Abstract
Autoimmune pancreatitis (AIP), in contrast to other benign chronic pancreatic diseases, can be cured with immunosuppressant drugs, thus the differentiation of AIP from pancreatic cancer is of particular interest in clinical practice. There is the possibility that some patients with AIP may develop pancreatic cancer, and this possibility contributes to increasing our difficulties in differentiating AIP from pancreatic cancer. We herein report the case of a 70-year-old man in whom pancreatic adenocarcinoma and AIP were detected simultaneously. We must carefully monitor AIP patients for the simultaneous presence of pancreatic cancer, even when a diagnosis of AIP is confirmed.
Key Words: Autoimmune pancreatitis, Pancreatitis, Pancreatic neoplasms, Therapy, Outcome, Azathioprine, Pathology
Introduction
Pancreatitis due to autoimmunity has not only been reported in Japan; in the last decade, the frequency of new diagnoses has increased all over the world [1, 2]. An autoimmune pathogenesis for this disease has been proposed because this condition is occasionally associated with antibodies or other autoimmune-associated diseases [2, 3, 4, 5, 6, 7, 8, 9]. The terms type 1 and type 2 autoimmune pancreatitis (AIP) have recently been introduced to describe the clinical profiles associated with lymphoplasmacytic sclerosing pancreatitis and idiopathic duct-centric pancreatitis [10]. The two entities share common histopathologic features, even if expert pathologists can accurately distinguish them on the basis of other unique histopathologic characteristics. In a clinical setting, both type 1 and type 2 AIP have a similar presentation, and they are characterized by obstructive jaundice, a pancreatic mass and a dramatic response to steroids, but differ in demography, serology, involvement of other organs and disease relapse rate. While type 1 is associated with elevation of nonspecific autoantibodies and serum IgG4 levels [11], type 2 does not have definitive serologic autoimmune markers. In addition, high serum IgG4 may also be found in patients with pancreatic cancer [11], and serum neoplastic markers such as CA19-9, SPAN-1 and DUPAN-2 may also be elevated in patients with AIP [12]; these findings sometimes render the diagnosis of AIP confusing. Since AIP, in contrast to other benign chronic pancreatic diseases, can be cured with immunosuppressant drugs [13], the differentiation of AIP from pancreatic cancer is of particular interest in clinical practice [14]. Two studies have also pointed out the possibility that some patients with AIP may develop pancreatic cancer [15, 16], and this contributes to increasing our difficulties in differentiating AIP from pancreatic cancer. We herein report the case of a patient in whom pancreatic adenocarcinoma and AIP were detected simultaneously.
Case Report
A 70-year-old man was admitted to our hospital in June 2010 for additional examination and suitable treatment for suspected pancreatic cancer. The patient was a light drinker (daily pure alcohol intake <40 g) and did not smoke. He had a history of HCV-related hepatitis which had been unsuccessfully treated with IFN; treatment had been discontinued due to serious depression. Familial history was negative for the presence of pancreatic and extrapancreatic cancer.
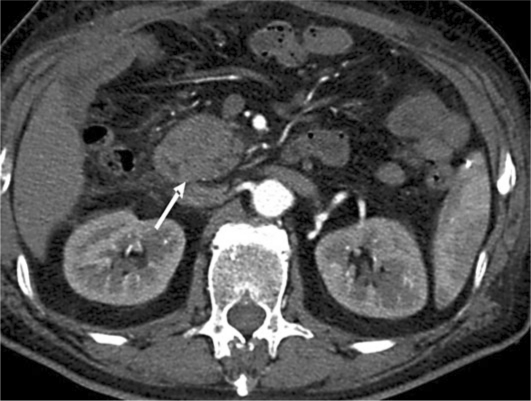
In April 2010, he became jaundiced and was admitted to another hospital. No other clinical symptoms were present. He underwent an abdominal computed tomography (CT) scan showing a mass in the pancreatic head measuring 5 × 3.5 cm, infiltrating the left gastric artery with compression and displacement of the portal vein; dilation of the common bile duct was also seen. Endoscopic retrograde pancreatography revealed severe narrowing of the duodenal lumen suggestive of infiltrating ductal adenocarcinoma; a duodenal biopsy showed flogistic tissue without signs of invasive neoplasia. An explorative laparotomy was performed in May 2010 and, during surgery, a bulky lesion in the head of the pancreas with vascular involvement was detected. Since the lesion caused massive infiltration of the duodenal wall and due to the close proximity of the mass to the inferior cava vein, hepatic artery and portal vein, a double bypass biliary-enteric and gastro-enteric bypass was performed. During surgery, a peritoneal biopsy was also performed because of the presence of small nodules on the peritoneal surface, but no signs of cancer were detected histologically, and cytological examination of the peritoneal lavage was also negative for the presence of malignant cells. A CT scan carried out in the postoperative period confirmed the pancreatic mass without dilation of the Wirsung duct (fig. 1); cytology of the pancreatic mass was carried out and no malignant cells were detected.
Fig. 1.
CT scan. This examination was carried out after a double bypass biliary-enteric and gastro-enteric bypass for jaundice and unresectable head pancreatic mass and it shows a pancreatic mass (arrow) without dilation of the Wirsung duct.
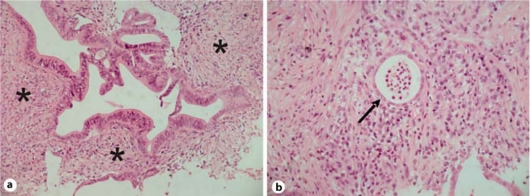
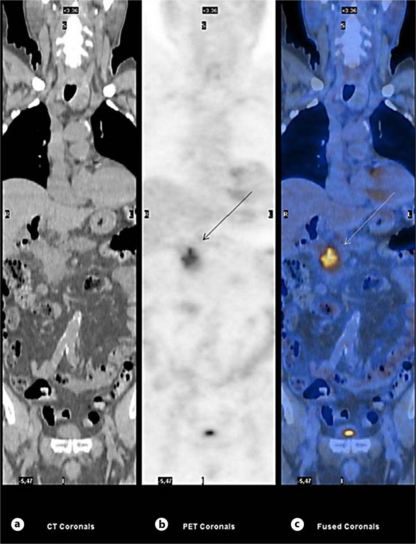
The patient was finally sent to our Unit in July 2010 for further investigation. On admission, performance status was good; he did not complain of abdominal pain and his body weight had increased after surgery. The results of the following blood chemistry examinations were abnormal: serum amylase 211 U/l (upper reference value 110 U/l), lipase 155 U/l (upper reference value 60 U/l), and alkaline phosphatases 333 U/l (normal reference range 98-280 U/l); γ-glutamyltranspeptidase 39 U/l (normal reference range 8-61 U/l) and glucose 103 mg/dl were within the normal reference ranges. The serum levels of CA19-9 and CEA were 1,128 U/ml (normal reference range 0-37 U/ml) and 4.5 ng/ml (normal reference range 0-5 ng/ml), respectively; IgG4 was 19 mg/dl (normal reference range 8-140 mg/dl). He underwent ultrasound-guided fine needle biopsy which disclosed chronic pancreatitis with lymphoplasmacytic infiltrate, a low number of eosinophils and intralobular and interlobular fibrosis, associated with granulocytic epithelial lesions and panIN 1A-B (fig. 2) suggestive of a diagnosis of AIP. Moreover, 18-fluorodeoxyglucose positron emission tomography (18 FDG-PET) showed a markedly increased glucose uptake in the head of the pancreas (SUV 6.1) and was unremarkable for differentiating pancreatic cancer from AIP (fig. 3).
Fig. 2.
Ultrasound-guided fine needle pancreatic biopsy (July 2010). The pathological examination reveals lymphoplasmacytic infiltrate (asterisks) (a) and granulocytic epithelial lesions (arrow) (b).
Fig. 3.
18 FDG-PET. a CT image (CT coronals). b Positron emission tomography acquisition (PET coronals) shows a markedly increased glucose uptake in the mass of the pancreas head (SUV 6.1) (arrow). c Fusion image of CT and PET (fused coronals); the arrow indicates the pancreatic head mass.
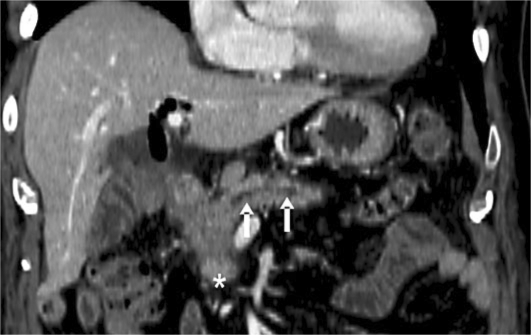
In August 2010, the patient was treated with azathioprine at a dosage of 100 mg/day and not with steroids because of the presence of viral hepatitis; two months later, his clinical condition remained good. However, the serum levels of the tumor markers continued to increase (CA19-9 >10,000 U/ml, CEA 39.5 ng/ml) and diabetes was diagnosed requiring metformin and insulin treatment. Another CT scan was carried out in October 2010, and this examination showed that the pancreatic mass had increased in volume (6 × 5.7 cm) and the main pancreatic duct was dilated behind the solid mass (fig. 4). Multiple hepatic lesions of a few millimeters, hyperdense in the arterial and the delayed phase, were also detected. The patient underwent another ultrasound-guided fine needle biopsy which showed the presence of intense fibrosis due to immunosuppressive therapy, the presence of a poor lymphoplasmacytic infiltrate and an intraductal papillary mucinous tumor; a ductal adenocarcinoma was also found (fig. 5). Azathioprine was discontinued and the patient underwent chemotherapy based on gemcitabine and oxaliplatin for 3 months and then on gemcitabine associated with capecitabine. After 8 months, the patient is still alive and continuing his chemotherapy.
Fig. 4.
CT scan carried out in October 2010. This examination shows the pancreatic mass (asterisk) and the dilation of the main pancreatic duct behind the lesion (arrows).
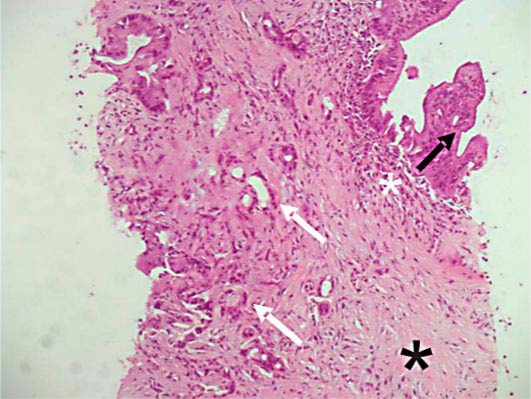
Fig. 5.
Ultrasound-guided fine needle pancreatic biopsy (October 2010). The pathological examination shows the presence of a poor periductal lymphoplasmacytic infiltrate (white asterisk) and marked fibrosis (black asterisk) and the presence of both intraductal papillary mucinous neoplasia (black arrow) and ductal adenocarcinoma (white arrows).
Discussion
Several hypotheses can be advanced regarding the possible relationship between pancreatic adenocarcinoma and AIP. It is possible that AIP can induce a carcinoma or, on the contrary, an adenocarcinoma may induce an inflammatory process mimicking that of AIP. The synchronous presence of adenocarcinoma and AIP cannot be excluded. All these hypotheses are supported by the data in the literature. In fact, it is possible that the obstruction of the pancreatic duct by the tumoral mass may determine an inflammatory process resembling that of AIP [17]. However, the localization of the ductal adenocarcinoma in the central part of the mass as in the present case and in the case reported by Motosugi et al. [17] is highly supportive of the hypothesis that AIP precedes adenocarcinoma formation. This also agrees with previous studies showing that pancreatic carcinoma develops several months or years after the diagnosis of AIP [15, 16, 18, 19, 20] and that patients with AIP may have a K-ras mutation [21]. At present, we cannot answer the question regarding the synchronous [17, 22] or metachronous [15, 16, 20, 23] appearance of AIP and pancreatic cancer. At present, the only effect of the reports on the association between AIP and pancreatic cancer is to add a new problem to the differential diagnosis between AIP and pancreatic cancer. Even if several guidelines suggest that steroid treatment may help in arriving at a diagnosis of AIP [24], our current policy is to initiate the treatment only after an accurate diagnosis of AIP has been reached by means of biopsy pathology [13]. Caution should be taken regarding the possible presence of an adenocarcinoma in an inflammatory pancreatic mass based solely on the results of a pancreatic biopsy [25]. On the other hand, the serum determination of tumoral markers does not add any useful information because they may also be abnormally high in patients with AIP without the presence of a pancreatic adenocarcinoma [26]; neither does 18 FDG-PET help in diagnosing the simultaneous presence of pancreatic adenocarcinoma and AIP in the case of the absence of concomitant extrapancreatic uptake by the salivary glands or kidneys [27, 28]. Finally, it is also possible that AIP may be associated with biliary malignancies [29]; thus, additional larger case studies are needed to clarify the association of synchronous or metachronous pancreatobiliary malignancies with AIP. In conclusion, the recommendations of Kawa et al. [30] should be kept in mind; we must carefully monitor AIP patients for the simultaneous presence of pancreatic cancer, even when a diagnosis of AIP is confirmed.
Disclosure Statement
The authors have no conflict of interest to declare.
Footnotes
This is an Open Access article licensed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs 3.0 License (www.karger.com/OA-license), applicable to the online version of the article only. Distribution for non-commercial purposes only.
References
- 1.Pearson RK, Longnecker DS, Chari ST, Smyrk TC, Okazaki K, Frulloni L, Cavallini G. Controversies in clinical pancreatology: autoimmune pancreatitis: does it exist? Pancreas. 2003;27:1–13. doi: 10.1097/00006676-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Kim KP, Kim MH, Lee SS, Seo DW, Lee SK. Autoimmune pancreatitis: it may be a worldwide entity. Gastroenterology. 2004;126:1214. doi: 10.1053/j.gastro.2004.02.044. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida K, Toki F, Takeuchi T, Watanabe S, Shiratori K, Hayashi N. Chronic pancreatitis caused by an autoimmune abnormality. Proposal of the concept of autoimmune pancreatitis. Dig Dis Sci. 1995;40:1561–1568. doi: 10.1007/BF02285209. [DOI] [PubMed] [Google Scholar]
- 4.Ectors N, Maillet B, Aerts R, Geboes K, Donner A, Borchard F, Lankisch P, Stolte M, Lüttges J, Kremer B, Klöppel G. Non-alcoholic duct destructive chronic pancreatitis. Gut. 1997;41:263–268. doi: 10.1136/gut.41.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kino-Ohsaki J, Nishimori I, Morita M, Okazaki K, Yamamoto Y, Onishi S, Hollingsworth MA. Serum antibodies to carbonic anhydrase I and II in patients with idiopathic chronic pancreatitis and Sjögren's syndrome. Gastroenterology. 1996;110:1579–1586. doi: 10.1053/gast.1996.v110.pm8613065. [DOI] [PubMed] [Google Scholar]
- 6.Okazaki K, Uchida K, Ohana M, Nakase H, Uose S, Inai M, Matsushima Y, Katamura K, Ohmori K, Chiba T. Autoimmune-related pancreatitis is associated with autoantibodies and a Th1/Th2-type cellular immune response. Gastroenterology. 2000;118:573–581. doi: 10.1016/s0016-5085(00)70264-2. [DOI] [PubMed] [Google Scholar]
- 7.Chutaputti A, Burrell MI, Boyer JL. Pseudotumor of the pancreas associated with retroperitoneal fibrosis: a dramatic response to corticosteroid therapy. Am J Gastroenterol. 1995;90:1155–1158. [PubMed] [Google Scholar]
- 8.Klimstra DS, Conlon KC, Adsay NV. Lymphoplasmacytic sclerosing pancreatitis with pseudotumor formation. Pathol Case Rev. 2001;6:94–99. [Google Scholar]
- 9.Romboli E, Campana D, Piscitelli L, Brocchi E, Barbara G, D'Errico A. Pancreatic involvement in systemic sarcoidosis. A case report. Dig Liver Dis. 2004;36:222–227. doi: 10.1016/j.dld.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 10.Chari ST, Kloeppel G, Zhang L, Notohara K, Lerch MM, Shimosegawa T. Histopathologic and clinical subtypes of autoimmune pancreatitis: the Honolulu consensus document. Pancreatology. 2010;10:664–672. doi: 10.1159/000318809. [DOI] [PubMed] [Google Scholar]
- 11.Morselli Labate AM, Pezzilli R. Usefulness of serum IgG4 in the diagnosis and follow-up of autoimmune pancreatitis: a systematic literature review and meta-analysis. J Gastroenterol Hepatol. 2009;24:15–36. doi: 10.1111/j.1440-1746.2008.05676.x. [DOI] [PubMed] [Google Scholar]
- 12.Mishima S, Mizuta Y, Yamao T, Yamakawa M, Akazawa Y, Mishima R, Ohba K, Masuda JI, Ohnita K, Isomoto H, Shikuwa S, Omagari K, Kohno S. Autoimmune pancreatitis with extreme elevation of DUPAN-2. Intern Med. 2007;46:377–381. doi: 10.2169/internalmedicine.46.6184. [DOI] [PubMed] [Google Scholar]
- 13.Pezzilli R, Cariani G, Santini D, Calculli L, Casadei R, Morselli-Labate AM, Corinaldesi R. Therapeutic management and clinical outcome of autoimmune pancreatitis. Scand J Gastroenterol. 2011 doi: 10.3109/00365521.2011.584896. in press. [DOI] [PubMed] [Google Scholar]
- 14.Pezzilli R, Casadei R, Calculli L, Santini D. Autoimmune pancreatitis. A case mimicking carcinoma. J Pancreas. 2004;5:527–530. [PubMed] [Google Scholar]
- 15.Uchida K, Yazumi S, Nishio A, Kusuda T, Koyabu M, Fukata M, Miyoshi H, Sakaguchi Y, Fukui T, Matsushita M, Takaoka M, Okazaki K. Long-term outcome of autoimmune pancreatitis. J Gastroenterol. 2009;44:726–732. doi: 10.1007/s00535-009-0049-3. [DOI] [PubMed] [Google Scholar]
- 16.Ghazale A, Chari S. Is autoimmune pancreatitis a risk factor for pancreatic cancer? Pancreas. 2007;35:376. doi: 10.1097/MPA.0b013e318073ccb8. [DOI] [PubMed] [Google Scholar]
- 17.Motosugi U, Ichikawa T, Yamaguchi H, Nakazawa T, Katoh R, Itakura J, Fujii H, Sato T, Araki T, Shimizu M. Small invasive ductal adenocarcinoma of the pancreas associated with lymphoplasmacytic sclerosing pancreatitis. Pathol Int. 2009;59:744–747. doi: 10.1111/j.1440-1827.2009.02437.x. [DOI] [PubMed] [Google Scholar]
- 18.Sakashita F, Tanahashi T, Yamaguchi K, Osada S, Sugiyama Y. A case of pancreatic tail cancer associated with autoimmune pancreatitis. Jpn J Gastroenterol Surg. 2006;39:78–83. [Google Scholar]
- 19.Kubota K, Iida H, Fujisawa T, Yoneda M, Inamori M, Abe Y, Kirikoshi H, Saito S, Ohshiro H, Kakuta Y, Nakajima A. Clinical factors predictive of spontaneous remission or relapse in cases of autoimmune pancreatitis. Gastrointest Endosc. 2007;66:1142–1151. doi: 10.1016/j.gie.2007.06.059. [DOI] [PubMed] [Google Scholar]
- 20.Fukui T, Mitsuyama T, Takaoka M, Uchida K, Matsushita M, Okazaki K. Pancreatic cancer associated with autoimmune pancreatitis in remission. Intern Med. 2008;47:151–155. doi: 10.2169/internalmedicine.47.0334. [DOI] [PubMed] [Google Scholar]
- 21.Kamisawa T, Tsuruta K, Okamoto A, Horiguchi S, Hayashi Y, Yun X, Yamaguchi T, Sasaki T. Frequent and significant K-ras mutation in the pancreas, the bile duct, and the gallbladder in autoimmune pancreatitis. Pancreas. 2009;38:890–895. doi: 10.1097/MPA.0b013e3181b65a1c. [DOI] [PubMed] [Google Scholar]
- 22.Witkiewicz AK, Kennedy EP, Kennyon L, Yeo CJ, Hruban RH. Synchronous autoimmune pancreatitis and infiltrating pancreatic ductal adenocarcinoma: case report and review of the literature. Hum Pathol. 2008;39:1548–1551. doi: 10.1016/j.humpath.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 23.Loos M, Esposito I, Hedderich DM, Ludwig L, Fingerle A, Friess H, Klöppel G, Büchler P. Autoimmune pancreatitis complicated by carcinoma of the pancreatobiliary system: a case report and review of the literature. Pancreas. 2011;40:151–154. doi: 10.1097/MPA.0b013e3181f74a13. [DOI] [PubMed] [Google Scholar]
- 24.Fantini L, Zanini N, Fiscaletti M, Calculli L, Casadei R, Campana D, Pezzilli R. Autoimmune pancreatitis: the classification puzzle. Adv Med Sci. 2007;52:71–75. [PubMed] [Google Scholar]
- 25.Hirano K, Fukushima N, Tada M, Isayama H, Mizuno S, Yamamoto K, Yashima Y, Yagioka H, Sasaki T, Kogure H, Nakai Y, Sasahira N, Tsujino T, Kawabe T, Fukayama M, Omata M. Diagnostic utility of biopsy specimens for autoimmune pancreatitis. J Gastroenterol. 2009;44:765–773. doi: 10.1007/s00535-009-0052-8. [DOI] [PubMed] [Google Scholar]
- 26.Kamisawa T, Egawa N, Nakajima H, Tsuruta K, Okamoto A, Kamata N. Clinical difficulties in the differentiation of autoimmune pancreatitis and pancreatic carcinoma. Am J Gastroenterol. 2003;98:2694–2699. doi: 10.1111/j.1572-0241.2003.08775.x. [DOI] [PubMed] [Google Scholar]
- 27.Lee TY, Kim MH, Park do H, Seo DW, Lee SK, Kim JS, Lee KT. Utility of 18F-FDG PET/CT for differentiation of autoimmune pancreatitis with atypical pancreatic imaging findings from pancreatic cancer. AJR Am J Roentgenol. 2009;193:343–348. doi: 10.2214/AJR.08.2297. [DOI] [PubMed] [Google Scholar]
- 28.Kamisawa T, Takum K, Anjiki H, Egawa N, Kurata M, Honda G, Tsuruta K. FDG-PET/CT findings of autoimmune pancreatitis. Hepatogastroenterology. 2010;57:447–450. [PubMed] [Google Scholar]
- 29.Hayashi M, Arisaka Y, Takeshita A, Tominaga Y, Ki T, Masuda D, Hirokawa F, Egashira Y, Tsuji M, Yamamoto K, Higuchi K, Tanigawa N. Differential diagnosis of pancreatobiliary carcinoma from autoimmune pancreatitis-related diseases: a report of three cases. J Gastrointest Cancer. 2010 doi: 10.1007/s12029-010-9209-1. Epub ahead to print. [DOI] [PubMed] [Google Scholar]
- 30.Kawa S, Hamano H, Ozaki Y, Ito T, Kodama R, Chou Y, Takayama M, Arakura N. Long-term follow-up of autoimmune pancreatitis: characteristics of chronic disease and recurrence. Clin Gastroenterol Hepatol. 2009;7:S18–S22. doi: 10.1016/j.cgh.2009.07.041. [DOI] [PubMed] [Google Scholar]