Abstract
Cancer is a leading cause of death in the world. Conventional treatments have severe side effects and low survival rate. It is important to discover new targets and therapeutic strategies to improve the clinical outcomes of cancer patients. Ion channels are specialized membrane proteins that play important roles in various physiological processes. Recent studies have shown that abnormal expression and/or activity of a number of ion channels e.g. voltage-gated K+, Na+, Ca2+ channels, TRP channels, and epithelial Na+/degenerin family of ion channels, are involved in the growth/proliferation, migration and/or invasion of cancer cells. In this review, we summarize the present knowledge about the roles of different ion channels in the development of cancer.
Keywords: Ion channels, cancer, therapeutic targets, voltage-gated K+, Na+, Ca2+ channels, TRP channels, epithelial Na+/degenerin family of ion channels
Introduction
Cancer, one of the leading causes of mortality in the world, is a devastating disease involving many steps of complex signaling pathways. Conventional treatments for cancer include surgery, chemotherapy and radiation. In addition to severe side effects, current available treatments have low survival rate and limited clinical outcomes for many cancers. It is essential to discover new targets and therapeutic strategies to increase the survival rate and improve the clinical outcomes of cancer patients. Collective studies have shown that ion channels, the specialized membrane proteins that conduct ion fluxes, are involved in the development of many diseases including cancers [1]. The roles of various ion channels in the growth/proliferation, migration and/or invasion of cancer cells, for example, have been well documented [2-8].
Ion channels can be divided into two major categories based on their gating mechanism: (1) Voltage-gated channels, which are opened by membrane potential changes. These channels conduct specific ions when they are activated. (2) Ligand-gated channels, which are opened by ligands, conduct cations or anions without high selectivity. Both voltage-gated and ligand-gated ion channels play important roles in physiological conditions such as electrical signaling, gene expression, hormone secretion, learning and memory [3,4]. In pathological conditions, the property or activity of ion channels may change. The abnormal property/activity of certain ion channels is able to promote the growth and proliferation of tumor cells [7].
In this review, we summarize the present knowledge about the roles of different ion channels in the growth/proliferation, migration, and invasion of tumor cells.
Voltage-gate ion channels (VGICs)
VGICs are widely expressed in excitable and non -excitable cells. In addition to be involved in a variety of physiological processes, VGICs are known to be involved in the development of a number of diseases, such as cardiac arrhythmias, epilepsy, hyperkalemia, and some hereditary diseases, etc. [3]. In addition, recent studies have shown that VGICs are involved in the onset, proliferation, migration and survival of various types of cancers [3].
According to the ion selectivity, VGICs are divided into three major types: voltage-gated potassium, voltage-gated sodium and voltage-gated calcium channels. All these sub-types share similar structural topology. The essential structure feature in VGICs is a tetramic association of a series of six transmembrane α-helical segments, numbered S1-S6, connected by both intracellular and extracellular loops, the inter-linkers of α-helical segments [1]. In addition to essential α-subunit, voltage-gated channels also have multifunctional accessory proteins called β -subunits, which modulate gating properties and assist in associations between different ion channels [3].
Voltage-gated potassium channels (VGKCs or Kv)
VGKCs are a large heterogeneous family of channels distributed widely throughout excitable cells such as neurons and cardiac myocytes, in which they control the membrane potential, and in non-excitable cells, in which they are involved in a number of physiological processes including volume regulation, apoptosis, immunomodulation and differentiation. In addition to physiological processes, Kv channels have been shown play a pivotal role in the proliferation, progression, and apoptosis of various cancer cells and have been considered as new targets for designing cancer treatment strategies [9].
Kv channels are divided into different subfamilies (Kv 1.1-1.8) based on their difference in the α-subunit [1], the subunit that contains essential voltage-sensor and pore-forming region. Accessory proteins β-subunits also exist in potassium channels. Their functions are thought to be independent of K+ channel conductance, but are involved in protein recruitment/ scaffolding and cell adhesion with extracellular matrix proteins. One of well-studied Kv channels associated with cancer development is human ether-a-go-go potassium channel (EAG1, KCNHI, Kv10.1). Lines of studies have shown that EAG1 is associated with the development of tumors both in patients and in animals [5,6,10-21]. Human EAG1 mRNA is almost exclusively expressed in normal brain. However, its ectopic expression has been linked to oncogenesis and tumor progression. The overexpression of EAG1 channels leads to uncontrolled cell proliferation. Over 75 % of tumors, including cell lines and primary tumors, for example, have been found to have overexpression of EAG1 proteins [16,18]. Farias and coworkers were the first to study the EAG channel activity in human tumors. They demonstrated the expression of EAG1 mRNA in several somatic cancer cell lines, and EAG1 protein in primary cultured cervical cancer cells and in cervical cancer biopsies [14]. Knockdown of EAG1 expression with siRNA and inhibition of EAG mediated currents decreased cell proliferation in cancer cell lines [14]. Studies by Mello and coworkers have shown that the expression level of EAG1 depends on the histological type, but no association was seen between expression of this protein and grade or tumor size [18]. Based on the high frequency of expression of EAG1 in primary tumors and the restriction of normal expression of the channel to the brain, EAG1 appears to be a promising cancer target either for diagnostic or therapeutic purposes [5,18]. Since normal cells expressing EAG1 are either protected by the blood-brain barrier or represent the terminal stage of differentiation, EAG1 based therapies are expected to produce only minor side effects [17].
The mechanism(s) of Kv mediated tumor progression and invasion remain unclear. Studies by Downie group suggest that EAG1 interferes with the cellular processes for maintaining oxygen homeostasis, increasing HIF-1 activity, and thereby VEGF secretion and tumor vascularization [19]. In MCF-7 breast cancer cell line, Kv are involved in both proliferation control and cell cycle progression according to the “membrane potential” model [22-24]. It has also been shown that Kv, like BK channels, are involved in glioma invasion and the formation of brain metastasis [25]. It is believed that activation of these channels, in concert with Cl-currents, affects net salt fluxes. Water is obliged to accompany these net salt fluxes, leading to cell shrinkage/flattening, conditions permissive to movement through the extracellular space of tissues.
In addition to EAG1 channels, other Kv channels have been found in tumor cells (Table 1). For example, the expression of Kv 2.1-Kv 9.3 channels has been found in uterine cancer cells and that these channels are involved in the proliferation of uterine cancer cells [26]. Similarly, the expression of Kv 1.3 potassium channels and large conductance Ca2+ and voltage-activated K+ channels has been reported in breast cancer cells [27-29].
Table 1.
Involvement of VGKCs in the pathology of cancer/tumor cells
| Major Kv channels | Cancer type (cell line) | Reference(s) |
|---|---|---|
| Kv 1.1 | MCF -7 human breast cancer cell line | [22] |
| Kv 2.1-2.9 | Uterine cancer cells | [26] |
| Kv 1.3 | Breast cancer cells | [27,28] |
| Kv 4.1 | Human breast epithelial cells | [30] |
| KATP channel | glioblastoma multiforme; allogeneic brain tumor model; uterine leiomyoma. | [32,33] |
| G-protein coupled inwardly rectifying potassium channel 1(GIRK1) | Breast carcinoma | [22,35] |
| HERG K+ channels | Leukemia cell lines K562, HL-60, and almost all the primary leukemia cells; gastric cancer cells | [36,37] |
| BKCa | Osteosarcoma; human glioma cells; human ovarian cancer cells; breast cancer (KCNMA1); MCF-7 human breast cancer cell line; LNCaP prostate cancer cells | [23, 25, 28, 31, 32, 34, 38, 39] |
| KCa3.1 | Leukocytes, mitogen-induced endothelial cells, vascular smooth muscle cells; human colon cancer cells | [40, 41] |
| TREK-1 (two-pore domain (K (2P)) potassium channel) | Prostate cancer | [42] |
| TASK3 (TWIK-related acidsensitive K+ channel, KCNK9) | Human glioma cell lines; glioma specimens; breast tumors | [22, 43] |
| Kir 4.1 | Human astrocytic tumors | [44, 45] |
Voltage-gated sodium channels (VGSCs or Nav)
VGSCs, gated by membrane depolarization, are primarily expressed in excitable cells, such as neurons and cardiac myocytes, in which they are responsible for the initiation of membrane depolarization by allowing rapid influx of Na+. The well known function of VGSCs is the generation of action potential in excitable tissues [1]. Mammalian VGSCs are composed of one single pore forming α-subunit associated with one or two auxiliary β- subunit. While α-subunits are responsible for current amplitude and density, the β- subunit modifies the kinetics and voltage-dependence of the channel gating [46]. So far, at least 9 functional members of VGSCα gene family have been studied in expression systems. They are designated Nav 1.1 - Nav 1.9 according to their phylogeny [47,48]. The distribution of Nav subunits are tissue specific. For example, Nav 1.4, Nav 1.5, Nax, and SCN1 β-subunit are expressed predominantly in the heart. Nav 1.1, Nav 1.2, Nav 1.3, Nav 1.8, SCN1β, and SCN3β-subunits are found predominantly in the brain [3]. According to the sensitivity to TTX, VGSCs are divided into TTX- sensitive (e.g. Nav 1.1-Nav 1.4, Nav 1.6 and Nav 1.7), which are blocked by nM TTX, and TTX- resistant (e.g. NaV1.5, NaV1.8 and NaV1.9), which are blocked by μM TTX [49]. In addition to producing action potentials in excitable cells, these channels also play important role in proliferation, migration, and adhesion of non-excitable cells including glia, fibroblasts, endothelial cells and T-lymphocytes [49-51]. It has been reported that VGSCs are associated with several channe-lopathies such as paralysis, epilepsy, multifocal motor neuropathy, hyperkalemia, long-QT syndrome and idiopathic ventricular fibrillation [1]. In addition, several lines of studies have demonstrated the role of sodium channels in several strongly metastatic carcinomas in controlling various steps of the metastatic cascade [4,52]. For example, high expression levels of VGSCs have been found in several aggressive carcinomas including prostate cancer [53-56], breast cancer [52,57,58], lymphoma [59], small cell lung cancer [60], non-small cell lung cancer [61], mesothelioma [62], neuroblastoma [63,64], melanoma [65], and cervical cancer [66]. Therefore, VGSCs may represent promising new targets for developing novel therapeutics for metastatic cancers.
The mechanism(s) of VGSCs in metastatic carcinomas is still under investigation. One possible explanation is that Na+ flows into the cell by sodium channel activation. Increased intracellular Na+ may affect downstream pathways, such as intracellular Ca2+ and protein kinases which implicated in cellular mobility [4].
Voltage-gated calcium channels (VGCCs or Cav)
VGCCs, expressed widely in excitable- and non-excitable tissues, are composed of five sub-units: α1, α2, β, δ, and γ. The α1 subunit contains voltage-sensor and channel pore, while α2, β, δ, and γ subunits are regulatory in channel function. VGCCs are classified into six subtypes based on the channel characteristics and their sensitivity to certain drugs: L, N, P, Q, R, and T types. L-type channel are characterized by long lasting activation, slow inactivation and dihydropyridine sensitivity. Although expressed in a variety of excitable and non-excitable tissues, the L-type channels are found predominantly in the brain, and in skeletal and cardiac muscle, where they play a role in coupling the afferent excitation to muscle contraction. N-type channels are slow inactivation channels, which are located in the presynaptic nerve terminals, controlling neurotransmitter release. P-type channels, also slow in inactivation, are found in the presynaptic terminals of cerebellar neurons, and in neuromuscular junctions. These channels can also control the release of neurotransmitters. Q-type channels play a similar role in the control of secretogogue release from cerebellar granule cells and pyramidal neurons. R-type channels, exist in cerebellar granule cells and in the dendrites of pyramidal neurons, are characterized by their fast gating properties. T-type channels are activated by small membrane depolarizations and have rapid inactivation. These channels are widely expressed in the brain, kidney, liver, bone, and heart. In addition to severe long QT syndromes, VGCCs are involved in other channelopathies including paralysis, Lambert-EatonMyasthenic Syndrome, migraines, and epilepsy. VGCCs are also found in some cancer cells and play an important role in cancer progression. For example, T- type Cav was found in human prostate cancer and up-regulated during neuroendocrine differentiation [67]. The L-type calcium channel subunit, Cav 1.2, was found in colon cancer cells [68]. Cav 1.2 expression increases with the differentiation of colon cells to cancer cells. P- and L- type Cav was found in small lung carcinomas [69]. Calcium spikes, which are caused by unspecified T-type calcium channels and play a role in membrane depolarization [70], have been shown to alter the motility of fibrosarcoma cells [71].
In summary, voltage-gated ion channels (VGICs) play an important role in the progression of cancer cells although the detailed cellular processes are still uncertain. In addition to affecting cell proliferation and migration, voltage-gated ion channels also play major roles in cell specific differentiation program for neuronal and non-neuronal cell types. Additionally, in some types of cancers, the peptide hormones and other growth factors in the control of VGICs are also very important. For example, Nav in prostate cancer cells are regulated by steroid hormone [3]. On the other hand, VGICs may act dependently in the physiological conditions. The depolarization of membrane potential that opens the VGSCs can in turn result in the opening of VGCCs. Ca2+ entry into the cells from VGCCs and increased intracellular Ca2+ may have multiple functions within the cell. In addition, the membrane depolarization will also activate VGKCs, which could then repolarize the membrane, effectively blocking activation of VGSCs or VGCCs. Normal cell growth and differentiation need the synchronization of VGICs. If this synchronization of VGICs is disturbed, cancer cell may be produced. A better understanding of these channels, their distribution and downstream effectors may help develop new therapeutic strategies through modulations of the channel activity in a selective manner.
Transient receptor potential (TRP) channels
Transient receptor potential (TRP), six-transmembrane cation-permeable channels, are widely expressed in mammalian tissues. They regulate intracellular Ca2+, Na+ concentrations and membrane voltage in both excitable and non-excitable cells. These channels, as signal integrators, can be activated by multiple distinct stimuli. When TRP channels open, Ca2+ and Na+ions flow into the cell, which couple their activity to downstream cellular signal amplification via calcium permeation and membrane depolarization [72]. Based on the amino acid sequence homology, mammalian TRP channels can be divided into 7 subfamilies: TRPA, TRPC, TRPM, TRPML, TRPN, TRPP, and TRPV [73,74]. Because Ca2+ is an essential regulator for cell cycle and proliferation [75], TRP channels play important roles in many physiological processes. In addition, they have been shown to be involved in a variety of pathological processes including tumor formation and metastasis.
TRPM subfamily
TRPM subfamily, also called melastatin-like transient receptor potential channels, comprised of eight members: TRPM1/3, TRPM4/5, TRPM6/7, TRPM2 and TRPM8 [72,76]. TRPM subfamily has important effects on various physiological and pathological processes through mediating cations entry and membrane depolarization.
TRPM1: TRPM1 is highly expressed in early stage melanomas but its expression declines with increases in the degree of aggressiveness of the melanoma [77,78]. Consistent with these results, several studies have suggested that TRPM1 may act as tumor suppressor [79-81]. Therefore, TRPM1 is identified as a prognostic marker for metastasis of localized melanoma [77]. Although its channel activity has not been described definitively, it has been proposed that TRPM1 may activate a Ca2+ permeable channel [82]. However, it is still not clear how TRPM1 reduces the growth rate of melanoma cells.
TRPM 7: Recent studies by our laboratory have demonstrated the expression of TRPM7 channels in FaDu and SCC25 cells, two human head and neck tumor cell lines [83]. In both cells, lowering extracellular Ca2+ induced a large non-desensitizing current with characteristics of TRPM7 channel currents inhibited by Gd3+, 2-aminoethoxydi-phenyl borate (2-APB), or intracellular Mg2+[84-87]. Consistent with the electrophysiological findings, the expression of TRPM7 proteins and mRNA was confirmed by Western blot, immunocytochemistry and reverse transcription-PCR techniques. Suppressing the activities of these channels inhibited the growth and proliferation of FaDu cells [83]. Similar to human head and neck cancer cells, TRPM7 channels were detected in both primary human glioma cells and human glioma cell lines (e.g. A172 cells)[88]. Suppressing these channels e.g. by channel inhibitors and/or siRNA, also inhibited the growth and proliferation of these cells. Taken together, our results suggest that activation of TRPM7 channels play an important role in the growth and proliferation of tumor cells.
TRPM 8: TRPM 8, a non-selective cation channel activated by cold stimuli (17-25°C) and menthol (cooling compounds) is highly expressed in sensory neurons[89-92]. In addition to sensing cold, they play a broader role in both physiological and pathological conditions including cancer. For example, TRPM 8 was first identified in prostate epithelial cells by Tsavaler group [93]. In normal prostate, the TRPM 8 level is moderate. However, the expression level of TRPM 8 increases dramatically in prostate cancer [94]. Similarly, the expression level of TRPM 8 becomes highly enriched in other primary human tumors such as breast, colon, lung and skin cancers [93]. In addition to Ca2+ entry across the plasma membrane, TRPM 8 also facilitates Ca2+ release from Ca2+ stores. Increased intracellular Ca2+- potentiates sensory synaptic transmission, causing cell proliferation and apoptosis [95-97]. In addition to be activated by cold stimuli, TRPM8 channel can also be activated by membrane potential changes. TRPM8 has been considered to be a promising target for therapeutic interventions in prostate cancer [98].
TRPV subfamily
TRPV (Vanilloid) channels have also been found to regulate cancer cell proliferation, apoptosis, angiogenesis, migration and invasion. TRPV6 gene is the first cloned TRPV subfamily from rat duodenum using an expression cloning strategy in Xenopus laevis oocytes [8]. Although it belongs to TRPV family, it is much less similar to other TRPVs [72]. TRPV6 channels show high Ca2+ selectivity and exhibit strong inward rectification in human embryonic kidney and rat basophilic leukemia cells [99-101]. The gating of TRPV6 channel is strongly dependent on the intracellular free Ca2+, and extracellular divalent cations [99,102]. TRPV6 channels can be activated by removing extracellular divalent cations and /or by lowing the intracellular Ca2+ [99,102]. Several studies have reported that TRPV6 mRNA is expressed in prostate adenocarcinoma, and colorectal cancer cell lines [103-105]. Its expression has been considered as a general marker for neoplasms since the expression level of TRPV6 mRNA correlates with the tumor grade and aggressiveness [104-106]. Expression of TRPV6 is also increased in prostate cancer and in a number of other cancers.
The expression of TRPV1, TRPC1, TRPC6, TRPM4, and TRPM5 is also increased in some cancers. Studies in human cell line and patients' specimens have shown that there were correlations between TRP mRNA expression levels and cancer progression. However, the molecular mechanisms underlying the modulation of cell proliferation by these proteins may be different even though all of them are Ca2+ permeable. In addition to intracellular Ca2+, physiological interaction with proteins may also be involved in cell proliferation [107,108]. In summary, TRP channels are novel targets for developing new diagnostics tools and therapeutic strategies for the treatment of various cancers.
Epithelial Na+ channel /degenerin family (ENaC/DEG)
Epithelial sodium channel (ENaC)/Degenerin are widely expressed, from epithelial, endothelia, osteoblasts, keratinocytes, taste cells, to lymphocytes, and brain tissues [109]. Na+ ions flow into the cell through ENaC via facilitated diffusion. The ENaC/DEG family usually has five subunits: α, β, γ, δ and ε, although typical ENaC channels are composed of α, β and γ subunits [110,111]. All subunits share the same structural topology with short intracellular N and C termini, two transmembrane domains and a large extracellular cystein-rich loop [109,112]. In addition to diseases such as salt-sensitive hypertension [113,114], pseudohypoaldosteronism [113], cystic fibrosis, chronic airway disease [115,116] and flu [117], ENaC/DEG family of channels also play an important role in the pathology of cancers. Kapoor and colleagues have reported that the presence of ENaC/DEG in glioblastoma cells. Knockdown of α, β and γ subunits inhibited migration of glioblastoma cells [63,118]. One possible mechanism underlying the involvement of ENaC/DEG in glioblastoma cell migration is that, Na+ flows into the cell via ENaC channel, which causes H2O into the cell resulting from the higher osmolity in the cell interior. This will cause cell swelling, a process required for lamellipodium expansion [119]. The presence ENaC has also been reported in human leukemic cell lines [120,121].
Conclusion
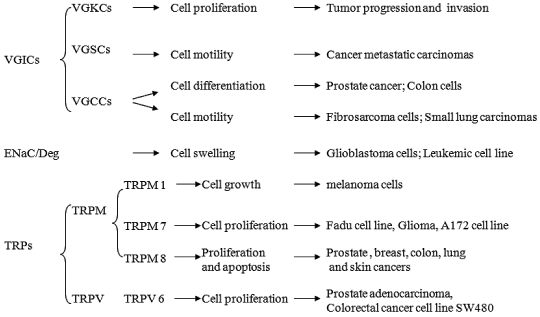
Membrane ion channels are essential for many physiological processes. However, they can also play an important role in the development of cancer (Figure 1). Although the detailed molecular mechanism(s) underlying the involvement of ion channels in proliferation, apoptosis, invasiveness, and metastatic spread of cancer cells are still under investigation, ion channels represent promising targets for developing novel and effective cancer therapies.
Figure 1.
Ion channels involved in cellular processes vital to the progression and development of cancer cells.
References
- 1.Lehmann-Horn F, Jurkat-Rott K. Voltage-gated ion channels and hereditary disease. Physiol Rev. 1999;79:1317–1372. doi: 10.1152/physrev.1999.79.4.1317. [DOI] [PubMed] [Google Scholar]
- 2.Le Guennec JY, Ouadid-Ahidouch H, Soriani O, Besson P, Ahidouch A, Vandier C. Voltage-gated ion channels, new targets in anti-cancer research. Recent Pat Anticancer Drug Discov. 2007;2:189–202. doi: 10.2174/157489207782497244. [DOI] [PubMed] [Google Scholar]
- 3.Fiske JL, Fomin VP, Brown ML, Duncan RL, Sikes RA. Voltage-sensitive ion channels and cancer. Cancer Metastasis Rev. 2006;25:493–500. doi: 10.1007/s10555-006-9017-z. [DOI] [PubMed] [Google Scholar]
- 4.Roger S, Potier M, Vandier C, Besson P, Le Guennec JY. Voltage-gated sodium channels: new targets in cancer therapy? Curr Pharm Des. 2006;12:3681–3695. doi: 10.2174/138161206778522047. [DOI] [PubMed] [Google Scholar]
- 5.Pardo LA, Stuhmer W. Eag1: an emerging oncological target. Cancer Res. 2008;68:1611–1613. doi: 10.1158/0008-5472.CAN-07-5710. [DOI] [PubMed] [Google Scholar]
- 6.Pardo LA, Contreras-Jurado C, Zientkowska M, Alves F, Stuhmer W. Role of voltage-gated potassium channels in cancer. J Membr Biol. 2005;205:115–124. doi: 10.1007/s00232-005-0776-1. [DOI] [PubMed] [Google Scholar]
- 7.Kunzelmann K. Ion channels and cancer. J.Membr.Biol. 2005;205:159–173. doi: 10.1007/s00232-005-0781-4. [DOI] [PubMed] [Google Scholar]
- 8.Bodding M. TRP proteins and cancer. Cell Signal. 2007;19:617–624. doi: 10.1016/j.cellsig.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Villalonga N, Ferreres JC, Argiles JM, Condom E, Felipe A. Potassium channels are a new target field in anticancer drug design. Recent Pat Anticancer Drug Discov. 2007;2:212–223. doi: 10.2174/157489207782497181. [DOI] [PubMed] [Google Scholar]
- 10.Pardo LA, del Camino D, Sanchez A, Alves F, Bruggemann A, Beckh S, Stuhmer W. Oncogenic potential of EAG K(+) channels. EMBO J. 1999;18:5540–5547. doi: 10.1093/emboj/18.20.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gutman GA, Chandy KG, Grissmer S, Lazdunski M, McKinnon D, Pardo LA, Robertson GA, Rudy B, Sanguinetti MC, Stuhmer W, WangX International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol Rev. 2005;57:473–508. doi: 10.1124/pr.57.4.10. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Ferreiro RE, Kerschensteiner D, Major F, Monje F, Stuhmer W, Pardo LA. Mechanism of block of hEag1 K+ channels by imipramine and astemizole. J Gen Physiol. 2004;124:301–317. doi: 10.1085/jgp.200409041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pardo LA. Voltage-gated potassium channels in cell proliferation. Physiology (Bethesda) 2004;19:285–292. doi: 10.1152/physiol.00011.2004. [DOI] [PubMed] [Google Scholar]
- 14.Farias LM, Ocana DB, Diaz L, Larrea F, Avila-Chavez E, Cadena A, Hinojosa LM, Lara G, Villanueva LA, Vargas C, Hernandez-Gallegos E, Camacho-Arroyo I, Duenas-Gonzalez A, Perez-Cardenas E, Pardo LA, Morales A, Taja-Chayeb L, Escamilla J, Sanchez-Pena C, Camacho J. Ether a go-go potassium channels as human cervical cancer markers. Cancer Res. 2004;64:6996–7001. doi: 10.1158/0008-5472.CAN-04-1204. [DOI] [PubMed] [Google Scholar]
- 15.Weber C, Mello DQ, Downie BR, Suckow A, Stuhmer W, Pardo LA. Silencing the activity and proliferative properties of the human EagI Potassium Channel by RNA Interference. J.Biol Chem. 2006;281:13030–13037. doi: 10.1074/jbc.M600883200. [DOI] [PubMed] [Google Scholar]
- 16.Stuhmer W, Alves F, Hartung F, Zientkowska M, Pardo LA. Potassium channels as tumour markers. FEBS Lett. 2006;580:2850–2852. doi: 10.1016/j.febslet.2006.03.062. [DOI] [PubMed] [Google Scholar]
- 17.Hemmerlein B, Weseloh RM, Mello DQ, Knotgen H, Sanchez A, Rubio ME, Martin S, Schliephacke T, Jenke M, Heinz JR, Stuhmer W, Pardo LA. Overexpression of Eag1 potassium channels in clinical tumours. Mol Cancer. 2006;5:41. doi: 10.1186/1476-4598-5-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mello DQ, Suarez-Kurtz G, Stuhmer W, Pardo LA. Ether a go-go potassium channel expression in soft tissue sarcoma patients. Mol Cancer. 2006;5:42. doi: 10.1186/1476-4598-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Downie BR, Sanchez A, Knotgen H, Contreras-Jurado C, Gymnopoulos M, Weber C, Stuhmer W, Pardo LA. Eag1 expression interferes with hypoxia homeostasis and induces angiogenesis in tumors. J Biol Chem. 2008;283:36234–36240. doi: 10.1074/jbc.M801830200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wadhwa S, Wadhwa P, Dinda AK, Gupta NP. Differential expression of potassium ion channels in human renal cell carcinoma. Int Urol Nephrol. 2009;41:251–257. doi: 10.1007/s11255-008-9459-z. [DOI] [PubMed] [Google Scholar]
- 21.Conti M. Targeting K+ channels for cancer therapy. J Exp Ther Oncol. 2004;4:161–166. [PubMed] [Google Scholar]
- 22.Ouadid-Ahidouch H. and Ahidouch, AK+ channel expression in human breast cancer cells: involvement in cell cycle regulation and carcinogenesis. J Membr Biol. 2008;221:1–6. doi: 10.1007/s00232-007-9080-6. [DOI] [PubMed] [Google Scholar]
- 23.Wonderlin WF. and Strobl JS. Potassium channels, proliferation and G1 progression. J Membr Biol. 1996;154:91–107. doi: 10.1007/s002329900135. [DOI] [PubMed] [Google Scholar]
- 24.Ouadid-Ahidouch H, Le BX, Roudbaraki M, Toillon RA, Delcourt P, Prevarskaya N. Changes in the K+ current-density of MCF-7 cells during progression through the cell cycle: possible involvement of a h-ether.a-gogo K+ channel. Receptors Channels. 2001;7:345–356. [PubMed] [Google Scholar]
- 25.Ransom CB, Liu X, Sontheimer H. BK channels in human glioma cells have enhanced calcium sensitivity. Glia. 2002;38:81–291. doi: 10.1002/glia.10064. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki T, Takimoto K. Selective expression of HERG and Kv2 channels influences proliferation of uterine cancer cells. Int J Oncol. 2004;25:153–159. [PubMed] [Google Scholar]
- 27.Jang SH, Kang KS, Ryu PD, Lee SY. Kv1.3 voltage-gated K(+) channel subunit as a potential diagnostic marker and therapeutic target for breast cancer. BMB Rep. 2009;42:535–539. doi: 10.5483/bmbrep.2009.42.8.535. [DOI] [PubMed] [Google Scholar]
- 28.Khaitan D, Sankpal UT, Weksler B, Meister EA, Romero IA, Couraud PO, Ningaraj NS. Role of KCNMA1 gene in breast cancer invasion and metastasis to brain. BMC Cancer. 2009;9:258. doi: 10.1186/1471-2407-9-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brevet M, Haren N, Sevestre H, Merviel P, Ouadid-Ahidouch H. DNA methylation of K(v) 1.3 potassium channel gene promoter is associated with poorly differentiated breast adenocarcinoma. Cell Physiol Biochem. 2009;24:25–32. doi: 10.1159/000227810. [DOI] [PubMed] [Google Scholar]
- 30.Jang SH, Choi C, Hong SG, Yarishkin OV, Bae YM, Kim JG, O'Grady SM, Yoon KA, Kang KS, Ryu PD, Lee SY. Silencing of Kv4.1 potassium channels inhibits cell proliferation of tumorigenic human mammary epithelial cells. Biochem Biophys Res Commun. 2009;384:180–186. doi: 10.1016/j.bbrc.2009.04.108. [DOI] [PubMed] [Google Scholar]
- 31.Han X, Xi L, Wang H, Huang X, Ma X, Han Z, Wu P, Ma X, Lu Y, Wang G, Zhou J, Ma D. The potassium ion channel opener NS1619 inhibits proliferation and induces apoptosis in A2780 ovarian cancer cells. Biochem Biophys Res Commun. 2008;375:205–209. doi: 10.1016/j.bbrc.2008.07.161. [DOI] [PubMed] [Google Scholar]
- 32.Black KL, Yin D, Konda BM, Wang X, Hu J, Ko MK, Bayan JA, Sacapano MR, Espinoza AJ, Ong JM, Irvin D, Shu Y. Different effects of KCa and KATP agonists on brain tumor permeability between syngeneic and allogeneic rat models. Brain Res. 2008;1227:198–206. doi: 10.1016/j.brainres.2008.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park SH, Ramachandran S, Kwon SH, Cha SD, Seo EW, Bae I, Cho C, Song DK. Upregulation of ATP-sensitive potassium channels for estrogen-mediated cell proliferation in human uterine leiomyoma cells. Gynecol Endocrinol. 2008;24:250–256. doi: 10.1080/09513590801893315. [DOI] [PubMed] [Google Scholar]
- 34.Sontheimer H. An unexpected role for ion channels in brain tumor metastasis. Exp Biol Med (Maywood) 2008;233:779–791. doi: 10.3181/0711-MR-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brevet M, Ahidouch A, Sevestre H, Merviel P, El Hiani Y, Robbe M, Ouadid-Ahidouch H. Expression of K+ channels in normal and cancerous human breast. Histol Histopathol. 2008;23:965–972. doi: 10.14670/HH-23.965. [DOI] [PubMed] [Google Scholar]
- 36.Li H, Liu L, Guo L, Zhang J, Du W, Li X, Liu W, Chen X, Huang S. HERG K+ channel expression in CD34+/CD38-/CD123(high) cells and primary leukemia cells and analysis of its regulation in leukemia cells. Int J Hematol. 2008;87:387–392. doi: 10.1007/s12185-008-0056-9. [DOI] [PubMed] [Google Scholar]
- 37.Shao XD, Wu KC, Guo XZ, Xie MJ, Zhang J, Fan DM. Expression and significance of HERG protein in gastric cancer. Cancer Biol Ther. 2008;7:45–50. doi: 10.4161/cbt.7.1.5126. [DOI] [PubMed] [Google Scholar]
- 38.Cambien B, Rezzonico R, Vitale S, Rouzaire-Dubois B, Dubois JM, Barthel R, Karimdjee BS, Mograbi B, Schmid-Alliana A, Schmid-Antomarchi H. Silencing of hSlo potassium channels in human osteosarcoma cells promotes tumorigenesis. Int J Cancer. 2008;123:365–371. doi: 10.1002/ijc.23511. [DOI] [PubMed] [Google Scholar]
- 39.Gessner G, Schonherr K, Soom M, Hansel A, Asim M, Baniahmad A, Derst C, Hoshi T, Heinemann SH. BKCa channels activating at resting potential without calcium in LNCaP prostate cancer cells. J Membr Biol. 2005;208:229–240. doi: 10.1007/s00232-005-0830-z. [DOI] [PubMed] [Google Scholar]
- 40.Chou CC, Lunn CA, Murgolo NJ. (KCa3.1: target and marker for cancer, autoimmune disorder and vascular inflammation? Expert Rev Mol Diagn. 2008;8:179–187. doi: 10.1586/14737159.8.2.179. [DOI] [PubMed] [Google Scholar]
- 41.De Marchi U, Sassi N, Fioretti B, Catacuzzeno L, Cereghetti GM, Szabo I, Zoratti M. Intermediate conductance Ca2+-activated potassium channel (KCa3.1) in the inner mitochondrial membrane of human colon cancer cells. Cell Calcium. 2009;45:509–516. doi: 10.1016/j.ceca.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 42.Voloshyna I, Besana A, Castillo M, Matos T, Weinstein IB, Mansukhani M, Robinson RB, Cordon-Cardo C, Feinmark SJ. TREK-1 is a novel molecular target in prostate cancer. Cancer Res. 2008;68:1197–1203. doi: 10.1158/0008-5472.CAN-07-5163. [DOI] [PubMed] [Google Scholar]
- 43.Meuth SG, Herrmann AM, Ip CW, Kanyshkova T, Bittner S, Weishaupt A, Budde T, Wiendl H. The two-pore domain potassium channel TASK3 functionally impacts glioma cell death. J Neurooncol. 2008;87:263–270. doi: 10.1007/s11060-008-9517-5. [DOI] [PubMed] [Google Scholar]
- 44.Tan G, Sun SQ, Yuan DL. Expression of Kir 4.1 in human astrocytic tumors: correlation with pathologic grade. Biochem Biophys Res Commun. 2008;367:743–747. doi: 10.1016/j.bbrc.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 45.Warth A, Mittelbronn M, Wolburg H. Redistribution of the water channel protein aquaporin-4 and the K+ channel protein Kir4.1 differs in low- and high-grade human brain tumors. Acta Neuropathol. 2005;109:418–426. doi: 10.1007/s00401-005-0984-x. [DOI] [PubMed] [Google Scholar]
- 46.Isom LL. Sodium channel beta subunits: any thing but auxiliary. Neuroscientist. 2001;7:42–54. doi: 10.1177/107385840100700108. [DOI] [PubMed] [Google Scholar]
- 47.Goldin AL, Barchi RL, Caldwell JH, Hofmann F, Howe JR, Hunter JC, Kallen RG, Mandel G, Meisler MH, Netter YB, Noda M, Tamkun MM, Waxman SG, Wood JN, Catterall WA. Nomenclature of voltage-gated sodium channels Neuron. 2000;28:365–368. doi: 10.1016/s0896-6273(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 48.Goldin AL. Resurgence of sodium channel research. Annu Rev Physiol. 2001;63:871–894. doi: 10.1146/annurev.physiol.63.1.871. [DOI] [PubMed] [Google Scholar]
- 49.Diss JK, Fraser SP, Djamgoz MB. Voltagegated Na+ channels: multiplicity of expression, plasticity, functional implications and pathophysiological aspects. Eur Biophys J. 2004;33:180–193. doi: 10.1007/s00249-004-0389-0. [DOI] [PubMed] [Google Scholar]
- 50.DeCoursey TE, Chandy KG, Gupta S, Cahalan MD. Voltage-dependent ion channels in T-lymphocytes. J Neuroimmunol. 1985;10:71–95. doi: 10.1016/0165-5728(85)90035-9. [DOI] [PubMed] [Google Scholar]
- 51.Smith P, Rhodes NP, Shortland AP, Fraser SP, Djamgoz MB, Ke Y, Foster CS. Sodium channel protein expression enhances the invasiveness of rat and human prostate cancer cells. FEBS Lett. 1998;423:19–24. doi: 10.1016/s0014-5793(98)00050-7. [DOI] [PubMed] [Google Scholar]
- 52.Fraser SP, Diss JK, Chioni AM, Mycielska ME, Pan H, Yamaci RF, Pani F, Siwy Z, Krasowska M, Grzywna Z, Brackenbury WJ, Theodorou D, Koyuturk M, Kaya H, Battaloglu E, De Bella MT, Slade MJ, Tolhurst R, Palmieri C, Jiang J, Latchman DS, Coombes RC, Djamgoz MB. Voltage-gated sodium channel expression and potentiation of human breast cancer metastasis. Clin Cancer Res. 2005;11:5381–5389. doi: 10.1158/1078-0432.CCR-05-0327. [DOI] [PubMed] [Google Scholar]
- 53.Parker SL, Tong T, Bolden S, Wingo PA. Cancer statistics, 1996. CA Cancer J Clin. 1996;46:5–27. doi: 10.3322/canjclin.46.1.5. [DOI] [PubMed] [Google Scholar]
- 54.Grimes JA, Fraser SP, Stephens GJ, Downing JE, Laniado ME, Foster CS, Abel PD, Djamgoz MB. Differential expression of voltageactivated Na+ currents in two prostatic tumour cell lines: contribution to invasiveness in vitro. FEBS Lett. 1995;369:290–294. doi: 10.1016/0014-5793(95)00772-2. [DOI] [PubMed] [Google Scholar]
- 55.Laniado ME, Lalani EN, Fraser SP, Grimes JA, Bhangal G, Djamgoz MB, Abel PD. Expression and functional analysis of voltageactivated Na+ channels in human prostate cancer cell lines and their contribution to invasion in vitro. Am J Pathol. 1997;150:1213–1221. [PMC free article] [PubMed] [Google Scholar]
- 56.Brackenbury WJ, Djamgoz MB. Activitydependent regulation of voltage-gated Na+ channel expression in Mat-LyLu rat prostate cancer cell line. J Physiol. 2006;573:343–356. doi: 10.1113/jphysiol.2006.106906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roger S, Besson P, Le Guennec JY. Involvement of a novel fast inward sodium current in the invasion capacity of a breast cancer cell line. Biochim Biophys Acta. 2003;16:107–111. doi: 10.1016/j.bbamem.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 58.Roger S, Le Guennec JY, Besson P. Particular sensitivity to calcium channel blockers of the fast inward voltage-dependent sodium current involved in the invasive properties of a metastastic breast cancer cell line. Br J Pharmacol. 2004;141:610–615. doi: 10.1038/sj.bjp.0705649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fraser SP, Diss JK, Lloyd LJ, Pani F, Chioni AM, George AJ, Djamgoz MB. T-lymphocyte invasiveness: control by voltage-gated Na+ channel activity. FEBS Lett. 2004;569:191–194. doi: 10.1016/j.febslet.2004.05.063. [DOI] [PubMed] [Google Scholar]
- 60.Blandino JK, Viglione MP, Bradley WA, Oie HK, Kim YI. Voltage-dependent sodium channels in human small-cell lung cancer cells: role in action potentials and inhibition by Lambert-Eaton syndrome IgG. J Membr Biol. 1995;143:153–163. doi: 10.1007/BF00234661. [DOI] [PubMed] [Google Scholar]
- 61.Roger S, Rollin J, Barascu A, Besson P, Raynal PI, Iochmann S, Lei M, Bougnoux P, Gruel Y, Le Guennec JY. Voltage-gated sodium channels potentiate the invasive capacities of human non-small-cell lung cancer cell lines. Int J Biochem Cell Biol. 2007;39:774–786. doi: 10.1016/j.biocel.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 62.Fulgenzi G, Graciotti L, Faronato M, Soldovieri MV, Miceli F, Amoroso S, Annunziato L, Procopio A, Taglialatela M. Human neoplastic mesothelial cells express voltagegated sodium channels involved in cell motility. Int J Biochem Cell Biol. 2006;38:1146–1159. doi: 10.1016/j.biocel.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 63.Kapoor N, Bartoszewski R, Qadri YJ, Bebok Z, Bubien JK, Fuller CM, Benos DJ. Knockdown of ASIC1 and epithelial sodium channel subunits inhibits glioblastoma whole cell current and cell migration. J Biol Chem. 2009;284:24526–24541. doi: 10.1074/jbc.M109.037390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ou S W, Kameyama A, Hao LY, Horiuchi M, Minobe E, Wang WY, Makita N, Kameyama M. Tetrodotoxin-resistant Na+ channels in human neuroblastoma cells are encoded by new variants of Nav1.5/SCN5A. Eur J Neurosci. 2005;22:793–801. doi: 10.1111/j.1460-9568.2005.04280.x. [DOI] [PubMed] [Google Scholar]
- 65.Allen DH, Lepple-Wienhues A, Cahalan MD. Ion channel phenotype of melanoma cell lines. J Membr Biol. 1997;155:27–34. doi: 10.1007/s002329900155. [DOI] [PubMed] [Google Scholar]
- 66.Diaz D, Delgadillo DM, Hernandez-Gallegos E, Ramirez-Dominguez ME, Hinojosa LM, Ortiz CS, Berumen J, Camacho J, Gomora JC. Functional expression of voltage-gated sodium channels in primary cultures of human cervical cancer. J Cell Physiol. 2007;210:469–478. doi: 10.1002/jcp.20871. [DOI] [PubMed] [Google Scholar]
- 67.Mariot P, Vanoverberghe K, Lalevee N, Rossier MF, Prevarskaya N. Overexpression of an alpha 1H (Cav3.2) T-type calcium channel during neuroendocrine differentiation of human prostate cancer cells. J Biol Chem. 2002;277:10824–10833. doi: 10.1074/jbc.M108754200. [DOI] [PubMed] [Google Scholar]
- 68.Wang XT, Nagaba Y, Cross HS, Wrba F, Zhang L, Guggino SE. The mRNA of L-type calcium channel elevated in colon cancer: protein distribution in normal and cancerous colon. Am J Pathol. 2000;157:1549–1562. doi: 10.1016/S0002-9440(10)64792-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oguro-Okano M, Griesmann GE, Wieben ED, Slaymaker SJ, Snutch TP, Lennon VA. Molecular diversity of neuronal-type calcium channels identified in small cell lung carcinoma. Mayo Clin Proc. 1992;67:1150–1159. doi: 10.1016/s0025-6196(12)61144-6. [DOI] [PubMed] [Google Scholar]
- 70.Su H, Alroy G, Kirson ED, Yaari Y. Extracellular calcium modulates persistent sodium current-dependent burst-firing in hippocampal pyramidal neurons. J Neurosci. 2001;21:4173–4182. doi: 10.1523/JNEUROSCI.21-12-04173.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang JB, Kindzelskii AL, Clark AJ, Petty HR. Identification of channels promoting calcium spikes and waves in HT1080 tumor cells: their apparent roles in cell motility and invasion. Cancer Res. 2004;64:2482–2489. doi: 10.1158/0008-5472.can-03-3501. [DOI] [PubMed] [Google Scholar]
- 72.Clapham DE. TRP channels as cellular sensors Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 73.Montell C, Birnbaumer L, Flockerzi V, Bindels RJ, Bruford EA, Caterina MJ, Clapham DE, Harteneck C, Heller S, Julius D, Kojima I, Mori Y, Penner R, Prawitt D, Scharenberg AM, Schultz G, Shimizu N, Zhu MX. A unified nomenclature for the superfamily of TRP cation channels. Mol Cell. 2002;9:229–231. doi: 10.1016/s1097-2765(02)00448-3. [DOI] [PubMed] [Google Scholar]
- 74.Clapham DE, Montell C, Schultz G, Julius D. International Union of Pharmacology. XLIII. Compendium of voltage-gated ion channels: transient receptor potential channels. Pharmacol Rev. 2003;55:591–596. doi: 10.1124/pr.55.4.6. [DOI] [PubMed] [Google Scholar]
- 75.Means AR. Calcium, calmodulin and cell cycle regulation. FEBS Lett. 1994;347:1–4. doi: 10.1016/0014-5793(94)00492-7. [DOI] [PubMed] [Google Scholar]
- 76.Fleig A, Penner R. The TRPM ion channel subfamily: molecular, biophysical and functional features. Trends Pharmacol Sci. 2004;25:633–639. doi: 10.1016/j.tips.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 77.Duncan LM, Deeds J, Hunter J, Shao J, Holmgren LM, Woolf EA, Tepper RI, Shyjan AW. Down-regulation of the novel gene melastatin correlates with potential for melanoma metastasis. Cancer Res. 1998;58:1515–1520. [PubMed] [Google Scholar]
- 78.Hunter JJ, Shao J, Smutko JS, Dussault BJ, Nagle DL, Woolf EA, Holmgren LM, Moore KJ, Shyjan AW. Chromosomal localization and genomic characterization of the mouse melastatin gene (Mlsn1) Genomics. 1998;54:116–123. doi: 10.1006/geno.1998.5549. [DOI] [PubMed] [Google Scholar]
- 79.Fang D, et al. Expression and Upregulation of alternatively spliced transcripts of melastatin, a melanoma metastasis-related gene, in human melanoma cells. Biochem Biophys Res Commun. 2000;279:53–61. doi: 10.1006/bbrc.2000.3894. [DOI] [PubMed] [Google Scholar]
- 80.Deeds J, Cronin F, Duncan LM. Patterns of melastatin mRNA expression in melanocytic tumors. Hum Pathol. 2000;31:1346–1356. [PubMed] [Google Scholar]
- 81.Duncan LM, Deeds J, Cronin FE, Donovan M, Sober AJ, Kauffman M, McCarthy JJ. Melastatin expression and prognosis in cutaneous malignant melanoma. J Clin Oncol. 2001;19:568–576. doi: 10.1200/JCO.2001.19.2.568. [DOI] [PubMed] [Google Scholar]
- 82.Xu XZ, Moebius F, Gill DL, Montell C. Regulation of melastatin, a TRP-related protein, through interaction with a cytoplasmic isoform. Proc Natl Acad Sci U.S.A. 2001;98:10692–10697. doi: 10.1073/pnas.191360198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jiang J, Li MH, Inoue K, Chu XP, Seeds J, Xiong ZG. Transient receptor potential melastatin 7-like current in human head and neck carcinoma cells: role in cell proliferation. Cancer Res. 2007;67:10929–10938. doi: 10.1158/0008-5472.CAN-07-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nadler MJ, Hermosura MC, Inabe K, Perraud AL, Zhu Q, Stokes AJ, Kurosaki T, Kinet JP, Penner R, Scharenberg AM, Fleig A. LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature. 2001;411:590–595. doi: 10.1038/35079092. [DOI] [PubMed] [Google Scholar]
- 85.Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, MacDonald JF, Tymianski MA. key role for TRPM7 channels in anoxic neuronal death. Cell. 2003;115:863–877. doi: 10.1016/s0092-8674(03)01017-1. [DOI] [PubMed] [Google Scholar]
- 86.Jiang X, Newell EW, Schlichter LC. Regulation of a TRPM7-like current in rat brain microglia. J Biol Chem. 2003;278:42867–42876. doi: 10.1074/jbc.M304487200. [DOI] [PubMed] [Google Scholar]
- 87.Prakriya M, Lewis RS. Separation and Characterization of Currents through Storeoperated CRAC Channels and Mg(2+)- inhibited Cation (MIC) Channels. J Gen Physiol. 2002;119:487–508. doi: 10.1085/jgp.20028551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li M, Shen JF, Branigan B, Li X, Si F, Chen J, Xinog ZG. TRPM7 channels in the growth and proliferation of human glioma cells. Abstract, Society for Neuroscience Annual Meeting. 2010 [Google Scholar]
- 89.Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 90.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 91.Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels Nature. 2004;430:748–754. doi: 10.1038/nature02732. [DOI] [PubMed] [Google Scholar]
- 92.Brauchi S, Orio P, Latorre R. Clues to understanding cold sensation: thermodynamics and electrophysiological analysis of the cold receptor TRPM8. Proc Natl Acad Sci U.S.A. 2004;101:15494–15499. doi: 10.1073/pnas.0406773101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tsavaler L, Shapero MH, Morkowski S, Laus R. Trp-p8, a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Cancer Res. 2001;61:3760–3769. [PubMed] [Google Scholar]
- 94.Prevarskaya N, Zhang L, Barritt G. TRP channels in cancer. Biochim Biophys Acta. 2007;1772:937–946. doi: 10.1016/j.bbadis.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 95.Tsuzuki K, Xing H, Ling J, Gu JG. Mentholinduced Ca2+ release from presynaptic Ca2+ stores potentiates sensory synaptic transmission. J Neurosci. 2004;24:762–771. doi: 10.1523/JNEUROSCI.4658-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Thebault S, Lemonnier L, Bidaux G, Flourakis M, Bavencoffe A, Gordienko D, Roudbaraki M, Delcourt P, Panchin Y, Shuba Y, Skryma R, Prevarskaya N. Novel role of cold/menthol-sensitive transient receptor potential melastatine family member 8 (TRPM8) in the activation of store-operated channels in LNCaP human prostate cancer epithelial cells. J Biol Chem. 2005;280:39423–39435. doi: 10.1074/jbc.M503544200. [DOI] [PubMed] [Google Scholar]
- 97.Zhang L, Barritt GJ. Evidence that TRPM8 is an androgen-dependent Ca2+ channel required for the survival of prostate cancer cells. Cancer Res. 2004;64:8365–8373. doi: 10.1158/0008-5472.CAN-04-2146. [DOI] [PubMed] [Google Scholar]
- 98.Zhang L, Barritt GJ. TRPM8 in prostate cancer cells: a potential diagnostic and prognostic marker with a secretory function? Endocr Relat Cancer. 2006;13:27–38. doi: 10.1677/erc.1.01093. [DOI] [PubMed] [Google Scholar]
- 99.Bodding M, Wissenbach U, Flockerzi V. The recombinant human TRPV6 channel functions as Ca2+ sensor in human embryonic kidney and rat basophilic leukemia cells. J Biol Chem. 2002;277:36656–36664. doi: 10.1074/jbc.M202822200. [DOI] [PubMed] [Google Scholar]
- 100.Vennekens R, Hoenderop JG, Prenen J, Stuiver M, Willems PH, Droogmans G, Nilius B, Bindels RJ. Permeation and gating properties of the novel epithelial Ca(2+) channel. J Biol Chem. 2000;275:3963–3969. doi: 10.1074/jbc.275.6.3963. [DOI] [PubMed] [Google Scholar]
- 101.Yue L, Peng JB, Hediger MA, Clapham DE. CaT1 manifests the pore properties of the calcium-release-activated calcium channel Nature. 2001;410:705–709. doi: 10.1038/35070596. [DOI] [PubMed] [Google Scholar]
- 102.Bodding M. and Flockerzi V. Ca2+ dependence of the Ca2+-selective TRPV6 channel. J Biol Chem. 2004;279:36546–36552. doi: 10.1074/jbc.M404679200. [DOI] [PubMed] [Google Scholar]
- 103.Peng JB, Chen XZ, Berger UV, Weremowicz S, Morton CC, Vassilev PM, Brown EM, Hediger MA. Human calcium transport protein CaT1. Biochem Biophys Res Commun. 2000;19(278):326–332. doi: 10.1006/bbrc.2000.3716. [DOI] [PubMed] [Google Scholar]
- 104.Pen JB, Zhuang L, Berger UV, Adam RM, Williams BJ, Brown EM, Hediger MA, Freeman MR. CaT1 expression correlates with tumor grade in prostate cancer. Biochem. Biophys Res Commun. 2001;282:729–734. doi: 10.1006/bbrc.2001.4638. [DOI] [PubMed] [Google Scholar]
- 105.Wissenbach U, Niemeyer BA, Fixemer T, Schneidewind A, Trost C, Cavalie A, Reus K, Meese E, Bonkhoff H, Flockerzi V. Expression of CaT-like, a novel calcium-selective channel, correlates with the malignancy of prostate cancer. J Biol Chem. 2001;276:19461–19468. doi: 10.1074/jbc.M009895200. [DOI] [PubMed] [Google Scholar]
- 106.Fixemer T, Wissenbach U, Flockerzi V, Bonkhoff H. Expression of the Ca2+-selective cation channel TRPV6 in human prostate cancer: a novel prognostic marker for tumor progression. Oncogene. 2003;22:7858–7861. doi: 10.1038/sj.onc.1206895. [DOI] [PubMed] [Google Scholar]
- 107.Wang H, Zhang Y, Cao L, Han H, Wang J, Yang B, Nattel S, Wang Z. HERG K+ channel, a regulator of tumor cell apoptosis and proliferation. Cancer Res. 2002;62:4843–4848. [PubMed] [Google Scholar]
- 108.Kagan A, Melman YF, Krumerman A, McDonald TV. 14-3-3 amplifies and prolongs adrenergic stimulation of HERG K+ channel activity. EMBO J. 2002;21:1889–1898. doi: 10.1093/emboj/21.8.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Benos DJ, Stanton BA. Functional domains within the degenerin/epithelial sodium channel (Deg/ENaC) superfamily of ion channels. J Physiol. 1999;520:631–644. doi: 10.1111/j.1469-7793.1999.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Canessa CM, Merillat AM, Rossier BC. Membrane topology of the epithelial sodium channel in intact cells. Am J Physiol. 1994;267:C1682–C1690. doi: 10.1152/ajpcell.1994.267.6.C1682. [DOI] [PubMed] [Google Scholar]
- 111.Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature. 1994;367:463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- 112.Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev. 2002;82:735–767. doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- 113.Snyder PM. The epithelial Na+ channel: cell surface insertion and retrieval in Na+ homeostasis and hypertension. Endocr Rev. 2002;23:258–275. doi: 10.1210/edrv.23.2.0458. [DOI] [PubMed] [Google Scholar]
- 114.Swift PA, MacGregor GA. The epithelial sodium channel in hypertension: genetic heterogeneity and implications for treatment with amiloride. Am J Pharmacogenomics. 2004;4:161–168. [PubMed] [Google Scholar]
- 115.Mall MA. Role of cilia, mucus, and airway surface liquid in mucociliary dysfunction: lessons from mouse models. J Aerosol Med Pulm Drug Deliv. 2008;21:13–24. doi: 10.1089/jamp.2007.0659. [DOI] [PubMed] [Google Scholar]
- 116.Matalon S, Lazrak A, Jain L, Eaton DC. Invited review: biophysical properties of sodium channels in lung alveolar epithelial cells. J Appl Physiol. 2002;93:1852–1859. doi: 10.1152/japplphysiol.01241.2001. [DOI] [PubMed] [Google Scholar]
- 117.Guggino WB, Guggino SE. Amiloridesensitive sodium channels contribute to the woes of the flu. Proc Natl Acad Sci U.S.A. 2000;97:9827–9829. doi: 10.1073/pnas.97.18.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Berdiev BK, Xia J, McLean LA, Markert JM, Gillespie GY, Mapstone TB, Naren AP, Jovov B, Bubien JK, Ji HL, Fuller CM, Kirk KL, Benos DJ. Acid-sensing ion channels in malignant gliomas. J Biol Chem. 2003;278:15023–15034. doi: 10.1074/jbc.M300991200. [DOI] [PubMed] [Google Scholar]
- 119.Schwab A. Ion channels and transporters on the move. News Physiol Sci. 2001;16:29–33. doi: 10.1152/physiologyonline.2001.16.1.29. 29-33. [DOI] [PubMed] [Google Scholar]
- 120.Mirshahi M, Mirshahi S, Golestaneh N, Nicolas C, Mishal Z, Agarwal MK. Mineralocorticoid hormone receptor and the epithelial sodium channel in a human leukemic cell line. Endocr Res. 1998;24:455–459. doi: 10.3109/07435809809032633. [DOI] [PubMed] [Google Scholar]
- 121.Mirshahi M, Mirshahi S, Golestaneh N, Mishal Z, Nicolas C, Hecquet C, Agarwal MK. Demonstration of the mineralocorticoid hormone receptor and action in human leukemic cell lines. Leukemia. 2000;14:1097–1104. doi: 10.1038/sj.leu.2401786. [DOI] [PubMed] [Google Scholar]