Abstract
Electrospray ionization is today the most widely used ionization technique in chemical and biochemical analysis. Interfaced with a mass spectrometer it allows the investigation of the molecular composition of liquid samples. With electrospray a large variety of chemical substances can be ionized. There is no limitation in mass which thus enables even the investigation of large noncovalent protein complexes. Its high ionization efficiency profoundly changed biomolecular sciences because proteins can be identified and quantified on trace amounts in a high throughput fashion. This review article focuses mainly on the exploration of the underlying ionization mechanism. Some ionization characteristics are discussed that are related to this mechanism. Typical spectra of peptides, proteins, and noncovalent complexes are shown and the quantitative character of spectra is highlighted. Finally the possibilities and limitations in measuring the association constant of bivalent noncovalent complexes are described.
With the discovery in the late 1980s of two soft ionization techniques, electrospray and matrix assisted laser desorption/ionization (MALDI)1, a very important and long-lasting limitation of mass spectrometers as analytical instruments was removed—the restriction in the molecular weight of the analytes. With MALDI and electrospray, molecules with masses beyond 1000 Da could be transferred into the gas phase and ionized with very high efficiency and without any obvious limitation in mass (1–3). It was not the first time that molecules with masses in the range of 10,000 Da were seen in a mass spectrometer, but the enormous transfer and ionization efficiency of these two methods opened entirely new areas of research in chemistry, biochemistry, and biology.
Electrospray, as a method to dissipate a liquid sample in a homogeneous form, is an old technique. Its underlying physical effect was first described by Sir Geoffrey Taylor (4). Very early, it was speculated by Malcolm Dole et al. that electrospray could be used to generate molecular beams of large molecules, but he could not underpin this speculation with convincing experiments; his experimental setup was too limited (5). Only in 1988 could John Fenn's group demonstrate that it was possible to transfer large molecules, such as proteins, as ions into the gas phase without breaking them apart (2, 3). Other research groups and companies already working with electrospray or similar spray techniques interfaced to mass spectrometers took up the discoveries rapidly, which was the beginning of the now broad use of mass spectrometers in biomolecular sciences.
Today, electrospray is the most widely used technique for the analysis of samples in liquid form. Because it ionizes molecules directly from the liquid phase, it is compatible with traditional chromatographic separation techniques widely used in analytical chemistry. Equally important is that it is the most universally known ionization method with very low chemical specificity. Ions released by electrospray are very stable and not in an excited state, which can lead to their rapid decay like many ions generated by MALDI. The ionization process is unlimited in mass (6). These characteristics, paired with its very high ionization efficiency, are the basis of the wide distribution of electrospray ion sources (7).
2. The Ionization Mechanism
The main focus of this article is to review the mechanistic description of the electrospray ionization process. Research into the ionization process must develop a deeper understanding of how the ions are generated with the ultimate aim of a mathematical description. Such an understanding is important for the optimal design of electrospray ion sources and their interfaces to mass spectrometers. Thus, ionization characteristics like the generated charge state and the transfer of molecular complexes into the gas phase can be manipulated.
The central question to answer is as follows: how can ions be generated from charged liquid droplets?
Immediately after the discovery that an electrospray ion source can generate large molecular ion beams, two models for the ionization process were proposed: the ion evaporation model (8, 9) and the charge residue model (5, 10). More recent research refers to these two models and gathers data to find a mathematical description to support either one or the other. In this evaluation process, it should be understood that a model remains a model; it never describes the reality in an absolute way. It is a simplification. A good model should explain experimental data and should have a good predictive power for experimental results in a qualitative or even quantitative way. A model is not true because it is believed to be true. It is even difficult to say that certain experimental findings confirm the model description. It is more accurate to say that the experimental data can be interpreted within the framework of a specific model.
Ion Evaporation Model
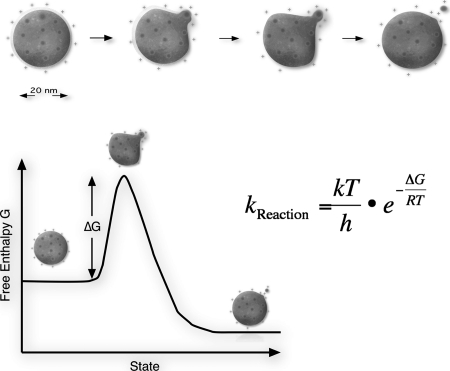
The ion evaporation model was originally developed by Iribarne and Thomson to explain the generation of atomic ions from randomly charged droplets produced by a spray atomizer (8, 9). Droplets shrink by evaporation until the field strength at their surface is sufficiently large that solvated ions can be expelled from the droplet (see Fig. 1). The energy gain in the strong electric field at the surface of the droplet compensates for the required energy to enlarge the surface of the droplet very rapidly when the solvated ion is expelled. There are three important characteristics of this process. The first is a geometric parameter: ion evaporation is expected to play a role when the droplet reaches a very small size of about 20 nm in diameter. Second, the reaction rate kinetic is heavily influenced by chemical properties of the ion. The ion evaporation rate constant depends exponentially on the difference in reaction free enthalpy that needs to be overcome when the ion is expelled from the droplet (see Fig. 1). Even though the ion is solvated by a small shell, this ΔG is a function of the physicochemical properties of the ion itself. Iribarne and Thomson realized that their model cannot explain the evaporation rates of different ions in their experiments. Ions with very different solvation energies had similar reaction rate constants, and simultaneously, other ions with very similar solvation energies had very different evaporation rates (9). Finally, at the onset of ion evaporation, the surface charge density of the droplet is below the maximal possible surface charge density at the Rayleigh limit (see below). In their early work, Fenn and coworkers favored the evaporation model over the charge residue model as an explanation for the generation of large ions from electrosprayed droplets (3).
Fig. 1.
The ion evaporation process. An individual ion leaves the charged droplet in a solvated state. The electric field strength at the surface of a droplet is so high that the energy required to increase the droplet surface is rapidly compensated by the gain because of Coulombic repulsion. kReaction, reaction rate constant; k, Boltzmann constant; T, temperature; h, Planck's constant; R, ideal gas constant.
Charge Residue Model
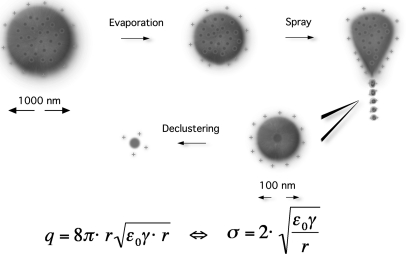
The charge residue model assumes that the electrospray process generates droplets that contain only one analytical ion (see Fig. 2). The ion is released when the solvent evaporates. A simple calculation shows that for a concentration of 1 pmol/μl, a droplet with a diameter of 200 nm contains on average less than one analyte molecule. Important characteristics of this model are that the ionization rate is strongly independent of the ion. The generation of very small droplets and the efficiency of solvent evaporation determine the ion current and not primarily the physicochemical properties of the ion. The ionization process is not limited by the mass of the analyte. Noncovalent complexes can be expected to survive the process because they are cooled by solvent evaporation and do not have to overcome an energy barrier with subsequent acceleration in an electric field. The available charge to the molecule depends on the Rayleigh stability limit because the final droplet comes from a spraying process caused by a Taylor Cone.
Fig. 2.
The charge residue process. A highly charged droplet shrinks by solvent evaporation until the field strength at the location with the highest surface curvature is so large that a Taylor Cone forms. From the tip of the Taylor Cone, other highly charged smaller droplets are emitted. This process can repeat itself until droplets are formed that contain only one analyte molecule. This molecule is released as an ion by solvent evaporation and declustering. The equation describes the maximum charge a droplet can carry before the Coulomb repulsion overcomes the surface tension. Locally, it is the condition for the formation of a Taylor Cone. q, droplet charge at the Rayleigh instability limit; r, droplet radius; ε0, electric permittivity of the surrounding medium; γ, surface tension; σ, surface charge density.
Electrospray Process
The starting point of the ionization process is the electrospray based dispersion of a liquid. The process is well understood. It is significant for the discussion of the ionization mechanism. When a potential is applied to a liquid held back in a nozzle, the liquid is pulled into an elliptic shape. The shape is formed in such a way that there is equilibrium between two dominating forces for every point of its surface. A surface tension derived force tries to pull the liquid back into the nozzle to minimize the energetically disfavored surface area. The electrostatic Coulomb attraction pulls the liquid to the counter electrode. The observation is that, at a certain voltage, the elliptical surface suddenly changes its shape to become a pointed cone. From the very tip of the cone, a spray is emitted. Sir Geoffrey Taylor showed that an equilibrium of surface tension derived forces and electrostatic forces can be reached for all points of its surface only for a liquid cone with an opening angel of 49.3° (4). Before the threshold voltage is reached, the equality between the two forces is met for a specific curvature radius at the apex of the fluid (10). However, the equation for the apex point shows that at a specific voltage, called the Taylor Cone voltage, the force balance becomes independent of the curvature radius; hence, the radius can theoretically become zero, which is the moment when the Taylor Cone forms and the electrospray is initiated (10).
The Taylor Cone description is a static description and does not include spraying behavior. A charged surface with an infinitesimally sharp tip would constitute a singularity for the electric field. Instead, the liquid starts to spray. This description explains why the newly generated droplets are charged close to their theoretical limit, the Rayleigh limit (11). The charge density at the apex always fulfills the condition that the electric field just counterbalances the surface tension; for a droplet, this is the definition of the Rayleigh stability limit.
Once the droplets are formed, their solvent starts to evaporate while they are in flight. Solvent molecules leave the droplet as neutral particles, leading to an increase in the field density at the surface of the droplets (12). In less than a few microseconds, the threshold field density is reached, and a new Taylor Cone forms on the droplet, which ejects highly charged small droplets (12–14). If the droplet is not perfectly spherical, this process will occur at an apex point of the droplet, which is the point with the smallest curvature radius. Here, the electric field density on the surface is the highest. The Rayleigh stability criterion is reached locally, not for the entire droplet, which is why droplets discharge via Taylor Cone emission at a total charging level below the Rayleigh limit for the entire droplet. The smaller the droplets and the higher the surface tension, the more spherical the droplet will be and the closer the discharging occurs to the total Rayleigh limit (13). This process occurs repeatedly on larger primary droplets and on secondary droplets because their charge density is already close to the Rayleigh limit when they are produced. The diameter of each secondary droplet is about 1/10th of the diameter of the ejecting droplet. Thus, a population of very small droplets is generated, which are most likely the major source of ions detected by a mass spectrometer. That the very small droplets are the major source of ions detectable by a mass spectrometer is reflected by an off-axis positioning of conventional electrospray sources. The outer rim of a spray plume consists of smaller droplets pushed there by electrostatic forces. Sampling this region in the mass spectrometer results in the highest ion intensities.
In summary, the Taylor Cone based spraying process can lead to a very fine dispersion of liquid without massive evaporation of the solvent beforehand. The formation of many droplets with diameters on the order of 200 nm or less appears to be realistic. The nano-electrospray source was built to generate this type of droplet as primary droplets and is one of the most efficient electrospray sources, with ionizations efficiencies of up to 100% (7, 10).
Ion Evaporation or Charge Residue Model for the Generation of Ions in the Electrospray Ionization Process
Ion evaporation certainly exists as an ionization mechanism. The high currents of atomic ions generated by liquid metal ion sources are explained by an ion evaporation mechanism (15). In a liquid metal ion source, a high voltage is applied to a liquid metal in a nozzle until a Taylor Cone forms. The electric field at its tip becomes so high that ions tunnel directly out of the liquid metal into the vacuum. They can be used to form a very intense and focused ion beam. The question is whether ion evaporation is responsible for electrospray generated ions under atmospheric conditions and, in particular, whether it is the mechanism for the formation of large molecular ions. At the very core of the theory of ion evaporation is the formula for the reaction rate constant (see Fig. 1). Ion currents should depend on the molecular solvation energy to an exponential degree. Even if the generated ion might be in a hydrated state, which reduces the differences between different species, some kind of dependence should be visible. For small ions, this dependence could not be observed clearly (9, 16). However, for larger molecular ions, hydrophobic molecules clearly have a higher ionization efficiency than hydrophilic ones. Hydrophobic molecules can even suppress the ion signal of hydrophilic analytes (16). It should be noted here that there is an alternative explanation for this effect within the charge residue model (see below).
Ion evaporation is a competitive mechanism for Taylor Cone based emission of charged droplets. If it occurs, it has to set in at a surface charge density that is below the Rayleigh stability limit. Ions of a defined globular shape should be charged less than solvent droplets of the same size at this limit. However, most proteins analyzed under structure conserving conditions carry a charge that corresponds to the charge of a droplet of the same size at its Rayleigh limit (17).
The charge residue model can explain many features of the electrospray ionization of large molecules. Via the Taylor Cone mechanism, it is possible to generate highly charged droplets that are small enough that they carry, on average, less than one analyte molecule (10). The unlimited mass scale and the occurrence on noncovalent complexes are a natural consequence of the process (6). The considerable independence of the ionization itself of chemical properties of the analyte is easily explained because the process depends more on the quality of the spray and the evaporation characteristics of the solvent. The high stability of the large ions generated with electrospray in comparison to other ionization techniques is explained by the exothermic solvent evaporation process (18). Multiple charging of ions simply occurs via charge distribution from the surface of the final droplet to the available charge retention sites on the molecule. One of the most significant observations in the discussion about the electrospray ionization process is certainly the one by Fernandez de la Mora that globular proteins electrosprayed under structure conserving conditions are most often charged up to the Rayleigh limit of solvent droplets of the same size (17). This is a strong indication that their predesolvated state is indeed a droplet generated via the Taylor Cone mechanism.
There are two observations that seem to conflict with the charge residue model: the higher ionization rate for hydrophobic molecules and the apparent tolerance of the electrospray ionization process for salt contamination of the sample. However, these effects can be explained within the framework of the charge residue model.
Standard electrospray ion sources operate at flow rates of 1 ml/min or beyond. Their flow rate is so high that their primary droplets are several microns in diameter, containing thousands of analyte molecules. After a certain evaporation time, these droplets undergo a Taylor Cone droplet emission process. These secondary droplets are about one order of magnitude smaller than the primary droplets and carry a charge already close to their own Rayleigh limit. Hence, these droplets might undergo a second Taylor Cone emission process, which finally generates the droplets that contain only one analyte molecule and give rise to the molecular ions observed. When a droplet undergoes one cycle of Taylor Cone based droplet emission, it loses 20–30% of its charge but only about 2% of its mass (11, 13). Considering the spray mechanism, most of the mass of secondary droplets comes from the surface of primary droplets. Only a small percentage of the total mass of primary droplets ends up in droplets that finally give rise to observable ions. Hence, surface active molecules will have a much higher chance of being observed in a mass spectrum than hydrophilic molecules (19). In contrast, when using a nano-electrospray, all of the liquid volume is dispersed in such a way that all contained analyte molecules can be desolvated.
The second argument against the charge residue model is the observed tolerance for nonvolatile contaminants. In particular, a sample analyzed with a nano-electrospray can contain a relatively high concentration of nonvolatile salt without the spectrum being dominated by it (20). Karas demonstrated that, when spraying a 10−5 molar insulin/10−2 molar NaCl solution with nano-electrospray, the [M+5H]5+ ion is still the dominating ion of all insulin ions in the spectrum. Some sodium adducts are visible but are not the major species. Only when increasing the NaCl concentration to 10−1 molar do the [M+iNa+(4-i)H]4+ for i = 1, …, 7 ions become more abundant (20). If insulin ions are ultimately generated by passive drying of a small droplet why is the spectrum is not dominated by NaCl-insulin clusters given the thousandfold higher abundance of NaCl?
A partial explanation can be found in the extensive dispersion of the liquid due to the Taylor Cone based spraying effect. With nano-electrospray, the initial droplets can be so small that they contain, on average, about one insulin molecule. The initial droplet is charged close to its Rayleigh stability limit and will undergo a spraying event after very little of its solvent has evaporated. The volume of the secondary droplets has only 1/1000 of the volume of the primary droplet. Thus, if the primary droplet contained one NaCl molecule and one insulin molecule, the probability that they both end up in the same droplet is only 1:1000. If the primary droplet dries down to generate the molecular ions, the spectrum would contain only the (insulin + Na)-cluster, whereas, if the secondary droplets dry down to form the ions, the insulin peak is a thousand times higher than the (insulin + Na) peak. This example demonstrates that the further the initial analyte is partitioned, the higher the relative tolerance toward nonvolatile contaminants. This explanation still builds on the charge residue model because it uses the specific properties of the Taylor Cone spraying process to generate droplets that are about one order of magnitude smaller in size and are still charged close to their Rayleigh stability limit.
In all of the discussions thus far, the solvent was assumed to be a homogeneous medium with a certain vapor pressure and surface tension. However, from a certain size downwards, the droplets must be seen more as molecular clusters consisting of an assembly of individual molecules and ions. The behavior of such nano-droplets has been studied by computer simulation (21). The fate of 10 nm sized NaI-formamide droplets in a strong external field was simulated. The computer model suggests that both processes ion evaporation and jetted emission of even smaller clusters can occur. After about 700 picoseconds, individual solvated Na+ ions are emitted from the droplet followed by jet formation and droplet emission after 1100 picoseconds.
In summary, the current consensus for the electrospray ionization process is that larger molecular ions, say above 1000 Da, are generated via passive desolvation from nano-droplets containing just one analytical ion according to the charge residue model. Smaller ions can be emitted from nano-droplets via field evaporation in a solvated state, as described by the ion evaporation model.
Electrospray Ionization Characteristics
Electrospray Mass Spectra
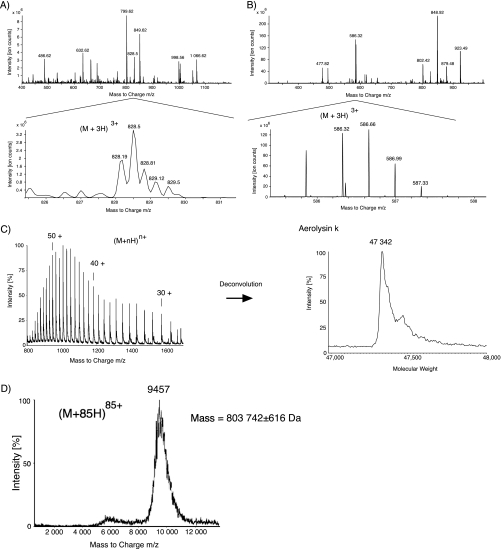
Fig. 3 shows four typical electrospray mass spectra recorded in proteomic experiments: a peptide mixture, one acquired with a quadrupole ion trap, the second with an orbitrap; a protein spectrum; and the spectrum of a large noncovalent complex, both acquired on a quadrupole time-of-flight mass spectrometer.
Fig. 3.
Typical electrospray mass spectra. (A and B) Show a peptide mixture and, in the detailed view, a triply charged ion recorded with an standard ion trap (A) and an higher resolving orbitrap (B). (C) shows a spectrum of a 47 kDa denatured protein. It is displayed by an entire series of peaks, one for each charge state of the protein. A deconvolution algorithm can construct a spectrum displaying the neutral mass of the protein. In (D), the spectrum of a GroEL chaperonin assembly, an 800 kDa large noncovalent complex, is shown (26). Its high m/z value of 9457 is remarkable. Noncovalent complexes are analyzed under structure conserving conditions and take up only a limited number of charges relative to their large mass.
Figs. 3A and 3B show a typical spectrum of a peptide mixture. These two examples demonstrate how different the resolution of mass spectrometers can be.
After a protein is denatured, it is typically seen with a series of different charge states in the form of H+ adducts when ionized with electrospray (Fig. 3C). Because a mass spectrometer measures the ion mass divided by its charge, the m/z value, a separate peak is recorded for every charge state. Software assists in the deconvolution of the spectrum to show the neutral mass of the protein.
Noncovalent complexes can have a very high mass and take up only a limited number of charges relative to their size. The consequence is that they are often seen with very high m/z values (see Fig. 3D). A protein-complex is analyzed in its native state and often includes a variable number of small adduct molecules, such as water or other components from the buffer. Thus, peaks from noncovalent complexes are often broader than is expected from the mass resolution of the instrument alone.
The charging of large molecules is a consequence of the ionization process and properties of the molecule itself. Charge agents are most often protons. They are added to available basic sides when sprayed in positive mode or removed from acidic sides when sprayed in negative mode. If no basic or acidic sides are available, the attachment of larger positive ions like sodium is observed. No protein can be charged higher than the number of available charge accepting sites. Beyond that, folded proteins, in particular if they are large, are often charged to the same level as droplets of the same size at their Rayleigh stability limit (17).
Smaller proteins can be seen with a lower charge state as suggested by this rule. A possible explanation for this subcharging is that the available number of charges in the final stages of desolvation is severely reduced by charge carrier field emission (22). A computer simulation of nano-droplets in strong external fields supports this idea (21). For smaller proteins, the evaporating droplet reaches a size of about 10 nm, at which ion evaporation of small molecules sets in before the protein starts to take over the charge carriers; this can limit the charge available to the protein.
Denatured proteins charge to a considerably higher degree than folded proteins. They are supercharged, which is made possible by their elongated shape. Their last fully hydrated state is not spherical but elliptical. A fluid ellipsoid can carry a higher charge than a sphere because its surface to volume ratio is higher. Computer simulations of the last steps of desolvation of a folded versus a denatured protein confirm this view (23).
Quantitative Evaluation of Electrospray Spectra
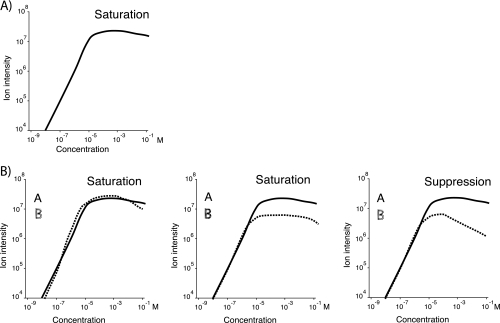
An important feature of an ionization technique is whether it can be used for quantitative measurements, which depends on whether the signal intensity corresponds to the concentration of the component in the sample. One practical limit is the stability of the spray. The ion signal will not reflect the analyte concentration if the spray is not stable over time. However, if the electrospray emitter is well built, the stability of the spray might be assured. The correlation between analyte concentration and signal intensity for single and double component solutions has been systematically studied by Tang and Kebarle (16). For single component solutions, the electrospray signal increases linearly with concentration over three orders of magnitudes before it levels off (see Fig. 4A). This saturation effect is probably caused by the exhaustion of analyte free nano-droplets generated by the spray, which give rise to molecular ions. A low flow electrospray source or even the nano-electrospray source will generate smaller initial droplets and generate more nano-droplets per sprayed volume. Using them a linear relationship between analyte concentration and ion signal can be observed over up to five orders of magnitude (24). This enlarged dynamic range is consistent with the assumption that the number of nano-droplets limits the achievable ion current.
Fig. 4.
Change of the electrospray ion signal with analyte concentration. A, The standard behavior of the electrospray ion signal with increasing analyte concentration. Over a range of three orders of magnitude, the signal grows linearly with concentration before it saturates. B, The signal dependence of a two component solution. The ion signals start to saturate simultaneously. They level off at the same or at different total ion intensities. If the components differ considerably in hydrophobicity, the more hydrophobic component can even suppress the hydrophilic one at high concentrations (B 3) (16).
When analyzing a two component system, the behavior is similar, but the saturation characteristics differ. Both components start to saturate simultaneously. They can saturate at the same level or at different total ion current levels. It is interesting that a suppression effect can be observed when two components with different hydrophilicity are mixed. At high concentrations, the hydrophobic component can suppress the ion signal of the hydrophilic one (see Fig. 4B), which can be interpreted as a competition effect for the nano-droplets, i.e. the origin of desolvated ions. Via the repetitive Taylor Cone based spraying mechanism, surface active components are more easily placed within a nano-droplet and can push hydrophilic components to the inside of larger droplets (19). From there they may never escape to become desolvated ions.
The linear response curve between the analyte concentration and electrospray ion signal is the basis of quantitative measurements in proteomics. When exploiting mass spectrometric measurements quantitatively, it should not be overlooked that the electrospray process is saturable and that hydrophobic components can suppress hydrophilic ones. In proteomic measurements, these potential sources of inaccuracy are often compensated by the fact that several peptides contribute to the determination of a protein concentration.
Binding Affinities of Binary Noncovalent Complexes
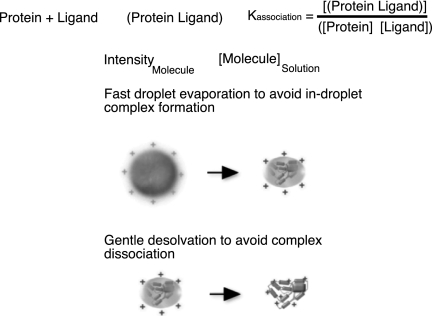
Noncovalent complexes survive the electrospray ionization process, but can physico-chemical properties be measured? It is of high interest to determine the binding affinities between components, in particular, of small ligands to proteins. Drug candidates are often selected from a library of compounds by maximizing the affinity they have for a target protein. The question is whether electrospray mass spectra can be used as a read-out to determine the association constant of a ligand to a particular protein using the titration method (see Fig. 5) (25). The titration method is based on changing the concentration of one of the components and measuring the concentration of reaction partners. At least three conditions have to be fulfilled to use mass spectra to determine the concentration of components.
Fig. 5.
Conditions for measuring the association constant of a binary protein complex. To use mass spectra as a read out in measuring the association constant of a protein complex with the titration method, at least three conditions have to be fulfilled. The concentrations of the components must remain below the saturation level so that the ion intensities reflect the molecular concentration in solution. The primary droplets have to be small so that all droplets generated evaporate fast and no in-droplet complexes are formed on the basis of higher concentrations. Finally, the desolvation of molecules in the interface region of mass spectrometers has to be gentle so that correctly formed complexes do not dissociate.
First, the concentrations have to remain below saturation levels (see Fig. 4), and the relative ionization efficiencies have to be determined. If the ligand is very small in comparison to the protein, it might be acceptable to assume that the protein and the (protein+ligand) complex have the same ionization efficiency (25).
Second, to avoid in-droplet complex formation, the transition from droplet to solvated molecule has to be fast and should involve as little solvent evaporation as possible. Nano-electrospray produces very small primary droplets, which evaporate in the micro-second range. True complexes cannot form in this time span. Loose associations between ligand and protein in the form of a cluster may occur. However, such clusters are not likely to withstand the desolvation process in the transmission region of the mass spectrometer (25).
Third, the desolvation conditions in the transmission region of the mass spectrometer when the last solvation shell is removed should be gentle enough not to destroy correctly formed ligand-protein complexes.
The stability of complexes in the gas phase is determined by other forces than those in solution. In solution, hydrophobic surfaces enhance the protein-ligand interaction because the surfaces avoid contact with the aqueous environment. Polar groups are often solvated and shielded by water molecules. In a vacuum, ionic interactions are much stronger than in solution because there are no water molecules that can attenuate the Coulombic forces. Thus, protein complexes whose stability is mostly based on hydrophobic surfaces might be much less stable in vacuum and might fall apart in the desolvation process (25).
In summary, if the experimental conditions are well chosen, the binding constants of binary complexes can be measured using electrospray mass spectrometry. However, there are cases in which the results do not reflect the in-solution kinetics of the complex formation despite the care taken; this is particularly true for complexes stabilized by hydrophobic surfaces.
Future Developments
The electrospray ionization process can now be considered to be well understood. Changes will be brought by using electrospray ionization sources to solve scientific problems. Instrument manufacturers are permanently working on increasing the ion transmission of interfaces and mass spectrometers to take full advantage of the high ionization efficiency of the electrospray process. This goes hand in hand with software development. The aim is to visualize the high complexity of proteomic samples. The tendency is clear—moving the analysis of biological experiments entirely into the computer by a deep and differential analysis of mass spectrometry based read outs of entire proteomes. The purpose is to understand the complexity of biological systems that currently defy human understanding. Only a computer assisted analysis will give a deeper insight of the inner workings of biological entities on the molecular level. Electrospray interfaced mass spectrometers are the tools to shed light onto the organism's constantly changing molecular networks.
Footnotes
1 The abbreviations used is:
- MALDI
- matrix assisted laser desorption/ionization.
REFERENCES
- 1. Karas M., Hillenkamp F. (1988) Laser desorption ionization of proteins with molecular masses exceeding 10,000 daltons. Anal. Chem. 60, 2299–2301 [DOI] [PubMed] [Google Scholar]
- 2. Meng C. K., Mann M., Fenn J. (1988) Of protons or proteins. Zeitschrift für Physik D Atoms, Molecules and Clusters 10, 361–368 [Google Scholar]
- 3. Fenn J. B., Mann M., Meng C. K., Wong S. F., Whitehouse C. M. (1989) Electrospray ionization for mass spectrometry of large biomolecules. Science 246, 64–71 [DOI] [PubMed] [Google Scholar]
- 4. Taylor G. (1964) Disintegration of water drops in an electric field. Proc. R. Soc. Lon. A 280, 383–397 [Google Scholar]
- 5. Dole M., Mack L. L., Hines R. L., Mobley R. C., Ferguson L. D., Alice M. B. (1968) Molecular beams of macroions. J. Chem. Phys. 49, 2240–2249 [Google Scholar]
- 6. Siuzdak G., Bothner B., Yeager M., Brugidou C., Fauquet C. M., Hoey K., Chang C. M. (1996) Mass spectrometry and viral analysis. Chem. Biol. 3, 45–48 [DOI] [PubMed] [Google Scholar]
- 7. Wilm M., Mann M. (1996) Analytical properties of the nanoelectrospray ion source. Anal. Chem. 68, 1–8 [DOI] [PubMed] [Google Scholar]
- 8. Iribarne J., Thompson B. (1976) On the evaporation of small ions from charged droplets. J. Chem. Phys. 64, 2287–2294 [Google Scholar]
- 9. Thompson B., Iribarne J. (1979) Field induced ion evaporation from liquid surfaces at atmospheric pressure. J. Chem. Phys. 71, 4451–4463 [Google Scholar]
- 10. Wilm M. S., Mann M. (1994) Electrospray and Taylor-Cone theory, Dole's beam of macromolecules at last? Int. J. Mass Spectrom. Ion Proc. 136, 167–180 [Google Scholar]
- 11. Smith J., Flagan R., Beauchamp J. (2002) Droplet evaporation and discharge dynamics in electrospray ionization. J. Phys. Chem. A 106, 9957–9967 [Google Scholar]
- 12. Grimm R. L., Beauchamp J. L. (2010) Evaporation and discharge dynamics of highly charged multicomponent droplets generated by electrospray ionization. J. Phys. Chem. A 114, 1411–1419 [DOI] [PubMed] [Google Scholar]
- 13. Gomez A., Tang K. (1994) Charge and fission of droplets in electrostatic sprays. Phys. Fluids 6, 404–414 [Google Scholar]
- 14. Hager D. B., Dovichi N. J., Klassen J., Kebarle P. (1994) Droplet electrospray mass spectrometry. Anal. Chem. 66, 3944–3949 [Google Scholar]
- 15. Swanson L. (1983) Liquid metal ion sources: Mechanism and applications. Nucl. Inst. Methods Phys. Res. 218, 347–353 [Google Scholar]
- 16. Tang L., Kebarle P. (1993) Dependence of ion intensity in electrospray mass spectrometry on the concentration of the analytes in the electrosprayed solution. Anal. Chem. 65, 3654–3668 [Google Scholar]
- 17. de la Mora F. J. (2000) Electrospray ionization of large multiply charged species proceeds via Dole's charged residue mechanism. Anal. Chim Acta 406, 93–104 [Google Scholar]
- 18. Touboul D., Jecklin M. C., Zenobi R. (2008) Ion internal energy distributions validate the charge residue model for small molecule ion formation by spray methods. Rapid Commun. Mass Spectrom. 22, 1062–1068 [DOI] [PubMed] [Google Scholar]
- 19. Cech N. B., Enke C. G. (2000) Relating electrospray ionization response to nonpolar character of small peptides. Anal. Chem. 72, 2717–2723 [DOI] [PubMed] [Google Scholar]
- 20. Juraschek R., Dülcks T., Karas M. (1999) Nanoelectrospray–more than just a minimized-flow electrospray ionization source. J. Am. Soc. Mass Spectrom. 10, 300–308 [DOI] [PubMed] [Google Scholar]
- 21. Luedtke W. D., Landman U., Chiu Y. H., Levandier D. J., Dressler R. A., Sok S., Gordon M. S. (2008) Nanojets, electrospray, and ion field evaporation: molecular dynamics simulations and laboratory experiments. J. Phys. Chem. A 112, 9628–9649 [DOI] [PubMed] [Google Scholar]
- 22. Hogan C. J., Jr., Carroll J. A., Rohrs H. W., Biswas P., Gross M. L. (2008) Charge carrier field emission determines the number of charges on native state proteins in electrospray ionization. J. Am. Chem. Soc. 130, 6926–6927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Konermann L. (2007) A minimalist model for exploring conformational effects on the electrospray charge state distribution of proteins. J. Phys. Chem. B 111, 6534–6543 [DOI] [PubMed] [Google Scholar]
- 24. Ejsing C. S., Duchoslav E., Sampaio J., Simons K., Bonner R., Thiele C., Ekroos K., Shevchenko A. (2006) Automated identification and quantification of glycerophospholipid molecular species by multiple precursor ion scanning. Anal. Chem. 78, 6202–6214 [DOI] [PubMed] [Google Scholar]
- 25. Peschke M., Verkerk U. H., Kebarle P. (2004) Features of the ESI mechanism that affect the observation of multiply charged noncovalent protein complexes and the determination of the association constant by the titration method. J. Am. Soc. Mass Spectrom. 15, 1424–1434 [DOI] [PubMed] [Google Scholar]
- 26. Robinson C. V., Gross M., Eyles S. J., Ewbank J. J., Mayhew M., Hartl F. U., Dobson C. M., Radford S. E. (1994) Conformation of GroEL-bound alpha-lactalbumin probed by mass spectrometry. Nature 372, 646–651 [DOI] [PubMed] [Google Scholar]