Abstract
Mesenchymal stem cells (MSCs) are being recognized as a viable cell source for cartilage repair and members of the transforming growth factor-beta (TGF-β) superfamily are a key mediator of MSC chondrogenesis. While TGF-β mediated MSC chondrogenesis is well established in in vitro pellet or hydrogel cultures, clinical translation will require effective delivery of TGF-βs in vivo. Here, we investigated the co-encapsulation of TGF-β3 containing alginate microspheres with human MSCs in hyaluronic acid (HA) hydrogels towards the development of implantable constructs for cartilage repair. TGF-β3 encapsulated in alginate microspheres with nanofilm coatings showed significantly reduced initial burst release compared to uncoated microspheres, with release times extending up to 6 days. HA hydrogel constructs seeded with MSCs and TGF-β3 containing microspheres developed comparable mechanical properties and cartilage matrix content compared to constructs supplemented with TGF-β3 continuously in culture media, whereas constructs with TGF-β3 directly encapsulated in the gels without microspheres had inferior properties. When implanted subcutaneously in nude mice, constructs containing TGF-β3 microspheres resulted in superior cartilage matrix formation to groups without TGF-β3 or with TGF-β3 added directly to the gel. However, calcification was observed in implanted constructs after 8 weeks of subcutaneous implantation. To prevent this, the co-delivery of parathyroid hormone-related protein (PTHrP) with TGF-β3 in alginate microspheres was pursued, resulting in partially reduced calcification. This study demonstrates that the controlled local delivery of TGF-β3 is essential to neocartilage formation by MSCs and that further optimization is needed to avert the differentiation of chondrogenically induced MSCs towards a hypertrophic phenotype.
Keywords: mesenchymal stem cells, growth factor delivery, hyaluronic acid, chondrogenesis, hypertrophy, hydrogel
Introduction
Mesenchymal stem cells (MSCs) have emerged as a clinically relevant cell source for regenerative medicine, especially for cartilage repair. MSCs undergo chondrogenesis and deposit a cartilage specific matrix in pellet cultures and in a variety of natural and synthetic scaffold materials in the presence of the appropriate growth factors [1, 2]. Transforming growth factor-beta (TGF-β) superfamily members are a key requirement for chondrogenesis of MSCs [3, 4] and studies have shown that TGF-β stimulates chondrocyte proliferation and prevents cartilage hypertrophy [5, 6]. Previous and our preliminary in vitro studies also indicate that continuous exposure to TGF-β3 is not needed and that exposure in the first week is the most critical to the chondrogenesis of MSCs [7]. Generally, the presentation of molecules such as TGF-β3 is simple in an in vitro setting, but is more complicated in an implantable system with challenges such as tissue and scaffold diffusion, foreign body response and proteolytic activity in an in vivo environment. However, it is of great interest to develop therapies where MSCs and a scaffold are implanted directly into cartilage defect sites for cartilage repair. This necessitates the development of a controlled release system to provide sustained local delivery of TGF-β3 in order to ensure therapeutic efficacy of the implanted MSC constructs.
Previously, we showed that as a natural polymer, hyaluronic acid (HA) hydrogels provide a stable 3D environment that is conducive to the chondrogenesis of MSCs in the presence of growth factors [8, 9]. However, these studies were performed with continuous exposure to chondrogenic media (containing TGF-β3) in vitro. Meanwhile, HA has also been used as a delivery carrier for a number of growth factors including IGF, PDGF, VEGF and FGF in both in vitro and in vivo studies [10–12]. These studies demonstrated that growth factors released from HA retained their bioactivity and promoted tissue development [11, 12].
Alginate microspheres, which are ionically crosslinked in the presence of multivalent cations, have been used widely for controlled delivery of growth factors due to advantages such as biocompatibility, high encapsulation efficiency, and mild fabrication conditions [13–15]. These mild conditions include the absence of organic solvent and high shear stresses together with the absence of acidic degradation products that can induce the denaturation and loss of biological activity of encapsulated proteins. Furthermore, alginate microspheres allow for high diffusion rates of macromolecules [16], and this diffusion can be controlled with a simple one step coating procedures [17, 18].
While MSCs are able to form cartilage-like tissue in vitro under chondrogenic induction by TGF-βs, transplantation to the uncontrolled in vivo environment commonly results in extensive calcification of the ECM [19, 20]. A recent study also reported that delivery of TGF-β1 with MSCs results in more subchondral trabecular bone formation in a rabbit osteochondral repair site [21]. To counter this aberrant hypertrophy, parathyroid hormone-related protein (PTHrP) has been employed to inhibit hypertrophy of chondrocytes or MSCs during chondrogenesis [22–26]. However, a recent study also showed that pretreatment of MSCs with molecules (such as PTHrP or FGF2) that suppress hypertrophy did not prevent mineralization of MSC pellets ectopically implanted in nude mice [23].
With these benefits and issues in mind, the objective of this study was to develop an alginate-based delivery vehicle for TGF-β3 and test its efficacy in inducing human MSC chondrogenesis and neocartilage formation within HA hydrogels in both in vitro and in vivo environments. HA hydrogels combined with alginate microspheres loaded with TGF-β3 forms a composite carrier which may help retain TGF-β3 bioactivity in the scaffold and promote chondrogenesis of MSCs when implanted.
Material and methods
Macromer synthesis
Methacrylated HA (MeHA) was synthesized as previously reported [27]. Briefly, methacrylic anhydride (methacrylic anhydride, 94%, FW: 154.17, Sigma) was added to a solution of 1 wt% HA (sodium hyaluronate powder, research grade, MW ~74 kDa, Lifecore) in deionized (DI) water, adjusted to a pH of 8 with 5 N NaOH, and reacted on ice for 24 h. The macromer solution was purified via dialysis (MW cutoff 6–8k) against deionized water for a minimum of 48 h with repeated changes of water. The final product was obtained by lyophilization and stored at −20 °C in powder form prior to use. The final macromer products were confirmed by 1H NMR to have a methacrylation of ~27%. Lyophilized macromers were dissolved in phosphate buffered saline (PBS) containing 0.05 wt% 2-methyl-1-[4-(hydroxyethoxy) phenyl]-2-methyl-1-propanone (I2959, Ciba) for polymerization.
Microsphere preparation
Alginate microspheres (MS) were prepared by adapting an emulsion/external gelation protocol [15]. Briefly, alginic acid sodium salt (from brown algae, Sigma) solution was combined with TGF-β3 (R&D Systems, MS+T group), PTHrP (PeproTech, MS+P group), or both TGF-β3 and PTHrP (MS+P+T group) to achieve a final alginate concentration of 2% (w/v). The mixture was then added to olive oil dropwise while homogenizing at 500 rpm with 1% (v/v) Tween 80 as surfactant. The emulsion was mixed for 3 minutes, followed by the dropwise addition of CaCl2 solution at a concentration of 200 mM. After a further 15 minutes of crosslinking, particles were then centrifuged at 1500 g for 5 minutes, the supernatant was removed and particles were subsequently washed 3 times in 2-propanol to remove the residue oil and then washed 4 times with sterile deionized water. The particles were then air-dried and resuspended in ultrapure water. Using a method modified from Srivastava et al., alginate microspheres were coated with a layer of self-assembled nanofilm consisting of poly(allylamine hydrochloride) (PAH, MW15 kDa) and poly(sodium 4-styrenesulfonate) (PSS, MW1 MDa) (Sigma, St. Louis, MO)[28].
Characterization of in vitro release of TGF-β3
Uncoated (Uncoated MS) or coated (Coated MS) alginate microspheres, HA hydrogel disks encapsulated with TGF-β3 directly without microspheres (T in HA) and HA hydrogel disks containing encapsulated coated microspheres (Coated MS in HA) were incubated in cell culture medium (DMEM, 1% ITS+Premix, 50 μg/ml L-proline, 0.1 μM dexamethasone, 0.9 mM sodium pyruvate, antibiotics) at 37°C and released TGF-β3 was quantified with an Elisa kit (R&D Systems). Containers used for the release study were pretreated with 1% BSA solution to minimize protein absorption. Cumulative release profiles were calculated by normalizing detected TGF-β3 to total amount detected after 28 days of release. Fresh alginate microspheres were dissolved in sodium citrate (55 mM) solution and the encapsulation efficiency of alginate microspheres was determined by normalizing the detected TGF-β3 to the total amount of TGF-β3 used in the microsphere fabrication process.
Sample preparation and in vitro culture
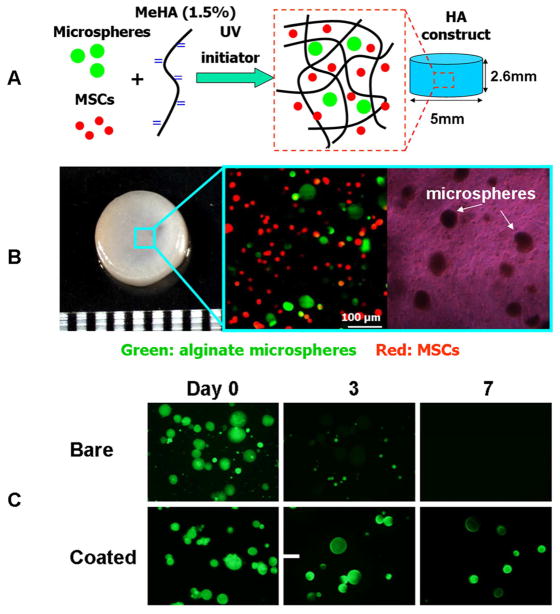
Human MSCs (Lonza) were expanded to passage 3 in growth media consisting of α-MEM with 16.7% FBS and 1% pen/strep. MSCs (20 million/ml) and alginate microspheres were photoencapsulated in 1.5% MeHA hydrogel disks (Ø5 mm, 2.6 mm thickness, Figure 1A,B) and cultured in standard media (DMEM, 1% ITS+Premix, 50 μg/ml L-proline, 0.1 μM dexamethasone, 0.9 mM sodium pyruvate, antibiotics) supplemented with ascorbate (50 μg/ml), which was changed three times per week [29]. Chondrogenic media (CM) consists of this standard media with the addition of 10 ng/ml of TGF-β3. In the in vitro study, HA constructs were prepared with (1) blank microspheres and cultured in CM media (MS-T+CM), (2) blank microspheres and cultured in standard media (MS-T-CM), (3) TGF-β3-loaded microspheres (MS+T, 100 ng TGF-β3/disk) and cultured in standard media, or (4) direct encapsulation of TGF-β3 (T only, 100 ng TGF-β3/disk) and culture in standard media. Cell viability was assessed using the LIVE/DEAD Assay Kit (Molecular Probes) where live cells are stained green with calcein- AM and dead cells stained red with ethidium homodimer.
Figure 1.
Photoencapsulation of alginate microspheres (4 mg/ml) and MSCs (20 million/ml) into methacrylated HA (MeHA) hydrogel disks (A); fabricated HA hydrogel disk and fluorescent and bright field microscopic images of MSCs (membrane labeled with red dye) and alginate microspheres (containing FITC-labeled protein) encapsulated in HA gels (B); release of encapsulated BSA-FITC from bare and coated alginate microspheres over 7 days in PBS (C), scale bar = 50 μm.
Subcutaneous implantation in nude mice
In the first in vivo study, MSC-laden (20 million/ml) HA hydrogel constructs (n = 8 for each group) were fabricated with (1) blank microspheres (MS-T), (2) direct encapsulation of TGF-β3 (T only, 100 ng TGF-β3/disk), or (3) TGF-β3-loaded microspheres (MS+T, 100 ng TGF-β3/disk) and then implanted on the following day. Four subcutaneous pockets were prepared on the backs of male nude mice (NCRNU, age 4 weeks; Taconic) and received implants. In the follow-up in vivo study, MSC-laden (20 million/ml) HA hydrogel disks (n = 8 for each group) were fabricated with (1) TGF-β3-loaded microspheres (MS+T, 100 ng TGF-β3/disk), (2) PTHrP-loaded microspheres (MS+P, 50 ng PTHrP/disk), or (3) TGF-β3+PTHrP-loaded microspheres (MS+T+P, 100 ng TGF-β3 and 50 ng PTHrP/disk) and then implanted on the following day. Samples were harvested 4 or 8 weeks later. Guidelines from Institutional Animal Care and Use Committee at the University of Pennsylvania were followed during all animal procedures.
Mechanical testing
At set time points, samples were removed from the culture or retrieved from the subcutaneous space of mouse and the bulk mechanical properties of construct disks were evaluated using a custom table top testing device as described previously [30]. Briefly, samples were first equilibrated in creep to a tare load of 2 grams by an impermeable loading platen in a loading chamber filled with phosphate buffered saline. From this offset, stress relaxation tests were performed with a single compression ramp at a speed of 10%/min until reaching 10% strain. The equilibrium Young’s modulus (EY) was determined by the equilibrium load obtained after 1000 sec of relaxation under unconfined compression at 10% strain. The Young’s moduli of the tested samples were considered undetectable if the equilibrium load at the end of stress relaxation was lower than the initial tare load, and these samples were plotted as having a Young’s modulus of zero.
Gene expression analysis
For gene expression analysis, samples were homogenized in Trizol Reagent (Invitrogen) with a tissue grinder, RNA was extracted according to the manufacturer’s instructions, and the RNA concentration was determined using an ND-1000 spectrophotometer (Nanodrop Technologies). One microgram of RNA from each sample was reverse transcribed into cDNA using reverse transcriptase (Superscript II, Invitrogen) and oligoDT (Invitrogen). Polymerase chain reaction (PCR) was performed on an Applied Biosystems 7300 Real-Time PCR system using Taqman primers and probes specific for GAPDH (housekeeping gene) and other genes of interest. Sequences of the primers and probes used are listed in Table 1. The relative gene expression was calculated using the ΔΔCT method, where fold difference was calculated using the expression 2ΔΔCt. Each sample was internally normalized to GAPDH, and each group was normalized to the expression levels of MSCs at the time of encapsulation (i.e., after expansion and before differentiation). Relative expression levels greater than 1 represent up-regulation with culture, while relative expression levels less than 1 represent down-regulation of that gene compared to that of initially encapsulated MSCs.
Table 1.
Sequences of primers and probes used for Real-Time PCR. Sequences related to gene type X collagen are proprietary to Applied Biosystems Inc. and not disclosed.
| Gene | Forward Primer | Reverse Primer | Probe |
|---|---|---|---|
| GAPDH | AGGGCTGCTTTTAACTCTGGTAAA | GAATTTGCCATGGGTGGAAT | CCTCAACTACATGGTTTAC |
| COL I | AGGACAAGAGGCATGTCTGGTT | GGACATCAGGCGCAGGAA | TTCCAGTTCGAGTATGGC |
| COL II | GGCAATAGCAGGTTCACGTACA | CGATAACAGTCTTGCCCCACTT | CTGCACGAAACATAC |
| Aggrecan | TCGAGGACAGCGAGGCC | TCGAGGGTGTAGCGTGTAGAGA | ATGGAACACGATGCCTTTCACCACGA |
Biochemical analysis
One-half of each construct was weighed wet, lyophilized, reweighed dry, and digested in 0.5 mg/ml Proteinase-K (Fisher Scientific) at 56°C for 16 hrs. The PicoGreen assay (Invitrogen, Molecular Probes) was used to quantify the DNA content of the constructs with Lambda phage DNA (0–1 mg/ml) as a standard [31]. For each sample, both the mass of the entire gel and the half gel used for DNA assay were measured. The total amount of DNA per sample was calculated by scaling the amount of DNA detected in the half gel by a weight ratio (total weight/half weight). The GAG content was measured using the dimethylmethylene blue (DMMB, Sigma Chemicals) dye-binding assay with shark chondroitin sulfate (0–50 mg/ml) as a standard [32]. The overall collagen content was assessed by measuring the orthohydroxyproline (OHP) content via dimethylaminobenzaldehyde and chloramine T assay. Collagen content was calculated by assuming a 1:7.5 OHP-to-collagen mass ratio [33]. The collagen and GAG contents were normalized to the disk wet weight.
Histological analysis
The remaining halves of the constructs were fixed in 4% formalin for 24 h, embedded in paraffin, and processed using standard histological procedures. The histological sections (8 μm thick) were stained for targets of interest using the Vectastain ABC kit and the DAB Substrate kit for peroxidase (Vector Labs). Briefly, sections were predigested in 0.5 mg/ml hyaluronidase for 30 min at 37°C and incubated in 0.5 N acetic acid for 4 h at 4°C to swell the samples prior to overnight incubation with primary antibodies at dilutions of 1:100, 1:200, and 1:3 for chondroitin sulfate (mouse monoclonal anti-chondroitin sulfate, Sigma), and type I (mouse monoclonal anti-collagen type 1, Sigma) and type II collagen antibodies (mouse monoclonal anti-collagen type II, Developmental Studies Hybridoma Bank), respectively. Non-immune controls underwent the same procedure without primary antibody incubation.
Statistical analysis
All data are presented as mean ± standard deviation. Statistica (Statsoft, Tulsa, OK) was used to perform statistical analyses using two-way ANOVA and the Tukey HSD post hoc test of the means (n= 4 samples per group) with culture duration and experimental groups as independent factors.
Results
Microsphere fabrication and release behavior
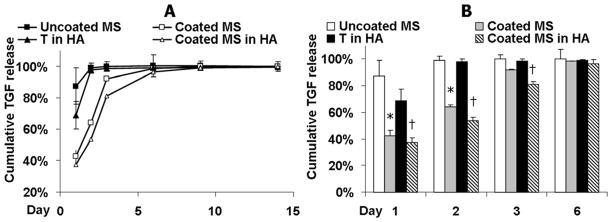
Alginate microspheres were fabricated using an emulsion process followed by coating. The coating process did not significantly change the size of the alginate microspheres, which had a mean diameter of 38.9±9.2 μm. Alginate microspheres with nanofilm coating exhibited improved retention of the encapsulated BSA-FITC molecules compared to the uncoated bare microspheres (Figure 1C). The encapsulation efficiency of TGF-β3 in the uncoated and coated microspheres was 79.6±5.6% and 85.4±9.5%, respectively. Furthermore, when TGF-β3 was encapsulated, the nanofilm coating reduced the initial burst release of the TGF-β3 from alginate microspheres in the first 2 days (Coated MS vs. Uncoated MS, Figure 2A, B). When TGF-β3 was directly encapsulated in acellular 1.5% (w/v) HA hydrogels without alginate microspheres, 68% of the total amount of TGF-β3 encapsulated was released after 1 day and the release profile plateaued after day 2 (T in HA, Figure 2A,B). By encapsulating coated alginate microspheres loaded with TGF-β3 into the HA hydrogel, the lowest initial burst release of 37% after 1 day was achieved and the TGF-β3 released remained lower than the “T in HA” group up to day 3 (Figure 2A,B).
Figure 2.
Release profile over 14 days (A) and 6 days (B) of TGF-β3 encapsulated in bare microspheres (Uncoated MS); coated microspheres (Coated MS); coated microspheres and further photoencapsulated in HA hydrogel (Coated MS in HA) and TGF-β3 directly encapsulated in HA hydrogel (T in HA). Cumulative release is calculated based on the total amount of TGF-β3 detected after 28 days. *p<0.05 vs. “Uncoated MS” at the same timepoints, †p<0.05 vs. “T in HA” (n=3).
In vitro neocartilage formation
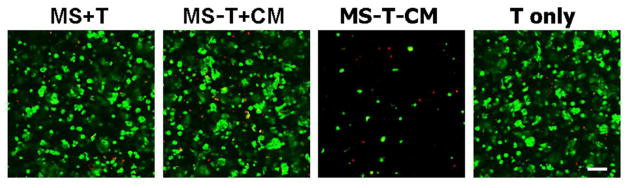
To evaluate the efficacy of released TGF-β3 in promoting chondrogenesis, human MSCs in HA hydrogels were assessed through day 28. Cells in all groups that received TGF-β3 remained nearly completely viable (MS+T, MS-T+CM and T only). However, there was a significant reduction in the number of viable cells (~ 90% decrease compared to “MS-T+CM” as estimated using Image J) in the absence of TGF-β3 in medium on day 28 (MS-T-CM, Figure 3).
Figure 3.
Viability staining of MSCs in HA hydrogel constructs after 28 days of in vitro culture for groups containing microspheres with TGF-β3 (MS+T), containing empty microspheres either in chondrogenic media (MS-T+CM) or in standard growth media (MS-T-CM), or with TGFβ-3 alone (T only). Green: live cells; Red: dead cells; scale bar = 100 μm.
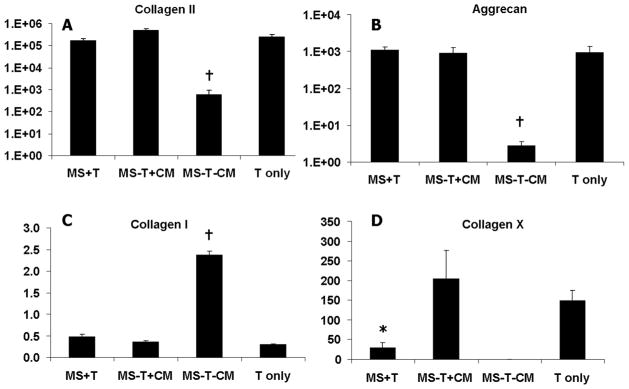
On day 7, all groups supplemented with TGF-β3 in the medium (MS-T+CM), in the HA hydrogel (T in only), or via microspheres (MS+T) exhibited similar degrees of upregulation in type II collagen expression. The expression of type I collagen remained unchanged from that of the pre-encapsulation MSCs in the presence of TGF-β3 (Figure 4). The “negative control” group (MS-T-CM), which received no TGF-β3 supplementation in medium, showed significantly lower collagen II and aggrecan expression and much higher collagen I expression compared to all other groups (Figure 4). Interestingly, the groups that were chondrogenically induced with a single initial supplementation of TGF-β3 loaded in alginate microspheres (MS+T) had lower collagen X expression than the “MS-T+CM” group which received a continuous supply of TGF-β3 in medium.
Figure 4.
Gene expression (in fold changes, first internally normalized to GAPDH and then normalized to monolayer cells prior to encapsulation) of selected chondrogenic and hypertrophic markers in MSC-laden HA hydrogel constructs after 7 days of in vitro culture. *p<0.05 vs. MS-T+CM at the same culture time; †p<0.05 vs. all other groups at the same culture time (n=4).
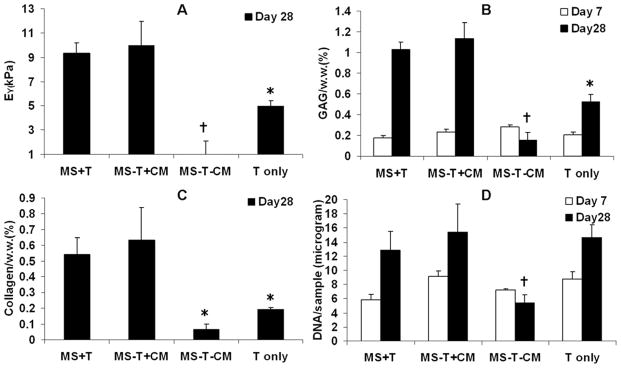
Tissue engineered cartilage disks that received a single initial dosage of TGF-β3 encapsulated in nanofilm coated microspheres (MS+T) developed similar levels of mechanical stiffness, collagen and GAG content compared to the “positive control” (MS-T+CM) which received continuous TGF-β3 supplementation in medium, whereas these values for the group containing directly encapsulated TGF-β3 without microspheres (T only) were all lower than the “MS-T+CM” group (Figure 5A-C). The “negative control” group (MS-T-CM) showed minimal cartilage matrix production and decreasing cell viability, as indicated by viability staining (Figure 3) and DNA content in the absence of TGF-β3 (Figure 5A–D).
Figure 5.
Young’s modulus (A), total collagen content normalized by sample wet weight (B), GAG content normalized by sample wet weight (C) and DNA content per sample (D) of MSC-laden HA hydrogel constructs after 7 or 28 days of in vitro culture (Young’s modulus and collagen content were not evaluated on day 7 due to low values). *p<0.05 vs. MS-T+CM at the same culture time; †p<0.05 vs. all other groups at the same culture time (n=4).
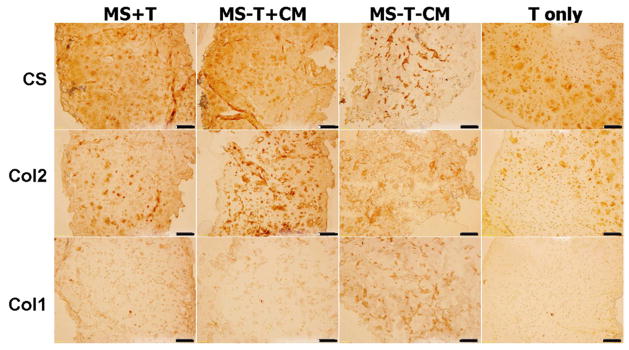
Immunohistochemical staining for type II collagen and chondroitin sulfate showed intense and distributed staining in all groups supplemented with TGF-β3 either in medium or via microspheres (MS-T+CM, T only, MS+T) on day 28, whereas the staining for type I collagen was low (Figure 6). There was only isolated matrix staining present in the negative control group (MS-T-CM) (Figure 6).
Figure 6.
Immunohistochemical staining for chondroitin sulfate (CS), type II (Col 2) and type I (Col 1) collagen on paraffin sections of MSC-laden HA hydrogel constructs after 28 days of in vitro culture, scale bar = 100 μm.
In vivo neocartilage formation
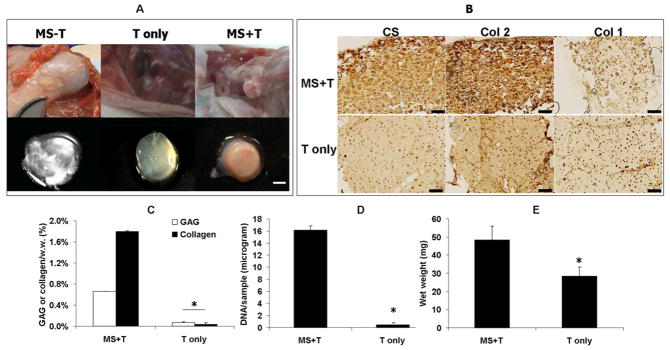
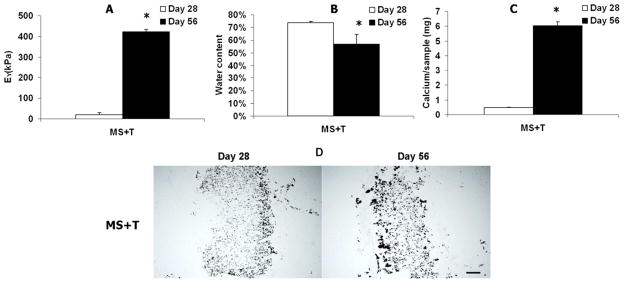
To test the in vivo efficacy of this delivery system, HA hydrogel disks were implanted in subcutaneous pockets of nude mice. After 56 days, out of 8 samples implanted per group, 1, 3 and 5 samples were recovered from the “MS-T”, “T only” and “MS+T” groups, respectively. The implants that contained coated alginate microspheres loaded with TGF-β3 (MS+T) were opaque and white and were palpably stiffer than implants that were loaded with TGF-β3 directly without alginate microspheres (T only) (which were translucent and soft) (Figure 7A). Only one intact implant was recovered from the group that did not have TGF-β3 encapsulated (MS-T). The implants recovered from this group were nearly transparent and very soft (Figure 7A). Furthermore, the “MS+T” groups developed significantly higher GAG, Collagen and DNA contents and implant wet weights compared to the “T only” group after 56 days of implantation (Figure 7B–D). The “MS+T” group had intense and distributed staining for type II collagen and chondroitin sulfate but faint staining for type I collagen (Figure 7E). In comparison, the staining of these markers in the “T only” group was faint and mostly localized in the pericellular region (Figure 7E). However, the “MS+T” group exhibited a significant increase in mechanical stiffness and calcium content accompanied by a decrease in water content of the implanted constructs (Figure 8AC). Von Kossa staining indicated extensive calcification in the “MS+T” constructs (Figure 8D).
Figure 7.
Harvested MSC-laden HA hydrogel implants after 56 days of subcutaneous implantation in nude mice. Out of 8 samples implanted per group, 1, 3 and 5 samples were recovered from the “MS-T”, “T only” and “MS+T” groups, respectively, scale bar = 2 mm (A). Immunohistochemical staining for chondroitin sulfate (CS), type II (Col 2) and type I (Col 1) collagen on paraffin sections of MSC-laden HA hydrogel constructs after 56 days of implantation, scale bar = 200 μm (B). GAG and total collagen content (C), DNA content (D) and wet weight (E) of the “T only” and “MS+T” group after 56 days of implantation. (“MS-T” was not presented due to limited number of samples recovered, *p<0.05 vs. “T only” (n=3~5)). .
Figure 8.
Young’s modulus (A), water content (B) and calcium content (C) of the implanted HA hydrogel constructs containing TGF-β3-loaded microspheres (MS+T) after 28 or 56 days of subcutaneous implantation in nude mice, *p<0.05 vs. Day 28 (n=4)). Von Kossa staining of the implanted HA constructs (MS+T), bar = 500 μm (D).
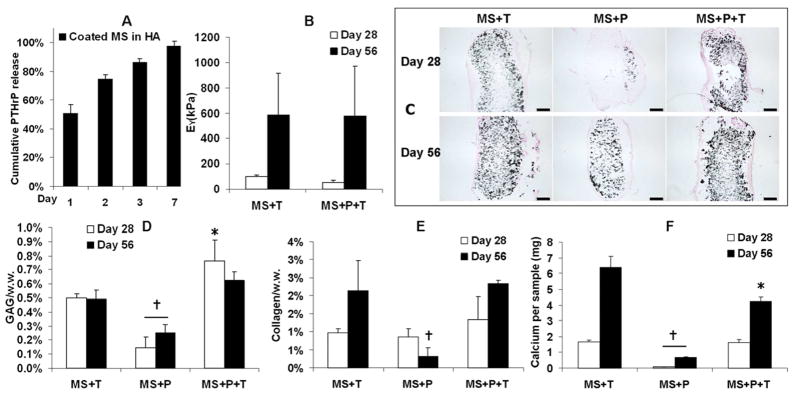
In a follow-up in vivo study the impact of PTHrP inclusion was examined after 56 days of implantation. In vitro release profile showed significant burst release of PTHrP out of alginate microspheres in the HA hydrogels (Figure 9A). Disks containing alginate microspheres loaded with only TGF-β3 (“MS+T”) or both TGF-β3 and PTHrP (“MS+P+T”) exhibited a dramatic increase in mechanical stiffness from day 28 (Figure 9B). The disks containing only PTHrP (“MS+P”) were translucent and soft with non-detectable mechanical stiffness (not shown). However, Von Kossa staining indicated extensive calcification in both “MS+T” and “MS+P+T” constructs on both day 28 and day 56 (Figure 9C). While cartilage matrix content of the “MS+P” group remained low, the “MS+P+T” group developed slightly higher GAG content and similar total collagen content compared to the “MS+T” group (Figure 9D,E). Importantly, calcium content of the “MS+P+T” group was moderately reduced compared to the “MS+T” group on day 56 (Figure 9F). The “MS+P” group had significantly less calcium content and Von Kossa staining than the other two groups (Figure 9C,F).
Figure 9.
Release profile of PTHrP encapsulated in coated microspheres and further photoencapsulated in HA hydrogel (Coated MS in HA) (A). Cumulative release is calculated based on the total amount of PTHrP detected. Young’s modulus (B), Von Kossa staining (C, bar = 500 μm), GAG content normalized by sample wet weight (D), total collagen content normalized by sample wet weight (E) and calcium content per sample (F) of the implanted MSC-laden HA hydrogel constructs after 28 or 56 days of subcutaneous implantation in nude mice (Young’s modulus of the “MS+P” was not detectable due to softness), *p<0.05 vs. “MS+T, †p<0.05 vs. all other groups at the same culture time (n=4).
Discussion
HA scaffolds are finding application as cell carriers in the repair of cartilage defects. Recent in vitro studies demonstrated successful chondrogenesis of MSCs photoencapsulated in HA hydrogels formed through the crosslinking of HA modified with methacrylate groups [8, 9, 34]. To improve the translation of these materials to in vivo applications, it is of great importance to develop a delivery vehicle capable of controlled local TGF-β3 release for the induction of MSC chondrogenesis. This is because applications of cartilage tissue engineering and regeneration using MSCs may be simplified by eliminating the step of in vitro culture of tissue engineered cartilage prior to implantation by directly implanting MSCs into defects. Several previous studies have showed that TGF-β1 delivered via microspheres enhanced articular chondrocytes proliferation and cartilage matrix deposition in hydrogel scaffolds in vitro [35, 36]. Recent studies also suggested that TGF-loaded microspheres embedded in hydrogel scaffold resulted in significant upregulation of chondrogenic gene expression by MSCs in vitro [37, 38]. Another recent study demonstrated that TGF-β3 delivered in collagen gel enhanced regeneration of articular cartilage in rabbit humeral joints [39]. In this study we demonstrated that an early dose of TGF-β3 delivered via a composite carrier consisting of nanofilm-coated alginate microspheres and HA hydrogel was sufficient to support human MSC chondrogenesis and neocartilage formation. This sustained delivery of TGF-β3 resulted in enhanced expression of chondrogenic genes and higher levels of cartilage specific matrix deposition by human MSCs in HA hydrogels compared to uncoated microspheres or a bolus encapsulation of TGF-β3 in vitro. The following in vivo study further showed that this TGF-β3 delivery is essential to neocartilage formation in vivo compared to a bolus encapsulation of TGF-β3.
Alginate gels have a wide pore size distribution due to the open lattice structure of the matrix [40], resulting in enzyme release from these matrices. Low molecular weight encapsulants (<20 kDa) diffuse freely in and out of the beads, and this diffusion from the alginate beads was found to be unaffected by the concentration of alginate or CaCl2 [41, 42]. The homodimeric recombinant human TGF-β3 has a predicted molecular mass of approximately 25 kDa. In this study most of the TGF-β3 encapsulated diffused out of bare alginate microspheres quickly within 2 days. The ionic nature of the molecule, i.e., cationic, anionic, and nonionic also influences the release behavior of drugs from alginate beads [43]. Recombinant human TGF-β3 used in this study is anionic at pH 7.2 (pI = 6.1) and is expected to be repelled by the negatively charged alginate matrix, resulting in faster release. Therefore, a self-assembled bilayer nanofilm coating made of polyelectrolytes of opposing charges was used to reduce the initial burst release of TGF-β3 from the alginate microspheres. One advantage of this nanofilm coating as a diffusion barrier is that its permeability can be tuned by adjusting the number of bilayers [28]. In this study, one bilayer of coating was sufficient to extend the release of TGF-β3 from alginate microspheres to a timeframe of around 4–5 days that is deemed necessary for MSC chondrogenesis. Multiple bilayers of coating could be used in future studies to further slow down the release of encapsulated molecules that are needed at later times.
It has been shown that compared with continuous supplementation, an initial transient exposure of TGF-β3 for 2 weeks enhanced the maturation of tissue engineered cartilage seeded with bovine chondrocytes and MSCs in vitro [29, 44]. From a molecular biology perspective, though TGF-β3 may be imperative for initiation of upregulation of sox9 expression (a crucial chondrogenic gene) it is not required for continued expression of chondrogenic markers such as type II collagen at later stages of chondrogenesis [45, 46]. Our recent preliminary studies also showed that while a brief exposure to TGF-β3 for less than 2 days is also less than optimal, supply of a high dosage of TGF-β3 (100 ng/ml) only in the first week of in vitro culture led to a similar amount of cartilage matrix accumulation in human MSC-seeded hydrogel constructs by week 9 compared to constructs receiving continuous TGF-β3 supplementation of regular dosage (10 ng/ml) (unpublished data). In this study, when TGF-β3 was encapsulated in the coated microspheres first and then encapsulated in the HA hydrogels (Coated MS in HA) the retention of TGF-β3 in the hydrogel was significantly improved compared to direct encapsulation of TGF-β3 in HA hydrogels (T in HA). When an identical amount of TGF-β3 was delivery either via coated microspheres (MS+T) or direct encapsulation without using microspheres (T only) into the MSC-laden HA hydrogel constructs, microsphere delivery resulted in significantly higher cartilage matrix content than direct encapsulation under in vitro or in vivo condition. This finding indicated that extending the exposure of TGF-β3 to about 6 days as seen in the “MS+T” group is essential to neocartilage formation by MSCs under both in vitro and in vivo conditions.
In this study, significant calcification was observed in the HA hydrogel constructs after 8 weeks of subcutaneous implantation in nude mice with only an initial administration of TGF-β3 via alginate microspheres. Co-delivering PTHrP with TGF-β3 using alginate microspheres resulted in only a modest reduction in calcium content on day 56 and failed to prevent extensive mineralization. This is likely due to the quick release of PTHrP, which has a smaller molecular weight (9.8 kD) than TGF-β3 (25 kD), within the first week similar to the release of TGF-β3 (Figure 9A). The effect of PTHrP on MSC hypertrophy inhibition in vivo might be optimized if its release from alginate microspheres can be delayed to the second week of implantation when MSCs begin to express hypertrophic markers [19]. This can be achieved by adding additional layers of nanofilm coatings, which has been shown to further slow down the release of encapsulated molecules compared to a single layer of coating [28]. Future studies should be focused on developing strategies to stabilize the chondrogenic phenotype of MSCs in vivo. One potential direction is to develop controlled release vehicles capable of delayed delivery of hypertrophy inhibiting molecules. It was also noted that though no blood vessels were found inside the implanted hydrogels, they were usually covered by a highly vascularized fibrous capsule (Figure 7A). It has been reported that hypertrophic chondrocytes secret vascular endothelial growth factor (VEGF) and vascularization is closely involved in the replacement of cartilage by bone during endochondral ossification [47]. A recent study employed suramin, an anti-angiogenic agent, to suppress hypertrophy of engineered cartilage seeded with periosteal cells in vivo [48]. Therefore, molecules capable of suppressing vascularization can also be incorporated in the alginate microspheres to prevent mineralization of MSCs ectopically. In addition, other therapeutic molecules involved in skeletal tissue remodeling can also be explored for potential application in regulating MSC hypertrophy. A recent study showed that pretreatment of MSCs with retinoid agonists substantially inhibited intramuscular and subcutaneous heterotopic ossification irreversibly after implantation in mice [49]. Lastly, previous studies demonstrated that articular chondrocytes secrete hypertrophy inhibition molecules such as PTHrP, FGF-2 and TGF-βs [26, 45]. A previous study by our group also showed that coculture of MSCs and a small fraction of articular chondrocytes in HA hydrogels not only enhanced chondrogenesis of MSCs but also reduced the expression of hypertrophic markers by MSCs [50]. Therefore, another potential strategy is to seed the MSC-laden constructs with articular chondrocytes that will serve as “living” drug delivery vehicles to supply hypertrophy regulating molecules continuously.
Conclusions
Our findings demonstrated that a single administration of TGF-β3 in nanofilm coated alginate microspheres encapsulated in HA hydrogels promoted chondrogenesis of encapsulated MSCs and resulted in superior neocartilage formation compared to a bolus direct encapsulation of TGF-β3 under both in vitro and in vivo conditions. This composite TGF-β3 delivery system will potentially simplify the proposed autologous MSC therapy for cartilage repair substantially by eliminating the step of in vitro culture, allowing direct implantation of MSCs into defect sites. However, future effort is needed to stabilize the chondrogenic phenotype and arrest hypertrophic differentiation of the implanted MSCs.
Acknowledgments
This work was supported by National Institutes of Health grant R01EB008722.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Erickson IE, Huang AH, Chung C, Li RT, Burdick JA, Mauck RL. Differential maturation and structure-function relationships in mesenchymal stem cell- and chondrocyte-seeded hydrogels. Tissue Eng Part A. 2009;15(5):1041–1052. doi: 10.1089/ten.tea.2008.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams CG, Kim TK, Taboas A, Malik A, Manson P, Elisseeff J. In vitro chondrogenesis of bone marrow-derived mesenchymal stem cells in a photopolymerizing hydrogel. Tissue Eng. 2003;9(4):679–688. doi: 10.1089/107632703768247377. [DOI] [PubMed] [Google Scholar]
- 3.Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238(1):265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 4.Tuli R, Tuli S, Nandi S, Huang X, Manner PA, Hozack WJ, et al. Transforming growth factor-beta-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogen-activated protein kinase and Wnt signaling cross-talk. J Biol Chem. 2003;278(42):41227–41236. doi: 10.1074/jbc.M305312200. [DOI] [PubMed] [Google Scholar]
- 5.Mello MA, Tuan RS. Effects of TGF-beta1 and triiodothyronine on cartilage maturation: in vitro analysis using long-term high-density micromass cultures of chick embryonic limb mesenchymal cells. J Orthop Res. 2006;24(11):2095–2105. doi: 10.1002/jor.20233. [DOI] [PubMed] [Google Scholar]
- 6.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113(6):685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 7.Buxton AN, Bahney CS, Yoo JU, Johnstone B. Temporal exposure to chondrogenic factors modulates human mesenchymal stem cell chondrogenesis in hydrogels. Tissue Eng Part A. 2010;17(3–4):371–380. doi: 10.1089/ten.tea.2009.0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung C, Burdick JA. Influence of three-dimensional hyaluronic acid microenvironments on mesenchymal stem cell chondrogenesis. Tissue Eng Part A. 2009;15(2):243–254. doi: 10.1089/ten.tea.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung C, Beecham M, Mauck RL, Burdick JA. The influence of degradation characteristics of hyaluronic acid hydrogels on in vitro neocartilage formation by mesenchymal stem cells. Biomaterials. 2009;30(26):4287–4296. doi: 10.1016/j.biomaterials.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parajo Y, D’Angelo I, Welle A, Garcia-Fuentes M, Alonso MJ. Hyaluronic acid/chitosan nanoparticles as delivery vehicles for VEGF and PDGF-BB. Drug Deliv. 2010;17(8):596–604. doi: 10.3109/10717544.2010.509357. [DOI] [PubMed] [Google Scholar]
- 11.Demirdogen B, Elcin AE, Elcin YM. Neovascularization by bFGF releasing hyaluronic acid-gelatin microspheres: in vitro and in vivo studies. Growth Factors. 2010;28(6):426–436. doi: 10.3109/08977194.2010.508456. [DOI] [PubMed] [Google Scholar]
- 12.Liu XW, Hu J, Man C, Zhang B, Ma YQ, Zhu SS. Insulin-like growth factor-1 suspended in hyaluronan improves cartilage and subchondral cancellous bone repair in osteoarthritis of temporomandibular joint. Int J Oral Maxillofac Surg. 2010;40(2):184–190. doi: 10.1016/j.ijom.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Silva CM, Ribeiro AJ, Ferreira D, Veiga F. Insulin encapsulation in reinforced alginate microspheres prepared by internal gelation. Eur J Pharm Sci. 2006;29(2):148–159. doi: 10.1016/j.ejps.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 14.van de Weert M, Hennink WE, Jiskoot W. Protein instability in poly(lactic-co-glycolic acid) microparticles. Pharm Res. 2000;17(10):1159–1167. doi: 10.1023/a:1026498209874. [DOI] [PubMed] [Google Scholar]
- 15.Jay SM, Saltzman WM. Controlled delivery of VEGF via modulation of alginate microparticle ionic crosslinking. J Control Release. 2009;134(1):26–34. doi: 10.1016/j.jconrel.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strand BL, Gaserod O, Kulseng B, Espevik T, Skjak-Baek G. Alginate-polylysine-alginate microcapsules: effect of size reduction on capsule properties. J Microencapsul. 2002;19(5):615–630. doi: 10.1080/02652040210144243. [DOI] [PubMed] [Google Scholar]
- 17.Gaserod O, Sannes A, Skjak-Braek G. Microcapsules of alginate-chitosan. II. A study of capsule stability and permeability. Biomaterials. 1999;20(8):773–783. doi: 10.1016/s0142-9612(98)00230-0. [DOI] [PubMed] [Google Scholar]
- 18.Amsden B, Turner N. Diffusion characteristics of calcium alginate gels. Biotechnol Bioeng. 1999;65(5):605–610. doi: 10.1002/(sici)1097-0290(19991205)65:5<605::aid-bit14>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 19.Pelttari K, Winter A, Steck E, Goetzke K, Hennig T, Ochs BG, et al. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006;54(10):3254–3266. doi: 10.1002/art.22136. [DOI] [PubMed] [Google Scholar]
- 20.Scotti C, Tonnarelli B, Papadimitropoulos A, Scherberich A, Schaeren S, Schauerte A, et al. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc Natl Acad Sci U S A. 2010;107(16):7251–7256. doi: 10.1073/pnas.1000302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo X, Park H, Young S, Kretlow JD, van den Beucken JJ, Baggett LS, et al. Repair of osteochondral defects with biodegradable hydrogel composites encapsulating marrow mesenchymal stem cells in a rabbit model. Acta Biomater. 2010;6(1):39–47. doi: 10.1016/j.actbio.2009.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim YJ, Kim HJ, Im GI. PTHrP promotes chondrogenesis and suppresses hypertrophy from both bone marrow-derived and adipose tissue-derived MSCs. Biochem Biophys Res Commun. 2008;373(1):104–108. doi: 10.1016/j.bbrc.2008.05.183. [DOI] [PubMed] [Google Scholar]
- 23.Weiss S, Hennig T, Bock R, Steck E, Richter W. Impact of growth factors and PTHrP on early and late chondrogenic differentiation of human mesenchymal stem cells. J Cell Physiol. 2010;223(1):84–93. doi: 10.1002/jcp.22013. [DOI] [PubMed] [Google Scholar]
- 24.Zhang M, Xie R, Hou W, Wang B, Shen R, Wang X, et al. PTHrP prevents chondrocyte premature hypertrophy by inducing cyclin-D1-dependent Runx2 and Runx3 phosphorylation, ubiquitylation and proteasomal degradation. J Cell Sci. 2009;122(Pt 9):1382–1389. doi: 10.1242/jcs.040709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo J, Chung UI, Yang D, Karsenty G, Bringhurst FR, Kronenberg HM. PTH/PTHrP receptor delays chondrocyte hypertrophy via both Runx2-dependent and -independent pathways. Dev Biol. 2006;292(1):116–128. doi: 10.1016/j.ydbio.2005.12.044. [DOI] [PubMed] [Google Scholar]
- 26.Fischer J, Dickhut A, Rickert M, Richter W. Human articular chondrocytes secrete parathyroid hormone–related protein and inhibit hypertrophy of mesenchymal stem cells in coculture during chondrogenesis. Arthritis Rheum. 2010;62(9):2696–2706. doi: 10.1002/art.27565. [DOI] [PubMed] [Google Scholar]
- 27.Smeds KA, Pfister-Serres A, Miki D, Dastgheib K, Inoue M, Hatchell DL, et al. Photocrosslinkable polysaccharides for in situ hydrogel formation. J Biomed Mater Res. 2001;54(1):115–121. doi: 10.1002/1097-4636(200101)54:1<115::aid-jbm14>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 28.Srivastava R, Brown JQ, Zhu H, McShane MJ. Stable encapsulation of active enzyme by application of multilayer nanofilm coatings to alginate microspheres. Macromol Biosci. 2005;5(8):717–727. doi: 10.1002/mabi.200500061. [DOI] [PubMed] [Google Scholar]
- 29.Byers BA, Mauck RL, Chiang IE, Tuan RS. Transient exposure to transforming growth factor beta 3 under serum-free conditions enhances the biomechanical and biochemical maturation of tissue-engineered cartilage. Tissue Eng Part A. 2008;14(11):1821–1834. doi: 10.1089/ten.tea.2007.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mauck RL, Soltz MA, Wang CC-B, Wong DD, Chao P-HG, Valhmu WB, et al. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122:252–260. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 31.McGowan KB, Kurtis MS, Lottman LM, Watson D, Sah RL. Biochemical quantification of DNA in human articular and septal cartilage using PicoGreen and Hoechst 33258. Osteoarthritis Cartilage. 2002;10(7):580–587. doi: 10.1053/joca.2002.0794. [DOI] [PubMed] [Google Scholar]
- 32.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883(2):173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 33.Hollander AP, Heathfield TF, Webber C, Iwata Y, Bourne R, Rorabeck C, et al. Increased damage to type II collagen in osteoarthritic articular cartilage detected by a new immunoassay. J Clin Invest. 1994;93(4):1722–1732. doi: 10.1172/JCI117156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erickson IE, Huang AH, Sengupta S, Kestle S, Burdick JA, Mauck RL. Macromer density influences mesenchymal stem cell chondrogenesis and maturation in photocrosslinked hyaluronic acid hydrogels. Osteoarthritis Cartilage. 2009;17(12):1639–1648. doi: 10.1016/j.joca.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elisseeff J, McIntosh W, Fu K, Blunk BT, Langer R. Controlled-release of IGF-I and TGF-beta1 in a photopolymerizing hydrogel for cartilage tissue engineering. J Orthop Res. 2001;19(6):1098–1104. doi: 10.1016/S0736-0266(01)00054-7. [DOI] [PubMed] [Google Scholar]
- 36.Park H, Temenoff JS, Holland TA, Tabata Y, Mikos AG. Delivery of TGF-beta1 and chondrocytes via injectable, biodegradable hydrogels for cartilage tissue engineering applications. Biomaterials. 2005;26(34):7095–7103. doi: 10.1016/j.biomaterials.2005.05.083. [DOI] [PubMed] [Google Scholar]
- 37.Park H, Temenoff JS, Tabata Y, Caplan AI, Raphael RM, Jansen JA, et al. Effect of dual growth factor delivery on chondrogenic differentiation of rabbit marrow mesenchymal stem cells encapsulated in injectable hydrogel composites. J Biomed Mater Res A. 2009;88(4):889–897. doi: 10.1002/jbm.a.31948. [DOI] [PubMed] [Google Scholar]
- 38.Bouffi C, Thomas O, Bony C, Giteau A, Venier-Julienne MC, Jorgensen C, et al. The role of pharmacologically active microcarriers releasing TGF-beta3 in cartilage formation in vivo by mesenchymal stem cells. Biomaterials. 2010;31(25):6485–6493. doi: 10.1016/j.biomaterials.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 39.Lee CH, Cook JL, Mendelson A, Moioli EK, Yao H, Mao JJ. Regeneration of the articular surface of the rabbit synovial joint by cell homing: a proof of concept study. Lancet. 2010;376(9739):440–448. doi: 10.1016/S0140-6736(10)60668-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DeGroot AR, Neufeld RJ. Encapsulation of urease in alginate beads and protection from alpha-chymotrypsin with chitosan membranes. Enzyme Microb Technol. 2001;29(6–7):321–327. [Google Scholar]
- 41.Scott CD, Woodward CA, Thompson JE. Solute diffusion in biocatalyst gel beads containing biocatalysis and other additives. Enzyme Microb Technol. 1989;11(5):258–263. [Google Scholar]
- 42.Tanaka H, Matsumura M, Veliky IA. Diffusion characteristics of substrates in Ca-alginate gel beads. Biotechnol Bioeng. 1984;26(1):53–58. doi: 10.1002/bit.260260111. [DOI] [PubMed] [Google Scholar]
- 43.You JO, Park SB, Park HY, Haam S, Chung CH, Kim WS. Preparation of regular sized Ca-alginate microspheres using membrane emulsification method. J Microencapsul. 2001;18(4):521–532. doi: 10.1080/02652040010018128. [DOI] [PubMed] [Google Scholar]
- 44.Huang AH, Stein A, Tuan RS, Mauck RL. Transient exposure to transforming growth factor beta 3 improves the mechanical properties of mesenchymal stem cell-laden cartilage constructs in a density-dependent manner. Tissue Eng Part A. 2009;15(11):3461–3472. doi: 10.1089/ten.tea.2009.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahmed N, Dreier R, Gopferich A, Grifka J, Grassel S. Soluble signalling factors derived from differentiated cartilage tissue affect chondrogenic differentiation of rat adult marrow stromal cells. Cell Physiol Biochem. 2007;20(5):665–678. doi: 10.1159/000107728. [DOI] [PubMed] [Google Scholar]
- 46.Furumatsu T, Tsuda M, Taniguchi N, Tajima Y, Asahara H. Smad3 induces chondrogenesis through the activation of SOX9 via CREB-binding protein/p300 recruitment. J Biol Chem. 2005;280(9):8343–8350. doi: 10.1074/jbc.M413913200. [DOI] [PubMed] [Google Scholar]
- 47.Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5(6):623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- 48.Emans PJ, van Rhijn LW, Welting TJ, Cremers A, Wijnands N, Spaapen F, et al. Autologous engineering of cartilage. Proc Natl Acad Sci U S A. 2010;107(8):3418–3423. doi: 10.1073/pnas.0907774107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shimono K, Tung WE, Macolino C, Chi AH, Didizian JH, Mundy C, et al. Potent inhibition of heterotopic ossification by nuclear retinoic acid receptor-gamma agonists. Nat Med. 2011;17(4):454–460. doi: 10.1038/nm.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bian L, Zhai DY, Mauck RL, Burdick JA. Coculture of human mesenchymal stem cells and articular chondrocytes reduces hypertrophy and enhances functional properties of engineered cartilage. Tissue Eng Part A. 2011;17(7–8):1137–1145. doi: 10.1089/ten.tea.2010.0531. [DOI] [PMC free article] [PubMed] [Google Scholar]