j.cub.2005.10.048. [DOI] [PubMed] [Google Scholar]
- 70.Eulalio A, Rehwinkel J, Stricker M, Huntzinger E, Yang SF, Doerks T, Dorner S, Bork P, Boutros M, Izaurralde E. Target-specific requirements for enhancers of decapping in miRNA-mediated gene silencing. Genes Dev. 2007;21:2558–2570. doi: 10.1101/gad.443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lai EC. Micro RNAs are complementary to 3' UTR sequence motifs that mediate negative post-transcriptional regulation. Nat Genet. 2002;30:363–364. doi: 10.1038/ng865. [DOI] [PubMed] [Google Scholar]
- 72.Lytle JR, Yario TA, Steitz JA. Target mRNAs are repressed as efficiently by microRNA-binding sites in the 5' UTR as in the 3' UTR. Proc Natl Acad Sci U S A. 2007;104:9667–9672. doi: 10.1073/pnas.0703820104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Olson P, Lu J, Zhang H, Shai A, Chun MG, Wang Y, Libutti SK, Nakakura EK, Golub TR, Hanahan D. MicroRNA dynamics in the stages of tumorigenesis correlate with hallmark capabilities of cancer. Genes Dev. 2009;23:2152–2165. doi: 10.1101/gad.1820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu DP, Song H, Xu Y. A common gain of function of p53 cancer mutants in inducing genetic instability. Oncogene. 2009 doi: 10.1038/onc.2009.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 76.Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, Rassenti L, Kipps T, Negrini M, Bullrich F, Croce CM. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 2002;99:15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M, Wojcik SE, Aqeilan RI, Zupo S, Dono M, Rassenti L, Alder H, Volinia S, Liu CG, Kipps TJ, Negrini M, Croce CM. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci U S A. 2005;102:13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, Labourier E, Reinert KL, Brown D, Slack FJ. RAS is regulated by the let-7 microRNA family. Cell. 2005;120:635–647. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 79.Michael MZ, SM OC, van Holst Pellekaan NG, Young GP, James RJ. Reduced accumulation of specific microRNAs in colorectal neoplasia. Mol Cancer Res. 2003;1:882–891. [PubMed] [Google Scholar]
- 80.Tam W, Dahlberg JE. miR-155/BIC as an oncogenic microRNA. Genes Chromosomes Cancer. 2006;45:211–212. doi: 10.1002/gcc.20282. [DOI] [PubMed] [Google Scholar]
- 81.Costinean S, Zanesi N, Pekarsky Y, Tili E, Volinia S, Heerema N, Croce CM. Pre-B cell proliferation and lymphoblastic leukemia/high-grade lymphoma in E(mu)-miR155 transgenic mice. Proc Natl Acad Sci U S A. 2006;103:7024–7029. doi: 10.1073/pnas.0602266103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Braconi C, Patel T. MicroRNA expression profiling: a molecular tool for defining the phenotype of hepatocellular tumors. Hepatology. 2008;47:1807–1809. doi: 10.1002/hep.22326. [DOI] [PubMed] [Google Scholar]
- 83.Huang YS, Dai Y, Yu XF, Bao SY, Yin YB, Tang M, Hu CX. Microarray analysis of microRNA expression in hepatocellular carcinoma and non-tumorous tissues without viral hepatitis. J Gastroenterol Hepatol. 2008;23:87–94. doi: 10.1111/j.1440-1746.2007.05223.x. [DOI] [PubMed] [Google Scholar]
- 84.Ladeiro Y, Couchy G, Balabaud C, Bioulac-Sage P, Pelletier L, Rebouissou S, Zucman-Rossi J. MicroRNA profiling in hepatocellular tumors is associated with clinical features and oncogene/tumor suppressor gene mutations. Hepatology. 2008 doi: 10.1002/hep.22256. [DOI] [PubMed] [Google Scholar]
- 85.Varnholt H, Drebber U, Schulze F, Wedemeyer I, Schirmacher P, Dienes HP, Odenthal M. MicroRNA gene expression profile of hepatitis C virus-associated hepatocellular carcinoma. Hepatology. 2008;47:1223–1232. doi: 10.1002/hep.22158. [DOI] [PubMed] [Google Scholar]
- 86.Wang Y, Lee AT, Ma JZ, Wang J, Ren J, Yang Y, Tantoso E, Li KB, Ooi LL, Tan P, Lee CG. Profiling microRNA expression in hepatocellular carcinoma reveals microRNA-224 up-regulation and apoptosis inhibitor-5 as a microRNA-224-specific target. J Biol Chem. 2008 doi: 10.1074/jbc.M707629200. [DOI] [PubMed] [Google Scholar]
- 87.Wong QW, Lung RW, Law PT, Lai PB, Chan KY, To KF, Wong N. MicroRNA-223 is commonly repressed in hepatocellular carcinoma and potentiates expression of Stathmin1. Gastroenterology. 2008;135:257–269. doi: 10.1053/j.gastro.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 88.Murakami Y, Yasuda T, Saigo K, Urashima T, Toyoda H, Okanoue T, Shimotohno K. Comprehensive analysis of microRNA expression patterns in hepatocellular carcinoma and non-tumorous tissues. Oncogene. 2006;25:2537–2545. doi: 10.1038/sj.onc.1209283. [DOI] [PubMed] [Google Scholar]
- 89.Kutay H, Bai S, Datta J, Motiwala T, Pogribny I, Frankel W, Jacob ST, Ghoshal K. Downregulation of miR-122 in the rodent and human hepatocellular carcinomas. J Cell Biochem. 2006;99:671–678. doi: 10.1002/jcb.20982. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 90.Gramantieri L, Ferracin M, Fornari F, Veronese A, Sabbioni S, Liu CG, Calin GA, Giovannini C, Ferrazzi E, Grazi GL, Croce CM, Bolondi L, Negrini M. Cyclin G1 is a target of miR-122a, a microRNA frequently down-regulated in human hepatocellular carcinoma. Cancer Res. 2007;67:6092–6099. doi: 10.1158/0008-5472.CAN-06-4607. [DOI] [PubMed] [Google Scholar]
- 91.Huang Q, Gumireddy K, Schrier M, le Sage C, Nagel R, Nair S, Egan DA, Li A, Huang G, Klein-Szanto AJ, Gimotty PA, Katsaros D, Coukos G, Zhang L, Pure E, Agami R. The microRNAs miR-373 and miR-520c promote tumour invasion and metastasis. Nat Cell Biol. 2008;10:202–210. doi: 10.1038/ncb1681. [DOI] [PubMed] [Google Scholar]
- 92.Voorhoeve PM, le Sage C, Schrier M, Gillis AJ, Stoop H, Nagel R, Liu YP, van Duijse J, Drost J, Griekspoor A, Zlotorynski E, Yabuta N, De Vita G, Nojima H, Looijenga LH, Agami R. A genetic screen implicates miRNA-372 and miRNA-373 as oncogenes in testicular germ cell tumors. Adv Exp Med Biol. 2007;604:17–46. doi: 10.1007/978-0-387-69116-9_2. [DOI] [PubMed] [Google Scholar]
- 93.Zender L, Hutker S, Liedtke C, Tillmann HL, Zender S, Mundt B, Waltemathe M, Gosling T, Flemming P, Malek NP, Trautwein C, Manns MP, Kuhnel F, Kubicka S. Caspase 8 small interfering RNA prevents acute liver failure in mice. Proc Natl Acad Sci U S A. 2003;100:7797–7802. doi: 10.1073/pnas.1330920100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Si ML, Zhu S, Wu H, Lu Z, Wu F, Mo YY. miR-21-mediated tumor growth. Oncogene. 2007;26:2799–2803. doi: 10.1038/sj.onc.1210083. [DOI] [PubMed] [Google Scholar]
- 95.Jiang J, Lee EJ, Gusev Y, Schmittgen TD. Realtime expression profiling of microRNA precursors in human cancer cell lines. Nucleic Acids Res. 2005;33:5394–5403. doi: 10.1093/nar/gki863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Iorio MV, Ferracin M, Liu CG, Veronese A, Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M, Menard S, Palazzo JP, Rosenberg A, Musiani P, Volinia S, Nenci I, Calin GA, Querzoli P, Negrini M, Croce CM. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65:7065–7070. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 97.Zhu S, Si ML, Wu H, Mo YY. MicroRNA-21 targets the tumor suppressor gene tropomyosin 1 (TPM1) J Biol Chem. 2007;282:14328–14336. doi: 10.1074/jbc.M611393200. [DOI] [PubMed] [Google Scholar]
- 98.Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449:682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 99.Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong C, Huang Y, Hu X, Su F, Lieberman J, Song E. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131:1109–1123. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 100.Tavazoie SF, Alarcon C, Oskarsson T, Padua D, Wang Q, Bos PD, Gerald WL, Massague J. Endogenous human microRNAs that suppress breast cancer metastasis. Nature. 2008;451:147–152. doi: 10.1038/nature06487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chowdhury TT, Akanji OO, Salter DM, Bader DL, Lee DA. Dynamic compression influences interleukin-1beta-induced nitric oxide and prostaglandin E2 release by articular chondrocytes via alterations in iNOS and COX-2 expression. Biorheology. 2008;45:257–274. [PubMed] [Google Scholar]
- 102.Karube Y, Tanaka H, Osada H, Tomida S, Tatematsu Y, Yanagisawa K, Yatabe Y, Takamizawa J, Miyoshi S, Mitsudomi T, Takahashi T. Reduced expression of Dicer associated with poor prognosis in lung cancer patients. Cancer Sci. 2005;96:111–115. doi: 10.1111/j.1349-7006.2005.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kumar MS, Lu J, Mercer KL, Golub TR, Jacks T. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat Genet. 2007;39:673–677. doi: 10.1038/ng2003. [DOI] [PubMed] [Google Scholar]
- 104.Suarez Y, Sessa WC. MicroRNAs as novel regulators of angiogenesis. Circ Res. 2009;104:442–454. doi: 10.1161/CIRCRESAHA.108.191270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bonauer A, Carmona G, Iwasaki M, Mione M, Koyanagi M, Fischer A, Burchfield J, Fox H, Doebele C, Ohtani K, Chavakis E, Potente M, Tjwa M, Urbich C, Zeiher AM, Dimmeler S. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science. 2009;324:1710–1713. doi: 10.1126/science.1174381. [DOI] [PubMed] [Google Scholar]
- 106.Chen Y, Gorski DH. Regulation of angiogenesis through a microRNA (miR-130a) that down-regulates antiangiogenic homeobox genes GAX and HOXA5. Blood. 2008;111:1217–1226. doi: 10.1182/blood-2007-07-104133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.WangS, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, Richardson JA, Bassel-Duby R, Olson EN. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15:261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Suarez Y, Fernandez-Hernando C, Pober JS, Sessa WC. Dicer Dependent MicroRNAs Regulate Gene Expression and Functions in Human Endothelial Cells. Circ Res. 2007 doi: 10.1161/01.RES.0000265065.26744.17. [DOI] [PubMed] [Google Scholar]
- 109.Poliseno L, Tuccoli A, Mariani L, Evangelista M, Citti L, Woods K, Mercatanti A, Hammond S, Rainaldi G. MicroRNAs modulate the angiogenic properties of HUVECs. Blood. 2006;108:3068–3071. doi: 10.1182/blood-2006-01-012369. [DOI] [PubMed] [Google Scholar]
- 110.Kuehbacher A, Urbich C, Zeiher AM, Dimmeler S. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ Res. 2007;101:59–68. doi: 10.1161/CIRCRESAHA.107.153916. [DOI] [PubMed] [Google Scholar]
- 111.Nicoli S, Standley C, Walker P, Hurlstone A, Fogarty KE, Lawson ND. MicroRNA-mediated integration of haemodynamics and Vegf signalling during angiogenesis. Nature. 464:1196–1200. doi: 10.1038/nature08889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Daubman S. MicroRNAs in angiogenesis and vascular smooth muscle cell function. Circ Res. 106:423–425. doi: 10.1161/RES.0b013e3181d61a0d. [DOI] [PubMed] [Google Scholar]
- 113.O'Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc-regulated microRNAs modulate E2F1 expression. Nature. 2005;435:839–843. doi: 10.1038/nature03677. [DOI] [PubMed] [Google Scholar]
- 114.Dews M, Homayouni A, Yu D, Murphy D, Sevignani C, Wentzel E, Furth EE, Lee WM, Enders GH, Mendell JT, Thomas-Tikhonenko A. Augmentation of tumor angiogenesis by a Myc-activated microRNA cluster. Nat Genet. 2006;38:1060–1065. doi: 10.1038/ng1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fang L, Deng Z, Shatseva T, Yang J, Peng C, Du WW, Yee AJ, Ang LC, He C, Shan SW, Yang BB. MicroRNA miR-93 promotes tumor growth and angiogenesis by targeting integrin-beta8. Oncogene. 30:806–821. doi: 10.1038/onc.2010.465. [DOI] [PubMed] [Google Scholar]
- 116.Lee DY, Deng Z, Wang CH, Yang BB. MicroRNA-378 promotes cell survival, tumor growth, and angiogenesis by targeting SuFu and Fus-1 expression. Proc Natl Acad Sci U S A. 2007;104:20350–20355. doi: 10.1073/pnas.0706901104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kahai S, Lee SC, Lee DY, Yang J, Li M, Wang CH, Jiang Z, Zhang Y, Peng C, Yang BB. MicroRNA miR-378 regulates nephronectin expression modulating osteoblast differentiation by targeting GalNT-7. PLoS One. 2009;4:e7535. doi: 10.1371/journal.pone.0007535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hua Z, Lv Q, Ye W, Wong CK, Cai G, Gu D, Ji Y, Zhao C, Wang J, Yang BB, Zhang Y. MiRNA-directed regulation of VEGF and other angiogenic factors under hypoxia. PLoS One. 2006;1:e116. doi: 10.1371/journal.pone.0000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jeyapalan Z, Deng Z, Shatseva T, Fang L, He C, Yang BB. Expression of CD44 3'-untranslated region regulates endogenous microRNA functions in tumorigenesis and angiogenesis. Nucleic Acids Res. 39:3026–3041. doi: 10.1093/nar/gkq1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lee DY, Jeyapalan Z, Fang L, Yang J, Zhang Y, Yee AY, Li M, Du WW, Shatseva T, Yang BB. Expression of versican 3'-untranslated region modulates endogenous microRNA functions. PLoS One. 5:e13599. doi: 10.1371/journal.pone.0013599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lee DY, Shatseva T, Jeyapalan Z, Du WW, Deng Z, Yang BB. A 3'-untranslated region (3'UTR) induces organ adhesion by regulating miR-199a* functions. PLoS One. 2009;4:e4527. doi: 10.1371/journal.pone.0004527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang Y, Cao L, Kiani C, Yang BL, Hu W, Yang BB. Promotion of chondrocyte proliferation by versican mediated by G1 domain and EGF-like motifs. J Cell Biochem. 1999;73:445–457. [PubMed] [Google Scholar]
- 123.Zhang Y, Cao L, Yang BL, Yang BB. The G3 domain of versican enhances cell proliferation via epidermial growth factor-like motifs. J Biol Chem. 1998;273:21342–21351. doi: 10.1074/jbc.273.33.21342. [DOI] [PubMed] [Google Scholar]
- 124.Zhang Y, Wu Y, Cao L, Lee V, Chen L, Lin Z, Kiani C, Adams ME, Yang BB. Versican modulates embryonic chondrocyte morphology via the epidermal growth factor-like motifs in G3. Exp Cell Res. 2001;263:33–42. doi: 10.1006/excr.2000.5095. [DOI] [PubMed] [Google Scholar]
- 125.Zheng PS, Reis M, Sparling C, Lee DY, La Pierre DP, Wong CK, Deng Z, Kahai S, Wen J, Yang BB. Versican G3 domain promotes blood coagulation through suppressing the activity of tissue factor pathway inhibitor-1. J Biol Chem. 2006;281:8175–8182. doi: 10.1074/jbc.M509182200. [DOI] [PubMed] [Google Scholar]
- 126.Sheng W, Wang G, Wang Y, Liang J, Wen J, Zheng PS, Wu Y, Lee V, Slingerland J, Dumont D, Yang BB. The roles of versican V1 and V2 isoforms in cell proliferation and apoptosis. Mol Biol Cell. 2005;16:1330–1340. doi: 10.1091/mbc.E04-04-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Du WW, Yang BB, Shatseva TA, Yang BL, Deng Z, Shan SW, Lee DY, Seth A, Yee AJ. Versican G3 promotes mouse mammary tumor cell growth, migration, and metastasis by influencing EGF receptor signaling. PLoS One. 5:e13828. doi: 10.1371/journal.pone.0013828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zheng PS, Vais D, Lapierre D, Liang YY, Lee V, Yang BL, Yang BB. PG-M/versican binds to P-selectin glycoprotein ligand-1 and mediates leukocyte aggregation. J Cell Sci. 2004;117:5887–5895. doi: 10.1242/jcs.01516. [DOI] [PubMed] [Google Scholar]
- 129.LaPierre DP, Lee DY, Li SZ, Xie YZ, Zhong L, Sheng W, Deng Z, Yang BB. The ability of versican to simultaneously cause apoptotic resistance and sensitivity. Cancer Res. 2007;67:4742–4750. doi: 10.1158/0008-5472.CAN-06-3610. [DOI] [PubMed] [Google Scholar]
- 130.Yee AJ, Akens M, Yang BL, Finkelstein J, Zheng PS, Deng Z, Yang B. The effect of versican G3 domain on local breast cancer invasiveness and bony metastasis. Breast Cancer Res. 2007;9:R47. doi: 10.1186/bcr1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wu YJ, La Pierre DP, Wu J, Yee AJ, Yang BB. The interaction of versican with its binding partners. Cell Res. 2005;15:483–494. doi: 10.1038/sj.cr.7290318. [DOI] [PubMed] [Google Scholar]
- 132.Zheng PS, Wen J, Ang LC, Sheng W, Viloria-Petit A, Wang Y, Wu Y, Kerbel RS, Yang BB. Versican/ PG-M G3 domain promotes tumor growth and angiogenesis. Faseb J. 2004;18:754–756. doi: 10.1096/fj.03-0545fje. [DOI] [PubMed] [Google Scholar]
- 133.Ota A, Tagawa H, Karnan S, Tsuzuki S, Karpas A, Kira S, Yoshida Y, Seto M. Identification and characterization of a novel gene, C13orf25, as a target for 13q31-q32 amplification in malignant lymphoma. Cancer Res. 2004;64:3087–3095. doi: 10.1158/0008-5472.can-03-3773. [DOI] [PubMed] [Google Scholar]
- 134.Yan HL, Xue G, Mei Q, Wang YZ, Ding FX, Liu MF, Lu MH, Tang Y, Yu HY, Sun SH. Repression of the miR-17-92 cluster by p53 has an important function in hypoxia-induced apoptosis. Embo J. 2009;28:2719–2732. doi: 10.1038/emboj.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Venturini L, Battmer K, Castoldi M, Schultheis B, Hochhaus A, Muckenthaler MU, Ganser A, Eder M, Scherr M. Expression of the miR-17-92 polycistron in chronic myeloid leukemia (CML) CD34+ cells. Blood. 2007;109:4399–4405. doi: 10.1182/blood-2006-09-045104. [DOI] [PubMed] [Google Scholar]
- 136.Wang Q, Li YC, Wang J, Kong J, Qi Y, Quigg RJ, Li X. miR-17-92 cluster accelerates adipocyte differentiation by negatively regulating tumor-suppressor Rb2/p130. Proc Natl Acad Sci U S A. 2008;105:2889–2894. doi: 10.1073/pnas.0800178105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Olive V, Bennett MJ, Walker JC, Ma C, Jiang I, Cordon-Cardo C, Li QJ, Lowe SW, Hannon GJ, He L. miR-19 is a key oncogenic component of mir-17-92. Genes Dev. 2009;23:2839–2849. doi: 10.1101/gad.1861409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ebi H, Sato T, Sugito N, Hosono Y, Yatabe Y, Matsuyama Y, Yamaguchi T, Osada H, Suzuki M, Takahashi T. Counterbalance between RB inactivation and miR-17-92 overexpression in reactive oxygen species and DNA damage induction in lung cancers. Oncogene. 2009 doi: 10.1038/onc.2009.201. [DOI] [PubMed] [Google Scholar]
- 139.Hayashita Y, Osada H, Tatematsu Y, Yamada H, Yanagisawa K, Tomida S, Yatabe Y, Kawahara K, Sekido Y, Takahashi T. A polycistronic microRNA cluster, miR-17-92, is overexpressed in human lung cancers and enhances cell proliferation. Cancer Res. 2005;65:9628–9632. doi: 10.1158/0008-5472.CAN-05-2352. [DOI] [PubMed] [Google Scholar]
- 140.Landais S, Landry S, Legault P, Rassart E. Oncogenic potential of the miR-106-363 cluster and its implication in human T-cell leukemia. Cancer Res. 2007;67:5699–5707. doi: 10.1158/0008-5472.CAN-06-4478. [DOI] [PubMed] [Google Scholar]
- 141.Petrocca F, Visone R, Onelli MR, Shah MH, Nicoloso MS, de Martino I, Iliopoulos D, Pilozzi E, Liu CG, Negrini M, Cavazzini L, Volinia S, Alder H, Ruco LP, Baldassarre G, Croce CM, Vecchione A. E2F1-regulated microRNAs impair TGFbeta-dependent cell-cycle arrest and apoptosis in gastric cancer. Cancer Cell. 2008;13:272–286. doi: 10.1016/j.ccr.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 142.Koralov SB, Muljo SA, Galler GR, Krek A, Chakraborty T, Kanellopoulou C, Jensen K, Cobb BS, Merkenschlager M, Rajewsky N, Rajewsky K. Dicer ablation affects antibody diversity and cell survival in the B lymphocyte lineage. Cell. 2008;132:860–874. doi: 10.1016/j.cell.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 143.1Sylvestre Y, De Guire V, Querido E, Mukhopadhyay UK, Bourdeau V, Major F, Ferbeyre G, Chartrand P. An E2F/miR-20a autoregulatory feedback loop. J Biol Chem. 2007;282:2135–2143. doi: 10.1074/jbc.M608939200. [DOI] [PubMed] [Google Scholar]
- 144.Mendell JT. miRiad roles for the miR-17-92 cluster in development and disease. Cell. 2008;133:217–222. doi: 10.1016/j.cell.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang L, Huang J, Yang N, Greshock J, Megraw MS, Giannakakis A, Liang S, Naylor TL, Barchetti A, Ward MR, Yao G, Medina A, O'Brien-Jenkins A, Katsaros D, Hatzigeorgiou A, Gimotty PA, Weber BL, Coukos G. microRNAs exhibit high frequency genomic alterations in human cancer. Proc Natl Acad Sci U S A. 2006;103:9136–9141. doi: 10.1073/pnas.0508889103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ivanovska I, Ball AS, Diaz RL, Magnus JF, Kibukawa M, Schelter JM, Kobayashi SV, Lim L, Burchard J, Jackson AL, Linsley PS, Cleary MA. MicroRNAs in the miR-106b family regulate p21/CDKN1A and promote cell cycle progression. Mol Cell Biol. 2008;28:2167–2174. doi: 10.1128/MCB.01977-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lu Y, Thomson JM, Wong HY, Hammond SM, Hogan BL. Transgenic over-expression of the microRNA miR-17-92 cluster promotes proliferation and inhibits differentiation of lung epithelial progenitor cells. Dev Biol. 2007;310:442–453. doi: 10.1016/j.ydbio.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Matsubara H, Takeuchi T, Nishikawa E, Yanagisawa K, Hayashita Y, Ebi H, Yamada H, Suzuki M, Nagino M, Nimura Y, Osada H, Takahashi T. Apoptosis induction by antisense oligonucleotides against miR-17-5p and miR-20a in lung cancers overexpressing miR-17-92. Oncogene. 2007;26:6099–6105. doi: 10.1038/sj.onc.1210425. [DOI] [PubMed] [Google Scholar]
- 149.Hossain A, Kuo MT, Saunders GF. Mir-17-5p regulates breast cancer cell proliferation by inhibiting translation of AIB1 mRNA. Mol Cell Biol. 2006;26:8191–8201. doi: 10.1128/MCB.00242-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ventura A, Young AG, Winslow MM, Lintault L, Meissner A, Erkeland SJ, Newman J, Bronson RT, Crowley D, Stone JR, Jaenisch R, Sharp PA, Jacks T. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell. 2008;132:875–886. doi: 10.1016/j.cell.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Xiao C, Srinivasan L, Calado DP, Patterson HC, Zhang B, Wang J, Henderson JM, Kutok JL, Rajewsky K. Lymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytes. Nat Immunol. 2008;9:405–414. doi: 10.1038/ni1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fontana L, Pelosi E, Greco P, Racanicchi S, Testa U, Liuzzi F, Croce CM, Brunetti E, Grignani F, Peschle C. MicroRNAs 17-5p-20a-106a control monocytopoiesis through AML1 targeting and M-CSF receptor upregulation. Nat Cell Biol. 2007;9:775–787. doi: 10.1038/ncb1613. [DOI] [PubMed] [Google Scholar]
- 153.Brown AG, Ross FM, Dunne EM, Steel CM, Weir-Thompson EM. Evidence for a new tumour suppressor locus (DBM) in human B-cell neoplasia telomeric to the retinoblastoma gene. Nat Genet. 1993;3:67–72. doi: 10.1038/ng0193-67. [DOI] [PubMed] [Google Scholar]
- 154.Kalachikov S, Migliazza A, Cayanis E, Fracchiolla NS, Bonaldo MF, Lawton L, Jelenc P, Ye X, Qu X, Chien M, Hauptschein R, Gaidano G, Vitolo U, Saglio G, Resegotti L, Brodjansky V, Yankovsky N, Zhang P, Soares MB, Russo J, Edelman IS, Efstratiadis A, Dalla-Favera R, Fischer SG. Cloning and gene mapping of the chromosome 13q14 region deleted in chronic lymphocytic leukemia. Genomics. 1997;42:369–377. doi: 10.1006/geno.1997.4747. [DOI] [PubMed] [Google Scholar]
- 155.Bullrich F, Fujii H, Calin G, Mabuchi H, Negrini M, Pekarsky Y, Rassenti L, Alder H, Reed JC, Keating MJ, Kipps TJ, Croce CM. Characterization of the 13q14 tumor suppressor locus in CLL: identification of ALT1, an alternative splice variant of the LEU2 gene. Cancer Res. 2001;61:6640–6648. [PubMed] [Google Scholar]
- 156.Rosenfeld N, Aharonov R, Meiri E, Rosenwald S, Spector Y, Zepeniuk M, Benjamin H, Shabes N, Tabak S, Levy A, Lebanony D, Goren Y, Silberschein E, Targan N, Ben-Ari A, Gilad S, Sion-Vardy N, Tobar A, Feinmesser M, Kharenko O, Nativ O, Nass D, Perelman M, Yosepovich A, Shalmon B, Polak-Charcon S, Fridman E, Avniel A, Bentwich I, Bentwich Z, Cohen D, Chajut A, Barshack I. MicroRNAs accurately identify cancer tissue origin. Nat Biotechnol. 2008;26:462–469. doi: 10.1038/nbt1392. [DOI] [PubMed] [Google Scholar]
- 157.Pasquinelli AE, Reinhart BJ, Slack F, Martindale MQ, Kuroda MI, Maller B, Hayward DC, Ball EE, Degnan B, Muller P, Spring J, Srinivasan A, Fishman M, Finnerty J, Corbo J, Levine M, Leahy P, Davidson E, Ruvkun G. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature. 2000;408:86–89. doi: 10.1038/35040556. [DOI] [PubMed] [Google Scholar]
- 158.Lee YS, Dutta A. The tumor suppressor microRNA let-7 represses the HMGA2 oncogene. Genes Dev. 2007;21:1025–1030. doi: 10.1101/gad.1540407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sampson VB, Rong NH, Han J, Yang Q, Aris V, Soteropoulos P, Petrelli NJ, Dunn SP, Krueger LJ. MicroRNA let-7a down-regulates MYC and reverts MYC-induced growth in Burkitt lymphoma cells. Cancer Res. 2007;67:9762–9770. doi: 10.1158/0008-5472.CAN-07-2462. [DOI] [PubMed] [Google Scholar]
- 160.Malumbres M, Barbacid M. RAS oncogenes: the first 30 years. Nat Rev Cancer. 2003;3:459–465. doi: 10.1038/nrc1097. [DOI] [PubMed] [Google Scholar]
- 161.Takamizawa J, Konishi H, Yanagisawa K, Tomida S, Osada H, Endoh H, Harano T, Yatabe Y, Nagino M, Nimura Y, Mitsudomi T, Takahashi T. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res. 2004;64:3753–3756. doi: 10.1158/0008-5472.CAN-04-0637. [DOI] [PubMed] [Google Scholar]
- 162.Yang Z, Mu Z, Dabovic B, Jurukovski V, Yu D, Sung J, Xiong X, Munger JS. Absence of integrin-mediated TGFbeta1 activation in vivo recapitulates the phenotype of TGFbeta1-null mice. J Cell Biol. 2007;176:787–793. doi: 10.1083/jcb.200611044. [DOI] [PMC free article] [PubMed] [Google Scholar]