Abstract
Leaves originate from stem cells located at the shoot apical meristem. The meristem is shielded from the environment by older leaves, and leaf initiation is considered to be an autonomous process that does not depend on environmental cues. Here we show that light acts as a morphogenic signal that controls leaf initiation and stabilizes leaf positioning. Leaf initiation in tomato shoot apices ceases in the dark but resumes in the light, an effect that is mediated through the plant hormone cytokinin. Dark treatment also affects the subcellular localization of the auxin transporter PIN1 and the concomitant formation of auxin maxima. We propose that cytokinin is required for meristem propagation, and that auxin redirects cytokinin-inducible meristem growth toward organ formation. In contrast to common wisdom over the last 150 years, the light environment controls the initiation of lateral organs by regulating two key hormones: auxin and cytokinin.
Keywords: light signaling, stem cells, organ initiation, cytokinin, auxin, shoot apical meristem
The plant shoot culminates in the shoot apical meristem, a dome-shaped organ that generates the aerial parts of the plant. Pluripotent stem cells are harbored in the central zone at the tip of the meristem, while organ initiation takes place below the tip in the peripheral zone (Carles and Fletcher 2003; Rieu and Laux 2009; Sablowski 2011). Because of its dynamic properties, the maintenance of the shoot apical meristem requires a precise coordination of growth and differentiation.
In the central zone, cytokinin has a role in the maintenance of the stem cell pool. The loss of meristem function in the stm mutant is rescued by exogenous cytokinin application as well as expression of a cytokinin biosynthesis gene from the STM promoter (Yanai et al. 2005). Rice log mutants have smaller shoot meristems. The LOG gene encodes a cytokinin biosynthesis enzyme, and its transcripts are localized in the shoot meristem tips (Kurakawa et al. 2007). A negative feedback loop involving the CLV ligand–receptor system limits expression of the homeobox gene WUS and thereby prevents accumulation of excess stem cells (Lenhard and Laux 2003). Local cytokinin perception by AHK4 and type A cytokinin response regulators maintains the WUS expression domain at a predictable distance from the L1 layer (Gordon et al. 2009). A computational model showed that, in a network in which cytokinin simultaneously activates WUS and represses CLV1, WUS expression increases steeply above a critical cytokinin concentration.
In the peripheral zone, a positive feedback loop between auxin and its transporter, PIN1, is required for organ patterning and initiation (Reinhardt et al. 2000, 2003; Heisler et al. 2005; de Reuille et al. 2006; Bayer et al. 2009). Treatment of tomato shoot apices with the auxin transport inhibitor NPA blocks organ formation, resulting in the formation of a radially symmetric pin-like structure. Similarly, mutations in the Arabidopsis PIN1 gene, which encodes an auxin efflux carrier, result in a pin-like shoot. The application of auxin to the flank of such pins induces organ formation (Okada et al. 1991; Reinhardt et al. 2000, 2003). PIN1 was detected predominantly in the epidermal L1 layer and vascular tissues of the developing primordia (Reinhardt et al. 2003). In the L1 layer, PIN1 localizes toward sites of incipient primordia, causing accumulation of auxin at these so-called convergence points (Reinhardt et al. 2000, 2003; Heisler et al. 2005; de Reuille et al. 2006; Bayer et al. 2009). The local auxin maxima generate the regular organ arrangement called phyllotaxis. Mathematical modeling supports a molecular mechanism in which the phyllotactic pattern is self-organized by positive feedback between auxin and PIN1 (Jönsson et al. 2006; Smith et al. 2006; Heisler et al. 2010).
Recent studies indicate cross-talk between auxin and cytokinin. In the Arabidopsis shoot apical meristem, WUS directly represses the transcription of type A ARR genes (ARR5, ARR6, ARR7, and ARR15), negative regulators of cytokinin signaling. Overexpression of a constitutively active form of ARR7 disrupts meristem activities similarly to wus mutants (Leibfried et al. 2005). These ARRs are under negative control of auxin. Accordingly, mutants in auxin biosynthetic enzymes, the auxin response regulator MP, or PIN1 have enhanced ARR expression. Silencing of ARR7 and ARR15 caused enlargement of the shoot apical meristem and restored organ formation in the mp mutant. Thus, ARR7 and ARR15 integrate cytokinin and auxin signals, connect them to the CLV–WUS network, and mediate shoot apical meristem activity.
In maize, a loss-of-function mutation in ABPH1, a type A ARR, caused enlargement of the shoot apical meristem and changed phyllotaxis (Giulini et al. 2004). In the abph1 mutant, PIN1 expression at the incipient primordia was reduced, indicating that ABPH1 is required for normal expression of PIN1. Maize PIN1 was rapidly induced by cytokinin, suggesting that ABPH1 acts as a positive regulator of PIN1 and auxin accumulation in leaf primordia (Lee et al. 2009). NPA treatment reduced ABPH1 expression. Therefore, in contrast to Arabidopsis, auxin enhances a type A ARR in maize, although the effect may be indirect. Despite this discrepancy, the ARRs appear to be part of a regulatory network that connects auxin and cytokinin signaling.
Auxin and cytokinin not only function as endogenous regulators of the shoot meristem, they are also involved in perceiving information from the environment and relaying it to a wide variety of developmental programs (Argueso et al. 2009; Shibasaki et al. 2009; Wolters and Jurgens 2009). Of the various environmental cues, light plays a particularly important role (Jiao et al. 2007). When mature plants compete with their neighbors, the decreased red/far red ratio of the incident radiation causes a shade avoidance response and leaf primordia transiently stop growing, accompanied by rapid arrest of leaf cell division. This response involves downstream activation of auxin signaling as well as auxin-inducible cytokinin degradation in the vascular procambium (Carabelli et al. 2007). Light also affects auxin biosynthesis, signaling, and transport (Bandurski et al. 1977; Jones et al. 1991; Behringer and Davies 1992; Gil et al. 2001; Salisbury et al. 2007; Laxmi et al. 2008; Stepanova et al. 2008; Tao et al. 2008; Halliday et al. 2009).
Surprisingly little is known about the effect of light on leaf initiation and leaf positioning in mature plants. The long-standing consensus has been that the shoot meristem, as the source of the all-important stem cells, is shielded from the “outward danger and vicissitudes” of the environment (Airy 1873), and that phyllotaxis is not affected by environmental cues. In a rigorous series of experiments published 40 years ago, it was shown that pea plants stopped leaf formation in the dark. Leaf formation resumed when the plants were returned into light (Low 1970). The arrest of leaf initiation in the dark could be due to the lack of energy, but it is also possible that light acts as an environmental signal of leaf initiation.
Microarray analysis of light- and dark-grown Arabidopsis seedlings showed that ∼1150 genes were up-regulated by light, whereas ∼800 genes were down-regulated by light (Ma et al. 2001). Some genes were regulated distinctly by light between adult leaves and seedlings. During light-induced greening of etiolated seedlings, microarray analysis also demonstrated rapid hormone responses in the shoot apex: Genes implicated in auxin and ethylene action were repressed, and genes associated with cytokinin and gibberellin actions were activated (Lopez-Juez et al. 2008).
Considering that light affects many hormonal pathways in different ways, we ask whether light modulates hormonal pathways to control organogenesis at the shoot apical meristem. Recently, we reported that the aux1 lax1lax2lax3 quadruple mutant, which is defective in auxin influx carriers, has a much stronger phyllotactic phenotype in short days than in long days (Bainbridge et al. 2008). This suggests that light has an influence on the shoot apical meristem by affecting auxin distribution. This prompted us to investigate the influence of light on auxin-dependent leaf initiation and positioning.
The common model plant Arabidopsis has a small shoot apical meristem that is deeply buried between rosette leaves, is virtually impossible to access, and cannot be grown in culture. Thus, most studies on Arabidopsis organ initiation concern the induction of floral meristems from the inflorescence apex, which is more easily accessed (Reddy et al. 2004; Heisler et al. 2005, 2010; Hamant et al. 2008). We use tomato as an experimental system because its vegetative shoot apical meristem is relatively large and therefore can be easily dissected, grows vigorously under defined culture conditions, and is well suited for a wide variety of micromanipulations. We show that light is strictly required for leaf initiation and stabilizes organ positioning, and that the light signal is transduced via cytokinin and PIN1 intracellular trafficking.
Results
Shoot apices stop producing leaf primordia in the absence of light
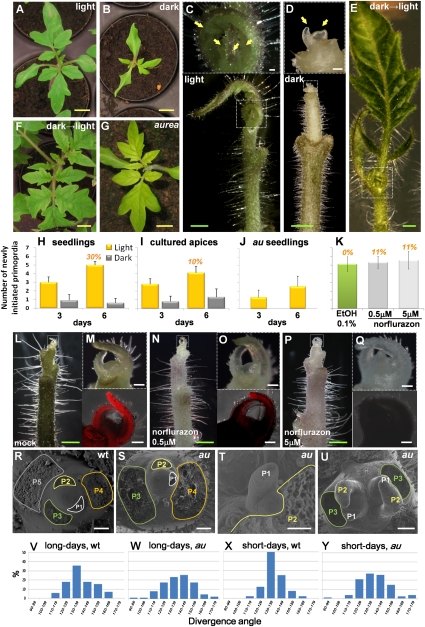
In order to investigate the effects of light on leaf initiation, we analyzed the number of newly initiated leaf primordia in long days and darkness. Soil-grown tomato seedlings produced approximately one primordium per day, while leaf initiation was arrested in the dark (Fig. 1A,B,H). The results supported the data from Low (1970): Shoot apices cease to make leaves in the dark, and light reverses the effects of dark and restarts leaf initiation. The lack of organ formation in the dark could be a photomorphogenic response or due to a lack of photosynthetic energy production. To avoid potential depletion of energy, we cultured shoot apices in the presence of sucrose (Fleming et al. 1997). When the apices were cultured in long days, primordium initiation continued (Fig. 1C,I). In contrast, when the apices were transferred into the dark, the production of leaf primordia arrested even in the presence of sucrose (Fig. 1D,I). When the dark-treated seedlings and apices were returned to the light, they resumed producing leaves (Fig. 1F,E), confirming that dark treatment did not affect the viability of the apices.
Figure 1.
Leaf initiation depends on light signaling. Soil-grown tomato seedlings were grown under 16-h days (A) or transferred to darkness for 6 d (B). Dissected shoot apices were kept in 16-h days (C) or transferred to darkness for 6 d (D); close-ups of the apices are shown above, and apices with stems are shown below. (E) Dark-treated apices that were returned to the light for an additional 10 d resumed vigorous growth. (F) Dark-treated seedlings that were returned to the light similarly resumed vigorous growth. (G) Seedlings of the aurea mutant were grown as the wild type in A. (H–J) Newly initiated primordia were counted. Numbers above bars indicate the percent of flowering. Error bars show SD (n = 10). The result was reproducible in three independent experiments. (Yellow arrows) Leaf primordia. (White dotted boxed regions) Shoot apical meristem. Note that leaf initiation stops in darkness in both soil-grown seedlings and apices cultured with sucrose. Moreover, the inhibitory effect of darkness is reversible. Apices cultured with 0.5 μM (N,O) and 5 μM (P,Q) norflurazon. (L,M) As controls, apices were cultured without norflurazon but with 0.1% EtOH. In L, N, and P, to confirm the effects of norflurazon, newly initiated leaves were removed. Note that not only the apex but also the stem is bleached. (M,O,Q) Close-ups of shoot apices in L, N, P; chlorophyll autofluorescence images of the apices are shown below. (K) Number of newly initiated leaves in control and norflurazon-treated apices. Numbers above bars indicate the percent of flowering. Error bars show SD (n > 8). The result was reproducible in three independent experiments. Note that inhibition of photosynthesis does not interfere with leaf initiation. Wild-type (R) and representative phenotypes of aurea seedlings (S–U). (S) Abnormal leaf positioning. (T) Smaller meristem. (U) In rare cases, the mutant apex developed two meristems. Divergence angles of wild-type seedlings in long days (V), aurea mutant seedlings in long days (W), wild-type plants in short days (X), and aurea mutant plants in short days (Y). The mutants display a more severe phenotype in short days than in long days. We underestimated the deviation from wild-type (wt) phyllotaxis because we were not able to dissect the ∼10% mutant plants with phenotypes too severe to allow meristem dissection. Yellow bars, 1 cm; green bars, 1 mm; white bars, 100 μm.
To ensure that the sucrose in the medium was sufficient as an energy source in the dark, we studied the effects of photosynthesis inhibitors on leaf initiation in cultured apices. Among various photosynthesis inhibitors tested (Supplemental Fig. 1A–F), we selected norflurazon, a pyridazinone herbicide that inhibits photosynthesis by blocking the synthesis of carotenoids (Guseinova et al. 2005). Shoot apices were cultured with 0.5 μM or 5 μM norflurazon. After 6 d with the inhibitor in long days, chlorophyll autofluorescence was absent, confirming that norflurazon was active (Fig. 1L–Q). These chlorotic apices produced as many new primordia as the control in both vegetative and inflorescence stages (Fig. 1K; Supplemental Fig. 1K,L). Thus, inactivation of photosynthesis does not inhibit leaf initiation.
If cessation of leaf initiation in the dark is a signaling response, photoreceptor mutants might be affected in this process. The tomato aurea (au) mutant has been characterized as a phytochrome photoreceptor-deficient mutant that is unable to synthesize the linear tetrapyrrole chromophore of phytochrome (Koornneef et al. 1985). The phenotype of au mutants depends on the developmental stages and is most severe during early stages, suggesting that it is a partial loss-of-function mutant (van Tuinen et al. 1996). The mutants exhibited shoot meristem abnormalities, such as irregular leaf positioning, smaller meristem size, and split meristems (Fig. 1R–U). Leaf initiation of au mutants was lower than in wild type (Fig. 1J), indicating a light signaling defect. In addition, phyllotaxis of the mutants was irregular especially in short days (Fig. 1V–Y). Thus, we conclude that light stabilizes phyllotaxis.
Darkness affects PIN1 membrane localization and auxin distribution
If light regulates organ initiation independently of photosynthesis, does it affect auxin transport-dependent auxin gradients in developing leaf primordia? To study the effect of light on auxin transport, we used transgenic tomato plants expressing an Arabidopsis PIN1-GFP construct under its own promoter (AtPIN1-GFP) (Bayer et al. 2009). The AtPIN1-GFP tomato plants were grown on soil under long days and transferred to the dark at the end of the day.
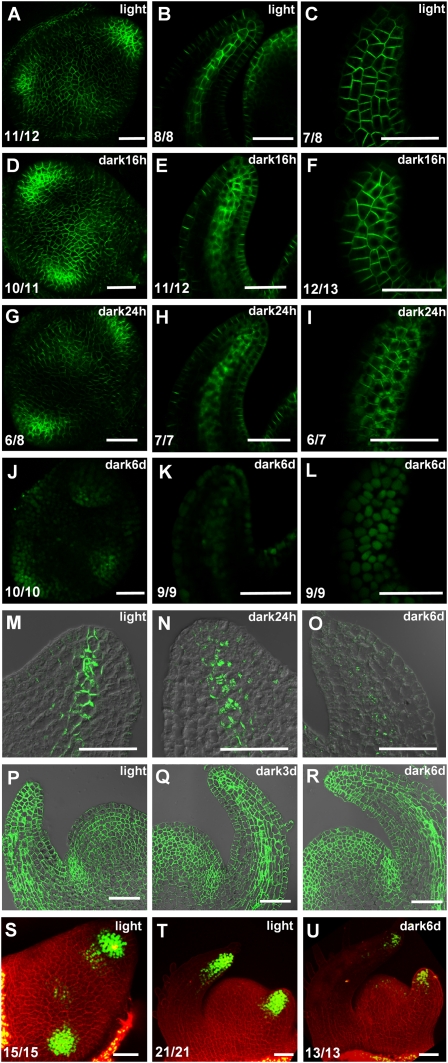
In the vegetative shoot apical meristem of light-grown seedlings, PIN1 was highly expressed in the L1 layer of the meristem and the incipient leaf primordium as well as in the provascular strands (Fig. 2A–C), in agreement with previous reports (Scarpella et al. 2006; Bayer et al. 2009). Interestingly, PIN1 was gradually internalized and lost from the plasma membrane (PM) in the dark. PIN1 internalization was first visible after 3–4 h (Supplemental Fig. 2A–C) and was evident in the entire provasculature by 16 h (Fig. 2E). At this time point, PIN1 had disappeared from the membrane in the basal part of the primordium; however, it remained at the tip. In contrast, in the epidermis, most of PIN1 remained polarized after 16 h of darkness, although slight internalization was also observed (Fig. 2D,F). The increase of internalized PIN1 signal and subsequent disappearance of polarized PIN1 signal on the PM started in the basal part of the provasculature, then spread to the tip of the primordium and finally to the epidermis and entire L1 of the shoot apical meristem (20 h [Supplemental Fig. 2D–F], 24 h [Fig. 2G–I], and 2 d [Supplemental Fig. 2G–I]). After 6 d of dark treatment, membrane localization of PIN1 had disappeared completely and the GFP signal was observed in large round structures, presumably vacuoles (Fig. 2J–L). In dark-cultured apices, although the speed of internalization was slower than in soil-grown seedlings, the polarized PIN1 was significantly reduced after 6 d (Supplemental Fig. 2J–O).
Figure 2.
Darkness affects expression and localization of PIN1 and DR5-YFP. Light-grown tomato PIN1-GFP seedlings (A–C) were transferred to dark for 16 h (D–F), 24 h (G–I), and 6 d (J–L). Maximal projections of transversal confocal sections (A,D,G,J), median longitudinal section of leaf primordia (B,E,H,K), and surface view of leaf epidermis (C,F,I,L). Immunolocalization of PIN1 protein in median longitudinal section of leaf primordia in light-grown plants (M), 24-h dark treated plants (N), or 6-d dark-treated plants (O). Immunolocalization of H+-ATPase in median longitudinal section of shoot apical meristem in light (P), 3-d dark-treated (Q), and 6-d dark-treated (R) plants. (S–U) DR5-YFP expression in tomato shoot apices. Light-grown seedlings (S,T) were transferred to dark for 6 d (U). Maximal projections of transversal confocal sections of the top view (S) and side view (T,U) of a shoot apical meristem. The green signal is AtPIN1-GFP protein in A–L, PIN1 protein in M–O, H+-ATPase protein in P–R, and DR5-YFP protein in S–U. Red signal is propidium-iodide (PI)-stained cell wall. The numbers in the bottom left corner show the number of apices that display the shown expression pattern out of the total number of samples. Bars, 50 μm.
Since GFP has been observed in the vacuole of dark-treated plants due to impaired degradation (Tamura et al. 2003), we ascertained that the internalization of PIN1-GFP is not an artifact of GFP stabilization. Immunofluorescence labeling of the endogenous PIN1 proteins using an antibody raised against the tomato PIN1 homolog confirmed that the PIN1-GFP expression patterns reflect the patterns of the endogenous PIN1 protein. In the light, PIN1 was highly expressed and polarized (Fig. 2M). In contrast, PIN1 was internalized in provasculature after 24 h of dark (Fig. 2N), and was reduced or completely disappeared after 6 d (Fig. 2O). Furthermore, we performed immunolocalization of another plasma membrane-localized protein, H+-ATPase (Morsomme et al. 1998). Importantly, H+-ATPase was stable after 3 d and 6 d of dark treatment (Fig. 2P–R), showing that light specifically affects PIN1.
In order to study how these changes in PIN1 distribution affect auxin maxima, we examined expression patterns of DR5-YFP in transgenic tomato plants. In the vegetative shoot apical meristem of light-grown seedlings, DR5 was expressed in the L1 layer and inner tissues of incipient primordia. In young bulging primordia, DR5 was expressed at the adaxial side and the tip of young leaf primordia (Fig. 2S,T). This DR5 signal gradually declined in the dark (Supplemental Fig. 2T–W). After 6 d of darkness, DR5 was strongly down-regulated in the entire shoot meristem (Fig. 2U). Thus, the arrest of leaf primordia was associated with the reduction of the levels of auxin and PIN1 expression. In addition, compared with older primordia, the expression of DR5 was higher in younger primordia in light-grown plants, and loss of PIN1 polarity progressed more slowly (Supplemental Fig. 2S,X–AA). Furthermore, in the dark-treated plants, both polarized PIN1 and DR5 signals tended to remain at the tip of primordia (Supplemental Fig. 2E,V,W). Therefore, there appears to be a correlation between the decrease of auxin concentrations and the PIN1 polarization. This is consistent with a previous report that auxin inhibits endocytosis of PIN1, thus increasing its levels at the PM (Paciorek et al. 2005).
Organ formation in the dark requires exogenous cytokinin
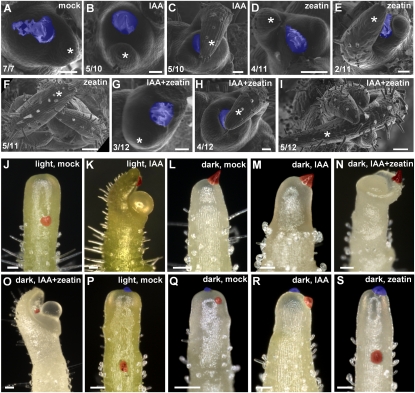
Arrest of leaf initiation in the dark was associated with decreased auxin signaling. If the light signal is transduced specifically by auxin, exogenous auxin treatment should restore leaf initiation in the dark. Therefore, we examined the effect of local auxin treatment to the apices in the dark. Dissected shoot apices were cultured for 6 d in the dark. All of the pre-existing primordia, except P1 (the youngest visible leaf primordium), were dissected, and then a small dot of IAA in lanolin paste was applied to the meristem. In all dark-cultured apices, microapplication of DMSO (mock) did not produce new primordia (Fig. 3A). IAA application did not produce new primordia either; note, however, that, in the same apices, the growth of pre-existing primordia was promoted (Fig. 3B,C). The result indicated that auxin is not sufficient to initiate new primordia in the dark. Thus, organogenesis requires at least two factors: auxin and light. It also suggested that the light signal is required after and/or in parallel with the establishment of an auxin gradient in primordia.
Figure 3.
Induction of primordium formation and meristem tip growth by auxin and cytokinin. (A–I) Microapplication of auxin and cytokinin to dark-cultured apices. Dissected tomato apices were precultured in the light and transferred to darkness for 6 d. Lanolin containing 1% DMSO (A), 10 mM IAA (B,C), 1 mM zeatin (D–F), or 10 mM IAA plus 1 mM zeatin (G–I) was applied in the dark. These apices were further cultured in the dark for 10 d. (White asterisks) Pre-existing primordia. (F,I) Note that apices treated with 10 mM IAA plus 1 mM zeatin and with 1 mM zeatin alone continued to grow in the dark. (A,B) However, apices treated with 1% DMSO or 10 mM IAA did not grow. (C,E,H) In addition, 10 mM IAA alone, 10 mM IAA plus 1 mM zeatin, and 1 mM zeatin alone promoted the development of pre-existing P1 and I1. The numbers in the bottom left corner show the number of apices that show the displayed phenotype out of the total number of samples. Thus cytokinin induces leaf initiation in the dark, and auxin promotes leaf initiation in the presence of cytokinin. (J–O) Microapplication of auxin and cytokinin to the flank of the meristems of tomato NPA pins. Dissected apices were cultured in the presence of NPA. Resulting pin-shaped apices (NPA pins) were precultured in the light or the dark, and microapplication of IAA and cytokinin was performed. Microapplication of 1% DMSO (mock) lanolin in the light (J), 10 mM IAA lanolin in the light (K), 1% DMSO lanolin in the dark (L), 10 mM IAA lanolin in the dark (M), 10 mM IAA plus 1 mM zeatin lanolin in the dark (N), and 10 mM IAA plus 10 mM zeatin lanolin in the dark (O). (P–S) Microapplication to the flank and the summit of the meristem of NPA pins. Microapplication of 1% DMSO lanolin to the flank and the summit in the light (P), 1% DMSO lanolin to the flank and the summit in the dark (Q), 10 mM IAA lanolin to the flank and 1% DMSO lanolin to the summit in the dark (R), and 1 mM zeatin lanolin to the summit and 1% DMSO lanolin to the flank in the dark (S). (A–I) Scanning electron microscope images. (J–S) Stereomicroscope images. Lanolin dots applied to the flank are colored red, and those applied to the summit are colored blue. Bars, 100 μm.
The result raises the question as to the signaling molecule that transduces the light signal and induces organ initiation. As a substance that might transduce the light signal, we considered cytokinin, which has important functions in the shoot apical meristem (Ori et al. 2000; Werner et al. 2003; Leibfried et al. 2005; Gordon et al. 2009; Lee et al. 2009). When cytokinin (zeatin) was applied to the summit of the meristem, 45% of the apices produced new primordia and continued to grow in the dark (Fig. 3D–F). Apices with applied cytokinin and auxin also produced new primordia at 42% frequency (Fig. 3G–I). Thus, in dark-grown apices, cessation of leaf initiation can be rescued by cytokinin alone. This suggested the involvement of cytokinin with the light-dependent leaf initiation pathway.
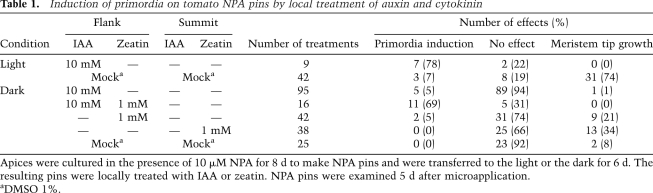
When tomato shoot apices were cultivated in the presence of NPA, leaf formation was completely inhibited, resulting in a pin-shaped shoot meristem (NPA pin). Organogenesis could be restored by exogenous auxin application (Reinhardt et al. 2000). Using this experimental system, we examined the effect of exogenous IAA on NPA pins in the dark. NPA pins were cultured in the dark, and then a small dot of IAA in lanolin paste was applied to the flank of the meristem. DMSO (mock) treatment did not induce organ initiation in either the light or the dark (Fig. 3J,L; Table 1). In contrast to light-grown NPA pins, microapplication of IAA did not induce organogenesis in the dark (Fig. 3K,M; Table 1; Supplemental Table 1). When zeatin and IAA were mixed with lanolin and applied locally to the flank of dark-cultured NPA pins, primordium formation was induced (Fig. 3N,O; Table1). The result confirmed the essential role of cytokinin.
Table 1.
Induction of primordia on tomato NPA pins by local treatment of auxin and cytokinin
Light promotes meristem tip growth by activating cytokinin signaling
The previous results show that exogenous cytokinin is essential for leaf initiation in the dark (Fig. 3F). They also suggest that light triggers activation of the cytokinin pathway. How, then, does cytokinin act? We showed previously that NPA completely blocks leaf formation in the light, but stem growth and meristem maintenance proceed normally (Reinhardt et al. 2000). In order to track meristem growth, both the summit and the flank of NPA pins were labeled with small dots of lanolin. After 5 d in the light, the two dots were separated by substantial growth (Fig. 3P; Table 1). Thus, the meristem tip grows in light-cultured NPA pins. In contrast, when dots of lanolin were applied to the summit and flank of dark-cultured NPA pins, the dots remained at their positions, indicating that there had essentially been no growth (Fig. 3Q). Similarly, microapplication of IAA did not induce meristem tip growth in the dark (Fig. 3R).
However, when zeatin was applied to the summit or flank of dark-cultured NPA pins, the distance between the lanolin dots increased like in the light-cultured NPA pins, showing that the meristem tip grew in the dark (Fig. 3S; Table 1). Note that this growth was not accompanied by organ induction. Thus, in dark-grown NPA pins, exogenous cytokinin induced apical growth. In addition, there were no obvious differences in cell shape between light-grown NPA pins and cytokinin-treated dark-cultured NPA pins, confirming that the effect of cytokinin is similar to that of light. We calculated the rate of meristem tip growth by measuring the distance between the summit and the lanolin dot in the flank (see the Supplemental Material). The rate of meristem tip growth per day was as follows: in light-cultured NPA pins: 48 ± 18 μm; in dark-cultured NPA pins with zeatin applied to the summit: 35 ± 15 μm. The results clearly show that, in the absence of light, cytokinin is required to promote meristem tip growth. In the absence of NPA, cytokinin induced organ initiation but not meristem tip growth in the dark (Fig. 3F). Together, this suggests that cytokinin promotes leaf initiation in the presence of active auxin transport.
Auxin redirects cytokinin-induced growth
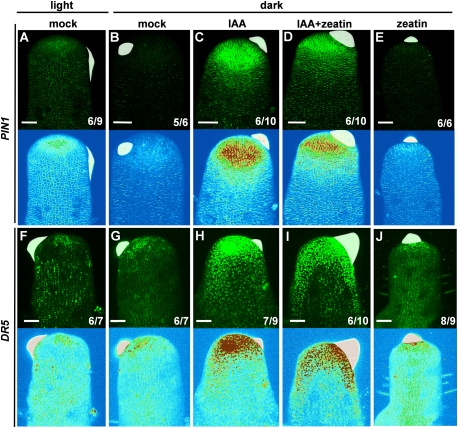
Next, we asked why auxin could not induce organ initiation in the dark. Microapplication of IAA alone promoted PIN1 and DR5 expression in the dark-cultured NPA pins (Fig. 4B,C,G,H). Similarly, both PIN1 and DR5 expression were up-regulated by IAA plus zeatin treatment in the dark (Fig. 4D,I). The longitudinal and transverse sections of DR5-expressing NPA pins showed up-regulation of the DR5 signal in the L1 layer and a gradient in the DR5 signal at the site of microapplication (Supplemental Fig. 3A,B). Notably, PIN1 and DR5 expression were up-regulated by IAA to the same extent in the absence or presence of cytokinin (Fig. 4, cf. C and D, H and I). Therefore, the results indicate that auxin promotes downstream signaling in the dark; however, for organ initiation, cytokinin is also required.
Figure 4.
Induction of auxin signaling by local auxin and cytokinin treatment on tomato NPA pins. Maximal projections of transversal confocal sections of NPA pins expressing PIN1-GFP (A–E) and DR5-YFP (F–J). Confocal image with GFP signal in green (top) and GEO look-up tables (bottom). In GEO look-up tables, blue indicates low intensity, and red indicates high intensity. Microapplication of 1% DMSO lanolin to the flank of a light-cultured NPA pin (A,F), 1% DMSO lanolin to the flank of a dark-cultured NPA pin (B,G), 10 mM IAA lanolin to the flank of a dark-cultured NPA pin (C,H), 10 mM IAA plus 1 mM zeatin lanolin to the flank of a dark-cultured NPA pin (D,I), and 1 mM zeatin lanolin to the summit of the meristem of a dark-cultured NPA pin (E,J). The numbers in the bottom right corner show the number of apices that display the shown expression pattern out of the total number of samples. Bars, 50 μm. Lanolin pastes are colored white.
According to Figure 3S, cytokinin treatment promotes meristem tip growth of NPA pins in the dark. Is activation of auxin signaling necessary for meristem tip growth? Application of auxin alone induced PIN1 and DR5 (Fig. 4C,H) but did not induce tip growth. Furthermore, microapplication of zeatin promoted neither DR5 nor PIN1 expression in the dark-cultured NPA pins (Fig. 4E,J). Therefore, auxin signaling is not necessary for induction of meristem tip growth. We conclude that (1) cytokinin induces growth, but (2) cytokinin in the absence of auxin causes the tip to grow, while, in its presence, the lateral organs initiate and grow out at the expense of tip elongation.
In addition, expression of PIN1 was higher in the light than in the dark (Fig. 4A,B). Expression of DR5 was low in the light and the dark (Fig. 4F,G). This suggests that light is required for PIN1 expression; however, as long as the auxin level is low, the meristem tip continues to grow without producing organs.
Light controls expression of key regulatory genes of the shoot apical meristem
The results so far show that light controls organogenesis via activation of cytokinin signaling. This signaling is likely to involve well-known regulators of meristem activity and organogenesis. Because no tomato lines carrying relevant reporter gene constructs are available, we switched to the Arabidopsis inflorescence meristem.
Compared with the apices in the light, the number of newly initiated flower primordia was lower in the dark. Local cytokinin treatment restored primordium initiation (Supplemental Fig. 4A). Furthermore, microapplication of IAA to the tip of the pin1 mutant induced organ formation in the light but not in the dark (Supplemental Fig. 4B–E). In contrast, microapplication of IAA plus zeatin induced organ formation in dark-cultured pin1 mutants (Supplemental Fig. 4F–H). These results confirmed that the light response in the shoot apical meristem is conserved between the vegetative tomato shoot meristem and the Arabidopsis inflorescence meristem.
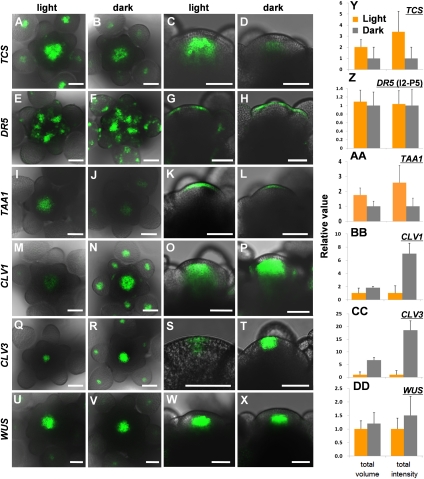
To determine whether alterations in meristem activity in the light and the dark were evident at the level of gene expression, we examined expression patterns of key regulatory genes. pTCS-GFP is a synthetic reporter to visualize cytokinin response, and it is activated in a domain similar to WUS (Müller and Sheen 2008; Gordon et al. 2009). In continuous-light-grown plants, TCS expression was confined to the center of the inflorescence and floral meristems (Fig. 5A,C). However, TCS expression was reduced in the dark (Fig. 5B,D). Compared with dark-treated plants, the total GFP fluorescence in the light was threefold higher (Fig. 5Y). This was due to both expansion of the expression domain and an increase in total signal intensity (Fig. 5Y). It suggests that the cytokinin signaling is decreased in the dark. Therefore, the light regulates meristematic activity by activation of cytokinin signaling.
Figure 5.
Darkness affects the expression of stem cell marker genes. Expression of TCS-GFP in the light (A,C) and the dark (B,D), DR5-GFP in the light (E,G) and the dark (F,H), TAA1-GFP in the light (I,K) and the dark (J,L), CLV1-GFP in the light (M,O) and the dark (N,P), CLV3-GFP in the light (Q,S) and the dark (R,T), and WUS-GFP in the light (U,W) and the dark (V,X) in inflorescence meristem in wild-type Arabidopsis plants. Maximal projections of transversal confocal sections (A,B,E,F,I,J,M,N,Q,R,U,V) and longitudinal confocal sections (C,D,G,H,K,L,O,P,S,T,W,X). Bars, 50 μm. (Y–DD) Total volume and total intensity of GFP-expressing regions are quantified (n > 10) in each condition. For DR5-GFP, expression in I2–P5 primordia was quantified. For other genes, expression in the central zone was quantified. When parametric statistics assumptions were met (normality and homogeneity of variances), a t-test was performed. In the remaining samples, a nonparametric test (Mann-Whitney's U-test) was performed. P-values are in parentheses; P < 0.05 was considered to be significant. The volume of TCS (P < 0.001), CLV3 (P < 0.001), CLV1 (P = 0.023), WUS (P = 0.211), DR5 (P = 0.684), and TAA1(P < 0.001) and the intensity of TCS (P < 0.001), CLV3 (P < 0.001), CLV1 (P < 0.001), WUS (P = 0.117), DR5 (P = 0.796), and TAA1 (P < 0.001). Therefore, there are significant differences in TCS, TAA1, CLV1, and CLV3 expression but no significant differences in WUS and DR5 expression between the light and the dark.
In the light, peaks of DR5-GFP were observed in the L1 layer of the inflorescence meristem at sites of incipient organ initiation, as has been observed previously (Fig. 5E,G; Heisler et al. 2005; Smith et al. 2006). In dark-grown apices, expression in the inflorescence meristem was not significantly changed at I2 to P5 primordia (Fig. 5G,H,Z), but the conspicuous difference was the strong signal in older flower primordia. (Fig. 5E,F). Expression of TAA1, a gene involved in auxin biosynthesis (Stepanova et al. 2008; Tao et al. 2008), was increased fivefold in the light (Fig. 5I–L,AA). This suggests that auxin biosynthesis in the shoot apical meristem requires light.
Cytokinin plays a critical role in establishing the WUS expression domain in the shoot apical meristem (Gordon et al. 2009). We analyzed expression of genes related to the CLV/WUS pathway. Surprisingly, we observed increased CLV3 and CLV1 expression in dark-treated plants (Fig. 5M–T,BB,CC). Compared with light-grown plants, the CLV1-GFP signal in dark-treated plants was sevenfold greater in total intensity, while the expression domain almost doubled (Fig. 5BB), and the CLV3-GFP signal was almost sevenfold greater in volume and 18-fold greater in intensity (Fig. 5CC). In contrast to the drastic up-regulation of the CLV signal, WUS expression did not significantly change between the light and the dark (Fig. 5U–X,DD). This suggests that the CLV pathway is more active in the dark. It also indicated that decreased TCS expression in the dark is not due to reduced viability of the meristem.
Discussion
It is generally assumed that the shoot apical meristem is shielded from the environment, and that leaf initiation and leaf positioning are autonomous processes. However, this quite reasonable assumption is based on very little data. The aim of this study was to determine whether light affects the initiation and positioning of organ primordia. We show that tomato vegetative shoot apices cease to grow in the absence of light, and that this is a photomorphogenic response rather than due to a lack of photosynthetic energy production. The photoreceptor mutant aurea also displayed defective phyllotaxis. While the average divergence angle remained close to the theoretical value of 137.5°, the variation in angles was increased considerably (Fig. 1V–Y). Thus, we arrive at the rather surprising conclusion that the precision of phyllotactic patterning is influenced by the environment.
If light acts as a morphogenic signal in the shoot apical meristem, where is it perceived? The day length signal is perceived in the leaves but induces flowering in the shoot apical meristem (Zeevaart 1976; Bernier and Périlleux 2005; Turck et al. 2008). The shoot apical meristem is covered by leaves, and thus the light intensity at the shoot apical meristem is likely to be low. However, there is plenty of evidence in the literature for extremely sensitive phytochrome-dependent light perception, and such a response may also occur in the shoot meristem. In our meristem culture system, apices resumed leaf initiation after return to the light (Fig. 1E). Therefore, even in the absence of leaves, shoot apices are able to produce primordia. Thus, the effect of light on organ initiation is likely to be a shoot apex-autonomous process.
An important finding is that light affects the establishment of efflux-dependent auxin gradients at the incipient primordium, a key hormonal event during organ initiation. In the absence of light, PIN1 was lost from the membrane and internalized, initially only in the provascular cells, and later uniformly in the meristem (Fig. 2; Supplemental Fig. 2). Unlike PIN1, H+-ATPase was stable in the dark in the meristem over 6 d, showing that the effect of light is not a general response. Thus, proper subcellular localization of PIN1, and thereby the establishment of PIN1-dependent auxin gradients, requires light. This finding is in line with the observation that PIN2 is internalized in dark-grown roots (Laxmi et al. 2008).
Our study uncovered cytokinin as an important factor involved in leaf initiation in addition to auxin. Inhibition of auxin transport specifically prevents the initiation of lateral organs, a defect that can be rescued by microapplication of IAA in the light (Reinhardt et al. 2000). However, in the dark, IAA application was ineffective in both the presence and absence of NPA, suggesting that auxin signaling is not sufficient to induce organs (Fig. 3M). Cytokinin can rescue the auxin-induced organogenesis in the absence of light (Fig. 3F). Cytokinin levels can substitute for the lack of light in other systems as well (Chaudhury et al. 1993; Chory et al. 1994; Muramoto et al. 2005; Lochmanová et al. 2008). The question that remains is which cytokinin-related process is regulated by light. Light may increase cytokinin levels (Mizuno et al. 1971; Qamaruddin and Tillberg 1989), promote cytokinin biosynthesis in the shoot meristem, negatively regulate cytokinin degradation (Carabelli et al. 2007), and affect type A ARRs (Sweere et al. 2001; Zheng et al. 2006). Light and cytokinin independently regulate the stability of HY5, a transcription factor promoting the expression of light-inducible genes by affecting COP1-mediated proteolysis (Vandenbussche et al. 2007).
Auxin and cytokinin interact in complex ways either antagonistically or synergistically, depending on the context (Dello Ioio et al. 2008; Müller and Sheen 2008). In the shoot apical meristem, cytokinin signaling antagonizes auxin-inducible organogenesis. The KNOX proteins, which activate cytokinin signaling, are absent from the incipient primordia, where auxin is high (Ori et al. 2000; Jasinski et al. 2005; Yanai et al. 2005). The cytokinin response regulators ARR7 and ARR15 are directly repressed by the auxin-responsive transcription factor MP (Zhao et al. 2010), indicating that auxin also negatively affects cytokinin signaling. In contrast, in maize, cytokinin promotes growth of the central zone and also triggers expression of the type A ARR, ABPH1, at incipient primordia to induce organogenesis (Lee et al. 2009). The amp1 mutant, which overproduces cytokinin, rescues the organogenesis defect of mp (Vidaurre et al. 2007). These studies suggest that auxin and cytokinin may act in concert.
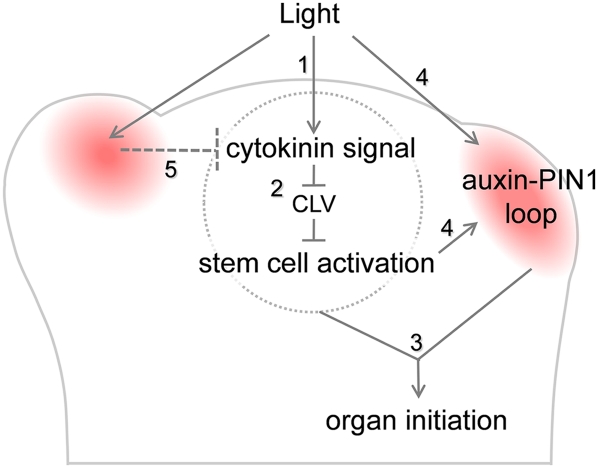
In our study, local application of cytokinin to NPA pins without auxin induced meristem tip growth but not organ initiation in the dark (Fig. 3S). Therefore, the primary effect of cytokinin is not the induction of organs, but, rather, meristem propagation. When auxin and cytokinin were applied together, this growth was redirected toward organogenesis. Thus, we propose that cytokinin promotes meristem growth to supply a source of stem cells as a prerequisite for leaf initiation. In the absence of NPA, cytokinin induced organ initiation in the dark. This suggests that, in the presence of active auxin transport, cytokinin promotes organ initiation by promoting the establishment of auxin gradients. Expression of the auxin biosynthetic enzyme TAA1 is higher in the light than in the dark. This suggests that cytokinin affects establishment of the auxin gradient via regulation of auxin biosynthesis. Thus, these results suggest two possible regulatory pathways for the effect of light on leaf initiation: (1) Light affects both cytokinin signaling and auxin signaling. These hormones act in parallel to promote organ initiation. (2) Light promotes cytokinin signaling, which in turn promotes auxin signaling to induce organ initiation (Fig. 6).
Figure 6.
A model for the role of light on organ initiation. Light promotes cytokinin signaling in the central zone (step 1), which relieves CLV-mediated inhibition of meristem propagation (step 2), thereby supplying a source of cells for organogenesis. (Step 3) This cytokinin-dependent meristem growth promotes organ initiation in concert with the auxin signaling pathway. Light is also required for the establishment of the PIN1–auxin loop (red area), as PIN1 is internalized in the absence of light. (Step 4) Light may affect auxin signaling, transport, or biosynthesis either directly or indirectly via cytokinin/stem cell activation. (Step 5) Based on previous reports (Ori et al. 2000; Jasinski et al. 2005; Yanai et al. 2005), auxin is likely to antagonize cytokinin in developing primordia.
The effect of light on cytokinin and auxin signaling is mirrored by the light-dependent expression of key regulatory genes. The CLV genes restrict stem cell identity and promote cell differentiation. In the dark, the expression of CLV1 and CLV3 was strongly up-regulated, whereas the expression of the cytokinin reporter TCS was reduced. We propose that light activates cytokinin signaling, which in turn promotes growth through reducing CLV expression (Fig. 6). An unexpected finding was that the enhanced expression of CLV1 and CLV3 in the dark was not accompanied by a reduction of WUS expression. This is consistent with a report that silencing of ARR7 and ARR15 induced strong up-regulation of CLV3 expression, whereas WUS expression was only moderately changed (Zhao et al. 2010). This suggests the potential involvement of other factors in the CLV/WUS pathway. On the other hand, Gordon et al. (2009) showed that cytokinin promoted the expression of WUS, CLV3, and TCS but suppressed the expression of CLV1. Taken together, these data hint at an unanticipated flexibility in the stem cell gene regulatory circuit. Constant expression of WUS between the light and the dark indicates that stem cell specification remains intact. Together with the fact that apical–basal patterning is not compromised, this hints at a general mechanism that allows a rapid resumption of growth and development after return to favorable environmental conditions. It will be interesting to study the expression of other meristem marker genes in photo-signaling mutants in Arabidopsis.
Our data show that light is required for the initiation and accurate positioning of lateral organs. As shown in Figure 6, we propose that light activates cytokinin signaling, which in turn activates growth through the inhibition of the CLV ligand/receptor system. Light is also required for the correct subcellular localization of the auxin transporter PIN1. In the presence of light, the activity of the auxin/PIN1 feedback loop redirects cytokinin-mediated growth toward lateral organ formation. Redirection of growth in response to a changing environment is a recurrent theme in plant development. The formation of lateral organs at the shoot apical meristem is no longer an exception.
Materials and methods
Plant material and growth conditions
Plants and cultured apices were grown under the following light conditions: long photoperiod (16 h light, irradiance 110 μE m−2 sec−1), continuous light (irradiance 110 μE m−2 sec−1), and continuous darkness. For tomato NPA pins, apices were cultured at 110 μE m−2 sec−1 light irradiance at 14 h light/10 h dark. In all light conditions, humidity was kept at 65% ± 10%, and temperature was kept at 20°C ± 2°C. All meristem manipulations in the dark were done under dim green safe light (attenuated green LED; OSRAM). The following lines of tomato (Solanum lycopersicum Mill) have been described previously: aureaw mutant (Koornneef et al. 1985), AtPIN1-GFP (Bayer et al. 2009), and the DR5-YFP line pDR5rev:3XVENUS-N7 (Shani et al. 2010). Arabidopsis TCS-GFP containing an enhanced version of the published construct (Müller and Sheen 2008), DR5-GFP, WUS-GFP (Grandjean et al. 2004), TAA1-GFP (Stepanova et al. 2008), and pin1-7 are in the Col-0 background. CLV3-GFP (Yadav et al. 2009) and CLV1-GFP (Gallois et al. 2002) are in the Landsberg erecta (Ler) background.
For in vitro tomato shoot meristem culture, shoot apices of 12-d-old long-day-grown seedlings were dissected and cultured as described (Fleming et al. 1997). Microapplications were performed essentially as described (Reinhardt et al. 2000). For details, see the Supplemental Material.
Microscopy
Confocal analysis was carried out using a Leica upright confocal laser-scanning microscope (Leica TCS SP5) with long-working-distance water immersion objectives (20×). The cell wall was stained with 0.2% propidium iodide (PI; Sigma-Aldrich) for 1–3 min. Light emitted at 620–690 nm was used to record chlorophyll autofluorescence or PI staining. For scanning electron microscopic analysis, meristems were viewed with an S-3500N variable pressure scanning electron microscope (Hitachi) equipped with a cool stage.
Fluorescent signals were quantified by MorphographX software (kindly made available by Richard Smith, University of Bern). The relative values of volume and the intensity of the GFP signal were calculated by dividing the values of dark-grown samples by the values of light-grown samples (CLV1, CLV3, and WUS) and by dividing the values of light-grown samples by the values of dark-grown samples (TCS, TAA1, and DR5).
Measurement of divergence angles
Wild-type and aurea tomato plants were grown in long days for 12 d or short days for 26 d. Top-view scanning electron microscope pictures of shoot apices were used for the measurements. Angles between the center point of the central zone and the P1–P5 leaf primordia were measured in at least 20 plants for each condition.
Immunolocalization
Samples were fixed in a 1:1 methanol:acetone mixture. Immunolocalizations were done on sections of wax-embedded plant material and performed as described previously (Bainbridge et al. 2008; Bayer et al. 2009). For immunolocalization of H+-ATPase, a 1:200 dilution of a rabbit anti-Nicotiana plumbaginifolia H+-ATPase was used (Morsomme et al. 1998).
Acknowledgments
We thank Drs. M. Koornneef, N. Ori, J. Traas, B. Müller, R. Sablowski, J. Alonso, M. Boutry, and V. Reddy for providing materials; and U. Klahre, D. Kierzkowski, B. Guenot, and S. Braybrook for discussions. R. Smith, J. Venail, and A. Dell'Olivo gave useful advice on MorphographX software, the use of the scanning electron microscope, and the statistical analysis. C. Ball and N. Signer provided expert plant care. This work was funded by grants from the Swiss National Science Foundation and SystemsX.ch, the Swiss Initiative in systems biology.
Footnotes
Supplemental material is available for this article.
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.631211.
References
- Airy H 1873. On leaf arrangement. Proc R Soc Lond 22: 298–307 [Google Scholar]
- Argueso CT, Ferreira FJ, Kieber JJ 2009. Environmental perception avenues: the interaction of cytokinin and environmental response pathways. Plant Cell Environ 32: 1147–1160 [DOI] [PubMed] [Google Scholar]
- Bainbridge K, Guyomarc'h S, Bayer E, Swarup R, Bennett M, Mandel T, Kuhlemeier C 2008. Auxin influx carriers stabilize phyllotactic patterning. Genes Dev 22: 810–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandurski RS, Schulze A, Cohen JD 1977. Photoregulation of the ratio of ester to free indole-3-acetic acid. Biochem Biophys Res Commun 79: 1219–1223 [DOI] [PubMed] [Google Scholar]
- Bayer EM, Smith RS, Mandel T, Nakayama N, Sauer M, Prusinkiewicz P, Kuhlemeier C 2009. Integration of transport-based models for phyllotaxis and midvein formation. Genes Dev 23: 373–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behringer FJ, Davies PJ 1992. Indole-3-acetic acid levels after phytochrome-mediated changes in the stem elongation rate of dark- and light-grown Pisum seedlings. Planta 188: 85–92 [DOI] [PubMed] [Google Scholar]
- Bernier G, Périlleux C 2005. A physiological overview of the genetics of flowering time control. Plant Biotechnol J 3: 3–16 [DOI] [PubMed] [Google Scholar]
- Carabelli M, Possenti M, Sessa G, Ciolfi A, Sassi M, Morelli G, Ruberti I 2007. Canopy shade causes a rapid and transient arrest in leaf development through auxin-induced cytokinin oxidase activity. Genes Dev 21: 1863–1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carles CC, Fletcher JC 2003. Shoot apical meristem maintenance: the art of a dynamic balance. Trends Plant Sci 8: 394–401 [DOI] [PubMed] [Google Scholar]
- Chaudhury AM, Letham S, Craig S, Dennis ES 1993. amp1—a mutant with high cytokinin levels and altered embryonic pattern, faster vegetative growth, constitutive photomorphogenesis and precocious flowering. Plant J 4: 907–916 [Google Scholar]
- Chory J, Reinecke D, Sim S, Washburn T, Brenner M 1994. A role for cytokinins in de-etiolation in Arabidopsis (det mutants have an altered response to cytokinins). Plant Physiol 104: 339–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dello Ioio R, Nakamura K, Moubayidin L, Perilli S, Taniguchi M, Morita MT, Aoyama T, Costantino P, Sabatini S 2008. A genetic framework for the control of cell division and differentiation in the root meristem. Science 322: 1380–1384 [DOI] [PubMed] [Google Scholar]
- de Reuille PB, Bohn-Courseau I, Ljung K, Morin H, Carraro N, Godin C, Traas J 2006. Computer simulations reveal properties of the cell–cell signaling network at the shoot apex in Arabidopsis. Proc Natl Acad Sci 103: 1627–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming AJ, McQueen-Mason S, Mandel T, Kuhlemeier C 1997. Induction of leaf primordia by the cell wall protein expansin. Science 276: 1415–1418 [Google Scholar]
- Gallois JL, Woodward C, Reddy GV, Sablowski R 2002. Combined SHOOT MERISTEMLESS and WUSCHEL trigger ectopic organogenesis in Arabidopsis development 129: 3207–3217 [DOI] [PubMed] [Google Scholar]
- Gil P, Dewey E, Friml J, Zhao Y, Snowden KC, Putterill J, Palme K, Estelle M, Chory J 2001. BIG: a calossin-like protein required for polar auxin transport in Arabidopsis. Genes Dev 15: 1985–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulini A, Wang J, Jackson D 2004. Control of phyllotaxy by the cytokinin-inducible response regulator homologue ABPHYL1. Nature 430: 1031–1034 [DOI] [PubMed] [Google Scholar]
- Gordon SP, Chickarmane VS, Ohno C, Meyerowitz EM 2009. Multiple feedback loops through cytokinin signaling control stem cell number within the Arabidopsis shoot meristem. Proc Natl Acad Sci 106: 16529–16534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean O, Vernoux T, Laufs P, Belcram K, Mizukami Y, Traas J 2004. In vivo analysis of cell division, cell growth, and differentiation at the shoot apical meristem in Arabidopsis. Plant Cell 16: 74–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guseinova IM, Suleimanov SY, Aliyev JA 2005. The effect of norflurazon on protein composition and chlorophyll organization in pigment protein complex of Photosystem II. Photosynth Res 84: 71–76 [DOI] [PubMed] [Google Scholar]
- Halliday KJ, Martínez-García JF, Josse EM 2009. Integration of light and auxin signaling. Cold Spring Harb Perspect Biol 1: a001586 doi: 10.1101/cshperspect.a001586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamant O, Heisler MG, Jonsson H, Krupinski P, Uyttewaal M, Bokov P, Corson F, Sahlin P, Boudaoud A, Meyerowitz EM, et al. 2008. Developmental patterning by mechanical signals in Arabidopsis. Science 322: 1650–1655 [DOI] [PubMed] [Google Scholar]
- Heisler MG, Ohno C, Das P, Sieber P, Reddy GV, Long JA, Meyerowitz EM 2005. Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Curr Biol 15: 1899–1911 [DOI] [PubMed] [Google Scholar]
- Heisler MG, Hamant O, Krupinski P, Uyttewaal M, Ohno C, Jönsson H, Traas J, Meyerowitz EM 2010. Alignment between PIN1 polarity and microtubule orientation in the shoot apical meristem reveals a tight coupling between morphogenesis and auxin transport. PLoS Biol 8: e1000516 doi: 10.1371/journal.pbio.1000516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinski S, Piazza P, Craft J, Hay A, Woolley L, Rieu I, Phillips A, Hedden P, Tsiantis M 2005. KNOX action in Arabidopsis Is mediated by coordinate regulation of cytokinin and gibberellin activities. Curr Biol 15: 1560–1565 [DOI] [PubMed] [Google Scholar]
- Jiao Y, Lau OS, Deng XW 2007. Light-regulated transcriptional networks in higher plants. Nat Rev Genet 8: 217–230 [DOI] [PubMed] [Google Scholar]
- Jones AM, Cochran DS, Lamerson PM, Evans ML, Cohen JD 1991. Red light-regulated growth. I. Changes in the abundance of indoleacetic acid and a 22-kilodalton auxin-binding protein in the maize mesocotyl. Plant Physiol 97: 352–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jönsson H, Heisler MG, Shapiro BE, Meyerowitz EM, Mjolsness E 2006. An auxin-driven polarized transport model for phyllotaxis. Proc Natl Acad Sci 103: 1633–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M, Cone JW, Dekens RG, O'Herne-Robers EG, Spruit CJP, Kendrick RE 1985. Photomorphogenic responses of long hypocotyl mutants of tomato. J Plant Physiol 120: 153–165 [Google Scholar]
- Kurakawa T, Ueda N, Maekawa M, Kobayashi K, Kojima M, Nagato Y, Sakakibara H, Kyozuka J 2007. Direct control of shoot meristem activity by a cytokinin-activating enzyme. Nature 445: 652–655 [DOI] [PubMed] [Google Scholar]
- Laxmi A, Pan J, Morsy M, Chen R 2008. Light plays an essential role in intracellular distribution of auxin efflux carrier PIN2 in Arabidopsis thaliana. PLoS ONE 3: e1510 doi: 10.1371/journal.pone.0001510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B-H, Johnston R, Yang Y, Gallavotti A, Kojima M, Travencolo BAN, Costa LF, Sakakibara H, Jackson D 2009. Studies of aberrant phyllotaxy1 mutants of maize indicate complex interactions between auxin and cytokinin signaling in the shoot apical meristem. Plant Physiol 150: 205–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibfried A, To JPC, Busch W, Stehling S, Kehle A, Demar M, Kieber JJ, Lohmann JU 2005. WUSCHEL controls meristem function by direct regulation of cytokinin-inducible response regulators. Nature 438: 1172–1175 [DOI] [PubMed] [Google Scholar]
- Lenhard M, Laux T 2003. Stem cell homeostasis in the Arabidopsis shoot meristem is regulated by intercellular movement of CLAVATA3 and its sequestration by CLAVATA1. Development 130: 3163–3173 [DOI] [PubMed] [Google Scholar]
- Lochmanová G, Zdráhal Z, Konecná H, Koukalová S, Malbeck J, Soucek P, Válková M, Kiran NS, Brzobohaty B 2008. Cytokinin-induced photomorphogenesis in dark-grown Arabidopsis: a proteomic analysis. J Exp Bot 59: 3705–3719 [DOI] [PubMed] [Google Scholar]
- Lopez-Juez E, Dillon E, Magyar Z, Khan S, Hazeldine S, de Jager SM, Murray JAH, Beemster GTS, Bogre L, Shanahan H 2008. Distinct light-initiated gene expression and cell cycle programs in the shoot apex and cotyledons of Arabidopsis. Plant Cell 20: 947–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low VHK 1970. Effects of light and darkness on the growth of peas. Aust J Biol Sci 24: 187–195 [Google Scholar]
- Ma L, Li J, Qu L, Hager J, Chen Z, Zhao H, Deng XW 2001. Light control of Arabidopsis development entails coordinated regulation of genome expression and cellular pathways. Plant Cell 13: 2589–2607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno K, Komamine A, Shimokoriyama M 1971. Vessel element formation in cultured carrot-root phloem slices. Plant Cell Physiol 12: 823–830 [Google Scholar]
- Morsomme P, Dambly S, Maudoux O, Boutry M 1998. Single point mutations distributed in 10 soluble and membrane regions of the Nicotiana plumbaginifolia plasma membrane PMA2 H+-ATPase activate the enzyme and modify the structure of the C-terminal region. J Biol Chem 273: 34837–34842 [DOI] [PubMed] [Google Scholar]
- Müller B, Sheen J 2008. Cytokinin and auxin interaction in root stem-cell specification during early embryogenesis. Nature 453: 1094–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramoto T, Kami C, Kataoka H, Iwata N, Linley PJ, Mukougawa K, Yokota A, Kohchi T 2005. The tomato photomorphogenetic mutant, aurea, is deficient in phytochromobilin synthase for phytochrome chromophore biosynthesis. Plant Cell Physiol 46: 661–665 [DOI] [PubMed] [Google Scholar]
- Okada K, Ueda J, Komaki MK, Bell CJ, Shimura Y 1991. Requirement of the auxin polar transport-system in early stages of Arabidopsis floral bud formation. Plant Cell 3: 677–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ori N, Eshed Y, Chuck G, Bowman JL, Hake S 2000. Mechanisms that control knox gene expression in the Arabidopsis shoot. Development 127: 5523–5532 [DOI] [PubMed] [Google Scholar]
- Paciorek T, Zazimalova E, Ruthardt N, Petrasek J, Stierhof YD, Kleine-Vehn J, Morris DA, Emans N, Jurgens G, Geldner N, et al. 2005. Auxin inhibits endocytosis and promotes its own efflux from cells. Nature 435: 1251–1256 [DOI] [PubMed] [Google Scholar]
- Qamaruddin M, Tillberg E 1989. Rapid effects of red light on the isopentenyladenosine content in Scots pine seeds. Plant Physiol 91: 5–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy GV, Heisler MG, Ehrhardt DW, Meyerowitz EM 2004. Real-time lineage analysis reveals oriented cell divisions associated with morphogenesis at the shoot apex of Arabidopsis thaliana. Development 131: 4225–4237 [DOI] [PubMed] [Google Scholar]
- Reinhardt D, Mandel T, Kuhlemeier C 2000. Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell 12: 507–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt D, Pesce ER, Stieger P, Mandel T, Baltensperger K, Bennett M, Traas J, Friml J, Kuhlemeier C 2003. Regulation of phyllotaxis by polar auxin transport. Nature 426: 255–260 [DOI] [PubMed] [Google Scholar]
- Rieu I, Laux T 2009. Signaling pathways maintaining stem cells at the plant shoot apex. Semin Cell Dev Biol 20: 1083–1088 [DOI] [PubMed] [Google Scholar]
- Sablowski R 2011. Plant stem cell niches: from signalling to execution. Curr Opin Plant Biol 14: 4–9 [DOI] [PubMed] [Google Scholar]
- Salisbury FJ, Hall A, Grierson CS, Halliday KJ 2007. Phytochrome coordinates Arabidopsis shoot and root development. Plant J 50: 429–438 [DOI] [PubMed] [Google Scholar]
- Scarpella E, Marcos D, Friml J, Berleth T 2006. Control of leaf vascular patterning by polar auxin transport. Genes Dev 20: 1015–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shani E, Ben-Gera H, Shleizer-Burko S, Burko Y, Weiss D, Ori N 2010. Cytokinin regulates compound leaf development in tomato. Plant Cell 22: 3206–3217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki K, Uemura M, Tsurumi S, Rahman A 2009. Auxin response in Arabidopsis under cold stress: underlying molecular mechanisms. Plant Cell 21: 3823–3838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RS, Guyomarc'h S, Mandel T, Reinhardt D, Kuhlemeier C, Prusinkiewicz P 2006. A plausible model of phyllotaxis. Proc Natl Acad Sci 103: 1301–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova AN, Robertson-Hoyt J, Yun J, Benavente LM, Xie D, Doležal K, Schlereth A, Jürgens G, Alonso JM 2008. TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 133: 177–191 [DOI] [PubMed] [Google Scholar]
- Sweere U, Eichenberg K, Lohrmann J, Mira-Rodado V, Bäurle I, Kudla J, Nagy F, Schäfer E, Harter K 2001. Interaction of the response regulator ARR4 with phytochrome B in modulating red light signaling. Science 294: 1108–1111 [DOI] [PubMed] [Google Scholar]
- Tamura K, Shimada T, Ono E, Tanaka Y, Nagatani A, Higashi S-i, Watanabe M, Nishimura M, Hara-Nishimura I 2003. Why green fluorescent fusion proteins have not been observed in the vacuoles of higher plants. Plant J 35: 545–555 [DOI] [PubMed] [Google Scholar]
- Tao Y, Ferrer JL, Ljung K, Pojer F, Hong FX, Long JA, Li L, Moreno JE, Bowman ME, Ivans LJ, et al. 2008. Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell 133: 164–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turck F, Fornara F, Coupland G 2008. Regulation and identity of florigen: FLOWERING LOCUS T moves center stage. Annu Rev Plant Biol 59: 573–594 [DOI] [PubMed] [Google Scholar]
- Vandenbussche F, Habricot Y, Condiff AS, Maldiney Rg, Straeten DVD, Ahmad M 2007. HY5 is a point of convergence between cryptochrome and cytokinin signalling pathways in Arabidopsis thaliana. Plant J 49: 428–441 [DOI] [PubMed] [Google Scholar]
- van Tuinen A, Hanhart CJ, Kerckhoffs LHJ, Nagatani A, Boylan MT, Quail PH, Kendrick RE, Koornneef M 1996. Analysis of phytochrome-deficient yellow-green-2 and aurea mutants of tomato. Plant J 9: 173–182 [Google Scholar]
- Vidaurre DP, Ploense S, Krogan NT, Berleth T 2007. AMP1 and MP antagonistically regulate embryo and meristem development in Arabidopsis. Development 134: 2561–2567 [DOI] [PubMed] [Google Scholar]
- Werner T, Motyka V, Laucou V, Smets R, Van Onckelen H, Schmulling T 2003. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 15: 2532–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters H, Jurgens G 2009. Survival of the flexible: hormonal growth control and adaptation in plant development. Nat Rev Genet 10: 305–317 [DOI] [PubMed] [Google Scholar]
- Yadav RK, Girke T, Pasala S, Xie M, Reddy GV 2009. Gene expression map of the Arabidopsis shoot apical meristem stem cell niche. Proc Natl Acad Sci 106: 4941–4946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanai O, Shani E, Dolezal K, Tarkowski P, Sablowski R, Sandberg G, Samach A, Ori N 2005. Arabidopsis KNOXI proteins activate cytokinin biosynthesis. Curr Biol 15: 1566–1571 [DOI] [PubMed] [Google Scholar]
- Zeevaart JAD 1976. Physiology of flower formation. Annu Rev Plant Physiol 27: 321–348 [Google Scholar]
- Zhao Z, Andersen SU, Ljung K, Dolezal K, Miotk A, Schultheiss SJ, Lohmann JU 2010. Hormonal control of the shoot stem-cell niche. Nature 465: 1089–1092 [DOI] [PubMed] [Google Scholar]
- Zheng B, Deng Y, Mu J, Ji Z, Xiang T, Niu Q-W, Chua N-H, Zuo J 2006. Cytokinin affects circadian-clock oscillation in a phytochrome B and Arabidopsis response regulator 4-dependent manner. Physiol Plant 127: 277–292 [Google Scholar]