Abstract
Background:
The evidence for the effectiveness of orthopaedic surgery to correct crouch gait in cerebral diplegic is insufficient. The crouch gait is defined as walking with knee flexion and ankle dorsiflexion through out the stance phase. Severe crouch gait in patients with spastic diplegia causes excessive loading of the patellofemoral joint and may result in anterior knee pain, gait deterioration, and progressive loss of function. We retrospectively evaluated the effect of surgery on the mobility and energy consumption at one year or more with the help of validated scales and scores.
Materials and Methods:
18 consecutive patients with mean age of 14.6 years with cerebral diplegia with crouched gait were operated for multilevel orthopaedic surgery. Decisions for surgery were made with the observations on gait analysis and physical examination. The surgical intervention consisted of lengthening of short muscle-tendon units, shortening of long muscles and correction of osseous deformities. The paired samples t test was used to compare values of physical examination findings, walking speed and physiological cost index. Two paired sample Wilcoxon signed rank test was used to compare functional walking scales.
Results:
After surgery, improvements in functional mobility, walking speed and physiological cost index were found. No patient was able to walk 500 meters before surgery while all were able to walk after surgery. The improvements that were noted at one year were maintained at two years.
Conclusions:
Multilevel orthopedic surgery for older children and adolescents with crouch gait is effective for improving function and independence.
Keywords: Cerebral palsy, crouch gait, multi level orthopedic surgery
INTRODUCTION
Crouch gait is commonly seen in diplegic cerebral palsy. It can develop due to the weakness of antigravity muscles which is inherent in diplegic cerebral palsy or weakness which is precipitated by intervention.1–3 The shortening of hamstrings or psoas muscles, lengthening of the quadriceps muscle tendon unit and musculoskeletal deformities like excessive femoral anteversion, fixed flexion deformity of knee, excessive external tibial torsion, and pes valgus are the other factors contributing to crouch gait.1,4–7 The development of an unfavorable body mass to strength ratio during adolescence years can also play a role.
In the recent years, an understanding of pathophysiology has improved leading to better management. Nonoperative options for the management of crouch gait are muscle-strengthening exercises8 and floor reaction ankle foot orthoses.9 Orthopedic surgeries to correct some or all the factors have also shown improvement in mobility.10,11 The evidence for the effectiveness of orthopedic surgery to correct crouch gait in patients with spastic diplegia is insufficient. The purpose of the present study is to evaluate the effect of surgery on mobility and energy consumption at 1 and 2 years postsurgery with the help of validated scales and scores.
MATERIALS AND METHODS
This consecutive, retrospective cohort study included a sample of 18 patients, who had spastic diplegic cerebral palsy and walked with a crouch gait, either independently or with the use of assistive devices and who underwent surgery between January 2006 and December 2007. Inclusion criteria for the study group were as follows: (1) crouch gait was defined as barefoot walking with knee flexion gait in which feet are dorsiflexed throughout the stance phase and (2) pain at the knee or deterioration in walking suggested by reduction in speed or in walking distance or increased knee flexion in stance phase in previous 2 years. Exclusion criteria were botulinum toxin A injections or muscle surgery within the preceding 12 months.
All patients were offered a combined surgical and rehabilitation program for the treatment of crouch gait. The surgical recommendations were determined by using set criteria for surgery [Table 1].
Table 1.
Criteria for various operative procedures
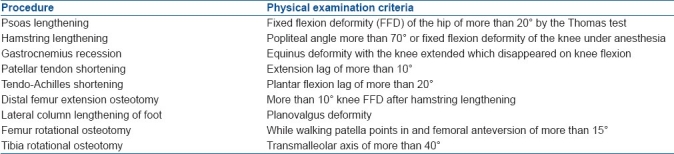
In those patients who had surgery for knee flexion deformity and/or planovalgus foot, a decision to lengthen gastrocnemius was made peroperatively after correcting the knee flexion deformity and planovalgus foot deformity. The Achilles tendon shortening decision was made after deciding the active plantarflexion lag. In the supine position keeping knees extended, the patient was asked to plantar flex the ankle and the angle of ankle plantar flexion was noted. Examiner tried to achieve further plantar flexion passively. The difference between these angles was defined as the active plantarflexion lag.
The soft-tissue surgical procedures were intramuscular lengthening of the psoas muscle at the pelvic brim,12 lengthening of the medial hamstrings with or without lateral hamstrings,13 Strayer's procedure14 for gastrocnemius recession, patellar tendon shortening to shorten the quadriceps, and tendo-Achilles shortening to shorten gastrosoleus.
Quadriceps was shortened at the patellar tendon level. Two transverse holes were made in the patella and in the proximal tibia distal to the tibial tubercle apophysis. A 1-mm diameter stainless steel wire (Synthes, Switzerland) was passed through transverse holes and tightened so that the inferior pole of the patella was at the level of the joint line with the knee extended. The patellar tendon was longitudinally incised. Half of the tendon was incised horizontally near the inferior pole of the patella while the remaining half was left intact. The distal cut end of the tendon was sutured to the patella. The intact half of the tendon was plicated.
For Achilles tendon shortening, the tendon was exposed with a 5–7 cm incision near its insertion. The tendon was divided by a sharp cut, made obliquely in the sagittal plane. Both ends of the divided tendon were secured by the Kessler suture technique. The proximal stump was pulled down to the original calcaneal insertion of the heel cord and fixed under maximal tension while holding the foot in 20° of plantarflexion. The distal part was then pulled up and fixed as proximally as possible. The shortening thus achieved was between 3 and 4 cm. The ankle and foot were immobilized in a cast in 20° of plantarflexion for 6 weeks to protect the tendon repair.
The osseous procedures were distal femur extension osteotomy with internal fixation, calcaneal lengthening15 for the correction of the planovalgus foot, internal rotation osteotomy of the tibia, and external rotation osteotomy of the femur.16 All long bone osteotomies were stabilized with internal fixation except two tibial osteotomies which were fixed with external fixation. Calcaneal osteotomies were fixed with two k-wires.
A first-generation cephalosporin was given at the time of induction of anesthesia and was continued for 24 h postoperatively. In all patients, intravenous analgesia was administered for first 24–48 h. Along with this, nonsteroidal antiinflammatory drugs were used.
A synthetic fiberglass cast was used to immobilize the knee joint in a fully extended position. Patients who underwent gastrocnemius lengthening, tendo-Achilles shortening, rotational osteotomy of the tibia, and bony procedure at the foot had casts with distal extension up to toes. Physical therapy began after 5 days when pain subsided and consisted of passive and active finger movement. Casts were removed 6 weeks after surgery. Radiographs of all osteotomy sites were made, and full weight-bearing was encouraged when healing at the osteotomy sites was demonstrated radiologically. Range-of-motion exercises were started after the removal of casts. Knee immobilizers were used at night only for the subsequent 6 month period to reduce the risk of recurrence of knee flexion deformity. The patients who underwent bony surgeries in the foot used custom made, molded polypropylene foot orthoses at the time of weight-bearing.
All patients were managed with an individually tailored, community based rehabilitation program. It consisted of five to six sessions of physical therapy starting 6 weeks postsurgery when the cast was removed and continued for further 12 weeks. Therapy gradually advanced from a passive to an assisted range-of-motion protocol and finally to a resistance exercise. Strengthening exercises were mainly for gluteus maximus, quadriceps, gastrosoleus, and hamstrings. To improve endurance, patients were encouraged to walk. The frequency of physical therapy sessions was reduced after 12 months. Formal physical therapy was stopped after 2 years.
All patients were reviewed at 3, 6, 9, 12, 18, and 24 months after surgery. Appropriate changes to orthoses, assistive devices, and the physical therapy program to optimize each subject's rehabilitation process were made. A two-dimensional video recording of the gait was performed at 1 and 2 years.
Outcome measures
A standardized physical examination was conducted preoperatively and at every follow up. The parameters relevant to the present study were the measurement of fixed flexion deformity at the hip and knee, the measurement of the hamstring length by the popliteal angle, and the measurement of the gastrocnemius and soleus length with the use of the Silfverskiold test. The protocol and reliability of these tests have been reported elsewhere.17
Three validated instruments to measure functional mobility were used: the functional mobility scale (FMS),18 the functional assessment questionnaire (FAQ),19 and the Gross motor function classification system (GMFCS).20
FMS uses three distances (5, 50, and 500 m) which represent typical distances walked by children at home, at school, and in the community. For each distance, a rating of 1–6 was assigned depending on the amount of assistance required for mobility (6 being the highest ability). Its usefulness as a clinical tool for quantifying the change after orthopedic surgery in children with cerebral palsy has been established.21
FAQ is a valid scale specific to the task of walking in children with chronic neuromuscular conditions and assists in the documentation of functional changes. It is a 10-level, walking scale encompassing a range of walking abilities from nonambulatory (level 1) to ambulatory in all community settings and terrains (level 10).
FMS and FAQ are sensitive to change in the cerebral palsy population with intervention and both are used as outcome measures after corrective surgery.10,11
In contrast, GMFCS is best suited to stratify patients with cerebral palsy based on broad functional levels. It is considered to be stable over time22 and nonresponsive to intervention.
Walking speed and physiological cost index (PCI) are two simple tools to measure energy consumption while walking. Both are responsive to interventions.23 The resting heart rate was calculated by palpating the radial pulse for 15 s and multiplying the value by 4. The subject then was made to walk barefoot along a 50-m walkway at a self-selected speed with the preferred assistive device. Immediately after the walk, the pulse rate was recorded. The time taken to complete the 50-m walk was recorded and the speed of walking was calculated. The PCI was calculated using the following formula:

Statistical analysis
All parameters were measured preoperatively, at 1 and finally at 2 years. The paired sample t-test was used to compare the preoperative and postoperative physical examination findings, walking speed, and PCI. The two-sample paired Wilcoxon signed rank test was used to compare FMS, FAQ, and GMFCS. The significance level was set at P<.05.
RESULTS
Eighteen subjects fulfilled eligibility criteria for the 2-year study period. The study group included 12 male and 6 female patients with a mean age of 14.6 years (range, 12-30 years) at the time of surgery. Five out of 18 subjects had previous surgeries. Three underwent bilateral hamstrings lengthening. Two patients had surgery to lengthen the gastrosoleus complex of whom one underwent bilateral tendo-Achillis lengthening and one underwent the bilateral Vulpius procedure.
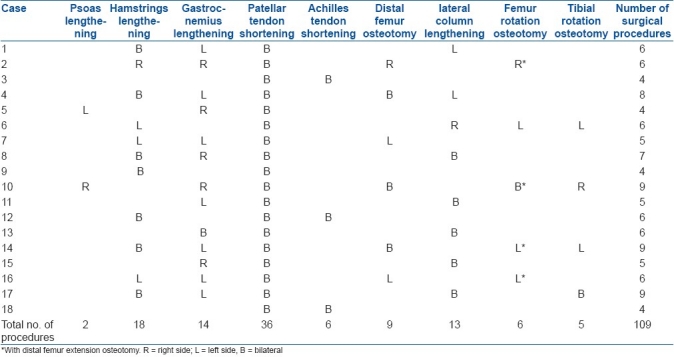
The surgical procedures for each subject are documented in Table 2. A total of 76 soft tissue procedures and 33 bony procedures were performed. In two distal femur extension osteotomies, torsion correction of the femur was also performed.
Table 2.
Surgical procedures for each subject
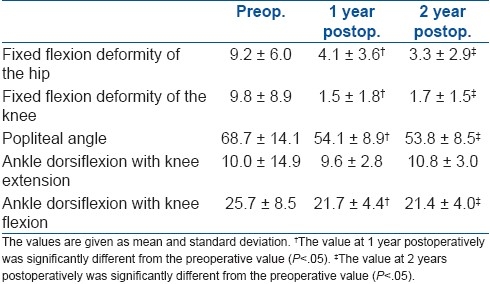
Preoperative, 1 year, and 2 year postoperative values for five physical examination parameters are described in Table 3. Data from both sides are added.
Table 3.
Preoperative, 1-year, and 2-year physical examination findings
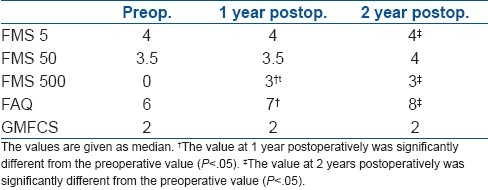
Values of FMS, for 5, 50, and 500 m, FAQ, and GMFCS are described in Table 4. Best improvement was seen in FMS 500 m. No person was able to walk the 500 m distance before surgery; however, all achieved the ability to walk 500 m at the end of follow-up. The median for FAQ which was 5 before surgery, improved to 6 at 1 year and 7 at 2 years.
Table 4.
Preoperative, 1-year, and 2-years values for functional scales
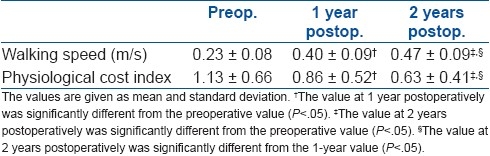
Preoperative, 1 year, and 2 year postoperative values for walking speed and PCI are described in Table 5. Progressive improvement was seen for these two parameters over 1 and 2-year follow-ups.
Table 5.
Preoperative, 1-year, and 2-year values for walking speed and physiological cost index
No additional surgery was performed to improve walking. In two patients (three sides), the encirclage wire broke within 2 months of surgery. These were revised, and the patients immobilized for further 6 weeks. In addition, three patients (three sides) had irritation from the wire knot which required removal after 12 months. In one patient with bilateral external fixation for tibial osteotomies, one side went for delayed union requiring the fixator to be continued for more than 6 weeks. This was removed at 12 weeks and the knee cast was given for further 4 weeks. Osteotomy united at 16 weeks.
DISCUSSION
Surgery for crouch gait is done mainly to improve walking. Walking function can be assessed by clinical scales. FMS and FAQ were used for assessing the change in the walking function. In the present study, walking improved when patients were assessed by these two tests.
Surgery improved the mobility of patients. An improvement in function is noteworthy because the natural history of the walking function of individuals with cerebral palsy is one of decline24,25 and a treatment that stabilizes or improves a current walking pattern or ability represents a positive outcome.25,26
With crouch being highly energy-consuming gait, the improvement in energy consumption suggests benefits to the patients. After surgery, the walking speed and PCI improved at 1 and 2 years. An improvement in the speed can be partly attributed to the increase in the height of the person as in the present study, walking speed data are not normalized to body height.27 During rehabilitation, the emphasis was on strengthening exercises and endurance training for walking. Strengthening exercises are beneficial for a person with crouch gait.8 So rehabilitation may have positively influenced results in this series. The various considerations to prevent progression of crouch gait are use of orthosis and strengthening exercises, deciding the right stage for surgical intervention, and a search for more efficient surgical management, in which patient hamstring lengthening to be carried out, whether to carry out quadriceps shortening above the patella or below the patella, how much shortening should be carried out, and long-term effect of patellar tendon shortening and tendo-Achillis shortening.
In conclusion, multilevel orthopedic surgery carried out with decisions based on physical examination can improve walking in diplegic cerebral palsy persons with crouch gait. The improvements that were noted at 1 year were maintained at 2 years.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Gage JR. Surgical treatment of knee dysfunction in cerebral palsy. Clin Orthop Relat Res. 1990;253:45–54. [PubMed] [Google Scholar]
- 2.Rab GT. Consensus. In: Sussman MD, editor. The diplegic child: Evaluation and management. Rosemont, IL: J Am Acad Orthop Surg; 1992. pp. 337–9. [Google Scholar]
- 3.de Morais Filho MC, Kawamura CM, Kanaji PR, Juliano Y. The relation of triceps surae surgical lengthening and crouch gait in patients with cerebral palsy. J Pediatr Orthop B. 2010;19:226–30. doi: 10.1097/BPB.0b013e3283387cdb. [DOI] [PubMed] [Google Scholar]
- 4.Gage JR. Treatment principles for crouch gait. In: Gage JR, editor. Treatment of gait problems in cerebral palsy. London: Mac Keith Press; 2004. pp. 382–9. [Google Scholar]
- 5.Schwartz M, Lakin G. The effect of tibial torsion on the dynamic function of the soleus during gait. Gait Posture. 2003;17:113–8. doi: 10.1016/s0966-6362(02)00058-9. [DOI] [PubMed] [Google Scholar]
- 6.Hicks J, Arnold A, Anderson F, Schwartz M, Delp S. The effect of excessive tibial torsion on the capacity of muscles to extend the hip and knee during single-limb stance. Gait Posture. 2007;26:546–52. doi: 10.1016/j.gaitpost.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gage JR, Schwartz M. Pathological gait and lever-arm dysfunction. In: Gage JR, editor. Treatment of gait problems in cerebral palsy. London: Mac Keith Press; 2004. pp. 180–204. [Google Scholar]
- 8.Damiano DL, Kelly LE, Vaughn CL. Effects of quadriceps femoris muscle strengthening on crouch gait in children with spastic diplegia. Phys Ther. 1995;75:658–71. doi: 10.1093/ptj/75.8.658. [DOI] [PubMed] [Google Scholar]
- 9.Rogozinski BM, Davids JR, Davis RB, 3rd, Jameson GG, Blackhurst DW. The efficacy of the floor-reaction ankle-foot orthosis in children with cerebral palsy. J Bone Joint Surg Am. 2009;91:2440–7. doi: 10.2106/JBJS.H.00965. [DOI] [PubMed] [Google Scholar]
- 10.Rodda JM, Graham HK, Nattrass GR, Galea MP, Baker R, Wolfe R. Correction of severe crouch gait in patients with spastic diplegia with use of multilevel orthopaedic surgery. J Bone Joint Surg Am. 2006;88:2653–64. doi: 10.2106/JBJS.E.00993. [DOI] [PubMed] [Google Scholar]
- 11.Stout JL, Gage JR, Schwartz MH, Novacheck TF. Extension osteotomy of distal femoral and patellar tendon advancement to treat persistent crouch gait in cerebral palsy. J Bone Joint Surg Am. 2008;90:2470–84. doi: 10.2106/JBJS.G.00327. [DOI] [PubMed] [Google Scholar]
- 12.Sutherland DH, Zilberfarb JL, Kaufman KR, Wyatt MP, Chambers HG. Psoas release at the pelvic brim in ambulatory patients with cerebral palsy: Operative technique and functional outcome. J Pediatr Orthop. 1997;17:563–70. doi: 10.1097/00004694-199709000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Herring JA. Disorders of the brain. In: Tachdjian MO, Herring JA, editors. Tachdjian's pediatric orthopaedics. 3rd edition. Philadelphia: Saunders; 2001. pp. 1158–73. [Google Scholar]
- 14.Strayer LM., Jr Recession of gastrocnemius; An operation to relieve spastic contracture of the calf muscles. J Bone Joint Surg Am. 1950;32:671–6. [PubMed] [Google Scholar]
- 15.Andreacchio A, Orellana CA, Miller F, Bowen TR. Lateral column lengthening as treatment for planovalgus foot deformity in ambulatory children with spastic cerebral palsy. J Pediatr Orthop. 2000;20:501–5. [PubMed] [Google Scholar]
- 16.Pirpiris M, Trivett A, Baker R, Rodda J, Nattrass GR, Graham HK. Femoral derotation osteotomy in spastic diplegia. Proximal or distal? J Bone Joint Surg Br. 2003;85:265–72. doi: 10.1302/0301-620x.85b2.13342. [DOI] [PubMed] [Google Scholar]
- 17.Keenan WN, Rodda J, Wolfe R, Roberts S, Borton DC, Graham HK. The static examination of children and young adults with cerebral palsy in the gait analysis laboratory: Technique and observer agreement. J Pediatr Orthop B. 2004;13:1–8. doi: 10.1097/00009957-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Graham HK, Harvey A, Rodda J, Nattrass GR, Pirpiris M. The functional mobility scale (FMS) J Pediatr Orthop. 2004;24:514–20. doi: 10.1097/00004694-200409000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Novacheck TF, Stout JL, Tervo R. Reliability and validity of the Gillette Functional Assessment Questionnaire as an outcome measure in children with walking disabilities. J Pediatr Orthop. 2000;20:75–81. [PubMed] [Google Scholar]
- 20.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214–23. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 21.Harvey A, Graham HK, Morris ME, Baker R, Wolfe R. The functional mobility scale: Ability to detect change following single event multilevel surgery. Dev Med Child Neurol. 2007;49:603–7. doi: 10.1111/j.1469-8749.2007.00603.x. [DOI] [PubMed] [Google Scholar]
- 22.Palisano RJ, Cameron D, Rosenbaum PL, Walter SD, Russell D. Stability of the gross motor function classification system. Dev Med Child Neurol. 2006;48:424–8. doi: 10.1017/S0012162206000934. [DOI] [PubMed] [Google Scholar]
- 23.Raja K, Joseph B, Benjamin S, Minocha V, Rana B. Physiological cost index in cerebral palsy: Its role in evaluating the efficiency of ambulation. J Pediatr Orthop. 2007;27:130–6. doi: 10.1097/01.bpb.0000242440.96434.26. [DOI] [PubMed] [Google Scholar]
- 24.Andersson C, Mattsson E. Adults with cerebral palsy: A survey describing problems, needs, and resources, with special emphasis on locomotion. Dev Med Child Neurol. 2001;43:76–82. doi: 10.1017/s0012162201. [DOI] [PubMed] [Google Scholar]
- 25.Bottos M, Feliciangeli A, Sciuto L, Gericke C, Vianello A. Functional status of adults with cerebral palsy and implications for treatment of children. Dev Med Child Neurol. 2001;43:516–28. doi: 10.1017/s0012162201000950. [DOI] [PubMed] [Google Scholar]
- 26.Gough M, Eve LC, Robinson RO, Shortland AP. Short-term outcome of multilevel surgical intervention in spastic diplegic cerebral palsy compared with the natural history. Dev Med Child Neurol. 2004;46:91–7. doi: 10.1017/s0012162204000192. [DOI] [PubMed] [Google Scholar]
- 27.Stansfield BW, Hillman SJ, Hazlewood ME, Lawson AM, Mann AM, Loudon IR, et al. Normalisation of gait data in children. Gait Posture. 2003;17:81–7. doi: 10.1016/s0966-6362(02)00062-0. [DOI] [PubMed] [Google Scholar]


