Abstract
Tea, the commonly consumed beverage, is gaining increased attention in promoting overall health. In specific, green tea is considered a healthful beverage due to the biological activity of its polyphenols namely catechins. Among the polyphenols Epigallo catechin 3 gallate and Epicatechin 3 Gallate are the most predominant catechins. The antioxidant, antimicrobial, anticollagenase, antimutagenic, and c hemopreventive properties of these catechins proved to be helpful in the treatment of chronic diseases like periodontal disease. Studies have demonstrated that the type of processing mainly effects the concentration of catechins. Several epidemiological studies have proved that green tea also has some general health benefitting properties like antihypertensive, reduction of cardiovascular risk, antibacterial, antiviral, and antifungal activity. The present review concentrates on the effects of green tea in periodontal and general health.
Keywords: Catechins, green tea, periodontal health
INTRODUCTION
Food stuff can be regarded functional if they satisfactorily demonstrate to affect beneficially one or more target functions in the body, beyond adequate nutritional effects to maintain health or reduction of risk of disease. Green tea has been proved to have many functional properties and at present its consumption is widely recommended.[1–3]
Green tea is widely consumed in China, Japan, Korea, and Morocco.[1,4] It has been considered as a healthy beverage since ancient times. The traditional Chinese medicine has recommended this plant for headaches, body aches, general pain, digestion, depression, as an energizer, and in general to prolong life.[5] Green tea also has many oral health benefits. It has cognitive function and positive impact on bone density, caries, periodontal disease, and diabetes.[6]
Since few years numerous epidemiological and clinical studies have revealed several physiological responses to green tea that may be relevant in promoting health and preventing or treating some chronic diseases.[5]
HISTORY
The word tea has been used to describe the shrub camellia sinensis.[7] It is the second most consumed beverage in the world aside from water, coffee, and carbonated soft drinks.[8] Tea originated in China as long as 2700 BC and became popular worldwide due to its economic and therapeutic aid in many illnesses.[7] Approximately 76–78% of the tea produced and consumed is black tea, 20–22% is green tea, and <2% is oolong tea.[1] The fresh leaves of the tea plant are picked as “two and a bud” for processing (also termed Flush). Green tea is obtained by macerating and heat drying this flush, whereas black tea is derived by fermentation of flush before heat drying. Both types of tea share many pharmacologically active components although at variable concentrations.[7]
COMPOSITION OF THE GREEN TEA
The chemical composition of green tea is complex. The components are illustrated in Tables 1 and 2, of which the flavonoids are more important in periodontal health.
Table 1.

Table 2.
Catechins[6]

Green tea also contains Gallic acid (GA) and other Phenolic acids such as chlorogenic acid, caffeic acid, and flavanoids such as kaempferol, myricetin, and quercetin.[11] Wu and Wei[4] indicated a cup of green tea (2.5 g of green tea leaves/200 ml of water) may contain 90 mg of Epigallocatechin -3 gallate (EGCG). Lin et al.[12] analyzed 31 commercial teas, and detected that the levels of EGCG and total catechins were in the following order: Green tea (old leaves) ≥green tea (young leaves) ≥an oolong tea ≥black tea and Pu-Erh tea. The amounts of catechins were always higher in green tea. EGCG and EGC were major catechins present with average contents of 7.358% and 3.955%, respectively; ECG and EC values are 0.910 and 3.556% respectively.[5]
PROCESSING OF GREEN TEA

CLASSIFICATION
Depending on the manufacturing process, teas are classified into three major types:[6,13]
Non-fermented green tea (produced by drying and steaming the fresh leaves to inactivate the polyphenol oxidase by nonoxidation).
Semi-fermented oolong tea (produced by partial fermentation of fresh leaves before drying)
Fermented black and red tea (Pu-Erh) by post harvest fermentation before drying and steaming.
MODES OF CONSUMPTION
As mouth wash
As local drug delivery
As chewing gum
FACTORS AFFECTING THE CONTENTS OF CATECHINS
Type of green tea (e.g., Blended, decaffeinated, instant).
Preparation of infusion (e.g. Amounts of the product used, brew time, temp).
Growing conditions (soil, climate, agricultural practices, fertilizers).
Geographical location.
McKay and Blumberg[6] reported that decaffeination slightly reduces the tea catechin content; also instant preparations and iced and ready-to-drink teas present less content of catechins.[16,17] The production of bottle green tea beverage encountered a brewing problem mainly caused by oxidation of catechins.[18]
BIOAVAILABILITY
Surprisingly, little is known about the bioavailability and absorption of catechins from tea beverages. In humans, EGCG may be less bioavailable than other green tea catechins. Peak plasma levels are reached within 2 to 4 h after ingestion.[19] Average peak plasma concentrations after single dose of 1.5 mmol of green tea were 5.0 μml/L-EGC,3.1 μmol/L -ECG, 1.3μmol/L -EGCG. After 24 h, plasma EGC, and EGCG returned to base line but plasma levels of ECG remained elevated.[20] The effect of green tea drinking may also differ by genotype.[21] There appear to be species differences in bioavailability of EGCG compared to other tea catechins. Addition of milk to tea does not alter catechin absorption. However, it may affect the antioxidant potential of tea depending upon milk fat content and volume of milk added.[22–26] Xu et al.[26] observed that epimerization reaction occurring in manufacturing canned and bottled tea drinks could not significantly affect antioxidant activity and bioavailability of total tea polyphenols. One hundred milliliter of green tea contains approximately 50–100 micrograms of tea catechins.
BIOLOGICAL ACTIVITY OF TEA COMPONENTS (CATECHINS)
Anti oxidative
Green tea polyphenols are responsible for its antioxidant activity either directly by scavenging of reactive oxygen and nitrogen species and chelating redox-active transition of metal ions like iron and copper or indirectly by inhibition of pro oxidant enzymes, redox sensitive transcription factors, and induction of antioxidant enzymes.[5,27,28]
Capacity to modulate the physical structure of cell membranes
This mechanism may be influenced by the influence of catechins with the cellular phospholipid palisade. EGCG has shown to induce apoptotic cell death and cell cycle arrest in tumor cells.[29–33]
Antimicrobial activity
EGC, EGCG, and ECG constitute the most important antibacterial agents on methicillin resistant Staphylococcus aureus, Helicobacter pylori and α-Hemolytic streptococcus.[34–36]
Anticariogenic activity
Catechins are found to be inhibitory against Streptococcus mutans and Streptococcus sobrin at minimum inhibitory concentration (MIC) ranging between 50–1000 μg/ml.[37]
EFFECTS OF GREEN TEA ON PERIODONTAL HEALTH
Kaneko et al.[38] 1993 found that a four-week regimen of mouth washing with a dilute catechin solution reduced the mouth odor (halitosis) associated with periodontal disease. Tea catechins especially EGCG deodorizes methyl mercaptain, the main cause of halitosis.[39] EGCG (active at 250–500 μg/ml) inhibited growth and adhesion of Porphyromonas gingivalis to buccal epithelial cells.[40] Hirasawa et al.[14] demonstrated bactericidal activity of green tea catechins against prevotella and P. gingivalis at concentration of 1 mg/ml. They found significant reduction in markers of gingivitis after the use of slow release buccal delivery system applied over a period of 8 weeks. More recent studies have shown that some virulence factors (toxic metabolites, protein tyrosine phosphatase, and gingipains) and aetiological agents of periodontal disease are neutralised by EGCG [Table 3].[41,42]
Table 3.
Studies on effects of green tea on periodontal health
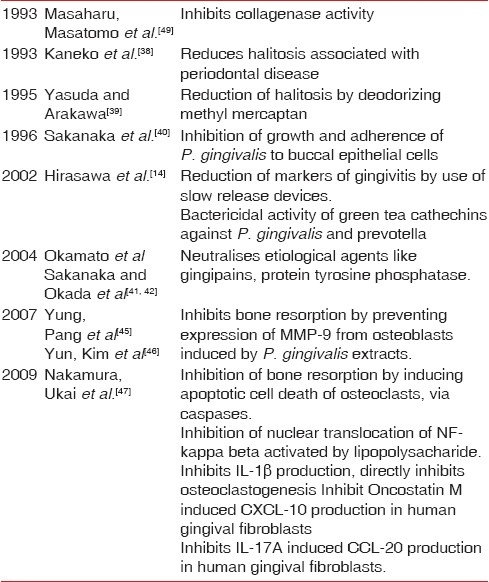
Effects on various periodontal pathogens
Green tea polyphenols inhibit the collagenase activity of oral bacteria. EGCGs the predominant component of green tea polyphenols inhibits both eukaryotic and prokaryotic cell derived collagenase activity. EGCG completely inhibits the growth of three strains of P. gingivalis at concentration of 250 or 500 μg/ml and that of P. melaninogenicus at MICs of 2000 μg/ml.[43] Other polyphenols were not as effective as EGCGs and their MICs against P. gingivalis and P. melaninogenicus were 1000 μg/ml or >2000 μg/ml. The numbers of adherent bacterial cells decrease in a dose dependent manner. This inhibitory effect was pronounced with polyphenols having a galloyl moiety linked with an ester linkage i.e. (EGCG, GCG, ECG) linkage with 3-OH of catechin structural molecules. The higher the concentration of EGCG and ECG the more severe the inhibition. This mechanism is mainly by binding of polyphenols to fimbriae of P. gingivalis. Inhibitory activity was observed against r-gingipain and to lesser extent against K-gingipain of P. gingivalis. Green tea catechins inhibit enzymatic activities of P. gingivalis, in a manner similar to that of Chlorhexidine, Doxycycline, and non-antimicrobial chemically modified tetracycline derivatives. EGCG inhibit protein tyrosine phosphatase activity in P. intermedia. It also possesses bactericidal activity against a variety of microorganisms like Helicobacer pylori and inhibits extracellular release of verotoxin from enterohaemorrhagic Escheresia coli. Epigallacto catechin gallate is synergistic with lactams for effective killing of methicillin-resistant Staphalococcus aureus. Epigallacto catechin gallate also inhibits the histamine release from rat basophilic leukemia cell, and exhibit selective immunomodulatory effect on cytokine formation.
Effects on host defense, human gingival cells, and inflammatory response
Green tea catechins were reported to be effective in preventing gingival and periodontal inflammation. EGCG inhibit the m-RNA expression of COX-2, MMP-1, IL-1, 6 and 8 by cultured cells. Effective concentration to achieve these effects was ≥1 μg/ml. EGCG inhibit the activation of NF-κB (Nuclear Factor–kappa B), which is one of the key positive regulators of COX- 2 expression.[44]
Effects on bone and bone cells
EGCG inhibited bone resorption by inducing the apoptotic cell death of osteoclasts and osteoclasts like multinucleated cells in a dose dependent manner (25–100 μm) mainly by involvement of caspases. Caspases are major regulatory enzymes in apoptotic pathway predominantly caspase 3. However, the mechanism by which EGCG modulates caspase activation is yet to be elucidated. EGCG is highly effective in inhibiting the survival of cell-derived osteoclasts in vitro. EGCG prevents increased matrix metalloproteinase expression from osteoblasts induced by P. gingivalis extracts. EGCG caused the reduction in MMP activities by inhibiting the gene expression of MMP-2 and MMP-9 via Mitogen Activated Protein Kinase) signaling pathway. EGCG inhibited the osteoclast formation induced by 1 α, 25 (OH)2 D 3 (10 -8M). Several signaling pathways could also be involved in EGCG-induced apoptosis of oseoclasts as well as caspase activity. EGCG has an effect on differentiated osteoclasts but not on undifferentiated cells, i.e., at appropriate dose inhibits osteoclasts but not osteogenic cells.[45,46] EGCG may not have advantage as a chemopreventive agent specific for osteoclasts. So these in vitro effects need to be studied in vivo to develop EGCG as a therapeutic agent for treatment of bone diseases.
Effect of catechin on lipopolysacharide induced bone resorption in vivo
The alveolar bone resorption and IL-1β expression induced by lipopolysacharide in gingival tissue were significantly decreased by injection or oral administration of green tea catechins (GTC). GTC inhibits nuclear translocation of NF-kappaβ activated by LPS.[47]
Effects on chondrocytes
EGCG inhibits IL-1β induced cartilage proteoglycan degradation and expression of MMP-1 and MMP-13 in human chondrocytes at micromolar concentration. Complete inhibition of MMP-1 and MMP-13 at a concentration of 100 μg EGCG was observed. This concentration can be achieved only by local administration and not by oral consumption. MMP-13 is more sensitive to the inhibitory effect even at lower conc. This inhibitory effect is by inhibition of IL-1β induced expression of m-RNAs signifying that the effect is at transcriptional level.[48] So EGCG may inhibit the activities of MMPs involved in the degradation of native collagen and this may have suppressive effects on the cartilage degradation in arthritic joints.
Effect on collagenase activity
Among the tea catechins ECG and EGCG with galloyl radical, showed the most potent inhibition effect on collagenase activity when an optimal concentration of tea catechins (100 μg/ml) was added to reaction mixture containing collagenase and collagen.[49] The steric structure of 3 galloyl radical is important for inhibition of collagenase activity. Tea catechins may aggregate rather than denature collagenase molecule which results in inhibition of enzyme activity (unpublished data).
Effect on cxcl-I0 (Cxc chemokine ligand 10) production from oncostatin-M stimulated human gingival gibroblasts
EGCG and ECG inhibits Oncostatin M (OS-M) induced CXCL-10 production in Human Gingival Fibroblasts (HGFS). CXC chemokine ligand 10 (CXCL 10) plays a pivotal role in recruitment of Th1 cells, and thus, in the development of periodontal disease.[50]
Effect on Ccl20 (Chemokine ligand 20) production in Il-17 A stimulated human gingival fibroblasts
Catechins (EGCG and ECG) inhibit IL- 17 A-induced CCL 20 production in human gingival fibroblasts.[51] Inhibition of P38 MAPK or extracellular signal regulated Kinase (ERK) decreased IL -17A induced CCL 20 production.
SCOPE FOR RESEARCH IN APPLICATION OF GREEN TEA CATECHINS FOR PERIODONTAL HEALTH
As green tea extract has so many effects on periodontal pathogens and periodontal tissues its application as local delivery systems like strips, chips, and fibers for the treatment of periodontal disease or in combination with regenerative materials to improve periodontal regeneration should be focused.
CONCLUSION
The changing trends in the etiopathogenesis and prevention of periodontal disease are innumerable. From the point of eradication of the periodontal pathogens to regeneration of complete periodontium various treatment modalities are suggested. Green tea extract may have numerous effects on periodontal pathogens and periodontal tissues. Greater the concentration of catechins better the health benefits. So the consumption of green tea in comparison to other beverages may be widely recommended.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Wu CD, Wei GX. Tea as a functional food for oral health. Nutrition. 2002;18:443–4. doi: 10.1016/s0899-9007(02)00763-3. [DOI] [PubMed] [Google Scholar]
- 2.Diplock AT, Aggett PJ, Ashwell M, Bornet F, Fern EB, Roberfroid MB. Scientific concepts of functional foods in Europe consensus document. Br J Nutr. 1999;81:1–27. [PubMed] [Google Scholar]
- 3.Roberfroid MB. Global view on functional foods: European perspectives. Br J Nutr. 2002;88:133–8. doi: 10.1079/BJN2002677. [DOI] [PubMed] [Google Scholar]
- 4.Zuo Y, Chen H, Deng Y. Simultaneous determination of catechins, caffeine and galic acids in Green, Oolong, Black and Pu-erh teas using HPLC with a photodiode array detector. Talanta. 2002;57:307–16. doi: 10.1016/s0039-9140(02)00030-9. [DOI] [PubMed] [Google Scholar]
- 5.Carmen C, Reyes A, Rafael G. Beneficial Effects of Green Tea- A Review. Journal of the American College of Nutrition. 2006;25:79–99. doi: 10.1080/07315724.2006.10719518. [DOI] [PubMed] [Google Scholar]
- 6.McKay DL, Blumberg JB. The role of tea in human health: an update. J Am Coll Nutr. 2002;21:1–13. doi: 10.1080/07315724.2002.10719187. [DOI] [PubMed] [Google Scholar]
- 7.Taylor PW, Hamilton-Miller JM, Stapleton PD. Antimicrobial properties of green tea catechins. Food Sci Technol Bull. 2005;2:71–81. doi: 10.1616/1476-2137.14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rietveld A, Wiseman S. Antioxidant effects of tea: evidence from human clinical trials. J Nutr. 2003;133:3275–84. doi: 10.1093/jn/133.10.3285S. [DOI] [PubMed] [Google Scholar]
- 9.Belitz DH, Grosch W. Food.sci.technol. Zaragoza: Acribia; 1997. Quimica de los Alimentos; pp. 71–81. [Google Scholar]
- 10.Vison J, Dabbagh Y, Serry M, Jand J. Plant flavonoids, especially tea flavonols, are powerful using an in vitro oxidation model for heart disease. J Agric Food Chem. 1995;43:2800–2. [Google Scholar]
- 11.USDA Database for the Flavonoid Contents of Selected Foods. Beltsville: US Department of Agriculture; 2003. USDA. [Google Scholar]
- 12.Lin YS, Tsai YJ, Tsay JS, Lin JK. Factors affecting the levels of tea polyphenols and caffeine in tea leaves. J Agric Food Chem. 2003;51:1864–73. doi: 10.1021/jf021066b. [DOI] [PubMed] [Google Scholar]
- 13.Willson KC. New York: CABI publishing; 1999. Coffee, Cocoa and Tea; pp. 71–81. [Google Scholar]
- 14.Hirasawa M, Takada K, Makimura M, Otake S. Improvement of periodontal status by green tea catechin using a local delivery system: a clinical pilot study. J Periodontal Res. 2002;37:433–8. doi: 10.1034/j.1600-0765.2002.01640.x. [DOI] [PubMed] [Google Scholar]
- 15.Newman, Takei, Klokkevold, Carranza . Fermin A carranza. 10th ed. Delhi: Elsevier; 2007. Carranza's Clinical periodontology. 2007, Elsevier, Delhi 10 th ed; p. 339. [Google Scholar]
- 16.Hakim IA, Harris RB, Weisgerber UM. Tea intake and squamous cell carcinoma of the skin: influence of type of tea beverages. Cancer Epidemiol Biomarkers Prev. 2000;9:727–31. [PubMed] [Google Scholar]
- 17.Arts IC, van De Putte B, Hollman PC. Catechin contents of foods commonly consumed in The Netherlands.2. Tea, wine, fruit juices, and chocolate milk. J Agric Food Chem. 2000;48:1752–7. doi: 10.1021/jf000026+. [DOI] [PubMed] [Google Scholar]
- 18.Wang LF, Kim DM, Park JD, Lee CY. Various antibrowning agents and green tea extract during processing and storage. J Food Process Press. 2003;27:213–25. [Google Scholar]
- 19.Yang CS, Chen L, Lee MJ, Balentine D, Kuo M, Schantz SP. Blood and urine levels of tea catechins after ingestion of different amounts of green tea by human volunteers. Cancer Epidemiol Biomarkers Prev. 1998;7:351–4. [PubMed] [Google Scholar]
- 20.Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003;43:89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- 21.Loktionov A, Bingham SA, Vorster H, Jerling JC, Runswick SA, Cummings JH. Apolipoprotein E genotype modulates the effect of black tea drinking on blood lipids and blood coagulation factors: a pilot study. Br J Nutr. 1998;79:133–9. doi: 10.1079/bjn19980024. [DOI] [PubMed] [Google Scholar]
- 22.Leenen R, Roodenburg AJ, Tijburg LB, Wiseman SA. A single dose of tea with or without milk increases plasma antioxidant activity in humans. Eur J Clin Nutr. 2000;54:87–92. doi: 10.1038/sj.ejcn.1600900. [DOI] [PubMed] [Google Scholar]
- 23.van het Hof KH, Kivits GA, Weststrate JA, Tijburg LB. Bioavailability of catechins from tea: the effect of milk. Eur J Clin Nutr. 1998;52:356–9. doi: 10.1038/sj.ejcn.1600568. [DOI] [PubMed] [Google Scholar]
- 24.Hollman PC, Van Het Hof KH, Tijburg LB, Katan MB. Addition of milk does not affect the absorption of flavonols from tea in man. Free Radic Res. 2001;34:297–300. doi: 10.1080/10715760100300261. [DOI] [PubMed] [Google Scholar]
- 25.Langley-Evans SC. Consumption of black tea elicits an increase in plasma antioxidant potential in humans. Int J Food Sci Nutr. 2000;51:309–15. doi: 10.1080/096374800426902. [DOI] [PubMed] [Google Scholar]
- 26.Xu JZ, Yeung SY, Chang Q, Huang Y, Chen ZY. Comparison of antioxidant activity and bioavailability of tea epicatechins with their epimers. Br J Nutr. 2004;91:873–81. doi: 10.1079/BJN20041132. [DOI] [PubMed] [Google Scholar]
- 27.Kim JH, Kang BH, Jeong JM. Antioxidant antimutagenic and chemopreventive activities of a phyto-extract mixture derived from various e vegetables, fruits and oriental herbs. Food Sci Biotechnol. 2003;12:631–8. [Google Scholar]
- 28.Skrzydlewsja E, Augustyniak A, Ostrowska J, Luczaj W, Tarasiuk E. Green tea protection against aging induced oxidative stress. Free Radic Biol Med. 2002;33:555. [Google Scholar]
- 29.Chung JY, Huang C, Meng X, Dong Z, Yang CS. Inhibition of activator protein 1 activity and cell growth by purified green tea and black tea polyphenols in H-ras-transformed cells: structure activity relationship and mechanisms involved. Cancer Res. 1999;59:4610–7. [PubMed] [Google Scholar]
- 30.Agarwal R, Katiyar SK, Zaidi SI, Mukhtar H. Inhibition of skin tumor promoter-caused induction of epidermal ornithine decarboxylase in SENCAR mice by polyphenolic fraction isolated from green tea and its individual epicatechin derivatives. Cancer Res. 1992;52:3582–8. [PubMed] [Google Scholar]
- 31.Spencer JP, Schroeter H, Kuhnle G, Srai SK, Tyrrell RM, Hahn U, et al. Epicatechin and its in vivo metabolite, 3’-O-methyl epicatechin, protect human fibroblasts from oxidative-stress-induced cell death involving caspase-3 activation. Biochem J. 2001;354:493–500. doi: 10.1042/0264-6021:3540493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caturla N, Vera-Samper E, Villalaín J, Mateo CR, Micol V. The relationship between the antioxidant and the antibacterial properties of galloylated catechins and the structure of phospholipid model membranes. Free Radic Biol Med. 2003;34:648–62. doi: 10.1016/s0891-5849(02)01366-7. [DOI] [PubMed] [Google Scholar]
- 33.Chen D, Daniel KG, Kuhn DJ, Kazi A, Bhuiyan M, Li L, et al. Green tea and tea polyphenols in cancer prevention. Front Biosci. 2004;9:2618–31. doi: 10.2741/1421. [DOI] [PubMed] [Google Scholar]
- 34.McNaught JG. On the action of cold or lukewarm tea on Bacillus typhosus. J R Army Med Corps. 1906;7:372–3. [Google Scholar]
- 35.Yam TS, Shah S, Hamilton-Miller JM. Microbiological activity of whole and fractionated crude extracts of tea (Camellia sinensis), and of tea components. FEMS Microbiol Lett. 1997;152:169–74. doi: 10.1111/j.1574-6968.1997.tb10424.x. [DOI] [PubMed] [Google Scholar]
- 36.Hara Y. New York, USA: Marcel Dekker; 2001. Green tea: helath benefits and applications; pp. 71–81. [Google Scholar]
- 37.Sakanaka S, Kim M, Taniguchi M, Yamamoto T. Antibacterial substances in Japanese green tea extract against Streptococcus mutans, a cariogenic bacterium. Agric Biol Chem. 1989;53:2307–11. [Google Scholar]
- 38.Kaneko K, Shimano N, Suzuki Y, Nakamukaim, Ikazaki R, Ishida N, et al. Effects of tea catechins on oral odor and dental plaque. J Oral Ther Pharmacol. 1993;12:189–97. [Google Scholar]
- 39.Yasuda H, Arakawa T. Deodorizing mechanism of (-)-epigallocatechin against methyl mercaptan. Biosci Biotechnol Biochem. 1995;59:1232–6. [Google Scholar]
- 40.Sakanaka S, Aizawa M, Kim M, Yamamoto T. Inhibitory effects of green tea polyphenols on growth and cellular adherence of an oral bacterium, Porphyromonas gingivalis. Biosci Biotechnol Biochem. 1996;60:745–9. doi: 10.1271/bbb.60.745. [DOI] [PubMed] [Google Scholar]
- 41.Okamoto M, Sugimoto A, Leung KP, Nakayama K, Kamaguchi A, Maeda N. Inhibitory effect of green tea catechins on cysteine proteinases in Porphyromonas gingivalis. Oral Microbiol Immunol. 2004;19:118–20. doi: 10.1046/j.0902-0055.2003.00112.x. [DOI] [PubMed] [Google Scholar]
- 42.Sakanaka S, Okada Y. Inhibitory effects of green tea polyphenols on the production of a virulence factor of the periodontal-disease-causing anaerobic bacterium Porphyromonas gingivalis. J Agric Food Chem. 2004;52:1688–92. doi: 10.1021/jf0302815. [DOI] [PubMed] [Google Scholar]
- 43.Sakanaka S, Aizawa M, Kim M, Yamamoto T. Inhibitory effects of green tea polyphenols on growth and cellular adherence of an oral bacterium, Porphyromonas gingivalis. Biosci Biotechnol Biochem. 1996;60:745–9. doi: 10.1271/bbb.60.745. [DOI] [PubMed] [Google Scholar]
- 44.Kou Y, Inaba H, Kato T, Tagashira M, Honma D, Kanda T, et al. Inflammatory responses of gingival epithelial cells stimulated with Porphyromonas gingivalis vesicles are inhibited by hop-associated polyphenols. J Periodontol. 2008;79:174–80. doi: 10.1902/jop.2008.070364. [DOI] [PubMed] [Google Scholar]
- 45.Yun JH, Pang EK, Kim CS, Yoo YJ, Cho KS, Chai JK, et al. Inhibitory effects of green tea polyphenol (-)-epigallocatechin gallate on the expression of matrix metalloproteinase-9 and on the formation of osteoclasts. J Periodontal Res. 2004;39:300–7. doi: 10.1111/j.1600-0765.2004.00743.x. [DOI] [PubMed] [Google Scholar]
- 46.Yun JH, Pang EK, Kim CS, Yoo YJ, Cho KS, Chai JK, et al. Inhibitory effects of green tea polyphenol (-)-epigallocatechin gallate on the expression of matrix metalloproteinase-9 and on the formation of osteoclasts. J Periodontal Res. 2007;42:212–8. doi: 10.1111/j.1600-0765.2004.00743.x. [DOI] [PubMed] [Google Scholar]
- 47.Nakamura H, Ukai T, Yoshimura A, Kozuka Y, Yoshioka H, Yoshinaga Y, et al. In vivo Green tea catechin inhibits lipopolysaccharide-induced bone resorption. J Periodontal Res. 2009;45:23–30. doi: 10.1111/j.1600-0765.2008.01198.x. [DOI] [PubMed] [Google Scholar]
- 48.Ahmed S, Wang N, Lalonde M, Goldberg VM, Haqqi TM. Green tea polyphenol epigallocatechin-3-gallate (EGCG) differentially inhibits interleukin-1 beta-induced expression of matrix metalloproteinase-1 and -13 in human chondrocytes. J Pharmacol Exp Ther. 2004;308:767–73. doi: 10.1124/jpet.103.059220. [DOI] [PubMed] [Google Scholar]
- 49.Makimura M, Hirasawa M, Kobayashi K, Indo J, Sakanaka S, Taguchi T, et al. Inhibitory effect of tea catechins on collagenase activity. J Periodontol. 1993;64:630–6. doi: 10.1902/jop.1993.64.7.630. [DOI] [PubMed] [Google Scholar]
- 50.Hosokawa Y, Hosokawa I, Ozaki K, Nakanishi T, Nakae H, Matsuo T. Catechins inhibit CXCL10 production form oncostatin M-stimulated human gingival fibroblasts. J Nutr Biochem. 2009;25:79–99. doi: 10.1016/j.jnutbio.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 51.Hosokawa Y, Hosokawa I, Ozaki K, Nakanishi T, Nakae H, Matsuo T. Catechins inhibit CCL20 production in IL-17A-stimulated human gingival fibroblasts. Cell Physiol Biochem. 2009;24:391–6. doi: 10.1159/000257431. [DOI] [PubMed] [Google Scholar]


