Abstract
Background:
The treatment options of bone loss with infections include bone transport with external fixators, vascularized bone grafts, non-vascularized autogenous grafts and vascularized allografts. The research hypothesis was that the graft length and intact ipsilateral fibula influenced hypertrophy and stress fracture. We retrospectively studied the graft hypertrophy in 15 patients, in whom vascularized fibular graft was done for post-traumatic tibial defects with infection.
Materials and Methods:
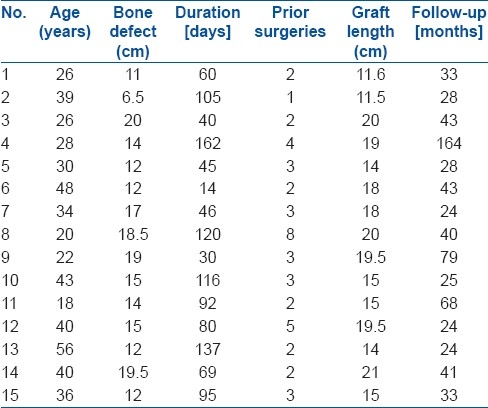
15 male patients with mean age 33.7 years (range 18 - 56 years) of post traumatic tibial bone loss were analysed. The mean bony defect was 14.5 cm (range 6.5 – 20 cm). The mean length of the graft was 16.7 cm (range 11.5 – 21 cm). The osteoseptocutaneous flap (bone flap with attached overlying skin flap) from the contralateral side was used in all patients except one. The graft was fixed to the recipient bone at both ends by one or two AO cortical screws, supplemented by a monolateral external fixator. A standard postoperative protocol was followed in all patients. The hypertrophy percentage of the vascularized fibular graft was calculated by a modification of the formula described by El-Gammal. The followup period averaged 46.5 months (range 24 – 164 months). The Pearson correlation coefficient (r) was worked out, to find the relationship between graft length and hypertrophy. The t-test was performed to find out if there was any significant difference in the graft length of those who had a stress fracture and those who did not and to find out whether there was any significant difference in hypertrophy with and without ipsilateral fibula union. The Chi square test was performed to identify whether there was any association between the stress fracture and the fibula union. Given the small sample size we have not used any statistical analysis to determine the relation between the percentage of the graft hypertrophy and stress fracture.
Results:
Graft union occurred in all patients in a mean time of 3.3 months, at both ends. At a minimum followup of 24 months the mean hypertrophy noted was 63.6% (30 – 136%) in the vascularized fibular graft. Ten stress fractures occurred in seven patients. The mean duration of the occurrence of a stress fracture in the graft was 11.1 months (2.5 – 18 months) postoperatively. The highest incidence of stress fractures was when the graft hypertrophy was less than 20%. The incidence of stress fractures reduced significantly after the graft hypertrophy exceeded 20%.
Conclusion:
In most cases hypertrophy of the vascularized fibular graft occurs in response to mechanical loading by protected weight bearing, and the amount of hypertrophy is variable. The presence or absence of an intact fibula has no bearing on the hypertrophy or incidence of stress fracture. The length of the fibular graft has no bearing on the hypertrophy or stress fracture.
Keywords: Vascularized fibular graft, hypertrophy of fibula, stress fracture fibula
INTRODUCTION
First described by Taylor in 1975, the vascularized fibular graft has become an important tool in the treatment of skeletal defects1 resulting from trauma, sepsis, or tumor resection. The problems are compounded by infection and poor condition of the soft tissues and previous multiple surgical procedures.2–4 The methods advocated in the treatment of infected bone gaps are bone transport with circular external fixators,5–7 vascularized bone grafts,8–10 non-vascularized autogenous graft,11,12 and vascularized allograft.13
One of the accepted methods to treat a post-traumatic tibial defect with infection is the vascularized fibular graft. The graft may be taken either from the contralateral or the ipsilateral limb.14–18 Vascularized bone grafts, by virtue of their inherent vascularity, unite more rapidly with the host bone and are more resistant to infection.19 Moreover, radical resection of the infected and necrotic bone and soft tissue can be undertaken. The ultimate function of the limb depends on the hypertrophy of the graft and the ability of the graft to withstand cyclical loading and fracture.20
The research hypothesis was that the graft length and intact ipsilateral fibula influenced hypertrophy and stress fracture. In this study, we have attempted to find out how much graft hypertrophy occurs at 24 months (minimum followup) and what degree of hypertrophy prevents or reduces the incidence of stress fractures in post-traumatic tibial defects with infection. We have also analyzed the relation of the graft length and influence of the intact or united fibula to hypertrophy and stress fracture.
MATERIALS AND METHODS
We retrospectively studied 15 patients with post traumatic infected tibial defects, who were treated with the vascularized fibular graft, between 1993 and 2006. All the patients were male and the average age was 33.7 years [range 18 – 56 years]. The location of the defect was in the middle third of tibia in eight cases, lower one-third of tibia in three cases, and upper third of tibia in four cases. The average bone defect was 14.5 cm [range 6.5 to 20 cm] and the graft length averaged 16.7 cm [range 11.5 – 21 cm]. The bony defect was measured with a scale at the time of the index procedure and depending on the amount of defect the appropriate length of the graft was harvested from the contralateral side In all cases except one, an osteoseptocutaneous flap (a bone flap with the attached overlying skin flap) was done. The vascularized fibular graft took care of the bone defect and the overlying skin flap took care of the soft tissue defect. In all cases the graft was placed as a single barrel strut, and harvested and fixed as one bone. In the presence of active infection, repeated surgical debridements were done in all cases along with appropriate antibiotics as dictated by the culture and sensitivity tests till the infection was controlled. The index procedure was done after the active infection was under control and when there was no pus drainage. The flap was based on the peroneal artery, which arose soon after the bifurcation from the posterior tibial artery. The limb vascularity was to be assessed if the peroneal artery was taken with the flap.
The surface marking of the fibula was done. The cutaneous perforator was marked using Doppler signals. The distal 7 to 8 cms of fibula above the ankle mortis was left intact. The skin paddle was marked to a breadth of about 8 to 9 cms. The skin was raised with the deep fascia. The fibular graft was fixed to the recipient tibia with one to two AO screws at each end of the graft and this was supplemented by a spanning external fixator with pins inserted into the tibia above and below the bone defect. Once the graft survived with respect to vascularity and there was no further danger to blood supply of vascularised fibular graft, the external fixator was removed and the above-knee plaster of Paris cast was put for a period of eight-to-twelve weeks, during which time the patient was non-weight-bearing and crutch walking. When the graft was seen to be uniting with the host bone, a gradual increase in protected weight bearing, with the aid of a Patellar Tendon Bearing cast or brace was started and this was continued till maximum graft hypertrophy (from 0-136%) was achieved. The period of followup ranged from 24 to 164 months [Table 1].
Table 1.
Clinical details of patients
The hypertrophy value was measured according to the formula described by El-Gammal et al.20 [Figure 1].
Figure 1.
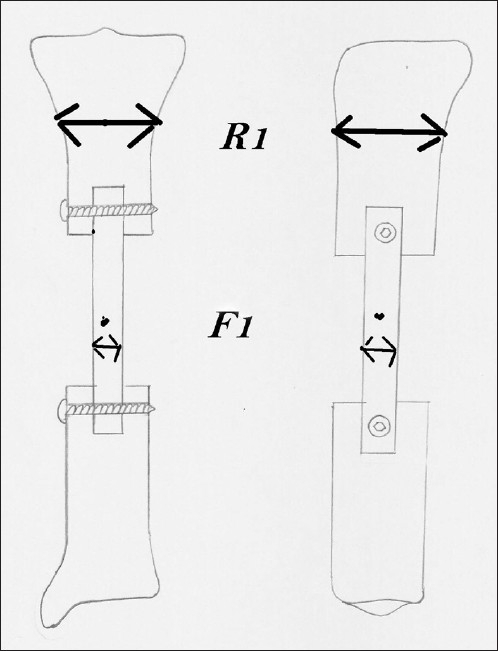
Diagram showing measurement of fibular width (F) and tibial width (R). The tibial width can be measures at any point on the tibia but the same point should be used for the particular patient
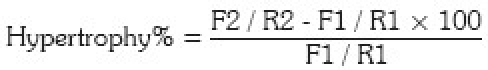
where F1 = mean immediate postoperative antero posterior and lateral width of the fibular graft, R1 = mean immediate postoperative antero posterior and lateral width of the recipient bone, F2 = mean antero posterior and lateral width of the fibular graft at 24 months and R2 = mean antero posterior and lateral width of the recipient bone on each followup X-rays.
The fibular graft width was measured at midpoint of the graft. The recipient bone width was the mean width of the recipient bone at a fixed point from the graft host junction according to the defect location. The same location was used for measurement in a particular patient in all the X-rays.
Statistical methods
Pearson correlation coefficient (r) was worked out to find the relationship between the graft length and hypertrophy. The t-test was performed to find out whether there was any significant difference in the graft length of those who had a stress fracture and those who had not, and to find out whether there was any significant difference in hypertrophy with and without fibula union. The Chi square test was performed to understand whether there was any association between a stress fracture and fibula union.
RESULTS
The average followup period is 46.5 months (range 24 - 164 months).
Graft union occurred in all patients in a mean time of 3.3 months (range 3-4 months) without any additional procedures [Table 2]. In 14 of the 15 cases (93%) significant hypertrophy was noted at 24 months and the mean hypertrophy achieved was 63.6% (0 – 136%) [Figure 2]. In one of the cases there was no graft hypertrophy at all, but an excellent callus had formed around the graft from the remnant periosteum and almost intact anteromedial cortex [Figure 3].
Table 2.
Time to union and hypertrophy
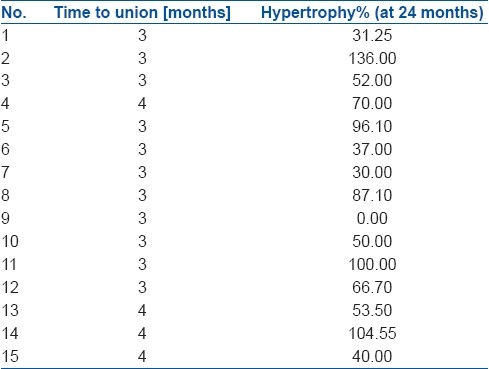
Figure 2.
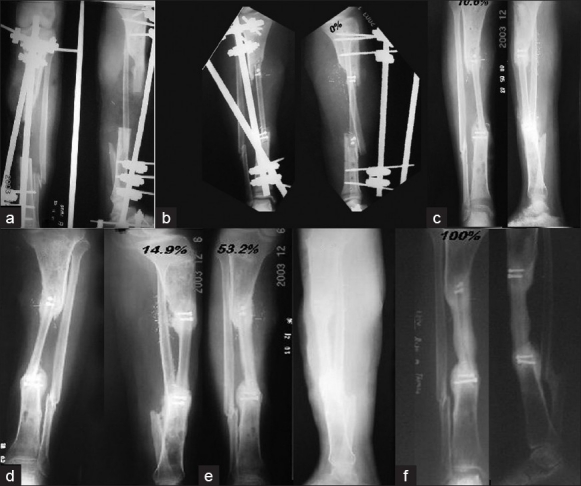
X-ray anteroposterior and lateral view of a twenty year old male showing (a) a 14 cm bone defect (b) immediate postoperative X-ray(c) four months later - minimal hypertrophy of 10.6% (d) eight months later - marginal increase in hypertrophy 14.9% (e) eleven months later - 53.2% increase of hypertrophy (f) 24 months - 100% hypertrophy.
Figure 3.
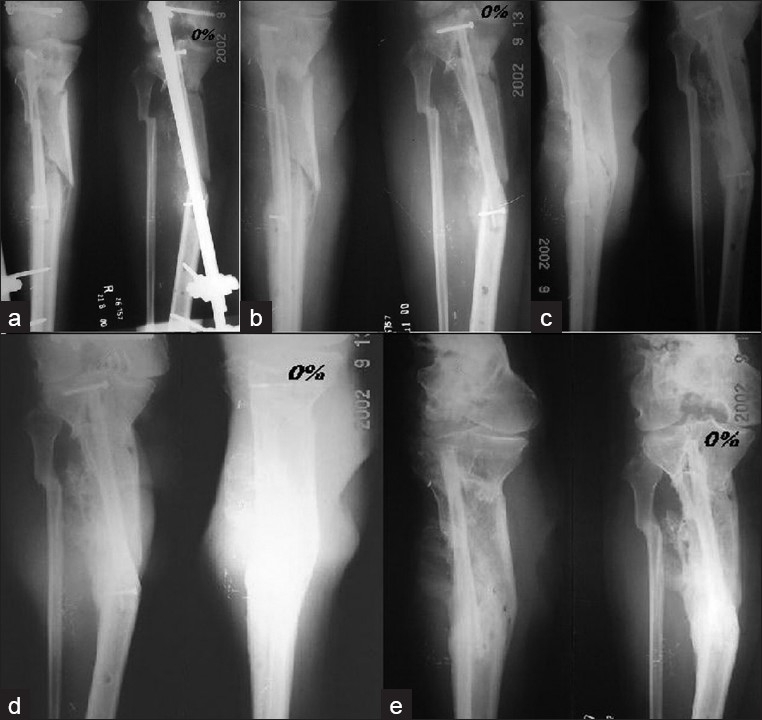
(a) Postoperative X-rays (anteroposterior view) of a 22 year old male showing proximal tibial hemicortical defect. (b) Three months – 0% hypertrophy. (c) Six months later – 0% hypertrophy – note callus formation from residual periosteum. (d) Twelve months later – 0% hypertrophy – note callus formation from residual periosteum. (e) Twenty-four months later – 0% hypertrophy – note solid bridging callus
Ten stress fractures occurred in seven patients in our series. The mean time of occurrence of stress fractures was 11.1 months (range 2.5 – 14 months) after the index procedure. The highest incidence of stress fractures was when the hypertrophy value was below 20%. The mean hypertrophy achieved was 63.6% (range 0 – 136%). The amount of hypertrophy each patient achieved is listed in Table 2. The patient who achieved 0% hypertrophy [patient 9] had solid callus formation with no hypertrophy and did not sustain any stress fractures [Figure 3]. Stress fractures occurred in patient numbers: 3, 5, 6, 8, 12, 14, 15. The incidence of stress fractures progressively decreased as the graft hypertrophied [Table 3].
Table 3.
Percentage of hypertrophy and stress fractures

As regards the graft length and hypertrophy, the Pearson correlation coefficient (r) was worked out and showed a negative, but statistically insignificant correlation, (r = 0.182, P value = 0.516). To determine the relation between the graft length and stress fracture, a t-test was performed, which indicated that the average graft length of those who had a fracture was significantly higher than that of those who had not at the 10% level (t-value = 1.817 P value = 0.092). In this series all the stress fractures were treated with an above-knee cast, for a period of six weeks. Out of ten stress fractures, nine united with this treatment. The one fracture that did not unite was in patient no: 14 (which occurred 14 months after the index procedure). This fracture went into a hypertrophic non-union, which was clinically stiff, but the patient could walk with protective patellar tendon brace without pain.
We also attempted to study if there was any role of the ipsilateral fibula in influencing graft hypertrophy. We found that the continuity or discontinuity of the ipsilateral fibula (11 had intact fibula and 4 did not have) had no bearing on the amount of graft hypertrophy. [t-test value = 0.902 P value = 0.384]. Also there was no significant association between the intact fibula and stress fracture. [Chi square test Chi square value = 1.759 P value = 0.185]
Thirteen patients returned to work, of which three changed occupation due to a stiff knee joint [n=2] and equinus deformity [n=1]. Knee stiffness occurred in three patients, all of whom had proximal tibial defects. One patient had shortening of two inches and another patient had a varus-procurvatum deformity after a stress fracture of the graft. There were no postoperative infections, which needed active treatment. There were four cases of residual discharging sinus, which needed curettage and screw removal. In one of our cases there was partial flap loss (loss of the skin paddle) due to either venous or arterial obstruction.
DISCUSSION
Our series consisted of post-traumatic tibia infected bone defects. We have noted graft hypertrophy in all our cases except one. Various articles have reported hypertrophy incidence ranging from 38 – 90%.20–24 Reduced hypertrophy was seen when the graft was by-passed with internal fixation,23 but some authors found no difference in the hypertrophy with regard to the method of fixation.20,21 When cancellous grafts were placed along the fibular graft no hypertrophy was seen after the cancellous graft ossified, due to stress shielding.24 In our series we noted a varying degree of hypertrophy, although we are not sure why this is so considering that we have used the same method of skeletal stabilization and a standard postoperative protocol. The only factor that was not standardized and not in the authors control was the amount of weight bearing the patient did on the affected limb. Probably this could be the reason for the varying degree of hypertrophy.
The most objective evaluation of hypertrophy of the vascularized fibular graft was shown by Ikeda et al.,25 with the aid of computed tomographic scanning. They had proven that hypertrophy occurred with mechanical loading, but the size of the medullary canal of the graft did not change and the portion of the graft in the medullary canal of the tibia did not hypertrophy, implying stress protection. Even on 15 years followup of the two cases, they showed no further hypertrophy than what was noted earlier, implying adequate strength to carry the physiological load had been achieved.26 We have used a similar method of graft fixation to the tibia and supplemental external fixation in our series. Although CT scanning is excellent method to measure graft hypertrophy objectively, we feel it is not practical to use it as a routine method, due to cost considerations. Falder27 noted graft hypertrophy in all his lower limb reconstructions and noted that the etiology had no bearing on the amount of hypertrophy of union of the graft to the tibia. We have noticed that in one of our cases there was extensive callus formation around the defect with the result that in this case no graft hypertrophy was seen. This could be taken as an example of stress shielding [Figure 3]. The callus probably arose from the residual periosteum. Similar lack of hypertrophy has been noted by Ikeda who showed on computed tomographic scanning, the absence of hypertrophy in those cases of tibial pseudoarthrosis, where there were either no bone defects or a partial cortical defect where there was bone to share the load.25,26
Stress fractures occurred 47% of our cases, at a mean time of 11.1 months, after the index procedure. Various articles have mentioned 20 – 60% incidence of stress fractures. The incidence of stress fractures was more when the graft was not by-passed with internal fixation.19,23,27,28 In our series the union rate for stress fractures with POP cast was 90%. Other authors have reported union rates varying form 0 – 83%.19,23,27,28 Application of an Ilizarov frame may reduce the incidence of stress fractures.27 We have noted, as in other series of vascularized fibular grafts, that graft length had no relation to stress fractures,23,27 unlike in non-vascularized grafts.29 It is opined that the graft should be protected, but mechanical loading should gradually be increased so as to stimulate graft remodeling.27 We are also in agreement with this view. Although bypassing internal fixation reduces the incidence of stress fractures, the amount of hypertrophy that occurs is also reduced.23
Regarding the union of the graft with the recipient bone we had no incidence of non-union in our series. Our mean union time was 3.3 months. Other authors have reported varying union rates ranging from 80 – 100%.19,20,23,27,28,30 The union rates were not influenced by the method of fixation,20,30 but reduced union rates were reported in infected cases by Han.30 As regards the time for union, variable times have been mentioned, ranging from three to eight months.20,22,25,27,28,30,31
We had not performed bone scintigraphy in any of our cases as most of our cases were osteoseptocutaneous flaps and the skin flap serves as a monitor for flap viability. Moreover, it may not be reliable to determine viability of the bone graft.14,32 That bone responds to loading by hypertrophy has been documented in our series as well as in literature. Bone responds by an increase in thickness, but there is no change in the mechanical properties of the bone.25,33,34
CONCLUSIONS
The hypertrophy of the vascularized fibular graft done for infected tibial bone defects occurs in response to mechanical loading by protected weight bearing. Stress fractures occur, but unite readily in a plaster cast and the incidence is markedly reduced when the graft hypertrophies more than 20%. The presence or absence of an intact ipsilateral fibula has no bearing on hypertrophy or the incidence of a stress fracture. The length of the fibular graft has no bearing on hypertrophy or stress fracture.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Taylor GI, Graeme D, Miller H, Ham FJ. The free Vascularized bone graft: A clinical extension of microvascular techniques. Plast Reconst Surg. 1975;55:533. doi: 10.1097/00006534-197505000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Bishop AT, Wood MB, Sheetz KK. Arthrodesis of the ankle with a free vascularized autogenous bone graft. J Bone Joint Surg Am. 1995;77:1867–75. doi: 10.2106/00004623-199512000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Arai K, Toh S, Yasumura M, Okamoto Y, Harata S. One bone forearm formation using vascularized fibula graft for massive bone defect of the forearm with infection: Case report. J Reconstr Microsurg. 2001;17:151–3. doi: 10.1055/s-2001-14345. [DOI] [PubMed] [Google Scholar]
- 4.Ring D, Jupiter JB, Toh S. Transarticular bony defects after trauma and sepsis: Arthrodesis using vascularized fibular transfer. Plast Reconstr Surg. 1999;104:426. doi: 10.1097/00006534-199908000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Dagher F, Roukoz S. Compound tibial fractures with bone loss treated by ilizarov technique. J Bone Joint Surg Br. 1991;73:316. doi: 10.1302/0301-620X.73B2.2005164. [DOI] [PubMed] [Google Scholar]
- 6.Paley D, Marr DC. Ilizarov bone transport treatment for tibial defects. J Orthop Trauma. 2000;14:76–85. doi: 10.1097/00005131-200002000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Catagni MA, Camagni M, Combi A, Ottaviani G. Medial fibula transport with the ilizarov frame to treat massive tibial bone loss. Clin Orthop Relat Res. 2006;448:208–16. doi: 10.1097/01.blo.0000205878.43211.44. [DOI] [PubMed] [Google Scholar]
- 8.Harrison DH. The osteocutaneous free fibular flap. J Bone Joint Surg Br. 1986;68:804. doi: 10.1302/0301-620X.68B5.3782250. [DOI] [PubMed] [Google Scholar]
- 9.Ikeda K, Yokoyama M, Okada K, Tomita K, Yoshimura M. Long term follow up of the vascularized Iliac Crest Graft. Microsurgery. 1998;18:419–23. doi: 10.1002/(sici)1098-2752(1998)18:7<419::aid-micr6>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 10.Buncke HJ, Furnas DW, Gordon L, Achauer BM. Free osteocutaneous flap from a rib to the tibia. Plast Reconstr Surg. 1977;59:799. doi: 10.1097/00006534-197706000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Steinlechner CW, Mkandawire NC. Non vascularized fibular transfer in the management of defects of long bones after sequestrectomy in children. J Bone Joint Surg Br. 2005;87:1259. doi: 10.1302/0301-620X.87B9.15734. [DOI] [PubMed] [Google Scholar]
- 12.Green S. Skeletal defects comparison of bone grafting and bone transport for segmental skeletal defects. Clin Orthop. Relat Res. 1994;301:50. [PubMed] [Google Scholar]
- 13.Hoffman GO, Kirschner MH, Buhren V, Land W. Allogenic vascularized transplantation of human femoral diaphysis under cyclosporin A immunosupression. Transl Int. 1995;8:418. doi: 10.1007/BF00337179. [DOI] [PubMed] [Google Scholar]
- 14.Weiland AJ, Moore JR, Daniel RK. Vascularized bone grafts: experience with 41 cases. Clin Orthop Relat Res. 1983;174:87–95. [PubMed] [Google Scholar]
- 15.Toh S, Tsubo K, Nishikawa S, Narita S, Kanno H, Harata S. Ipsilateral pedicle vascularized fibula grafts for reconstruction of tibial defects and non-unions. Reconstr Microsurg. 2001;17:487. doi: 10.1055/s-2001-17751. [DOI] [PubMed] [Google Scholar]
- 16.Yokoyamma K, Itoman M, Nakamura K, Tsukamoto T, Saita Y, Aoki S. Free vascualrized fibular graft vs.ilizarov method for post traumatic tibial bone defect. Reconstr Microsurg. 2001;17:17. doi: 10.1055/s-2001-12684. [DOI] [PubMed] [Google Scholar]
- 17.Keating JF, Simpson AH, Robinson CM. The management of fractures with bone loss. J bone Joint Surg Br. 2005;87:142. doi: 10.1302/0301-620x.87b2.15874. [DOI] [PubMed] [Google Scholar]
- 18.Chacha PB, Ahmed M, Daruwala JS. Vascular pedicle graft of the ipsilateral fibula for non-union of the tibia with a large defect. J Bone Joint Surg Br. 1981;63:244. doi: 10.1302/0301-620X.63B2.7217150. [DOI] [PubMed] [Google Scholar]
- 19.Chew WY, Low CK, Tan SK. Long-term results of free vascularized fibular graft: A clinical and radiographic evaluation. Clin Orthop Relat Res. 1995;311:258–61. [PubMed] [Google Scholar]
- 20.El-Gammal TA, El-Sayed AM, Kotb MM. Hypertrophy after free vascularized fibular transfer to lower limb. Microsurgery. 2002;22:367. doi: 10.1002/micr.10066. [DOI] [PubMed] [Google Scholar]
- 21.Lazar E, Rosenthal DI, Jupiter J. Free vascularized fibular grafts: Radiographic evidence of remodeling and hypertrophy. AJR Am J Roentgenol. 1993;161:613. doi: 10.2214/ajr.161.3.8352118. [DOI] [PubMed] [Google Scholar]
- 22.Wada T, Usui M, Nagoya S, Isu K, Yamawaki S, Ishii S. Resection arthrodesis of the Knee with a Vascularized Fibular Graft. J Bone Joint Surg Br. 2000;82:489–93. doi: 10.1302/0301-620x.82b4.10574. [DOI] [PubMed] [Google Scholar]
- 23.de Boer HH, Wood MB. Bone changes in the vascularized fibular graft. J Bone Joint Surg Br. 1989;71:374–8. doi: 10.1302/0301-620X.71B3.2722923. [DOI] [PubMed] [Google Scholar]
- 24.Bos KE, Besselaar PP, vd Eijken LW, Raaymakers EL. Failure of hypertrophy in re vascularized fibula grafts due to stress protection. Microsurgery. 1996;17:366–70. doi: 10.1002/(SICI)1098-2752(1996)17:7<366::AID-MICR4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 25.Ikeda K, Tomita K, Hashimoto F, Morikawa S. Long-term follow-up of vascularized bone grafts for the reconstruction of tibial nonunion: Evaluation with computed tomographic scanning. J Trauma. 1992;32:693–7. doi: 10.1097/00005373-199206000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Yoshimura M, Ikeda K, Hashimoto F, Okada K, Tomita K. Fate of the vascularized fibular graft: Report of two cases with 15 year follow-up. J Trauma Injury Infect Crit Care. 1999;47:777–9. doi: 10.1097/00005373-199910000-00029. [DOI] [PubMed] [Google Scholar]
- 27.Falder S, Sinclair JS, Rogers CA, Townsend PL. Long-term behaviour of the free vascularized fibula following reconstruction of large bony defects. Br J Plast Surg. 2003;56:571–84. doi: 10.1016/s0007-1226(03)00186-3. [DOI] [PubMed] [Google Scholar]
- 28.Hsu RW, Wood MB, Sim FH, Chao EY. Free vascularized fibular grafting for reconstruction after tumor resection. J Bone Joint Surg Br. 1997;79:36–42. doi: 10.1302/0301-620x.79b1.6818. [DOI] [PubMed] [Google Scholar]
- 29.Enneking WF, Lady JL, Burchardt H. Autogenous cortical bone grafts in reconstruction of segmental skeletal defects. J Bone Joint Surg Am. 1980;62:1039–57. [PubMed] [Google Scholar]
- 30.Han CS, Wood MB, Bishop AT, Cooney WP. Vascularized bone transfer. J Bone Joint Surg Am. 1992;74:1441–9. [PubMed] [Google Scholar]
- 31.Imran Y, Zulmi W, Halim AS. Skeletal union following long bone reconstruction using vascularized fibula graft. Singapore Med J. 2003;44:286–7. [PubMed] [Google Scholar]
- 32.Berggren A, Weiland AJ, Ostrup LT. Bone scintigraphy in evaluating the viability of composite bone grafts revascularized by micro vascular anastomoses, conventional autogenous bone grafts, and free non revascularized periosteal grafts. J Bone Joint Surg Am. 1982;64:799–809. [PubMed] [Google Scholar]
- 33.Woo SL, Kuei SC, Amiel D, Gomez MA, Hayes WC, White FC, et al. The effect of prolonged physical training on the properties of long bone: A study of wolfs law. J Bone Joint Surg Am. 1981;63:780–7. [PubMed] [Google Scholar]
- 34.Goodship AE, Lanyon LE, McFie H. Functional adaptation of bone to increased stress. J Bone Joint Surg Am. 1979;61:539–46. [PubMed] [Google Scholar]


