Abstract
Cell cycle regulation in hematopoietic stem cells (HSCs) is tightly controlled during homeostasis and in response to extrinsic stress. p53, a well-known tumor suppressor and transducer of diverse stress signals, has been implicated in maintaining HSC quiescence and self-renewal. However, the mechanisms that control its activity in HSCs, and how p53 activity contributes to HSC cell cycle control, are poorly understood. Here, we use a genetically engineered mouse to show that p53 C-terminal modification is critical for controlling HSC abundance during homeostasis and HSC and progenitor proliferation after irradiation. Preventing p53 C-terminal modification renders mice exquisitely radiosensitive due to defects in HSC/progenitor proliferation, a critical determinant for restoring hematopoiesis after irradiation. We show that fine-tuning the expression levels of the cyclin-dependent kinase inhibitor p21, a p53 target gene, contributes significantly to p53-mediated effects on the hematopoietic system. These results have implications for understanding cell competition in response to stresses involved in stem cell transplantation, recovery from adverse hematologic effects of DNA-damaging cancer therapies, and development of radioprotection strategies.
Keywords: p53, C-terminal modification, HSC, radiosensitivity, cell cycle, apoptosis
In its most-studied role, p53 acts as a tumor suppressor that responds to DNA damage, oncogenic, and other cellular stresses to maintain genomic stability (for review, see Toledo and Wahl 2006). However, accumulating evidence reveals other functions for p53 in cell metabolism, the inflammatory response, angiogenesis, reproduction, embryogenesis, and stem cell renewal (Vousden and Prives 2009). These recent observations suggest that p53 may have evolved due to its roles in biological development with tumor suppression being an additional benefit (Aranda-Anzaldo and Dent 2007).
p53 is critical for maintaining the balance between cell survival and apoptosis during development and homeostasis, often in response to stress. The hematopoietic system is particularly vulnerable to genotoxic stresses such as radiation, which activates p53 and results in dose-dependent acute marrow failure due to apoptosis in highly proliferative progenitors and mature blood cells (Gudkov and Komarova 2003). The p53-regulated proapoptotic gene puma is also recently shown to be critical in modulating the function of hematopoietic stem and progenitor cells in response to high-dose irradiation (Shao et al. 2010; Yu et al. 2010). Hematopoietic system restoration relies on the hematopoietic stem cells (HSCs) that reside in the bone marrow (BM). HSCs sustain hematopoiesis through self-renewal, proliferation, and generation of differentiated progeny of distinct blood cell lineages. Radiation-induced damage in the HSC compartment, or forcing HSCs to undergo rapid proliferation, can lead to HSC exhaustion and long-term myelosuppression (Cheng et al. 2000; Wang et al. 2006), which is a common side effect observed in cancer patients receiving chemotherapy and extensive radiation therapy.
Several recent studies using mouse models suggest a critical role for the p53 pathway in HSC self-renewal and quiescence, as p53-null mice exhibit an increased pool of HSCs and their HSCs are more resistant to radiation-induced senescence (TeKippe et al. 2003; Meng et al. 2003; Wang et al. 2006; Chen et al. 2008; Liu et al. 2009). However, precise regulation of p53 activity is likely to be important in determining the response of HSCs and proliferative progenitors to irradiation. Thus, insufficient p53 activation should favor cell survival, but put cells at risk for loss of genomic integrity. In contrast, excessive p53 activation could compromise steady-state hematopoiesis and its recovery following exogenous marrow insult by causing too many cells to be eliminated. While insight has been gained into the impact of p53 itself on radiation sensitivity in the hematopoietic system, such studies have commonly used p53-deficient mice, which are of limited utility as they tend to develop tumors very rapidly (within 3 mo of age) and the heterozygotes quickly succumb to radiation-induced lymphomas (Kemp et al. 1994). This precludes evaluating the mechanisms of radioresistance, which requires dynamic and prolonged observation of radiation effects on hematopoietic cell kinetics. Moreover, p53-null models do not enable analyses of the roles of factors that control p53 level or activity, and these are likely to be critically important. For example, slight increases in p53 activity caused by reduced expression of p53 inhibitors Mdm2 and Mdmx render mice more radiosensitive (Mendrysa et al. 2003; Terzian et al. 2007). Conversely, mice encoding a stable form of Mdmx are impressively radioresistant despite modest reduction of p53 activity (Wang et al. 2009). However, whether the differences in radiosensitivity associated with altered p53 activity in these models reside in mature blood cells, the proliferative pool, the HSC compartment, or a combination of these, and the molecular mechanisms by which p53 activation elicits the observed phenotypes, remain to be determined.
In vitro and in vivo studies show that p53 activity is determined to a significant extent by mechanisms that regulate its abundance and stability. Mdm2 and Mdmx reduce p53 activity by binding to the N-terminal p53 transactivation domain (TAD) and by promoting ubiquitin-dependent p53 degradation (for review, see Wade et al. 2010). Control of p53 degradation is thought to partially require ubiquitylation of highly conserved C-terminal lysine residues (Rodriguez et al. 2000). The same lysines can also be acetylated by coactivators such as p300 and CBP to promote transactivation of target genes (for review, see Kruse and Gu 2009). In vitro studies suggest a model in which p53 is activated by damage-mediated kinases that induce phosphorylation in N-terminal serines to produce a conformational change leading to Mdm2/Mdmx dissociation and p300/CBP recruitment (Appella and Anderson 2001). These factors then acetylate the C terminus to stabilize p53 and enhance its transcriptional activity. However, in vivo, these modifications appear to be dispensable during embryogenesis, and do not significantly affect p53 activity in mouse embryonic fibroblasts (MEFs) (Krummel et al. 2005; Feng et al. 2005). This does not address tissue and condition-specific effects of these residues in vivo, nor whether such effects would be manifested through regulation of a subset of p53 target genes. Evidence of the importance of lysine modification for p53 in vivo functions comes from recent studies showing that mutation of K120 impairs puma activation (Tang et al. 2006), and that a K120R/K164R double mutant reduces induction of puma and p21, but not mdm2 (Tang et al. 2008). Thus, lysine modifications in the DNA-binding region appear to play a key role in differential gene regulation.
These observations led us to examine more deeply the impact of modification of the conserved seven C-terminal lysine residues in a knock-in model in which they were replaced with arginine (7KR) to prevent ubiquitylation or acetylation (Krummel et al. 2005). Here, we show that the p537KR mutation plays a critical role in regulating p53 transcriptional activity on a subset of genes in the hematopoietic system. This, in turn, affects maintenance of HSCs during unchallenged homeostatic growth, in the setting of BM transplantation, and after radiation exposure. Notably, the p537KR mutation causes extraordinary radiosensitivity, which is partially rescued by loss of a single p21 allele, implicating C-terminal modification in controlling p21 transcription in the HSC and progenitor pools that play critical roles in radiation responses.
Results
Wild-type (WT) and p537KR mutant mice have similar life spans
We first asked whether p537KR elicits a premature aging phenotype, as reported previously for some mouse mutants with elevated p53 activity (e.g., see García-Cao et al. 2002; Tyner et al. 2002). As such, we followed a large cohort of p537KR and WT animals to ascertain potential aging phenotypes. The data clearly show that the life span is similar between mutant mice and their WT littermates, and that the mutant mice do not display obvious signs of premature aging (Supplemental Fig. S1).
Preventing p53 C-terminal modification engenders exquisite radiosensitivity due to multiple hematopoietic system defects
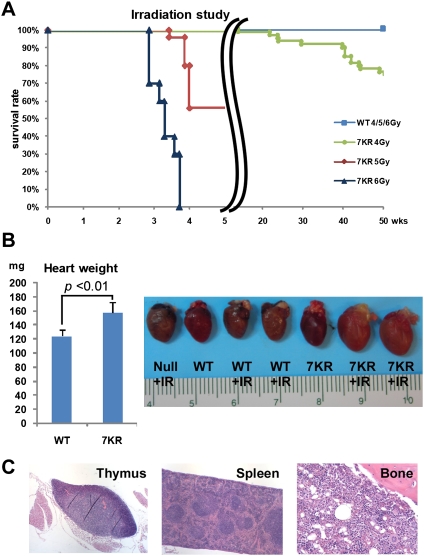
To determine whether p537KR mice have altered biological responses to DNA damage, mutant and wild-type (WT) mice were subjected to a range of sublethal doses of whole-body γ-irradiation (4, 5, and 6 Gy). As expected, all WT mice survived these exposures. In contrast, p537KR mice in the same genetic background exhibited a striking dose-dependent response to irradiation: Only ∼55% of p537KR mice were alive at 4 wk after 5 Gy irradiation, while 100% of p537KR mice died within 4 wk following 6 Gy irradiation (Fig. 1A).
Figure 1.
Preventing C-terminal modification of p53 engenders exquisite radiosensitivity, particularly in the hematopoietic system. (A) Kaplan-Meier radiation survival curves in WT mice and p537KR (7KR) mice after 4, 5, and 6 Gy of whole-body irradiation showing dose-dependent mortality in p537KR mice (4 Gy: n = 58, P < 0.001; 5 Gy: n = 25, P < 0.001; 6 Gy: n = 10, P < 0.001). (B) Increased cardiac weights and enlarged hearts in irradiated p537KR mice 4 wk after 5 Gy of irradiation. Error bars represent the SEM from four animals. The representative hearts from nonirradiated and irradiated mice are shown in the picture. (C) H&E staining of thymus, spleen, and BM from a p537KR mouse that died after exposure to 5 Gy of whole-body irradiation.
After irradiation, the mutant mice exhibited pallor of the carcass, low hematocrit, and enlarged hearts (Fig. 1B). Based on prior studies (Fauci et al. 2008), these observations are consistent with death resulting from heart failure secondary to severe anemia. Indeed, pathological evaluation showed that the radiation-induced lesions in p537KR mice were confined to the hematopoietic organs (Fig. 1C; Supplemental Fig. S2). Importantly, no significant differences were observed between WT and p537KR mice in other organs including radiosensitive tissues such as the gastrointestinal tract (Supplemental Fig. S2; data not shown).
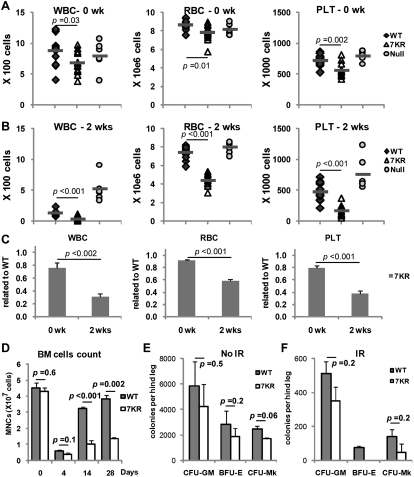
To expand on these results, we compared complete blood counts (CBCs) in WT and p537KR mice prior to and 2 wk following 5 Gy irradiation. This time point was chosen because myeloablation in mice has been shown to occur within 2 wk of irradiation followed by recovery by ∼4 wk (Uchida et al. 1994). We found that p537KR mice exhibit slightly lower blood counts at baseline (mutant = 80%–90% of WT levels) (Fig. 2A,C). Consistent with previous studies (Wang et al. 2009), we observed a marked decrease in white blood cell (WBC) counts and a mild decrease in red blood cell (RBC) and platelet counts in WT mice 2 wk post-irradiation (Fig. 2B). These changes are p53-dependent, as mild-to-no changes in CBCs occurred in p53-null mice (Fig. 2B). On the other hand, p537KR mice exhibited more severe decreases in all three blood lineages 2 wk post-irradiation (mutant ∼30%–50% of WT) (Fig. 2B,C).
Figure 2.
Irradiation causes long-term defects in p537KR hematopoietic progenitor cells. (A) White blood cells (WBC) red blood cells (RBC), and and platelets (PLT) in WT, p537KR, and p53-null mice prior to irradiation. WT and 7KR: n = 13; null: n = 6. (B) Blood cell counts at 2 wk after 5 Gy of whole-body irradiation. (C) The fold changes of blood cell counts in p537KR mice related to WT mice 2 wk post-irradiation. (D) Mononucleated cell (MNCs) count in BM at baseline and 4, 14, and 28 d post-irradiation revealed insufficient recovery of BM cells in irradiated p537KR mice. (E) BM cells were isolated from nonirradiated mice and seeded for granulocytic/macrophage (CFU-GM), erythroid (BFU-E), and megakaryocytic (CFU-MK) progenitor cell colony-forming assay. (F) BM cells were immediately isolated from animals that were exposed to irradiation for progenitor colony-forming assay. Note: No colonies were found when 5 × 106 p537KR BM cells were seeded for BFU-E assay. The error bars in D, E, and F represent SEM from three independent experiments.
Histology of the BM and spleen, the major hematopoietic organs in mice, revealed no visible differences between WT and p537KR mice prior to irradiation (Supplemental Fig. S2). Two weeks after 5 Gy irradiation, WT BM and spleens exhibited moderate to robust intra- and extramedullary hematopoiesis, which gradually abated to near baseline by 4 wk as blood counts returned to normal (Supplemental Fig. S2). p537KR mice exhibited more dramatic changes in hematopoietic organs. As such, some mutants showed robust intra- and extramedullary hematopoiesis in response to the severe radiation-induced pancytopenia, while others displayed severe atrophy of the hematopoietic BM and splenic red pulp (Supplemental Fig. S2). Both observations are consistent with increased radiosensitivity in the hematopoietic system of the p537KR mice. At 4 wk, mutant mice showed intra- and extramedullary hematopoiesis, indicative of continued stimulation from persistent pancytopenia. Importantly, hematopoiesis was not sufficient to compensate for the marked pancytopenia in time to ensure survival of half of the mutant mice. Consistent with the survival curve (Fig. 1A), 6 Gy irradiation translated histopathologically as a more severe atrophy in the BM and spleen (Supplemental Fig. S2).
The peripheral pancytopenia observed in irradiated p537KR mice could reflect impaired hematopoietic stem and progenitor cell survival, proliferation, or differentiation defects, increased blood cell destruction, or a combination of these. Importantly, responses measured by peripheral blood cell counts can underestimate a progenitor cell phenotype, as the post-progenitor cell hematopoietic compartment can hyperproliferate to compensate for profound deficiencies in marrow stem or progenitor cells (Kaushansky et al. 2002). As such, we evaluated total BM counts before and after 5 Gy irradiation to further understand the factors involved in the increased radiosensitivity of p537KR mice. The total BM counts in WT and p537KR mice were not significantly different prior to irradiation (Fig. 2D). Consistent with previous studies (Wang et al. 2006), the total BM cells from WT mice dropped significantly by 4 d after irradiation, and then started to recover by 2 wk post-irradiation. Four weeks after irradiation, total BM cells increased to ∼85% of baseline levels. Importantly, total BM cells in p537KR mice decreased to similar levels as WT at 4 d but remained significantly lower at 2 and 4 wk post-irradiation (Fig. 2D).
We further quantified hematopoietic progenitor cells by culturing marrow cells from WT and p537KR mice in semisolid medium containing various growth factor combinations. Figure 2E shows that, compared with WT animals, p537KR mouse marrow may contain slightly fewer granulocytic/macrophage (CFU-GM), erythroid (BFU-E), and megakaryocytic (CFU-MK) progenitor cells prior to irradiation (differences are not statistically significant). Immediately following irradiation and isolation of marrow cells, we observed a 10- to 20-fold reduction of colony-forming cells of all cell types in both WT and p537KR mice, again with slightly fewer colony-forming cells in p537KR mice (Fig. 2F). In contrast, and consistent with lower total BM cell counts at 2 and 4 wk post-irradiation (Fig. 2D), the mutant mice exhibited more severe reductions in hematopoietic colony-forming progenitor cells when marrows were isolated 2 and 4 wk post-irradiation (Supplemental Fig. S3).
p537KR BM cells compete poorly with WT cells in transplantation and are more radiosensitive
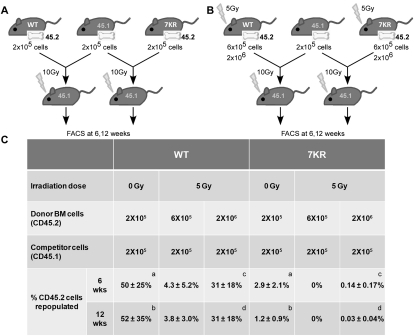
The radiosensitivity observed in the colony formation assays implies a defect in the progenitor and/or stem cell compartments. We therefore used competitive BM transplantation to address whether the long-term stem cell repopulating abilities of the WT and mutant differ. BM cells from donor mice (WT or p537KR) expressing the CD45.2 leukocyte cell surface marker were transplanted into lethally irradiated recipient mice in fixed ratios with WT competitor BM cells expressing the CD45.1 leukocyte cell surface marker (Fig. 3A). Twelve weeks after transplantation, at which time only long-term HSCs (LT-HSCs) contribute to hematopoiesis, mice were sacrificed and leukocytes from peripheral blood and BM were analyzed for expression of CD45.1 and CD45.2 using flow cytometry. As expected, WT donor cells (CD45.2) and competitor cells (CD45.1) transplanted in equal amounts (2 × 105) into lethally irradiated recipients competed almost equally to repopulate the peripheral blood compartment (Fig. 3C). In contrast, under the same experimental conditions, p537KR CD45.2 donor cells only contributed to 1.2% of the reconstituted peripheral blood cell populations at 12 wk post-transplantation.
Figure 3.
p537KR HSCs exhibit sensitivity to transplantation and to irradiation. BM from nonirradiated (A) and 4-d post-irradiated (B) WT or p537KR donor mice expressing the CD45.2 leukocyte cell surface marker were transplanted into lethally irradiated recipient mice in fixed ratios with WT competitor marrow cells expressing CD45.1. (C) p537KR HSCs did not compete as well as WT for long-term repopulation under both nonirradiated and irradiated conditions. At 6 wk and 12 wk post-transplantation, peripheral blood was withdrawn from the recipients and analyzed for the percentage of CD45.2 cells in total leukocytes. Results show mean ± SD from at least five animals. a, b: P < 0.05. c, d: P < 0.001.
We further characterized the radiation-induced defect in p537KR HSCs by performing the same competitive repopulation assays with irradiated WT and p537KR cells. BM cells were isolated 4 d post-irradiation, as this corresponds to the time of maximal radiation-induced BM cell lethality (Wang et al. 2006). Since irradiation significantly affects multiple hematopoietic cell types and can induce HSC senescence (Meng et al. 2003), we used three or 10 times more irradiated WT or p537KR cells than nonirradiated competitor cells to ensure the presence of some viable HSCs from irradiated donors for engraftment. Under these experimental conditions, irradiation of WT donor cells produced a 10- to 15-fold reduction in transplantation efficiency as compared with nonirradiated WT donor cells (Fig. 3C).
Identical analysis using p537KR donor cells revealed such a profound defect in p537KR HSCs that none of the reconstituting cells were derived from the mutant animals when threefold more irradiated p537KR cells were cotransplanted with competitor WT cells. Even with 10-fold more irradiated p537KR donor cells, mutant HSCs were not capable of competing in the reconstitution of the hematopoietic compartment (Fig. 3C). These data reveal a profound defect in the ability of p537KR HSCs to compete with WT HSCs for repopulation, and that this phenotype is magnified by irradiation.
Unchallenged p537KR BM contains fewer HSCs
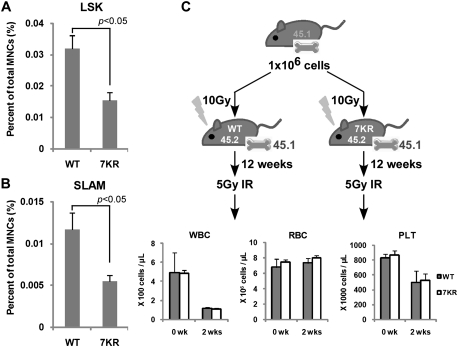
In addition to a reduced competitive fitness of the mutant HSCs, we also explored the possibility that nonirradiated p537KR BM contains fewer HSCs. We estimated HSC numbers by enumerating Lin−Scal1+cKit+ (LSK) cells, a population of cells previously shown to be highly enriched for HSCs (Wognum et al. 2003). We observed that p537KR BM contains approximately two-thirds the number of LSK cells as compared with WT BM (Fig. 4A; Supplemental Fig. S4). We confirmed this result by sorting for an independent set of HSC cell surface markers, referred to as SLAM markers (Lin−CD41−CD48−CD150+), as this yields a population that contains 40%–50% HSCs as determined by limiting dilution long-term repopulation analysis (Kiel et al. 2005). As observed for LSK cells, SLAM cells from p537KR marrows were ∼40% the abundance of similarly isolated cells from WT BM (Fig. 4B; Supplemental Fig. S4).
Figure 4.
The hematopoietic defects in p537KR mice reside in HSCs/progenitor cells and not in the stem cell niche. (A) BM cells were isolated from nonirradiated mice and stained with antibodies against hematopoietic lineage (Lin) markers and cell surface marks Sca1 and c-Kit and analyzed by flow cytometry. Lin−Sca1+c-Kit+ subpopulation were gated as HSC-enriched LSK cells. Cell plots are shown in Supplemental Figure S4. (B) Antibody cocktail lineage markers CD41, CD48, and CD150 were used for the analysis. The Lin−CD41−CD48−CD150+ subpopulation was gated as HSC-enriched SLAM cells. Cell plots are shown in Supplemental Figure S4. (C) Introducing WT HSCs into p537KR mice by BM transplantation overcame the radiosensitivity of the animals. BM cells expressing CD45.1 were transplanted into lethally irradiated WT or p537KR mice. Twelve weeks later, the chimeric mice were exposed to 5 Gy of irradiation followed by CBC analysis. (A–C) Error bars represent the SEM from three animals.
The radiosensitivity of p537KR animals resides in the HSC and not in the stem cell niche
We next determined whether irradiation induces changes in the HSC niche(s) in p537KR animals, as this could also contribute to their reduced abundance and to their radiosensitivity. We investigated this possibility by transplanting 1 × 106 WT (CD45.1) BM cells into either WT or p537KR mice exposed to the lethal dose of 10 Gy whole-body radiation (Fig. 4B). We observed that 100% of these lethally irradiated p537KR mice survived after transplantation of WT BM. FACS analyses showed that donor cells (CD45.1) reconstituted the entire hematopoietic system by 6 wk post-transplantation (Supplemental Fig. S5). The rescued mice are thus chimeric in that their hematopoietic compartment is fully reconstituted by WT HSCs, while the marrow niche and all other cells in these animals are p537KR. CBC analysis showed that these WT:p537KR chimeras resisted 5 Gy of whole-body irradiation like WT:WT chimaeras (Fig. 4C). This restoration of radioresistance was not due to an effect of the age of the animals at the time of analysis, as 23-wk-old p537KR mice (slightly older than the irradiated chimeras) were still sensitive to the same dose of irradiation (Supplemental Fig. S6). This result demonstrates that introducing WT HSCs by BM transplantation is sufficient to rescue the radiosensitivity of p537KR animals.
p537KR function and radiosensitivity are affected by Mdm2
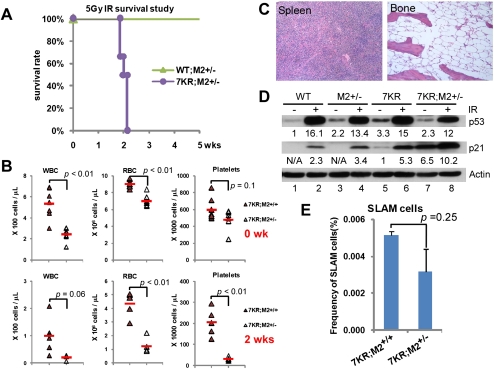
Previous studies of the impact of Mdm2 and Mdmx expression on p53 activity revealed that the p53 pathway is exquisitely sensitive to p53 protein levels and basal activity, which can be modulated by p53-negative regulators (Mendrysa et al. 2003; Terzian et al. 2007; Wang et al. 2009). Since Mdm2 heterozygous mice are known to have elevated p53 activity (Terzian et al. 2007), we compared their radiosensitivity with p537KR mice. Interestingly, while half of p537KR mice died from 5 Gy ionizing radiation (IR), all Mdm2+/− mice survived. Moreover, loss of one Mdm2 allele in a p537KR background significantly increased p537KR radiosensitivity (Fig. 5A). CBC and histopathological evaluation revealed that p537KR;Mdm2+/− mice exhibited a more severe radiation-induced pancytopenia concomitant with severe atrophy in the spleen and BM (Fig. 5B,C). p537KR;Mdm2+/− BM also had slightly fewer HSC-enriched SLAM cells at baseline when compared with p537KR;Mdm2+/+ littermates (Fig. 5E). It is unlikely that genetic background differences underlie any of these effects, as CBC analyses showed similar responses in the backcrossed p537KR and p537KR;Mdm2+/+ genotypes (Supplemental Fig. S7).
Figure 5.
Mdm2 dependence of radiosensitivity in p537KR mice. (A) Kaplan-Meier radiation survival curves. WT;Mdm2+/− (n = 9) and p537KR;Mdm2+/− (n = 6) mice under the same genetic background were exposed to 5 Gy whole-body irradiation. Losing one allele of Mdm2 enhanced the radiosensitivity of p537KR mice. P < 0.001. (B) CBC analysis of p537KR;Mdm2+/+ and p537KR;Mdm2+/− mice before irradiation (n = 9) and 2 wk after 5 Gy whole-body irradiation (n = 5) showed severe pancytopenia in p537KR;Mdm2+/− mice. (C) Histopathological evaluation revealed that p537KR;Mdm2+/− mice that died from irradiation exhibited severe atrophy in the spleen and BM. (D) Western analysis of p53 and p21 abundance in spleens isolated from WT, Mdm2+/− (M2+/−), p537KR (7KR), and p537KR;Mdm2+/− (7KR;M2+/−) mice prior to or 3 h after 5 Gy of whole-body irradiation. Actin blot provides a loading control. Intensities of p53 and p21 signals were normalized to Actin signals and are presented as relative fold differences. (E) p537KR;Mdm2+/− mice exhibited a slightly lower frequency of HSC-enriched SLAM cells when compared with p537KR;Mdm2+/+ mice. Error bars represent the SEM from three animals.
We next asked whether the relative radiosensitivity of the various genotypes analyzed above correlated with basal p53 protein levels. Surprisingly, the basal levels of p53 in splenocytes from the different genotypes (Fig. 5D) showed no direct correlation with the severity of the radiation response. At baseline, p53 level was slightly higher in p537KR mice than in WT or Mdm2+/− animals, while it was, if anything, slightly lower in the more radiosensitive p537KR;Mdm2+/− mice (Fig. 5D; Supplemental Fig. S8). In contrast, we observed a significant increase in basal p21 protein levels in p537KR mice, and this was substantially stronger in p537KR;Mdm2+/− mice. Furthermore, the p21 level in the p537KR;Mdm2+/− mice was the highest of any genotype after irradiation, and this correlates with their exquisite radiosensitivity. Thus, the p21 levels parallel the relative radiosensitivity of each genotype analyzed. This raises the possibility that the p537KR mutant activates a different transcriptional program than p53WT, and that this program may be further modulated by changes in Mdm2 levels.
p537KR exhibits differential transcriptional activity
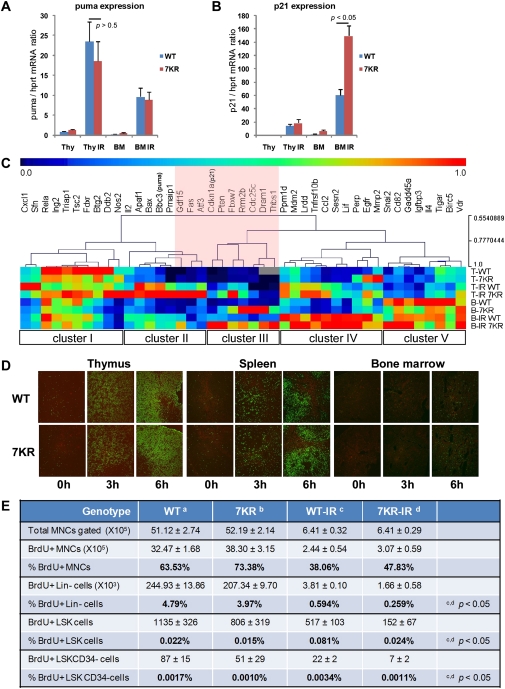
Recent studies show that post-translational modifications of different lysine residues in p53 can elicit differential effects on gene regulation that have significant biological consequences (Tang et al. 2008; W Gu, pers. comm.). Therefore, we investigated whether the K-to-R mutations we introduced into p53 C-terminal lysines also elicited differential effects on target gene transcription that contribute to the radiosensitivity of the mutant animals. As a first analysis, quantitative PCR (qPCR) measurements revealed that the induced levels of proapoptotic puma and noxa in the thymus and BM were very similar in WT and p537KR after 5 Gy of whole-body irradiation, (Fig. 6A; data not shown). Consistent with our previous studies (Krummel et al. 2005), we found that p21 expression was slightly higher in irradiated p537KR thymus compared with WT thymus (Fig. 6B; Supplemental Fig. S9). Furthermore, p21 was expressed at even higher levels in BM than in thymus, and is reproducibly expressed at twofold higher levels in the p537KR BM than in WT BM at baseline and after irradiation (Fig. 6B; Supplemental Fig. S9).
Figure 6.
Differential gene expression and cell cycle kinetics in p537KR mice. (A) The p53-inducible proapoptotic gene puma was expressed at higher levels in thymus than in BM after irradiation. The level of puma expression was similar between WT and p537KR mice. (B) The p53-inducible cell cycle arrest gene p21 was highly expressed in BM after irradiation, and showed even greater expression in p537KR animals. (A,B) Error bars represent the SEM from three animals. (C) Expression of 43 p53 target genes from thymus (T) and BM (B) prior to irradiation or 3 h post-irradiation (IR) was detected using microfluidic qPCR analysis. Relative expressions of each gene from all samples were scaled from 0 to 1, followed by hierarchical clustering analysis. (Cluster I) Repressive or less responsive genes. (Cluster II) Highly IR-induced genes in thymus. (Cluster III) Genes highly affected by 7KR mutations in BM. (Cluster IV) Highly IR-induced genes in BM. (Cluster V) Highly expressed genes in BM. The analysis suggests that several genes (highlighted in pink) are differentially affected by the 7KR mutations. (D) Thymi, spleens, and BM were isolated from mice at 0, 3, and 6 h after 5 Gy of whole-body irradiation. Apoptosis was detected by TUNEL staining (green). (E) Nonirradiated mice or mice exposed to 5 Gy of irradiation were injected with 2 mg of BrdU followed by 2-d administration of BrdU water (1 mg/mL). BMs were then isolated and stained with antibodies against lineage (Lin) markers; cell surface markers Sca1, c-kit, and CD34; and BrdU, followed by flow analysis. BrdU incorporated cell in Lin−, LSK (Lin−Sca1+c-kit+) and LSKCD34− subcell populations were analyzed. Error bars represent the SD from three animals.
We further studied the transcriptional response elicited by p53 in BM and thymus in the different genotypes using a microfluidic chip that enables qPCR analysis of 43 p53 target genes. This provided a greater sampling of genes to explore and quantify genotype- and tissue-related differences in transcriptional activity. Cluster analysis allowed us to identify gene clusters that were expressed differentially in tissues and/or in response to irradiation (Fig. 6C). For example, cluster IV contains a subset of genes that were highly induced by irradiation in the BM, while cluster II contains genes that were highly induced in the thymus. We noted that several proapoptotic genes (apaf1, bax, puma, and noxa) are present in cluster II. Importantly, these genes were induced to similar levels in WT and p537KR animals. This result suggested that apoptosis might be induced to similar high levels in the WT and mutant animals in the thymus, and to similar but lower levels in the BM. We investigated this directly by terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end-labeling (TUNEL) assay. Figure 6D shows that cells in the thymus and spleen start to undergo apoptosis at 3 h post-irradiation and that the number of apoptotic cells increases by 6 h. In contrast, few BM cells were apoptotic at this time point. More importantly, there was no measurable difference in apoptotic frequency of cells in WT and p537KR mice (Supplemental Fig. S10).
In addition to the cluster analysis, we identified several genes (Fig. 6C, highlighted in pink) whose expression was strongly and differentially affected by the 7KR mutations. Consistent with the above analyses, we observed higher p21 expression in irradiated p537KR thymus than in irradiated WT thymus (Fig. 6C; Supplemental Fig. S11) and even higher p21 levels in irradiated p537KR BM (Fig. 6C; Supplemental Fig. S11). Indeed, these analyses revealed p21 to be one of the most significantly up-regulated p53-inducible genes we analyzed in irradiated p537KR BM.
We next determined whether the significant differences in p21 expression correlated with IR-induced proliferation differences between WT and p537KR BM cells. BrdU was administered to mice for 2 d, marrow was isolated, and cells of different hematopoietic lineages were analyzed for BrdU incorporation by flow cytometry. Interestingly, we observed a slightly higher number of BrdU-positive (BrdU+) total BM-MNCs (BM mononucleated cells) in p537KR mice relative to WT mice at baseline (Fig. 6E). However, the number of BrdU+ LSK cells, and the more quiescent HSC-enriched LSKCD34− subpopulation, was lower in p537KR than in WT mice. Upon irradiation, the number of BrdU+Lin− cells, BrdU+LSK cells, and BrdU+LSKCD34− cells were significantly lower in p537KR mice than in WT mice (Fig. 6E). Taken together, the data reveal differences in the hematopoietic cell cycle kinetics in unirradiated p537KR and WT animals, with the mutant manifesting greater sensitivity to radiation-induced withdrawal from the cell cycle.
p21 gene dosage affects radiosensitivity of p537KR mice
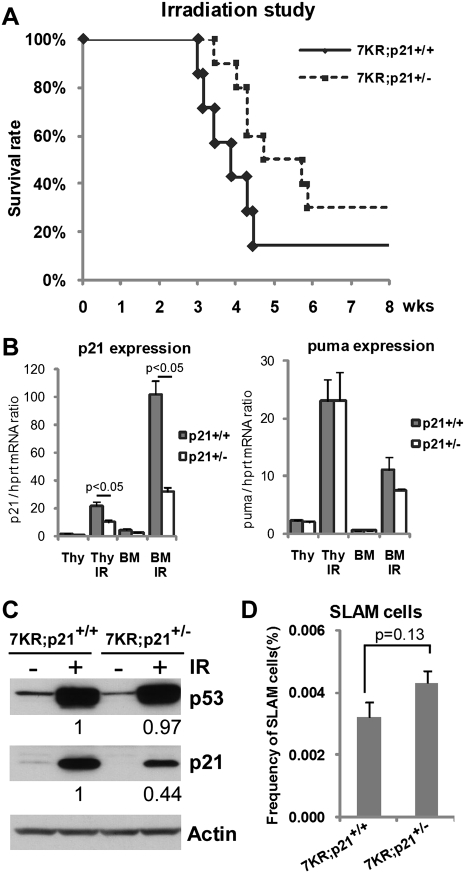
Several lines of evidence indicate that puma-mediated apoptosis and p21-mediated cell cycle arrest contribute to HSC abundance and functionality after irradiation (Cheng et al. 2000; Meng et al. 2003; Shao et al. 2010; Yu et al. 2010). While WT and p537KR mice exhibited similar levels of puma induction (Fig. 6B,C) and apoptosis at early times after irradiation (Figs. 2D, 6D; Supplemental Fig. S10), p21 was significantly and differentially expressed in p537KR BM cells. We determined whether the increased p21 induction was functionally relevant to the radiosensitivity of p537KR mice by determining whether reducing p21 gene dosage would reduce the p537KR radiosensitivity. We crossed p537KR mice with p21+/− mice to obtain p537KR;p21+/− mice, and found a significant increase in radioresistance in these mice. For example, we observed no mortality in p537KR;p21+/− mice at least 6 wk after exposure to 5 Gy radiation (Supplemental Fig. S12). When mice were exposed to 6 Gy radiation, almost 100% of p537KR;p21+/+ mice died within 4 wk of irradiation, while 50% of p537KR;p21+/− littermates survived during the same time period. By 8 wk after irradiation, 30% of p537KR;p21+/− mice were still alive (Fig. 7A). Western blot analysis and gene expression confirmed that p21 levels were lower in irradiated p537KR;p21+/− mice relative to irradiated p537KR;p21+/+ mice (Fig. 7B,C). When HSC-enriched SLAM cells were analyzed, we observed slightly more SLAM cells in p537KR;p21+/− BM (Fig. 7D), although the increase did not achieve statistical significance. Together, our data demonstrate the importance of proper C-terminal p53 modification to ensure that the p53-dependent cell cycle arrest function is appropriately regulated. Furthermore, they reveal the importance of properly regulated p53-induced cell cycle arrest for hematopoietic system recovery after genotoxic stress. They also indicate that p21 contributes to, but is likely not the only factor responsible for, the increased radiosensitivity of p537KR mice.
Figure 7.
p21 gene dosage affects radiosensitivity of p537KR mice. (A) Kaplan-Meier radiation survival curves. Littermate p537KR;p21+/+ (n = 7) and p537KR;p21+/− (n = 10) mice were exposed to 6 Gy of whole-body irradiation. Losing one allele of p21 partially rescued the sensitivity of p537KR mice to 6 Gy irradiation. P = 0.06. (B) Analysis of gene expression in thymus and BM showed that p21 expression was reduced by half in p537KR;p21+/− mice (p21+/−), while puma expression was not affected. Error bars represent the SEM from three animals. (C) Western analysis showed reduced p21 levels in irradiated p537KR;p21+/− mice, while p53 levels were unchanged. (D) p537KR;p21+/− mice exhibited slight lower frequency of HSC-enriched SLAM cells when compared with p537KR;p21+/+ mice. Error bars represent the SEM from three animals.
Discussion
Our data provide the first evidence that post-translational modifications at highly conserved lysines in the p53 C-terminal regulatory domain are critically important for fine-tuning p53 activity to properly maintain hematopoietic system homeostasis and enable appropriate responses to DNA damage. The inability to post-translationally modify these sites leads to altered expression of a subset of genes that prevents the timely re-entry of HSCs and progenitors into the cell cycle to enable recovery from radiation-induced myelosuppression. A combination of gene expression and genetic strategies reveal that this aberrant response is due in part to elevated expression of the cyclin-dependent kinase inhibitor p21, although other factors are also likely involved.
Our studies reveal a differential capacity for p537KR to regulate a subset of target genes in subtypes of cells in the hematopoietic system following genotoxic stress. Our data show that p53-induced proapoptotic genes, including puma, bax, noxa, and apf1, are expressed at relatively high levels in fully differentiated cells (thymocytes and splenocytes) after irradiation. In contrast, genes that are involved in the cell cycle, such as p21, pten, and fbxw7, are highly expressed in hematopoietic stem or progenitor cells (BM and Lin− cells) after irradiation (Fig. 5). The ability of p53 to differentially regulate genes is likely to be dependent on additional factors such as its binding affinity to the distinct p53 response elements in each promoter as well as promoter chromatin structure, binding of other regulatory proteins, availability of cofactors, etc. (for review, see Espinosa 2008). Here, we provide the first in vivo evidence that modifications in the highly conserved C-terminal regulatory domain contribute to differential, tissue-specific gene regulation. The subset of genes that were altered by the 7KR mutations was distinct from that identified in Mdm2+/− cells (Fig. 6C; Supplemental Fig. S13), suggesting that induction of different p53 response programs can be regulated by C-terminal modifications. The importance of this region in p53 transcriptional control is consistent with structural studies indicating that the loose, positively charged residues within this highly conserved regulatory domain facilitate target gene identification by enabling p53 to encircle and slide along DNA (Jayaraman and Prives 1995; Weinberg et al. 2004; Tidow et al. 2007). Our data demonstrate that p21 is one of the most exquisitely sensitive p53 response genes affected by p53 C-terminal modification in hematopoietic cells. Interestingly, DNA sequence and biochemical analyses indicate that the p21 gene contains one of the strongest p53-binding sites (Inga et al. 2002), and the gene may be poised to express rapidly due to both a special configuration of the transcriptional apparatus and the presence of preloaded polymerase II (Espinosa et al. 2003). Together, such factors likely enable p21 to manifest significant changes in the face of subtle alterations in p53 abundance or binding. It is possible that other genes that are hyperresponsive to p537KR exhibit similar properties.
Control of p53 abundance and activity are clearly critical for survival and response to oncogenic and genotoxic stresses (Wade et al. 2010). Mdm2 plays key roles in both levels of control by virtue of its ubiquitin ligase activity and its ability to bind to the N-terminal TAD and inhibit p53-dependent effects on transcription. Our data clearly show that preventing C-terminal lysine modifications targeted by Mdm2 for ubiquitylation is apparently not sufficient to completely stabilize p53, as p537KR levels still increase after irradiation (Fig. 5D). This may be due to the use of alternative ubiquitylation sites, as indicated by our previous study showing that p537KR is aberrantly ubiquitylated (Krummel et al. 2005). However, the activity of the p537KR mutant is still exquisitely sensitive to Mdm2, as removing only one Mdm2 allele significantly increased radiosensitivity of p537KR mice but not p53WT mice in response to 5 Gy irradiation (Fig. 5A). Given this result, we were surprised to find that p53 levels do not strictly correlate with Mdm2 in the case of p537KR animals. Thus, while p53WT increased about twofold, at baseline, in Mdm2+/− animals, p537KR levels were, if anything, slightly less abundant in p537KR;Mdm2+/− than p537KR;Mdm2+/+ animals at baseline and after irradiation (Fig. 5D, lanes 5–8). Interestingly, Mendrysa et al. 2003, 2006) have made similar observations using mice that express ∼30% of the WT level of Mdm2. In their analyses, the thymus in Mdm2puro/Δ7–12 mice was smaller, and they observed increased p53 activity with similar to lower p53 levels. They also reported that the mutant thymus and spleen contained reduced numbers of leukocytes, indicating how p53 activity can affect cellular composition in these organs. Furthermore, their analyses showed significant decreases of WBCs in Mdm2puro/Δ7–12 mice than Mdm2puro/+ and Mdm2+/− mice. These results are similar to what we observed in p537KR;Mdm2+/−, p537KR, and Mdm2+/− mice (Fig. 5B; Supplemental Fig. S7). Thus, the altered cellular composition in the p537KR;Mdm2+/− spleens might contribute to p53 levels that are significantly different from WT, especially when employing methods such as Western blotting that detect protein levels in mixed cell populations. It is also possible that p537KR;Mdm2+/− cells with low p53 levels were positively selected through development. While the exact mechanism underlying the observed difference requires further investigation, it seems even at WT levels of expression, the C terminally altered p537KR protein is more efficient than p53WT at regulating a subset of genes in BM, such as p21.
Radiosensitivity in the hematopoietic system is determined by the severity of irradiation-induced apoptosis in mature blood cells during the acute phase, and by the ability of HSCs and progenitors to repopulate and replace the damaged cells during the recovery phase. The proapoptotic gene puma and cell cycle arrest gene p21 are two well-studied p53-inducible genes reported previously to contribute to radiosensitivity and HSC regulation. While puma deficiency protects HSCs from high-dose radiation-induced damage (Shao et al. 2010; Yu et al. 2010), the role of p21 in HSC regulation remains controversial owing to conflicting reports that the expanded HSC pool in p21-deficient mice is exhausted over time due to inappropriate cell cycle entry (Cheng et al. 2000; van Os et al. 2007). Other studies reporting increased radiosensitivity in Atm- and p21-deficient mice (Wang et al. 1997; Ito et al. 2007) suggest that the loss of cell cycle control in such animals generates defects in G1/S boundary control that could result in unscheduled S-phase entry and p53-dependent and -independent death processes. On the other hand, overexpressing p21, which was observed in irradiated p537KR BM, could result in a significant number of HSCs or progenitor cells undergoing cell cycle arrest. This could, in turn, cause a delay or insufficient repopulation of hematopoietic cells during the recovery phase after irradiation or other forms of genotoxic myelosuppression. Consistent with this, we found significantly less BrdU incorporation in irradiated p537KR HSC and progenitor compartments (Fig. 6E) and low BM cellularity 2 and 4 wk after irradiation (Figs. 2D; Supplemental Fig. S2). Intriguingly, we also observed greater BrdU incorporation in p537KR total BM-MNCs relative to WT during homeostasis (Fig. 6E). Since p537KR mice contain similar numbers of total BM cells at baseline (Fig. 2D), it is conceivable that the reduced HSC frequency in p537KR mice (Fig. 4A,B) might trigger a positive compensating stimulus that results in more progenitor or terminally differentiated cells entering cell cycle to generate sufficient mature cells to sustain hematopoiesis (Jankovic et al. 2007; Madan et al. 2009). In our study, reducing p21 levels by losing one allele of p21 completely rescued radiosensitivity of p537KR mice at 5 Gy, and partially rescued at 6 Gy of γ-irradiation, suggesting that complete restoration of HSC function in p537KR mice after irradiation likely requires additional factors. While puma and other proapoptotic genes clearly contribute to p53-dependent radiosensitivity, such genes were not differentially up-regulated in the p537KR mutant (Fig. 6C), raising the possibility that other genes that are expressed at greater levels in the mutant may contribute to its radiosensitive phenotype. Candidates for genes include those in Figure 6C that are highly up-regulated in the p537KR mutant (highlighted in pink).
We also observed that p537KR BM exhibited extremely low repopulating efficiency in the competitive BM transplant assay even in the absence of irradiation. It is possible that transplant-associated stress (Lahav et al. 2005) cooperates with the elevated basal p53 activation of p537KR HSCs and progenitors to produce the observed low repopulating efficiency of nonirradiated p537KR BM (Fig. 3). A homing defect preventing p537KR HSCs from accessing the hematopoietic niche could also contribute to their low repopulating efficiency. We have no direct evidence for such a deficiency, and note that p537KR cells were present in both circulating peripheral blood and BM 6 and 12 wk after transplant. However, we cannot rule out the possibility that p537KR cells might exhibit different homing kinetics, which causes a delay in HSC niche engraftment. Recently, p53 activity level has been demonstrated to be a critical determinant of the ability of stem cells to compete in the setting of BM transplantation (Bondar and Medzhitov 2010; Marusyk et al. 2010). Similar to our observations, Bondar and Medzhitov (2010) found that cell cycle regulation plays an important role in p53-dependent HSC competition. Our data add a mechanistic function to explain roles for p53 in transplantation competitiveness by showing that p21 levels can profoundly influence HSC/progenitor fitness, and that the p53 C-terminal regulatory domain appears to be one element at which signals elicited during transplantation are processed. This competition model implies that cells with compromised p53 function may gain selective advantages and contribute to tumor development or evolution.
Together, our and other studies (Mendrysa et al. 2003; Terzian et al. 2007; Wang et al. 2009) reveal the exquisite sensitivity of the hematopoietic system to modest elevations or reductions in p53 activity. By extension, transient antagonism of p53 activity could be protective in situations where environmental or chemotherapeutic genotoxins would otherwise be cytotoxic in normal tissues and stem cells (Gudkov and Komarova 2007). Furthermore, we and others previously showed that suppressing p53 activity enhances reprogramming of differentiated cells to pluripotent stem cells (Kawamura et al. 2009; Krizhanovsky and Lowe 2009). An implication of the data shown here is that transient p53 antagonism could also be beneficial for in vitro stem cell culturing to prevent stress-induced stem cell senescence, as BM transplants often require HSC enrichment or expansion ex vivo. In vivo, inappropriate repression of p53 C-terminal acetylation in PML-RAR oncoprotein-associated acute promyelocytic leukemia (APL) (Insinga et al. 2004) may compromise p53 activity sufficiently to enable rare leukemic stem cells to self-renew, generate leukemic blasts, and fuel leukemia progression. Interestingly, acute leukemias rarely contain p53 mutations but do exhibit compromised p53 function (Dino et al. 1994). The enhanced understanding of p53 regulation in HSC homeostasis and recovery from genotoxic injury afforded by these analyses suggest routes to the development of therapeutic approaches to spare or protect the hematopoietic system.
Materials and methods
Mice and irradiation procedure
C57Bl/6-Ly5.2 and C57Bl/6-Ly5.1 mice were purchased from Taconic. p537KR mice were generated previously (Krummel et al. 2005) and backcrossed to C57Bl/6 background. p537KR mice that were used in the study are in B6 or a mixed B6/129 background. Mdm2+/− mice (kind gift of G. Lozano) are in a mixed B6/129 background. The p21+/− mice used here (K Tinkum, D Piwnica-Worms, and H Piwnica-Worms, in prep.) are in a mixed B6/129 background; Supplemental Figure S14 shows that this p21 knockout allele expresses no detectable p21 following irradiation. For the irradiation study, age- and gender-matched mice were exposed to whole-body irradiation with a 60Co γ-irradiator at an average rate of 0.5 Gy/min. All mice used in the study were between 8 and 12 wk of age. All mouse maintenance and procedures were approved by the Animal Care and Use Committee of the Salk Institute.
Histochemical analysis
Tissues were isolated from mice and fixed in 10% neutral buffered formalin (Protocol) for 24 h. Bones were decalcified in Cal-Ex solution (Thermo-Fisher). Fixed tissues were embedding in paraffin. Paraffin sections were deparaffinized and heated in 10 mM citrate buffer (pH 6) in a microwave for antigen retrieval. The sections were then stained with H&E for pathological analysis. Apoptosis was determined by TUNEL assay using In Situ Cell Death Detection kit, Fluorescein (Roche).
Colony-forming cell assay
BM cells were flushed from femurs and tibias using IMDM medium (Gibco) containing 2%FBS. Cells were triturated and filtered through a nylon screen to obtain a single-cell suspension. An aliquot of cells were stained with Turk solution for MNC counting. BM-MNCs (5 × 105∼5 × 106) were plated for colony-forming cell assay using MethoCult and MegaCult-C (Stem Cell Technologies) following the manufacturer's instructions. Cells were incubated at 37°C with 5% CO2 and >95% humidity. Colonies of CFU-GM and BFU-E were scored on day 7 and colonies of CFU-Mk were scored on day 12.
Competitive long-term reconstitution assay
Donor BM-MNCs were isolated from WT or p537KR mice (C57Bl/6-Ly5.2) and mixed with 2 × 105 competitive BM-MNCS isolated from congenic C57Bl/6-Ly5.1 mice. The mixture was retro-orbitally injected into lethally irradiated recipients (C57Bl/6-Ly5.1). Twelve weeks post-transplants, reconstitution of donor leukocytes were analyzed by staining blood cells or BM with antibodies against leukocyte cell surface markers CD45.1 and CD45.2 (BD Pharmingen) using flow cytometry.
Western blot analysis
Cells or tissues were lysed in RIPA buffer (50 mM Tris-HCl at pH 7.4, 150 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate, 1% NP40, 10 mM Na3VO4, 100 mM NaF, protease inhibitor cocktails [complete tablet, Roche]). Protein extracts were analyzed by Western blotting using antibodies specific for p53 (1C12, Cell Signaling), p21 (BD Pharmigen), and Actin (Sigma).
Real-time RT–PCR
Total RNA from thymus or BM was isolated using RNeasy mini kit (Qiagen) and subjected to real-time qPCR as described previously (Krummel et al. 2005).
Microfluidic qPCR
Total RNA from thymus or BM was isolated using an RNeasy mini kit (Qiagen). The first strand cDNA was synthesized using SuperScript III kit (Invitrogen) followed by specific target amplification using primer pair sets specific for p53-induced genes and hprt and gapdh as controls. Preamplified gene expression assays were provided by Fluidigm Assay Design. Specific target amplification PCR products were loaded onto a 48.48 Dynamic Array Integrated Fluidic Circuits (Fluidigm), and qPCR thermal cycling was performed using a BioMark instrument (Fluidigm) following the manufacturer's instructions. Data were analyzed using Fluidigm Melting Curve Analysis software (Fluidigm). Expression of p53-induced genes was normalized to the average signals of control genes hprt and gapdh, and relative expression of each gene from all the samples was scaled from 0 to 1. Hierarchical clustering was performed using MeV 4.4 software (TM4 microarray software suite).
Flow cytometry
Antibodies are purchased from BD Pharmingen or mentioned elsewhere. BM cells were incubated with FITC or biotin-conjugated antibodies against lineage markers including B220, CD3, Gr1, Mac1, Ter119, biotin, or PerCP-Cy5.5-conjugated anti-c-Kit; PE- or APC-conjugated anti-Sca1 (Biolegend); APC-Cy7-conjugated stretavidin; FITC-conjugated anti-CD41 and anti-CD48; PE-conjugated anti-CD150 (Biolegend), and FITC-conjugated anti-CD34 (eBioscience). Cells were analyzed using FACScan, LSRI, or LSRII (Becton-Dickinson), and data were analyzed using CellQuest Pro and FlowJo software.
BrdU incorporation
Mice were intraperitoneally injected with 2 mg of BrdU (Sigma) followed by the administration of BrdU (1 mg/mL) in the drinking water for 2 d. Mice were sacrificed and BM cells were isolated for the analysis of BrdU incorporation using APC BrdU flow kit (BD Biosciences).
Statistical analysis
We used the two-way t-test for analyzing differences between two groups. Data are represented as mean ± SEM unless otherwise noted, with values of P < 0.05 considered significant.
Acknowledgments
We thank Daphne Chen and Daniel Kim for mouse colony assistance and BM extraction, Dr. Grant Barish for the help with BM transplantation, and Rose Rodewald for technical assistance. We thank Dr. Alain Mir from Fluidigm Corporation for designing primers and his assistance in microfluidic chip analysis. This work was supported by grants from NCI (grants CA100845 and CA61449 to G.M.W., and CA094056 to D.P.W.) and the Cancer Center Core Grant for Core Facility support (grant 5 P30 CA014195). A.B. and J.-H.M. acknowledge support from the NCI (U01 CA84244) and the DOE Low Dose Program.
Footnotes
Supplemental material is available for this article.
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.2024411.
References
- Appella E, Anderson CW 2001. Post-translational modifications and activation of p53 by genotoxic stresses. Eur J Biochem 268: 2764–2772 [DOI] [PubMed] [Google Scholar]
- Aranda-Anzaldo A, Dent MAR 2007. Reassessing the role of p53 in cancer and ageing from an evolutionary perspective. Mech Ageing Dev 128: 293–302 [DOI] [PubMed] [Google Scholar]
- Bondar T, Medzhitov R 2010. p53-mediated hematopoietic stem and progenitor cell competition. Cell Stem Cell 6: 309–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Felicia ME, Keyvan K, Stephanie OO, Marie JD, Michael AE, Neal SY 2008. Enrichment of hematopoietic stem cells with SLAM and LSK markers for the detection of hematopoietic stem cell function in normal and Trp53 null mice. Exp Hematol 36: 1236–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T, Rodrigues N, Shen H, Yang Y-g, Dombkowski D, Sykes M, Scadden DT 2000. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science 287: 1804–1808 [DOI] [PubMed] [Google Scholar]
- Dino T, Letizia L, Andrea B, Lilla C, Rossella C, Fausto G, Anna Teresa M, Pier Giuseppe P, Antonino N 1994. Analysis of p53 gene mutations in acute myeloid leukemia. Am J Hematol 46: 304–309 [DOI] [PubMed] [Google Scholar]
- Espinosa JM 2008. Mechanisms of regulatory diversity within the p53 transcriptional network. Oncogene 27: 4013–4023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa JM, Verdun RE, Emerson BM 2003. p53 Functions through stress- and promoter-specific recruitment of transcription initiation components before and after DNA damage. Mol Cell 12: 1015–1027 [DOI] [PubMed] [Google Scholar]
- Fauci A, Braunwald E, Martin JB 2008. Harrison's principles of internal medicine. McGraw-Hill Companies, New York [Google Scholar]
- Feng L, Lin T, Uranishi H, Gu W, Xu Y 2005. Functional analysis of the roles of posttranslational modifications at the p53 C terminus in regulating p53 stability and activity. Mol Cell Biol 25: 5389–5395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Cao I, García-Cao M, Martín-Caballero J, Criado L, Klatt P, Flores J, Weill J, Blasco M, Serrano M 2002. ‘Super p53' mice exhibit enhanced DNA damage response, are tumor resistant and age normally. EMBO 21: 6225–6235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudkov AV, Komarova EA 2003. The role of p53 in determining sensitivity to radiotherapy. Nat Rev Cancer 3: 117–129 [DOI] [PubMed] [Google Scholar]
- Gudkov AV, Komarova EA 2007. Dangerous habits of a security guard: the two faces of p53 as a drug target. Hum Mol Genet 16: R67–R72 doi: 10.1093/hmg/ddm052 [DOI] [PubMed] [Google Scholar]
- Inga A, Storici F, Darden TA, Resnick MA 2002. Differential transactivation by the p53 transcription factor is highly dependent on p53 level and promoter target sequence. Mol Cell Biol 22: 8612–8625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insinga A, Monestiroli S, Ronzoni S, Carbone R, Pearson M, Pruneri G, Viale G, Appella E, Pelicci P, Minucci S 2004. Impairment of p53 acetylation, stability and function by an oncogenic transcription factor. EMBO 23: 1144–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Takubo K, Arai F, Satoh H, Matsuoka S, Ohmura M, Naka K, Azuma M, Miyamoto K, Hosokawa K, et al. 2007. Regulation of reactive oxygen species by Atm is essential for proper response to DNA double-strand breaks in lymphocytes. J Immunol 178: 103–110 [DOI] [PubMed] [Google Scholar]
- Jankovic V, Ciarrocchi A, Boccuni P, DeBlasio T, Benezra R, Nimer SD 2007. Id1 restrains myeloid commitment, maintaining the self-renewal capacity of hematopoietic stem cells. Proc Natl Acad Sci 104: 1260–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman L, Prives C 1995. Activation of p53 sequence-specific DNA bindingby short single strands of DNA requires the p53 C-terminus. Cell 81: 1021–1029 [DOI] [PubMed] [Google Scholar]
- Kaushansky K, Fox N, Lin NL, Liles WC 2002. Lineage-specific growth factors can compensate for stem and progenitor cell deficiencies at the postprogenitor cell level: an analysis of doubly TPO- and G-CSF receptor-deficient mice. Blood 99: 3573–3578 [DOI] [PubMed] [Google Scholar]
- Kawamura T, Suzuki J, Wang YV, Menendez S, Morera LB, Raya A, Wahl GM, Belmonte JCI 2009. Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature 460: 1140–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp CJ, Wheldon T, Balmain A 1994. p53-deficient mice are extremely susceptible to radiation-induced tumorigenesis. Nat Genet 8: 66–69 [DOI] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz ÖH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ 2005. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121: 1109–1121 [DOI] [PubMed] [Google Scholar]
- Krizhanovsky V, Lowe SW 2009. Stem cells: the promises and perils of p53. Nature 460: 1085–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummel KA, Lee CJ, Toledo F, Wahl GM 2005. The C-terminal lysines fine-tune P53 stress responses in a mouse model but are not required for stability control or transactivation. Proc Natl Acad Sci 102: 10188–10193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse J-P, Gu W 2009. Modes of p53 regulation. Cell 137: 609–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahav M, Uziel O, Kestenbaum M, Fraser A, Shapiro H, Radnay J, Szyper-Kravitz M, Avihai S, Hardan I, Shem-Tov N, et al. 2005. Nonmyeloablative conditioning does not prevent telomere shortening after allogeneic stem cell transplantation. Transplantation 80: 969–976 [DOI] [PubMed] [Google Scholar]
- Liu Y, Elf SE, Miyata Y, Sashida G, Liu Y, Huang G, Di Giandomenico S, Lee JM, Deblasio A, Menendez S, et al. 2009. p53 regulates hematopoietic stem cell quiescence. Cell Stem Cell 4: 37–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan V, Madan B, Brykczynska U, Zilbermann F, Hogeveen K, Dohner K, Dohner H, Weber O, Blum C, Rodewald H-R, et al. 2009. Impaired function of primitive hematopoietic cells in mice lacking the mixed-lineage-leukemia homolog Mll5. Blood 113: 1444–1454 [DOI] [PubMed] [Google Scholar]
- Marusyk A, Porter CC, Zaberezhnyy V, DeGregori J 2010. Irradiation selects for p53-deficient hematopoietic progenitors. PLoS Biol 8: e1000324 doi: 10.1371/journal.pbio.1000324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendrysa SM, McElwee MK, Michalowski J, O'Leary KA, Young KM, Perry ME 2003. mdm2 is critical for inhibition of p53 during lymphopoiesis and the response to ionizing irradiation. Mol Cell Biol 23: 462–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendrysa SM, O'Leary KA, McElwee MK, Michalowski J, Eisenman RN, Powell DA, Perry ME 2006. Tumor suppression and normal aging in mice with constitutively high p53 activity. Genes Dev 20: 16–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng A, Wang Y, Van Zant G, Zhou D 2003. Ionizing radiation and busulfan induce premature senescence in murine bone marrow hematopoietic cells. Cancer Res 63: 5414–5419 [PubMed] [Google Scholar]
- Rodriguez MS, Desterro JMP, Lain S, Lane DP, Hay RT 2000. Multiple C-terminal lysine residues target p53 for ubiquitin-proteasome-mediated degradation. Mol Cell Biol 20: 8458–8467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao L, Sun Y, Zhang Z, Feng W, Gao Y, Cai Z, Wang ZZ, Look AT, Wu WS 2010. Deletion of proapoptotic Puma selectively protects hematopoietic stem and progenitor cells against high-dose radiation. Blood 115: 4707–4714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Luo J, Zhang W, Gu W 2006. Tip60-dependent acetylation of p53 modulates the decision between cell-cycle arrest and apoptosis. Mol Cell 24: 827–839 [DOI] [PubMed] [Google Scholar]
- Tang Y, Zhao W, Chen Y, Zhao Y, Gu W 2008. Acetylation is indispensable for p53 activation. Cell 133: 612–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- TeKippe M, Harrison D, Chen J 2003. Expansion of hematopoietic stem cell phenotype and activity in Trp53-null mice. Exp Hematol 31: 521–527 [DOI] [PubMed] [Google Scholar]
- Terzian T, Wang Y, Van Pelt CS, Box NF, Travis EL, Lozano G 2007. Haploinsufficiency of Mdm2 and Mdm4 in tumorigenesis and development. Mol Cell Biol 27: 5479–5485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidow H, Melero R, Mylonas E, Freund SMV, Grossmann JG, Carazo JM, Svergun DI, Valle M, Fersht AR 2007. Quaternary structures of tumor suppressor p53 and a specific p53–DNA complex. Proc Natl Acad Sci 104: 12324–12329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo F, Wahl GM 2006. Regulating the p53 pathway: in vitro hypotheses, in vivo veritas. Nat Rev Cancer 6: 909–923 [DOI] [PubMed] [Google Scholar]
- Tyner SD, Venkatachalam S, Choi J, Jones S, Ghebranious N, Igelmann H, Lu X, Soron G, Cooper B, Brayton C, et al. 2002. p53 mutant mice that display early ageing-associated phenotypes. Nature 415: 45–53 [DOI] [PubMed] [Google Scholar]
- Uchida N, Aguila HL, Fleming WH, Jerabek L, Weissman IL 1994. Rapid and sustained hematopoietic recovery in lethally irradiated mice transplanted with purified Thy-1.1lo Lin-Sca-1+ hematopoietic stem cells. Blood 83: 3758–3779 [PubMed] [Google Scholar]
- van Os R, Kamminga LM, Ausema A, Bystrykh LV, Draijer DP, van Pelt K, Dontje B, de Haan G 2007. A limited role for p21Cip1/Waf1 in maintaining normal hematopoietic stem cell functioning. Stem Cells 25: 836–843 [DOI] [PubMed] [Google Scholar]
- Vousden KH, Prives C 2009. Blinded by the light: the growing complexity of p53. Cell 137: 413–431 [DOI] [PubMed] [Google Scholar]
- Wade M, Wang YV, Wahl GM 2010. The p53 orchestra: Mdm2 and Mdmx set the tone. Trends Cell Biol 20: 299–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YA, Elson A, Leder P 1997. Loss of p21 increases sensitivity to ionizing radiation and delays the onset of lymphoma in atm-deficient mice. Proc Natl Acad Sci 94: 14590–14595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Schulte BA, LaRue AC, Ogawa M, Zhou D 2006. Total body irradiation selectively induces murine hematopoietic stem cell senescence. Blood 107: 358–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YV, Leblanc M, Wade M, Jochemsen AG, Wahl GM 2009. Increased radioresistance and accelerated B cell lymphomas in mice with Mdmx mutations that prevent modifications by DNA-damage-activated kinases. Cancer Cell 16: 33–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg RL, Freund SMV, Veprintsev DB, Bycroft M, Fersht AR 2004. Regulation of DNA binding of p53 by its C-terminal domain. J Mol Biol 342: 801–811 [DOI] [PubMed] [Google Scholar]
- Wognum AW, Eaves AC, Thomas TE 2003. Identification and isolation of hematopoietic stem cells. Arch Med Res 34: 461–475 [DOI] [PubMed] [Google Scholar]
- Yu H, Shen H, Yuan Y, XuFeng R, Hu X, Garrison SP, Zhang L, Yu J, Zambetti GP, Cheng T 2010. Deletion of Puma protects hematopoietic stem cells and confers long-term survival in response to high-dose γ-irradiation. Blood 115: 3472–3480 [DOI] [PMC free article] [PubMed] [Google Scholar]