Abstract
The covalent attachment of lysine 63-linked polyubiquitin to the zinc finger domain of IKBKG/NEMO (also known as IKKγ) is necessary for full activation of NF-κB. Impairments of this biochemical mechanism explain the deleterious effects of hypomorphic NEMO mutations on NF-κB signaling function in humans suffering from X-linked ectodermal dysplasia and immunodeficiency. Nevertheless, the biological function of the NEMO zinc finger domain in the regulation of mitogen-activated protein kinase (MAPK) activity is poorly understood. Here we show that dendritic cells from patients with EDI caused by a C-terminal E391X deletion of the zinc finger of NEMO exhibit impaired MAPK activation in response to lipopolysaccharide (LPS) stimulation. Interestingly, DCs from patients with a C417R missense mutation within the zinc finger domain of NEMO in which ubiquitination of NEMO is preserved are also defective in JNK and ERK activity following LPS stimulation. Our findings indicate that the structural integrity of the NEMO ZF domain is more important than its polyubiquitination for full activation of the MAPK. Furthermore, phosphorylation and polyubiquitination of upstream TAK1 were significantly reduced in the E391X zinc finger deleted patients, indicating that the NEMO zinc finger may play an important role in assembling the proximal signaling complex for MAPK activation.
Keywords: IKBKG, NEMO, IKKγ, MAPK, toll-like receptor, ubiquitination
Introduction
Hypohidrotic ectodermal dysplasia combining immune deficiency (EDI or HED-ID) is a X-linked disorder characterized by abnormalities in hair, teeth, and skin, as well as impaired immune responses and increased susceptibility to microbial pathogen infection [Orange, et al., 2004]. EDI is caused by hypomorphic mutations of IKBKG/NEMO (MIM# 300248, also called IKKγ), a crucial regulatory subunit of the IκB kinase (IKK) complex [Doffinger, et al., 2001; Jain, et al., 2001; Zonana, et al., 2000]. Approximately 50% of patients with the EDI phenotype have missense or deletion mutations in the C-terminal zinc finger (ZF) domain of NEMO [Hanson, et al., 2008]. The ZF domain is believed to interact with specific factors and recruit IKK to the upstream signaling complex, thus regulating IKK activity [Brummelkamp, et al., 2003; Saito, et al., 2004]. Mutations and/or deletions in the ZF motif block NF-κB activation downstream of pathways activated by genotoxic stress [Huang, et al., 2002], CD40 [Jain, et al., 2001; Jain, et al., 2004], T-cell receptor (TCR) [Zhou, et al., 2004], and TNFα [Makris, et al., 2002], suggesting that an intact ZF is required for full NF-κB induction.
Mitogen activated protein kinase (MAPK) plays an essential role in inducing expression of genes that regulate innate and adaptive immunity. The host defense against microbial pathogens is mediated by Toll-like receptor (TLR), and signaling through TLR induces both the NF-κB and MAPK intracellular signaling pathways. Upon stimulation, MAPKs such as c-Jun N-terminal kinase (JNK), p38, and extracellular signal-regulated kinase (ERK) are rapidly phosphorylated by activated upstream mitogen activated protein kinase kinase (MAP2K) and mitogen activated protein kinase kinase kinase (MAP3K). Activation of these MAPK cascades ultimately leads to activation of transcription factor AP-1. Overexpression of NEMO has been shown to activate the AP-1 pathway [Shifera, et al., 2008] and NEMO-deficient mouse embryonic fibroblasts and B-cell lines exhibit reduced phospho-JNK and phospho-P38 activities following stimulation by IL-1 and a TLR9 antagonist, CpG [Yamamoto, et al., 2006]. However, the role of NEMO and its specific domains in the regulation of MAPK in human primary cells and in related diseases is not established.
Protein modification by ubiquitination has emerged as an important proximal regulatory mechanism for immune signaling. Macrophages from mice deficient in Ubc13, a K63-linked E2 ubiquitin conjugation enzyme, exhibit impaired MAPK activation following stimulation with various TLR agonists, indicating that K63-linked ubiquitination is important for MAPK activation [Wang, et al., 2001; Yamamoto, et al., 2006]. In addition, activation of TGF®-activated kinase 1 (TAK1), which is crucial for MAPK activation [Sato, et al., 2005; Wang, et al., 2001], is initiated by polyubiquitination followed by auto-phosphorylation and is dependent on the binding of ubiquitin acceptors to ubiquitinated TRAF6 [Kishimoto, et al., 2000; Reiley, et al., 2007; Wang, et al., 2001]. Several studies showed that the lysine 399 residue [Zhou, et al., 2004] in the ZF domain of NEMO is the site of K63-linked polyubiquitination in various pathways of NF-κB induction such as those induced by TCR [Zhou, et al., 2004], TLR [Ni, et al., 2008], TNFα [Tang, et al., 2003], and CD40 [Temmerman, et al., 2006]. Previous studies also suggest that NEMO polyubiquitination may play a role in TLR-induced MAPK activation in a murine pre-B cell line [Yamamoto, et al., 2006]. Overexpression of the human equivalent of K399, the ubiquitination-defective K392R (p.Lys392Arg) NEMO mutant, in a murine pre-B-cell line decreased JNK activity following stimulation with TLR9 agonist [Yamamoto, et al., 2006]. However, a later study of knock-in NEMO K392R mice showed no effect on MAPK activation in LPS stimulated bone marrow derived macrophages [Ni, et al., 2008].
We examined MAPK activities in cells of patients with two different EDI mutations, E391X (p.Glu391X) and C417R (p.Cys417Arg), that affect the NEMO ZF domain (Fig 1). The E391X mutation results in a C-terminal truncation and deletion of the entire ZF domain, whereas the C417R mutation is a missense mutation within the ZF domain. We showed that although PMA/Ionomycin-induced polyubiquitination of NEMO was defective in E391X T-cells, MAPK activation was largely unaffected. In contrast, both TLR4-induced MAPK activation and NEMO polyubiquitination were impaired in E391X dendritic cells (DCs). Interestingly, DCs of the C417R patient, in which the K399 residue in the ZF and polyubiquitination are preserved, also demonstrated a reduction in MAPK activity. Our results indicate that NEMO polyubiquitination is not sufficient; instead, the structural integrity of NEMO ZF domain is essential for full activation of the MAPK in LPS-treated dendritic cells. Furthermore, polyubiquitination and phosphorylation of TAK1 were defective in LPS-activated E391X DCs. Our findings indicate that an intact NEMO ZF domain is required for modification of upstream TAK1 by ubiquitin ligases and its subsequent activation to induce MAPK.
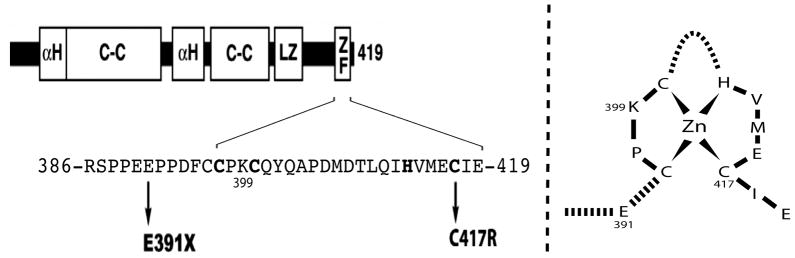
Fig 1. NEMO mutations and zinc finger structure in EDI patients.
Left: domains of NEMO: αH, alpha-helix; C-C, coiled-coil; LZ, leucine zipper; ZF, zinc finger. The conserved CCHC residues coordinating zinc are in bold. The E391X mutation leads to ZF truncation. The C417R mutation destabilizes the ZF structure [Cordier, et al., 2008] but retains the polyubiquitination site K399 in the ZF domain. Right: proposed ZF secondary structure.
Materials and Methods
Patients, mutations, and protocols
Patients were studied at the Clinical Center, NIAID, NIH (protocol 89-1-0158). The diagnosis of EDI was established by medical and family history, and confirmed by sequencing the IKBKG/NEMO gene as described previously [Jain, et al., 2001]. Unaffected family members or unrelated immunologically healthy volunteers served as controls. The Institutional Review Board of NIAID approved the open protocol and informed consent was obtained from all patients or their parents before enrollment in the study. A nonsense mutation at codon 391 glutamic acid (p.Glu391X, E391X) in two siblings [Cheng, et al., 2009] and a cysteine to arginine (p.Cys417Arg, C417R) mutation in a separate patient have been previously reported (GenBank reference sequence NP_003630.1) [Jain, et al., 2001; Jain, et al., 2004; Orange, et al., 2004]. The E391X mutation produces a truncated NEMO protein with complete deletion of the ZF including the K399 polyubiquitination site, while the C417R mutation partially disrupts the CCHC type ZF structure [Cordier, et al., 2008] by replacing the last key cysteine with arginine, but retains K399 (Fig 1). All three patients presented with life-threatening infections from infancy, hypohidrosis, and abnormal dentition. The symptoms of the E391X patients are much more severe than those of the C417R patient, requiring frequent hospitalization for bacterial infections, in particular Streptococcus pneumoniae infection.
Cell preparation and stimulation
IL-12 and TNFα production in peripheral blood mononuclear cell (PBMCs) from patients or healthy donors was measured as described previously [Jain, et al., 2001]. TNFα and IL-12p70 levels were measured by ELISA (R&D) from PBMCs treated with lipopolysaccharide (LPS) (1 μg/ml, Sigma-Aldrich) and 50 ng/ml IFN-γ (Peprotech) for 36 hours. Immature DCs were generated by stimulating elutriated or CD14 positive monocytes separated from PBMCs with IL-4 (R&D Systems, Minneapolis, MN) and granulocyte-macrophage colony-stimulating factor (GM-CSF, Berlex Laboratories) for 8 days [Temmerman, et al., 2006]. DCs (2 × 106/ml) derived from patients or healthy donors were stimulated with 2 μg/ml LPS (Sigma, St Louis, MO) for the indicated time. T-cells were blasted by plating PBMCs on anti-CD3 (OKT3) coated plates with anti-CD28 (2 μg/ml, BD Pharmingen) overnight, and transferred to IL-2 containing medium (100 U/ml) for 5 days. Dead cells were removed using the Dead Cell Removal Kit (Miltenyi Biotech). Cells were rested in serum-free medium supplemented with Nutridoma-SP (Roche Diagnostics) overnight prior to stimulation with PMA (20 ng/ml) and ionomycin (1 μM).
Electrophoresis mobility shift assay (EMSA), immunoprecipitation, and western blotting
EMSA was performed as previously described [Jain, et al., 2004]. To study protein ubiquitination, DCs were lysed in IP buffer containing 50 mM Tris-Cl (pH 7.5), 150 mM NaCl, 1% Nonidet P-40, 0.5% deoxycholate, 10 mM N-ethylaleimide (Sigma-Aldrich), 1 mM DTT, 10% glycerol, and protease inhibitor cocktail with EDTA (Roche Diagnostics). After addition of SDS to 1%, lysates were denatured by boiling, diluted 10-fold in IP buffer, immunoprecipitated by rabbit specific antibodies (Santa Cruz Biotech) and analyzed by western blotting as previously described [Temmerman, et al., 2006]. Polyubiquitinated proteins were probed using anti-ubiquitin antibodies (Santa Cruz Biotech and Imgenex). TAK1 polyubiquitination and phosphorylation assays were performed in the presence of 2.5 nM calyculin A (Cell Signaling Technology) for 10 minutes before LPS stimulation. Phospho-specific antibodies (Cell Signaling Technology) were used for western blot analyses of MAPK and TAK1 activities.
Results
Cytokine production, NEMO protein expression, and NF-κB binding activity in dendritic cells with deleted (E391X) or partially destabilized (C417R) NEMO zinc finger domain
The major defects of the NEMO E391X and C417R EDI patients appear to be in B cells and antigen presenting cells such as dendritic cells and macrophages [Jain, et al., 2004; Temmerman, et al., 2006]. B cells from these EDI patients were naïve with invariable expression of surface IgD and IgM, and failed to undergo immunoglobulin class switch recombination in response to CD40L plus IL-4 in vitro [Jain, et al., 2001; Jain, et al., 2004]. As in previous reports on the C417R mutant [Jain, et al., 2001], monocytes separated from PBMCs of E391X patients produced reduced levels of TNFα (Fig 2a) and failed to produce any IL-12 (Fig 2b) in response to LPS + IFNγ, Pam3Cys + IFNγ (data not shown), or CD40L + IFNγ (data not shown), indicating an essential role of the zinc finger of NEMO in regulating normal B cell and APC functions.
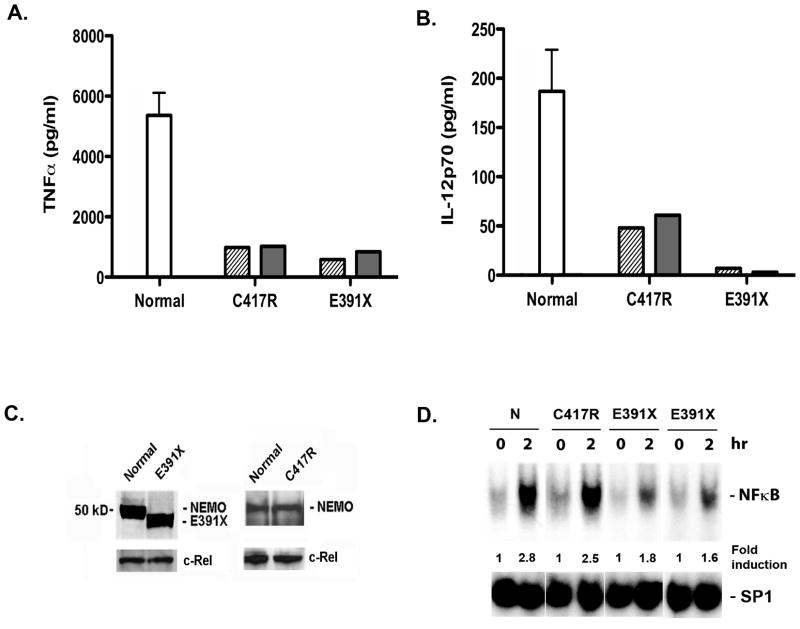
Fig 2. Cytokine production, NEMO protein expression, and NF-κB binding activity in dendritic cells with deleted or mutated zinc finger domain.
(A) TNFα and (B) IL-12 production in patient monocytes after LPS/IFN-γ treatment. Open bar, normal; closed and hatched bars, two E391X siblings and two experiments on the same C417R patient. (C) Western analysis of NEMO protein in PBMCs. (D) EMSA analysis of NF-κB binding activity in DCs of two E391X siblings and C417R patient after LPS treatment. Fold inductions were quantitated using ImageJ software normalized by Sp1.
Since it has been suggested that mutations in the ZF domain of NEMO affect protein stability [Doffinger, et al., 2001], we examined NEMO protein expression in PBMCs of C417R and E391X patients. As expected, a truncated form of NEMO protein was detected in E391X patients with an expression level comparable to that of normal control (Fig 2c). We also observed normal expression levels of C417R protein (Fig 2c), suggesting that mutations in NEMO ZF did not affect protein stability in PBMCs. NF-κB binding activity assessed by EMSA after LPS stimulation (Fig 2d) was comparable in DCs from normal controls and C417R patients as previously reported [Temmerman, et al., 2006], but was reduced in E391X DCs (Fig 2d). Consistent with these findings, the increased susceptibility of E391X patients to systemic inflammation and intestinal pathology [Cheng, et al., 2009] was likely caused by the TNF-mediated response in epithelial cells as a result of severely impaired NF-κB activity [Nenci, et al., 2007; Pasparakis, et al., 2002]. Since both C417R and E391X EDI patients are extremely vulnerable to diseases caused by pyogenic bacteria and mycobacteria, it is reasonable to conclude that there are defects in other intracellular signaling pathways induced by the TLR agonist LPS, such as MAPK cascades. This is supported by our observation that LPS-stimulated C417R DCs exhibit normal NF-κB DNA-binding, but impaired production of IL-12 (Fig 2b and Fig 2d) [Temmerman, et al., 2006]. Moreover, although DCs of E391X patients retain more than 50% NF-κB binding activity compared with controls (Fig 2d), IL-12 production in these cells is completely abolished (Fig 2b). Promoter analysis of the IL-12p40 gene reveals that both AP-1 and NF-κB binding sites are required for IL-12 production in LPS-stimulated human monocytes [Ma, et al., 2004], strengthening our proposal that MAPK pathways may also be defective in EDI patients.
Mutations in NEMO zinc finger impair JNK and ERK activities in LPS stimulated dendritic cells
We next measured MAPK activation following TLR4 stimulation in DCs prepared from patients and normal controls. Modest impairments in phosphorylation of JNK1/2 and ERK1/2, but not p38, were observed in LPS-stimulated C417R DCs (Fig 3a). Despite some individual variations in p38 activity, the defects in phosphorylation of both JNK1/2 and ERK1/2 were much more prominent in E391X DCs (Fig 3b) than in C417R DCs (Fig 3a). These findings indicated that an intact NEMO ZF is required for full activation of MAPK following TLR4 stimulation.
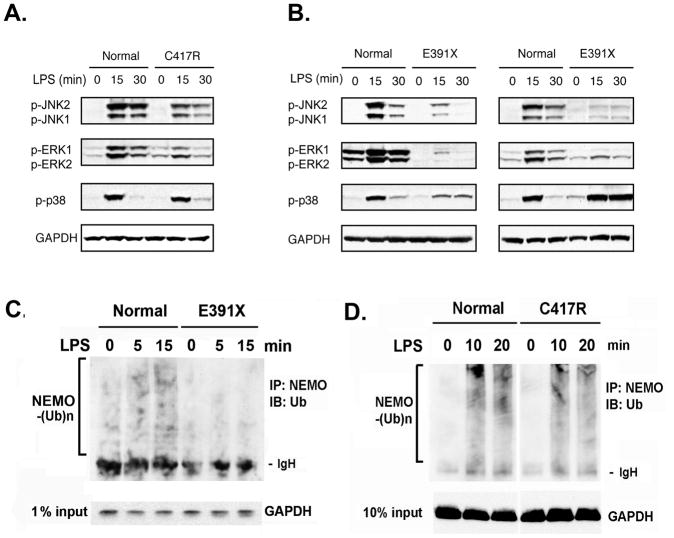
Fig 3. An intact NEMO ZF domain is more important than its polyubiquitination for full activation of MAPK in LPS-treated dendritic cells.
Western blot analysis of MAPK activities in DCs from patients with the C417 missense mutation (A) and the E391X ZF deletion (B) after LPS treatment. Polyubiquitination of NEMO in E391X ZF deleted (C) and C417R missense (D) patients after LPS stimulation. (E) Bone marrow derived macrophages from NEMO K392R mice were stimulated with LPS and MAPK activities were analyzed by western blot.
Defective JNK and ERK activations despite normal NEMO polyubiquitination in LPS-treated dendritic cells
We assessed NEMO polyubiquitination in cells of EDI patients with the NEMO E391X and C417R mutations in an attempt to find a correlation between NEMO polyubiquitination and MAPK activation. High molecular weight polyubiquitin conjugates of NEMO were present in lysates from controls but not in lysates prepared from NEMO ZF-deleted E391X DCs after LPS treatment (Fig 3c). While it is possible that the decreased MAPK activity in E391X DCs is due to deletion of the polyubiquitination site K399, C417R DCs were also defective in MAPK activation (Fig 3a) but exhibited normal levels of NEMO polyubiquitination (Fig 3d). We therefore concluded that the NEMO polyubiquitination is not sufficient; instead, the structural integrity of the NEMO ZF domain is more important for full induction of MAPK in human monocyte-derived dendritic cells upon TLR engagement. Consistent with our result, the murine K392R knock-in mutation, though devoid of NEMO polyubiquitination, has previously been reported exhibiting normal JNK and p38 activations upon LPS treatment [Ni, et al., 2008].
MAPK activation is largely unaffected in T cells of EDI patients with NEMO ZF deletion after PMA/ionomycin treatment
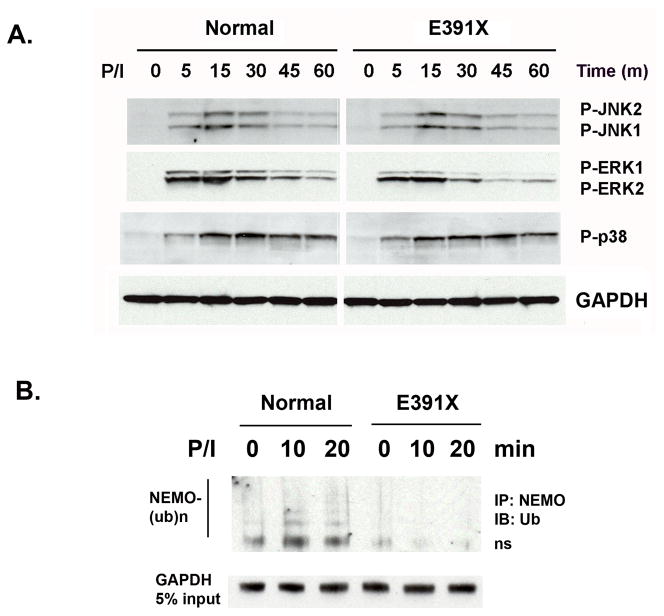
Although MAPK activation is impaired in LPS-stimulated DCs from EDI patients, the MAPK activities in E391X ZF-deleted T cells appear to be largely unaffected except for a mild decrease in ERK1 phosphorylation after PMA/Ionomycin stimulation (Fig 4a), despite the fact that polyubiquitination of NEMO is impaired (Fig 4b). We conclude that unlike LPS-stimulated DCs, in primary T cells activated through PMA/Ionomycin MAPK activation is much less dependent on the ZF of NEMO and the polyubiquitination of NEMO is not essential for MAPK induction. This observation is consistent with the finding that MAPK induction was normal in PMA/ionomycin stimulated thymocytes of a K392R knock-in mouse [Ni, et al., 2008]. Taken together, these results suggest that NEMO regulation of MAPK is cell type- and pathway-specific and could influence specific MAPK signaling pathways in different cell lineages.
Fig 4. Deletion of the ZF significantly impairs NEMO polyubiquitination but has a minimal effect on MAPK activities in PMA/ionomycin stimulated T-cells.
(A) Western blot analysis of MAPK activities in EDI E391X T-cells after PMA/ionomycin treatment. (B) Polyubiquitination of NEMO in T-cells from E391X patient after PMA/ionomycin stimulation.
The NEMO ZF domain is important for upstream TAK1 activity
TAK1 has been shown to be the crucial MAP3K in the MAPK signaling cascade of the TLR pathway [Sato, et al., 2005; Wang, et al., 2001]. Numerous reports have demonstrated that, proximal to the NEMO/IKK complex in TLR signaling, polyubiquitinated constituents such as IRAK1 and TRAF6 can recruit TAK1 molecule to the ubiquitination machinery and activate TAK1 polyubiquitination and subsequent auto-phosphorylation. However, the role of NEMO in the assembly of the upstream MAP3K complex is unclear. We therefore investigated TAK1 activity in NEMO ZF mutant cells by performing TAK1 polyubiquitination and phosphorylation assays on LPS-stimulated E391X DCs. After TLR4 engagement, both TAK1 polyubiquitination (Fig 5a) and phosphorylation (Fig 5b) were reduced in E391X ZF-deleted cells compared with the normal controls. The remaining low level of phospho-TAK1 in the ZF-deleted E391X cells in Fig 5b could account for the residual phospho-JNK activity observed in Fig 3b. These results suggested that, besides its essential role in IKK activation, the ZF domain of NEMO has an additional role in regulation of proximal TAK1 activity in the TLR pathway.
Fig 5. Deletion of NEMO ZF affects proximal TAK1 activity.
(A) Polyubiquitination of TAK1 in E391X DCs after LPS treatment. (B) Phosphorylation of TAK1 in E391X DCs after LPS treatment. The film was scanned and signals were measured using ImageJ software; the numbers depict fold induction calculated based on the intensity of the corresponding zero time point.
Discussion
NEMO hypomorphic mutations exhibit various clinical phenotypes. It has been hypothesized that mutations affecting different domains of NEMO can lead to defects in specific pathways that result in distinct disease states. NEMO has emerged as a nexus of assembly of multiple pathways and may play a role in regulating crosstalk between different pathways, such as those induced by NF-kB, MAPK, apoptosis, and genotoxic stress. Understanding how NEMO integrates into these pathways might be an important contribution to the development of therapeutic drugs for immunodeficiency and cancer.
Although it has been shown that NEMO deficiency leads to impairment of TLR9 and CD40-induced MAPK signaling in a murine pre-B-cell line [Matsuzawa, et al., 2008; Yamamoto, et al., 2006], evidence of NEMO participation in MAPK regulation in human primary cells is lacking. In this study we describe two different EDI NEMO mutations (C417R and E391X) that affect the ZF structure differently. The C417R mutation only partially disrupts ZF stability [Cordier, et al., 2008] due to a missense mutation of one of the four zinc coordinating molecules (Fig 1), whereas the E391X mutation causes a complete deletion of the ZF. Our data demonstrate that the severity of the defect in TLR4-induced MAPK activation is in accordance with the degree of structural impairment of the ZF domain. Interestingly, the MAPK signaling pathway has been shown to be important for host defense against Streptococcus pneumoniae, the susceptible phenotype also observed in the case of MAPK activation deficiency [Lim, et al., 2007]. Mice deficient in plasminogen activator inhibitor-1, which is a target gene of JNK and MAPK [Arndt, et al., 2005], are also susceptible to severe S. pneumoniae infection [Lim, et al., 2007]. The marked impairment in signal-induced JNK activity in patients with the E391X NEMO ZF deletion mutation may therefore account for their predisposition to S. pneumoniae infection of deep-seated organs such as bone and liver. The disease state is also consistent with differences in the degree of the mutation between the full ZF deletion of E391X and the less defective C417R mutation.
Regulation of cross talk between NF-κB and MAPK pathways is not clearly understood. PMA/ionomycin mediated activation of T cells mimics T cell receptor signaling that has been shown to activate MAPK through PKCθ/vav, a NEMO-independent mechanism [Villalba, et al., 2000]. This could explain the minimal defects in MAPK induction in the T cells of E391X ZF deletion patients following PMA/ionomycin stimulation, and suggest that NEMO’s role in MAPK regulation is restricted to certain cell lineages.
We also observed distinct influences of the NEMO ZF on the induction of different MAPKs. It has been proposed that ERK is activated by an upstream kinase Tpl2 [Dumitru, et al., 2000] via a pathway mediated by a NF-κB member p105, in which IKK/NEMO participates in the phosphorylation and degradation of the inhibitor p105 to release active Tpl2 [Waterfield, et al., 2004]. This could explain why we observed reduced ERK activities in both LPS-treated DCs from NEMO EDI patients.
Because JNK activation is impaired in the C417R patient despite the fact that NEMO polyubiquitination is unaffected, we therefore proposed that polyubiquitination of NEMO is not important for full induction of JNK. To strengthen the hypothesis, we stimulated bone marrow derived macrophages from the K392R knock-in mouse [Ni, et al., 2008] with LPS and observed only a slight decrease in ERK activation, whereas JNK and p38 activities are intact (unpublished data). The murine K392R mutation has been shown previously to be defective in NEMO polyubiquitination upon LPS treatment although the JNK and p38 activities were preserved [Ni, et al., 2008]. This provided evidence that the polyubiquitination of NEMO is not essential for activation of JNK and p38. Consistent with our finding, the defect in ERK activation is likely due to the impact of the K392R mutation on IKK activity via Tpl2 [Ni, et al., 2008; Waterfield, et al., 2004]. These results indicate that the structure of the ZF domain rather than its polyubiquitination is important for full induction of MAPK.
Our findings indicate that an alteration of the NEMO ZF adversely affects both JNK and p38 phosphorylation following TLR4 stimulation, although the degree of impairment in p38 activity is less prominent. TAK1 polyubiquitination has been shown to be important for distal MAPK signaling [Fan, et al., 2010; Reiley, et al., 2007]. While a direct association between NEMO and TAK1 has not been demonstrated, we have found that NEMO and TAK1 are components of the TLR4 signaling complex (unpublished observation) and our results suggest that an alteration in the NEMO zinc finger adversely affect TAK1 polyubiquitination. In addition, our findings indicate that JNK activity is more associated with TAK1 polyubiquitination than p38 activity. TAK1 activity is dependant on the E2 enzyme Ubc13 [Wang, et al., 2001] and similar observations were noted in mice lacking Ubc13 [Yamamoto, et al., 2006]. B cells deficient in Ubc13 exhibited severe impairment of JNK activity following LPS or bacterial lipoproteins (BLP) stimulation, whereas p38 induction was less affected [Yamamoto, et al., 2006]. While the precise mechanism by which the zinc finger domain affects TAK1 polyubiquitination awaits further study, we propose that the NEMO ZF may serve as a nexus of assembly that allows TAK1 recruitment to the membrane receptor and subsequent modification by ubiquitin ligases such as Ubc13. In support of this model, CD40-induction of phospho-TAK1 was abolished in a NEMO-deficient pre-B-cell line [Matsuzawa, et al., 2008]. The NEMO ZF has been shown to play an important role in the non- covalent binding of K63-linked polyubiquitin chains to the NEMO ubiquitin-binding (NUB/UBAN/NOA) motif [Laplantine, et al., 2009]. The NEMO NUB domain facilitates recruitment of the IKK complex to the upstream signaling molecules modified by polyubiquitin. Thus it will be interesting to determine whether NEMO hypomorphs with mutations in the NEMO NUB domain also manifest defects in MAPK and MAP3K activities following TLR4 signaling.
Acknowledgments
We thank Warren Strober for helpful discussions, Dean Ballard and Eugene Oltzfor providing the NEMO K392R knock-in mouse, and Mary Derry for editorial assistance. This research was supported in part by the Intramural NIH Research Programs of the NIH Clinical Center and the National Institute of Allergy and Infectious Disease. This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Disclosure statement: The authors declare no conflict of interest.
References
- Brummelkamp TR, Nijman SM, Dirac AM, Bernards R. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-kappaB. Nature. 2003;424(6950):797–801. doi: 10.1038/nature01811. [DOI] [PubMed] [Google Scholar]
- Cheng LE, Kanwar B, Tcheurekdjian H, Grenert JP, Muskat M, Heyman MB, McCune JM, Wara DW. Persistent systemic inflammation and atypical enterocolitis in patients with NEMO syndrome. Clin Immunol. 2009;132(1):124–31. doi: 10.1016/j.clim.2009.03.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordier F, Vinolo E, Veron M, Delepierre M, Agou F. Solution structure of NEMO zinc finger and impact of an anhidrotic ectodermal dysplasia with immunodeficiency-related point mutation. J Mol Biol. 2008;377(5):1419–32. doi: 10.1016/j.jmb.2008.01.048. [DOI] [PubMed] [Google Scholar]
- Doffinger R, Smahi A, Bessia C, Geissmann F, Feinberg J, Durandy A, Bodemer C, Kenwrick S, Dupuis-Girod S, Blanche S, et al. X-linked anhidrotic ectodermal dysplasia with immunodeficiency is caused by impaired NF-kappaB signaling. Nat Genet. 2001;27(3):277–85. doi: 10.1038/85837. [DOI] [PubMed] [Google Scholar]
- Dumitru CD, Ceci JD, Tsatsanis C, Kontoyiannis D, Stamatakis K, Lin JH, Patriotis C, Jenkins NA, Copeland NG, Kollias G, et al. TNF-alpha induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell. 2000;103(7):1071–83. doi: 10.1016/s0092-8674(00)00210-5. [DOI] [PubMed] [Google Scholar]
- Fan Y, Yu Y, Shi Y, Sun W, Xie M, Ge N, Mao R, Chang A, Xu G, Schneider MD, et al. Lysine 63-linked polyubiquitination of TAK1 at lysine 158 is required for tumor necrosis factor alpha- and interleukin-1beta-induced IKK/NF-kappaB and JNK/AP-1 activation. J Biol Chem. 2010;285(8):5347–60. doi: 10.1074/jbc.M109.076976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson EP, Monaco-Shawver L, Solt LA, Madge LA, Banerjee PP, May MJ, Orange JS. Hypomorphic nuclear factor-kappaB essential modulator mutation database and reconstitution system identifies phenotypic and immunologic diversity. J Allergy Clin Immunol. 2008;122(6):1169–1177. e16. doi: 10.1016/j.jaci.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TT, Feinberg SL, Suryanarayanan S, Miyamoto S. The zinc finger domain of NEMO is selectively required for NF-kappa B activation by UV radiation and topoisomerase inhibitors. Mol Cell Biol. 2002;22(16):5813–25. doi: 10.1128/MCB.22.16.5813-5825.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A, Ma CA, Liu S, Brown M, Cohen J, Strober W. Specific missense mutations in NEMO result in hyper-IgM syndrome with hypohydrotic ectodermal dysplasia. Nat Immunol. 2001;2(3):223–8. doi: 10.1038/85277. [DOI] [PubMed] [Google Scholar]
- Jain A, Ma CA, Lopez-Granados E, Means G, Brady W, Orange JS, Liu S, Holland S, Derry JM. Specific NEMO mutations impair CD40-mediated c-Rel activation and B cell terminal differentiation. J Clin Invest. 2004;114(11):1593–602. doi: 10.1172/JCI21345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto K, Matsumoto K, Ninomiya-Tsuji J. TAK1 mitogen-activated protein kinase kinase kinase is activated by autophosphorylation within its activation loop. J Biol Chem. 2000;275(10):7359–64. doi: 10.1074/jbc.275.10.7359. [DOI] [PubMed] [Google Scholar]
- Lim JH, Stirling B, Derry J, Koga T, Jono H, Woo CH, Xu H, Bourne P, Ha UH, Ishinaga H, et al. Tumor suppressor CYLD regulates acute lung injury in lethal Streptococcus pneumoniae infections. Immunity. 2007;27(2):349–60. doi: 10.1016/j.immuni.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Ma W, Gee K, Lim W, Chambers K, Angel JB, Kozlowski M, Kumar A. Dexamethasone inhibits IL-12p40 production in lipopolysaccharide-stimulated human monocytic cells by down-regulating the activity of c-Jun N-terminal kinase, the activation protein-1, and NF-kappa B transcription factors. J Immunol. 2004;172(1):318–30. doi: 10.4049/jimmunol.172.1.318. [DOI] [PubMed] [Google Scholar]
- Makris C, Roberts JL, Karin M. The carboxyl-terminal region of IkappaB kinase gamma (IKKgamma) is required for full IKK activation. Mol Cell Biol. 2002;22(18):6573–81. doi: 10.1128/MCB.22.18.6573-6581.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa A, Tseng PH, Vallabhapurapu S, Luo JL, Zhang W, Wang H, Vignali DA, Gallagher E, Karin M. Essential cytoplasmic translocation of a cytokine receptor-assembled signaling complex. Science. 2008;321(5889):663–8. doi: 10.1126/science.1157340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nenci A, Becker C, Wullaert A, Gareus R, van Loo G, Danese S, Huth M, Nikolaev A, Neufert C, Madison B, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446(7135):557–61. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- Ni CY, Wu ZH, Florence WC, Parekh VV, Arrate MP, Pierce S, Schweitzer B, Van Kaer L, Joyce S, Miyamoto S, et al. Cutting edge: K63-linked polyubiquitination of NEMO modulates TLR signaling and inflammation in vivo. J Immunol. 2008;180(11):7107–11. doi: 10.4049/jimmunol.180.11.7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orange JS, Jain A, Ballas ZK, Schneider LC, Geha RS, Bonilla FA. The presentation and natural history of immunodeficiency caused by nuclear factor kappaB essential modulator mutation. J Allergy Clin Immunol. 2004;113(4):725–33. doi: 10.1016/j.jaci.2004.01.762. [DOI] [PubMed] [Google Scholar]
- Pasparakis M, Courtois G, Hafner M, Schmidt-Supprian M, Nenci A, Toksoy A, Krampert M, Goebeler M, Gillitzer R, Israel A, et al. TNF-mediated inflammatory skin disease in mice with epidermis-specific deletion of IKK2. Nature. 2002;417(6891):861–6. doi: 10.1038/nature00820. [DOI] [PubMed] [Google Scholar]
- Reiley WW, Jin W, Lee AJ, Wright A, Wu X, Tewalt EF, Leonard TO, Norbury CC, Fitzpatrick L, Zhang M, et al. Deubiquitinating enzyme CYLD negatively regulates the ubiquitin-dependent kinase Tak1 and prevents abnormal T cell responses. J Exp Med. 2007;204(6):1475–85. doi: 10.1084/jem.20062694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Kigawa T, Koshiba S, Sato K, Matsuo Y, Sakamoto A, Takagi T, Shirouzu M, Yabuki T, Nunokawa E, et al. The CAP-Gly domain of CYLD associates with the proline-rich sequence in NEMO/IKKgamma. Structure. 2004;12(9):1719–28. doi: 10.1016/j.str.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji J, Yamamoto M, Kawai T, Matsumoto K, Takeuchi O, Akira S. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol. 2005;6(11):1087–95. doi: 10.1038/ni1255. [DOI] [PubMed] [Google Scholar]
- Shifera AS, Friedman JM, Horwitz MS. IKK gamma (NEMO) is involved in the coordination of the AP-1 and NF-kappa B pathways. Mol Cell Biochem. 2008;310(1–2):181–90. doi: 10.1007/s11010-007-9679-z. [DOI] [PubMed] [Google Scholar]
- Tang ED, Wang CY, Xiong Y, Guan KL. A role for NF-kappaB essential modifier/IkappaB kinase-gamma (NEMO/IKKgamma) ubiquitination in the activation of the IkappaB kinase complex by tumor necrosis factor-alpha. J Biol Chem. 2003;278(39):37297–305. doi: 10.1074/jbc.M303389200. [DOI] [PubMed] [Google Scholar]
- Temmerman ST, Ma CA, Borges L, Kubin M, Liu S, Derry JM, Jain A. Impaired dendritic-cell function in ectodermal dysplasia with immune deficiency is linked to defective NEMO ubiquitination. Blood. 2006;108(7):2324–31. doi: 10.1182/blood-2006-04-017210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba M, Coudronniere N, Deckert M, Teixeiro E, Mas P, Altman A. A novel functional interaction between Vav and PKCtheta is required for TCR-induced T cell activation. Immunity. 2000;12(2):151–60. doi: 10.1016/s1074-7613(00)80168-5. [DOI] [PubMed] [Google Scholar]
- Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. TAK1 is a ubiquitin- dependent kinase of MKK and IKK. Nature. 2001;412(6844):346–51. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- Waterfield M, Jin W, Reiley W, Zhang M, Sun SC. IkappaB kinase is an essential component of the Tpl2 signaling pathway. Mol Cell Biol. 2004;24(13):6040–8. doi: 10.1128/MCB.24.13.6040-6048.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Okamoto T, Takeda K, Sato S, Sanjo H, Uematsu S, Saitoh T, Yamamoto N, Sakurai H, Ishii KJ, et al. Key function for the Ubc13 E2 ubiquitin-conjugating enzyme in immune receptor signaling. Nat Immunol. 2006;7(9):962–70. doi: 10.1038/ni1367. [DOI] [PubMed] [Google Scholar]
- Zhou H, Wertz I, O'Rourke K, Ultsch M, Seshagiri S, Eby M, Xiao W, Dixit VM. Bcl10 activates the NF-kappaB pathway through ubiquitination of NEMO. Nature. 2004;427(6970):167–71. doi: 10.1038/nature02273. [DOI] [PubMed] [Google Scholar]
- Zonana J, Elder ME, Schneider LC, Orlow SJ, Moss C, Golabi M, Shapira SK, Farndon PA, Wara DW, Emmal SA, et al. A novel X-linked disorder of immune deficiency and hypohidrotic ectodermal dysplasia is allelic to incontinentia pigmenti and due to mutations in IKK-gamma (NEMO) Am J Hum Genet. 2000;67(6):1555–62. doi: 10.1086/316914. [DOI] [PMC free article] [PubMed] [Google Scholar]