Abstract
The neonatal Fc receptor (FcRn) is responsible for transporting maternal IgGs to fetus/newborns and maintaining the homeostasis of IgGs in adults. FcRn resembles class I major histocompatibility complex in structure, and is composed of a transmembrane heavy chain and an invariant beta 2 microglobulin. Changes in the affinity of IgGs to FcRn lead to changes in the half-life of engineered IgGs and Fc fusion proteins. Longer half-life of therapeutic antibodies means lower dose and longer interval between administering. For some diagnostic agents including imaging or radio-labelled agents a shorter half life in circulation results in lower non-specific binding and decreased side effects. Therefore, studying the interaction of FcRn and therapeutic antibodies has direct clinical implications. A reliable method to prepare soluble and functional FcRn protein is essential for such studies. In this study, we describe a new method to express in mammalian cells soluble human FcRn (sFcRn) as a single-chain soluble fusion protein. The highly hydrophilic beta 2 microglobulin was joined with the hydrophobic heavy chain via a 15 amino acid linker. The single-chain fusion protein format not only improved the expression level of the heavy chain but also simplified the purification process. The sFcRn maintained its pH-dependent binding to IgG. This method typically yielded ~1 mg/100 ml culture without optimization, and is easy to scale up for production of large quantities.
Keywords: neonatal Fc receptor, FcRn, IgG half-life
Introduction
The neonatal Fc receptor (FcRn) is critically important for the regulation of the pharmacokinetics of antibodies and albumin in vivo. Similar to the class I major histocompatibility complex (MHC I) molecules, FcRn protein is composed of a ~50 kDa transmembrane alpha chain (or heavy chain) and a ~14 kDa beta 2 microglobulin (B2M) noncovalently linked at the 1:1 molar ratio. The B2M chain is also the light chain for MHC I molecules. FcRn was first recognized as the receptor that transports maternal IgGs from milk to the young across the infant intestinal epithelial cells, hence the name neonatal Fc receptor. FcRn also delivers IgGs across the materno-fetal barrier during the gestation [1]. FcRn is responsible for regulating the IgG homeostasis in humans and animals through its unique pH-dependent association with IgG. FcRn binds to the Fc portion of IgG at the acidic pH 6.0–6.5, but the binding is weak at the physiological pH 7.0–7.4 [2]. This binding feature allows FcRn to bind to IgG in endosomes and thus protect IgG from degradation, the IgG is then released into extracellular matrix where pH is neutral [3–5]. Through this repeated sequestering-releasing cycle FcRn protects IgGs from catabolism. In mice with B2M gene deleted, IgGs have six-fold shorter half-lives in circulation and the mice exhibit ten-fold lower endogenous IgG levels [6]. The function of FcRn is conserved in many species studied, including human [7], mouse [6, 8, 9], rat [1], cattle [10], and chicken [11].
Currently, there is a great interest in increasing the half-life of marketed therapeutic antibodies and antibodies in development [12]. Better affinity to FcRn at certain ranges means a longer half-life and better tissue bio-distribution for antibodies in non-human primates. Much of work is ongoing on engineering both IgGs and Fc-fusion based therapeutic proteins to improve their affinities to FcRn. FcRn protein is the critical reagent in such attempts, and it is not commercially available. Being able to obtain a large quantity of soluble, functional FcRn with relative ease could save much time and resources.
Currently published/patented methods to produce recombinant human or mouse FcRn can be classified into five categories: (1) in stably transfected mammalian cells [7], (2) in insect cells with a dual promoter baculoviral vector [9], (3) in mammalian cells transfected with two separate vectors [12], (4) in Pichia pastoris [13], (5) in E. coli [14]. The first three methods place the alpha chain and the beta 2 chain sequences either in separate vectors or in a dual-promoter vector for expression in mammalian cells or insect cells. In all three cases, the two chains are expressed separately and the heterodimer is purified away from the monomers [7, 9, 12]. These methods have been widely used in many studies and have played an important role in the advance in our understanding of IgG kinetics and FcRn functions. However, in both mammalian and insect cell expression systems the expression levels of the heavy chain are generally low, whereas the expression of the small beta 2 chain is high, which complicates the downstream purification process and reduces the overall yield of the functional protein. The fourth method is recently reported, and has a similar design as the first three methods but protein is expressed in the Pichia system. The FcRn produced in Pichia is shown not to be glycosylated [13]. The fifth method produces the two chains separately in E. coli and purifies the heavy chain under the denaturing condition; the heavy chain is then refolded in the presence of the beta 2 chain [14]. Although the authors justify the function of recombinant FcRn derived from E. coli, in many cases a method that produces FcRn with correct post-translational modification is preferred, because multiple lines of evidence indicate that the type of carbohydrates on FcRn could affect its stability and interaction with IgGs [4, 15].
We have tried to follow the method of expressing FcRn in two separate mammalian vectors, as well as expressing it in a dual promoter baculoviral vector in insect cells. In both cases we obtained an abundant amount of B2M, but limited amount of the heavy chain protein, which was not enough for our studies. We also tried to express the heavy chain fused with glutathione S-transferase (GST) tag at the C-terminus with mammalian vectors according to the previous report [16]. It is reported that expression of mouse FcRn in insect cells with the dual promoter vector pAcUW51 yields 15 milligrams of protein per liter culture [17]. We do not know the reason of the low expression of the heavy chain by these methods in our hands; it may be explained by the difference in culture conditions among labs. The first method mentioned above developed by P. Pjorkman’s group utilizes a stable CHO cell line with glutamine synthetase gene selection technology, which is owned by Lonza. Unfortunately we were not able to obtain the license from Lonza, which is required to use the stable cell line.
We designed a method by using commercially available reagents for the expression of soluble FcRn in mammalian cells in a single fusion protein format. Our method of producing human FcRn allows the 1:1 molar ratio of the heavy chain and B2M when expressed, and it is fully functional. The method we have designed employs simple one-step purification and is easy to scale up for producing large quantities of highly pure FcRn. The design is based on the generation of a single chain-like fusion protein with a short linker connecting the two components of the FcRn.
Materials and Methods
Constructions of FcRn expression vector
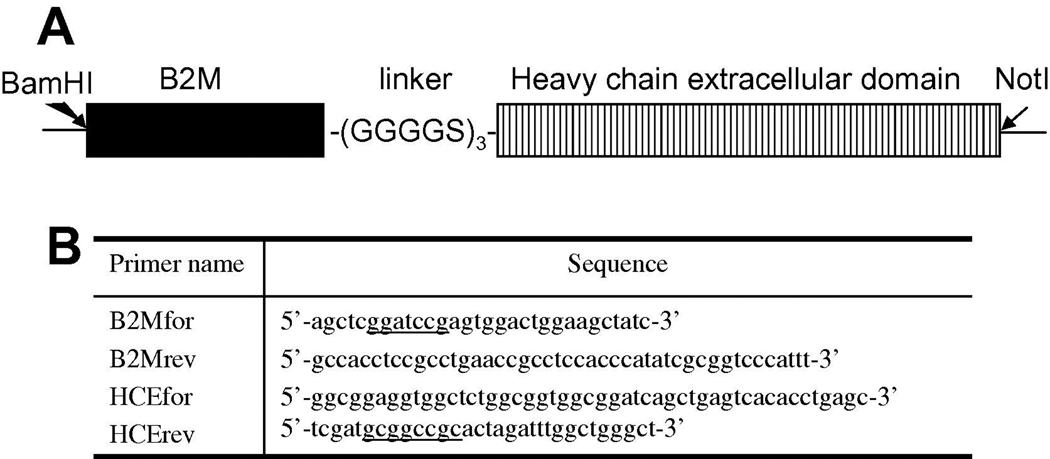
The full-length DNA sequences of the heavy chain and beta 2 microglobulin chain (B2M) of human FcRn were synthesized by GenScript. The sequences are based on reported sequences in Genebank U12255 and NM_004048, and codons were optimized for the expression in mammalian cells. The single fusion FcRn protein expression vector was constructed in pSecTag2A (Invitrogen). Briefly, B2M DNA coding sequence was amplified with primers: B2Mfor and B2Mrev (Figure 1B). The N-terminal sequence for the signal peptide of B2M was not amplified. Similarly, the coding sequence for the heavy chain excluding the signal peptide and transmembrane domain was amplified with primers HCEfor and HCErev. Primer B2Mrev and HCEfor each encodes 2 copies of the linker (GGGGS) and they share a stretch of 12-nucleotide complementary sequence. Therefore, the two PCR products were joined together in an overlapping PCR, and the joined product further amplified in the presence of primers B2Mfor and HCErev. The final PCR product was gel purified and restriction digested with BamHI and NotI. The digested product was purified and ligated with pSecTag2A that has been digested with the same two restriction enzymes. The vector pSecTag2A has Ig kappa chain leader sequence as the secretion signal and this sequence is cleaved off before the recombinant protein is secretion. The expression vector pSecTag2A-FcRn encodes a recombinant protein with mature B2M sequence connected through 3(GGGGS) to the mature sequence of the heavy chain, followed by myc and His6 tags carried over from the vector (Figure 1A).
Figure 1. Schematic diagram of the single fusion protein human sFcRn for expression in mammalian cells.
A, The B2M extracellular domain minus leader peptide is represented by the black bar. The stitched bar stands for the extracellular domain of the heavy chain. The two domains are connected with 3 copies of linker GGGGS sequence. The whole insert was flanked with BamHI and NotI restriction sites, which were used to subclone into pSecTag2A expression vector.
B, Primers for cloning human sFcRn as a single fusion protein into pSecTag2A. Primers B2Mrev and HCEfor have 12 nucleotides complementary to each other, through which the two PCR products were connected in an overlapping PCR. The sequences for restriction sites are underlined.
Expression and purification of sFcRn
The cell line for the expression is 293 FreeStyle cells (293FS) (Invitrogen), engineered from HEK293 cells. 293FS cells were cultured in the serum-free and antibiotics-free medium, FreeStyle 293 Expression Medium (Invitrogen). Cells at a density of 1 million/ml were transfected with pSecTag2A-FcRn DNA with CellFectin reagent (Invitrogen) according to the product instruction. A typical transfection was performed with 100 ml culture, 100 µg plasmid DNA and 120 µl CellFectin; a larger transfection is possible by simply scaling up the volume of reagents. Transfected cells were maintained on an orbital shaker rotating at 125 rpm in a CO2 incubator at 37°C and 8% CO2. Five days after the transfection, the culture medium was collected and filtered. The supernatant was concentrated to ¼ volume, and diafiltered against PBS on a tangential force flow device (TFF) with 8 kDa membrane cassette (Millipore). The concentrated sample was loaded onto a 1 ml Ni-chelating HiTrap column (GE Healthcare) equilibrated with PBS+0.5 M NaCl, and washed with 5 column volumes of 40 mM imidazole in PBS with 0.5 M NaCl, and finally eluted with 200 mM imidazole in PBS with 0.5 M NaCl. This purification step usually gave the product sufficiently pure for most of our studies. A polishing step with Superdex75 column (GE Healthcare) could be added to further purify the protein. Superdex75 10/300 GL (bed volume 23.5 ml) was equilibrated with PBS and the fractions from Ni-chelating column were loaded and eluted with PBS at a flow rate 1 ml/min. The final protein sample was dialyzed against PBS. The purity of sFcRn was examined on a 4–12% NuPAGE (Invitrogen).
Binding of sFcRn to IgG on ELISA assay
A TRAIL receptor 1 (or death receptor DR4) was coated on ELISA plate wells at 50 ng/well in PBS overnight. The protein does not have His tag and functioned as the capture antigen. The wells were blocked with 4% milk/PBS (MPBS) for 1 hour at 37°C. An anti-DR4 fully human IgG (m921) developed in our lab was added to the wells and captured by the antigen (2 hours at 37°C). Negative control wells received BSA or an isotype control IgG in MPBS. All steps after this were performed in two pHs in parallel: pH 6.0 and pH 7.4. The regular PBS at pH 7.4 was used for making MPBS and wash buffer PBST (PBS with 0.05% Tween 20). PBS with pH adjusted to 6.0 with HCl was used to make MPBS6.0 and PBST6.0. MPBS and MPBS6.0 were used to dilute sFcRn and secondary antibody. PBST and PBST6.0 were used for washing. Purified sFcRn was diluted in MPBS or MPBS6.0 and added to wells with m921 captured. The sFcRn was incubated in wells for 2 hours at 37°C. After extensive washes with corresponding wash buffers, anti-His tag mAb conjugated with HRP at 1:1000 was incubated with wells for 1 hour at 37°C. The wells were washed for 4 times with PBST or PBST6.0. The binding of sFcRn to IgG was detected with the addition of substrate ABTS (Roche), and signals were read at 405 nm wavelength. All samples were repeated in quadruplicate wells.
Binding of sFcRn on IgG column
One hundred ml of conditioned medium from post-transfection 5-day 293FS culture was divided into two parts, one was adjusted to pH 6.0 with HCl and the other was adjusted to 7.4. Two mini-columns were packed with hIgG-sepharose (GE Healthcare), and equilibrated with PBS6.0 or PBS, respectively. The two supernatant samples with secreted sFcRn were filtered though 0.45 µm filter, and loaded to the corresponding hIgG column. After all 50 ml sample was loaded, the columns were washed with 5 column volumes of PBS6.0 or PBS, respectively, until the absorbance 280 of the wash was back to the baseline. Finally the bound protein was eluted from the columns with pH 8.0 buffer (50 mM Tris, pH 8.0, 100 mM NaCl). An aliquot of each eluted sample was run on gel and transferred to PVDF membrane for Western blot with anti-His tag mAb.
Surface plasmon resonance analysis
Binding of sFcRn to human IgG and scFv was tested in BiacoreX100 instrument. Purified sFcRn was diluted in 10 mM Na-acetate buffer, pH 5.0, and immobilized on a CM5 sensor chip with amine coupling kit at approximately 1130 response units (RU). The reference flow cell was treated with the amine coupling reagent without exposing to sFcRn. The running buffer was PBS6.0 with 0.005% Tween 20. m921 hIgG, diluted with the running buffer, was let flow through the cells. After 10 minutes of dissociation, the chip was regenerated with pH 8.0 buffer (50 mM Tris, pH 8.0, 100 mM NaCl). m921 was tested at 75, 150 300, 600 and 1200 nM concentrations, followed by a repeat at 300 nM to monitor the regeneration efficiency. A single chain format of m921 was tested at a wider concentration range from 150 to 4800 nM. The sensorgram was analyzed with BiaEvaluation software, and data were fitted to the 1:1 binding model.
Results
Design and expression of sFcRn as a single-chain fusion protein
When we tried to produce recombinant human FcRn protein in mammalian cells and insect cells by separate vectors or dual promoter vector, we found that B2M soluble expression was very high, whereas the heavy chain usually was not efficiently secreted. Even the intracellular amount of the recombinant heavy chain protein was much lower compared with the intracellular amount of B2M. It is possible that the poor secretion of the heavy chain suppressed the synthesis of the protein. Analyzing the primary sequence of the two chains showed that in the heavy chain there are two hydrophobic patches within amino acids 175–184, and amino acids 217–227, while the B2M is overall hydrophilic. We reasoned that if the two subunits are fused as a single protein, the hydrophilic nature of the B2M may help folding and secretion of the heavy chain. It is known that in the MHC I molecules, which share similarities to FcRn, B2M functions not only as a structural component but also as a chaperone-like molecule for MHC I folding [18]. When B2M is deleted in mice, MHC I molecules are not expressed efficiently on cell surface [19]. Because in native FcRn the B2M subunit makes contact with the alpha 3 domain (closer to the C-terminus) of the heavy chain, the B2M domain needs to be flexible rather than rigid in the recombinant protein, so that it will be able to find its association partner alpha 3 domain. We decided to connect B2M (amino acids 16–120) with the heavy chain (amino acids 24–297) through a 15 amino acid-linker, specifically three copies of GGGGS (Figure 1A). This linker lacks secondary structure, and is successfully used for constructing single chain variable fragments of antibodies. The transmembrane domain and the intracellular domain of the heavy chain were not included in this construct. The mammalian expression vector used, pSecTag2A, has Ig kappa chain leader peptide as the signal peptide to direct protein secretion, and we have found this leader peptide to be sufficiently strong to direct the secretion of most proteins. Therefore, the original signal peptides of the two subunits were not included in our design of the recombinant FcRn. The final sFcRn will have additional amino acids on the C-terminus, ARRGGPEQKLISEEDLNSAVDHHHHHH (c-myc tag sequence underlined), which gives a total of 419 amino acids.
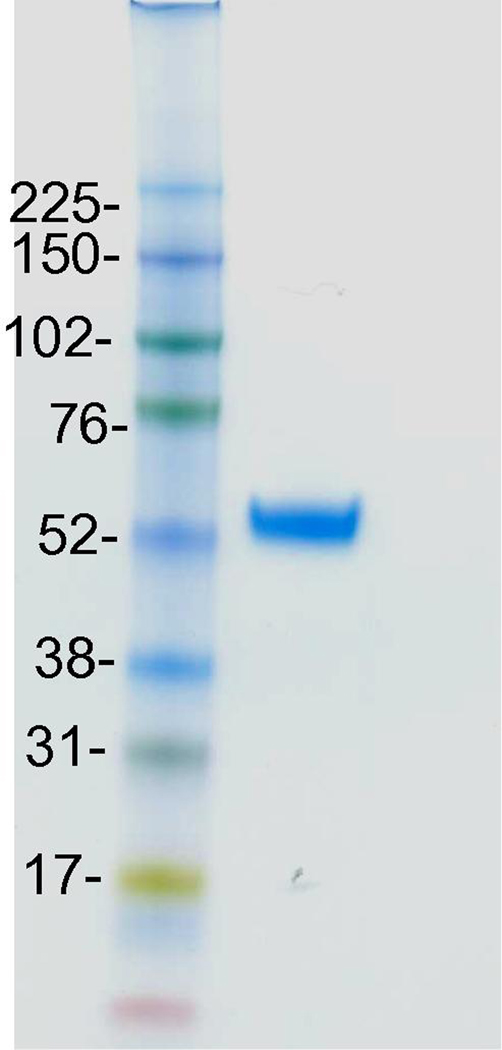
After expression in 293FS culture, and sFcRn purified from the conditioned medium, the final yield of the sFcRn was found to be reasonable, at ~1 mg/100 ml culture (Table 1). The purity of sFcRn was >95%, and sFcRn migrated as a single protein of ~54 kDa on 4–12% NuPAGE (Figure 2), slightly larger than the calculated molecular weight from 419 amino acids. This is possibly due to glycosylations on the heavy chain.
Table 1.
Purification of sFcRn from the conditioned 293FS supernatant.
| Steps | Total Protein | sFcRn | Purity | Yield |
|---|---|---|---|---|
| Conditioned supernatant | 15.9 mg | 3.00 mg | 19% | |
| Concentrated supernatant | 6.4 mg | 2.80 mg | 44% | 93% |
| Fractions from Ni chelating column | 1.36 mg | 1.30 mg | 95% | 43% |
| Final product after dialysis | 1.36 mg | 1.30 mg | 96% | 43% |
The estimation reflects a typical procedure from 100 ml 293FS conditioned supernatant.
Figure 2. NuPAGE image of purified sFcRn.
The 4~12% gel picture shows a typical image of sFcRn purified from conditioned 293FS supernatant with a Ni-chelating column. Protein molecular weight standards are indicated on the left in kDa.
pH-dependent binding of sFcRn to human IgG
The hallmark of FcRn function is its binding to IgG at the acidic pH and releasing at the neutral pH. We analyzed the pH-dependence of recombinant FcRn/IgG binding with ELISA and column methods.
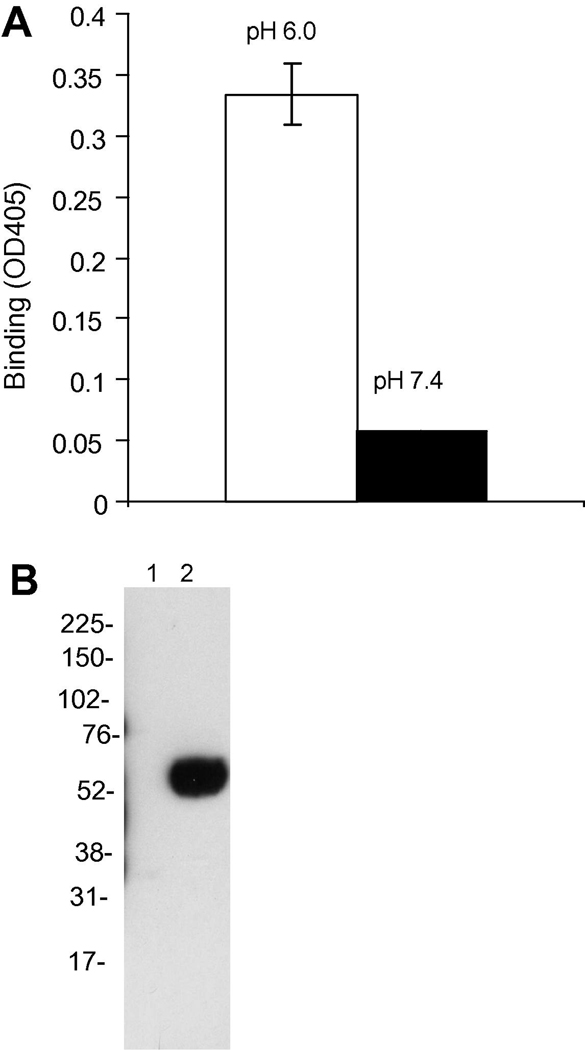
In the ELISA method, human IgG m921 was captured by its antigen DR4 onto the wells. sFcRn at 400 nM was added for binding to IgG at two pHs, 6.0 and 7.4. The binding of sFcRn was consistently detected at pH 6.0, but not at pH 7.4 (Figure 3A). It was found that direct coating of IgG to ELISA wells gave very low bindings, implying that the orientation of IgG molecule and availability of the Fc portion is critical.
Figure 3. sFcRn binds to human IgG in a pH-dependent fashion.
A, pH-dependent binding of sFcRn to IgG shown with ELISA. m921 IgG was captured by its cognate antigen DR4 that is pre-coated on wells. Bindings of sFcRn to IgG were tested under pH 6 and pH 7.4 in parallel.
B, pH-dependent binding of sFcRn to human IgG conjugated to sepharose. The conditioned medium with sFcRn expressed was adjusted to pH 6.0 or 7.4, and loaded to two human IgG-sepharose columns pre-equilibrated with the PBS buffer at pH 6.0 or 7.4. After extensive washing with the corresponding equilibration PBS buffer, the bound protein was eluted with a pH 8.0 buffer. The content of the eluted sample was analyzed with Western blot using anti-Histidine tag mAb.
The pH-dependent binding of sFcRn was further tested with a human IgG-sepharose column. Conditioned 293FS supernatant containing sFcRn was adjusted to pH 6 or 7.4, and loaded separately to hIgG-columns. After extensive washing with pH 6.0 buffer, any bound protein was released with pH 8.0 buffer. The presence of any sFcRn protein in the eluted sample was detected with anti-His tag Western blot. It was found that adjusting pH of starting supernatant to 6.0 allowed binding of sFcRn to the IgG column, whereas sFcRn at pH 7.4 did not bind to the IgG column (Figure 3B). These results agreed with the ELISA results, and together they demonstrated that the recombinant FcRn shows pH-dependent binding to human IgG.
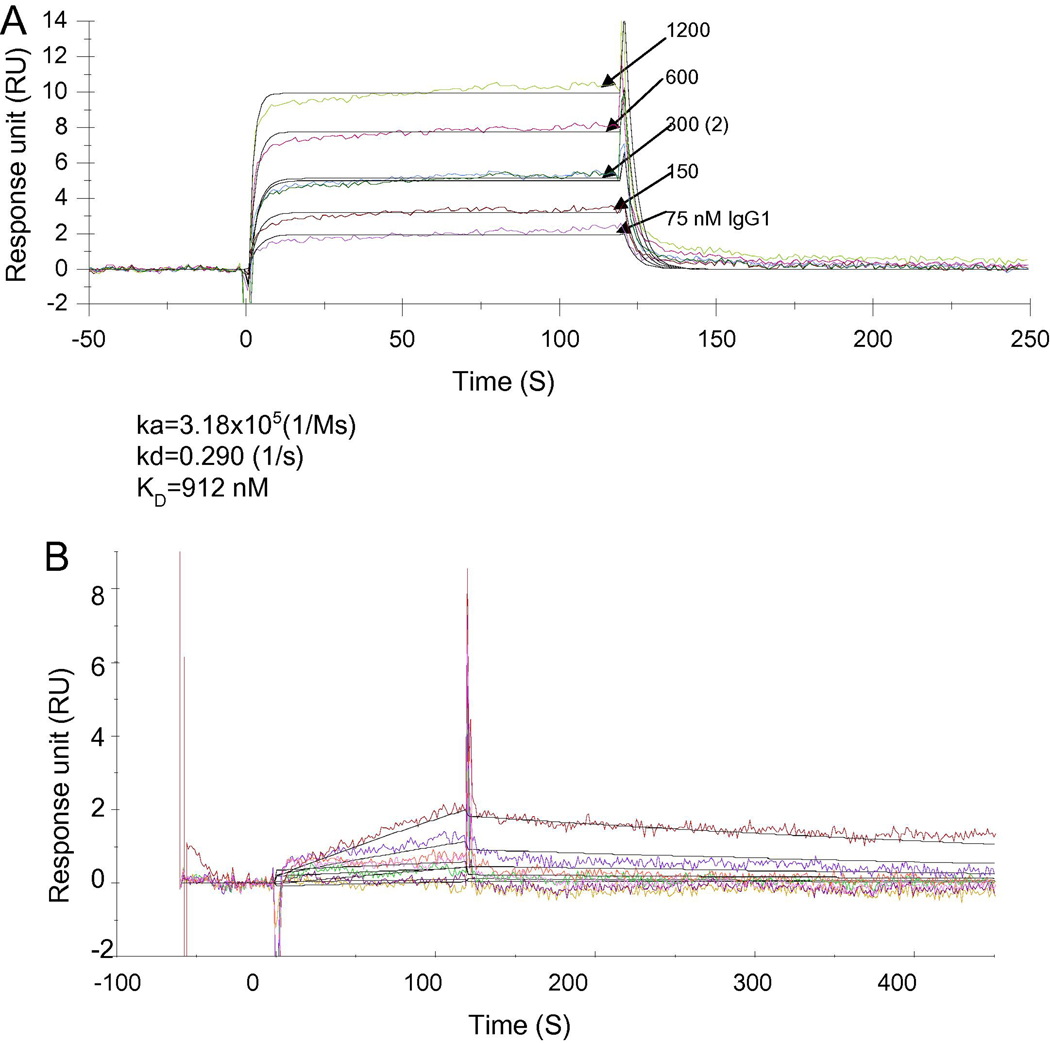
The binding of human FcRn to IgG is reported at an equilibrium dissociation constant (KD) of approximately 1000 nM [12, 20]. It is important to know if the sFcRn prepared in our study behaves as sFcRn reported. We immobilized sFcRn on a Biacore CM5 chip and analyze the binding kinetics of human IgG (m921) and a control sample scFv, which lacks the Fc fragment. The binding was performed under pH 6.0, and the chip was regenerated with pH 8.0 buffer. The IgG sensorgram fitted well with the 1:1 binding model. Indeed, the IgG showed concentration dependent binding to sFcRn (Figure 4A), and the calculated KD is approximately 912 nM. The binding has a fast on-rate (ka=3.18×105 1/Ms) and a fast off-rate (kd=0.290 1/s). During the test, the chip was efficiently regenerated with pH 8.0 buffer, as was indicated by the overlapping of the two 300 nM IgG binding curves. m921 IgG diluted in pH 7.4 PBS buffer did not show any binding to sFcRn (data not shown). This is another good indication of pH-dependent binding of IgG to immobilized sFcRn. The scFv did not bind even at 4.8 µM concentration (Figure 4B). Interestingly, an Fc fusion protein showed slightly lower binding affinity (KD =1.36 µM) than IgG (data not shown). Previous reports [13, 20] indicate that Fc fusion proteins generally have lower affinities (KD ranging from 1900 to 4000 nM) to human FcRn, implying the other moiety of the fusion protein may indirectly influence the structural environment of the binding region. Our observations are consistent with the binding kinetics of FcRn reported in the literature.
Figure 4. Kinetics of IgG1 Binding to sFcRn.
A, The purified sFcFn was immobilized on a CM5 chip at 1130 RU, and binding of m921 IgG was tested with Biacore X100 using PBS6.0 with 0.005% Tween 20 as the running buffer. m921 was tested at following concentrations: 75, 150 300, 600, 1200 and 300 nM. At the end of each binding cycle, the CM5 chip was regenerated with pH 8.0 buffer to remove IgG. The 300 nM concentration was repeated after 1200 nM to check the regeneration efficiency. m921 IgG has on-rate ka=3.18×105 (1/Ms), off-rate kd=0.290 (1/s), and equilibrium dissociation rate constant KD=912 nM.
B, The single chain format of m921 was tested on the sFcRn-coated chip under pH6.0 PBS with 0.005% Tween 20 at concentrations 150, 300, 600, 1200, and 4800 nM. Single chain had no binding to sFcRn even at 4800 nM.
Discussion
FcRn is critical in maintaining IgG homeostasis, and transporting maternal IgGs. A good correlation between the affinity for FcRn and the half-life of IgG exists. Mutations in Fc leading to higher affinity of IgG to human FcRn at pH 6.0 improve the half-life of IgGs in the circulation [12, 17, 21–23]. An application of this correlation is to engineer therapeutic antibodies in the market and under development, so that patients need fewer administering of the antibodies without compromising their efficacies. In the diagnosis field the opposite may be desired, namely a shorter half-life and quick clearance will decrease patients’ exposure to radiolabelled and imaging agents. The application can be extended to therapeutic Fc-fusion proteins and antigens. For abovementioned work, the need for an abundant source of function FcRn is clear and present. However, to our knowledge, there is no commercial source of either human or mouse FcRn protein. We designed an expression vector where the B2M is physically connected to the heavy chain, and helps the secretion of the heavy chain. We showed here that soluble functional FcRn could be produced in the mammalian cell system with a single protein format, therefore allows a 1:1 molar ratio in the mature protein. The hydrophilic nature of B2M was powerful enough to lead the folding and secretion of the otherwise difficult to secret heavy chain extracellular domain. We obtained ~1 mg final product from 100 ml transiently transfected culture, and we believe there is room for optimization. We are currently trying to establish a stable cell line for constitutive expression of sFcRn. This design should also be valid for producing soluble mouse FcRn protein and MHC I molecules. It is possible that B2M may help folding and surface expression of the whole heavy chain, not only the extracellular domain of the heavy chain. The length of the linker between the two domains could also be varied.
The pH dependent binding of sFcRn to IgGs was shown by three different approaches: ELISA, binding in columns, and Biacore. In the ELISA test, it was found that coating of IgG or FcRn directly on plates only gave very weak signals. IgG captured by antigen pre-coated in wells consistently gave much stronger signals, indicating the orientation of the IgG is important, and/or the amount of Fc available is important. Up to 1 µg of sFcRn was added in each well for binding to IgGs. After all, the affinity between human IgG and human FcRn is fairly low, at ~900 nM to 1 µM [20]. When the conditioned supernatant was flowed through the hIgG-sepharose column at pH 6.0 and 7.4, a small portion of sFcRn was recovered from the column at pH 6.0, while much of the sFcRn flew through the column. This is in agreement with the low affinity profile of the binding. Nevertheless, on both ELISA and column methods, sFcRn did not bind to IgGs at pH 7.4. For surface plasmon resonance studies, sFcRn, instead of IgG, was immobilized and the binding was shown to be pH-dependent.
This method of producing recombinant human FcRn could be another option among the existing methods. It may also be applied to produce MHC I molecules.
Acknowledgment
We thank members of our groups for helpful discussions. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This Research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Abbreviation list
- FcRn
neonatal Fc receptor
- B2M
beta 2 microglobulin chain
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Simister NE, Rees AR. Isolation and characterization of an Fc receptor from neonatal rat small intestine. Eur. J. Immunol. 1985;15:733–738. doi: 10.1002/eji.1830150718. [DOI] [PubMed] [Google Scholar]
- 2.Raghavan M, Bonagura VR, Morrison SL, Bjorkman PJ. Analysis of the pH dependence of the neonatal Fc receptor/immunoglobulin G interaction using antibody and receptor variants. Biochemistry. 1995;34:14649–14657. doi: 10.1021/bi00045a005. [DOI] [PubMed] [Google Scholar]
- 3.Raghavan M, Gastinel LN, Bjorkman PJ. The class I major histocompatibility complex related Fc receptor shows pH-dependent stability differences correlating with immunoglobulin binding and release. Biochemistry. 1993;32:8654–8660. doi: 10.1021/bi00084a037. [DOI] [PubMed] [Google Scholar]
- 4.Ghetie V, Ward ES. Multiple roles for the major histocompatibility complex class I- related receptor FcRn. Ann. Rev. Immunol. 2000;18:739–766. doi: 10.1146/annurev.immunol.18.1.739. [DOI] [PubMed] [Google Scholar]
- 5.He W, Ladinsky MS, Huey-Tubman KE, Jensen GJ, McIntosh JR, Bjorkman PJ. FcRn-mediated antibody transport across epithelial cells revealed by electron tomography. Nature. 2008;455:542–546. doi: 10.1038/nature07255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghetie V, Hubbard JG, Kim JK, Tsen MF, Lee Y, Ward ES. Abnormally short serum half-lives of IgG in beta 2-microglobulin-deficient mice. Eur. J. Immunol. 1996;26:690–696. doi: 10.1002/eji.1830260327. [DOI] [PubMed] [Google Scholar]
- 7.Gastinel LN, Simister NE, Bjorkman PJ. Expression and crystallization of a soluble and functional form of an Fc receptor related to class I histocompatibility molecules. Proc. Natl Acad Sci USA. 1992;89:638–642. doi: 10.1073/pnas.89.2.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim JK, Tsen MF, Ghetie V, Ward ES. Localization of the site of the murine IgG1 molecule that is involved in binding to the murine intestinal Fc receptor. Eur. J. Immunol. 1994;24:2429–2434. doi: 10.1002/eji.1830241025. [DOI] [PubMed] [Google Scholar]
- 9.Popov S, Hubbard JG, Kim J, Ober B, Ghetie V, Ward ES. The stoichiometry and affinity of the interaction of murine Fc fragments with the MHC class I-related receptor, FcRn. Mol. Immunol. 1996;33:521–530. doi: 10.1016/0161-5890(96)00004-1. [DOI] [PubMed] [Google Scholar]
- 10.Kacskovics I, Kis Z, Mayer B, West AP, Jr, Tiangco NE, Tilahun M, Cervenak L, Bjorkman PJ, Goldsby RA, Szenci O, Hammarstrom L. FcRn mediates elongated serum half-life of human IgG in cattle. Int. Immunol. 2006;18:525–536. doi: 10.1093/intimm/dxh393. [DOI] [PubMed] [Google Scholar]
- 11.Tesar DB, Cheung EJ, Bjorkman PJ. The chicken yolk sac IgY receptor, a mammalian mannose receptor family member, transcytoses IgY across polarized epithelial cells. Mol. Biol. Cell. 2008;19:1587–1593. doi: 10.1091/mbc.E07-09-0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeung YA, Leabman MK, Marvin JS, Qiu J, Adams CW, Lien S, Starovasnik MA, Lowman HB. Engineering human IgG1 affinity to human neonatal Fc receptor: impact of affinity improvement on pharmacokinetics in primates. J. Immunol. 2009;182:7663–7671. doi: 10.4049/jimmunol.0804182. [DOI] [PubMed] [Google Scholar]
- 13.Lee CH, Choi DK, Choi HJ, Song MY, Kim YS. Expression of soluble and functional human neonatal Fc receptor in Pichia pastoris. Protein Expr. Purif. 2010;71:42–48. doi: 10.1016/j.pep.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Andersen JT, Justesen S, Berntzen G, Michaelsen TE, Lauvrak V, Fleckenstein B, Buus S, Sandlie I. A strategy for bacterial production of a soluble functional human neonatal Fc receptor. J. Immunol. Methods. 2008;331:39–49. doi: 10.1016/j.jim.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Sanchez LM, Penny DM, Bjorkman PJ. Stoichiometry of the interaction between the major histocompatibility complex-related Fc receptor and its Fc ligand. Biochemistry. 1999;38:9471–9476. doi: 10.1021/bi9907330. [DOI] [PubMed] [Google Scholar]
- 16.Shields RL, Namenuk AK, Hong K, Meng YG, Rae J, Briggs J, Xie D, Lai J, Stadlen A, Li B, Fox JA, Presta LG. High resolution mapping of the binding site on human IgG1 for Fc gamma RI, Fc gamma RII, Fc gamma RIII, and FcRn and design of IgG1 variants with improved binding to the Fc gamma R. J. Biol. Chem. 2001;276:6591–6604. doi: 10.1074/jbc.M009483200. [DOI] [PubMed] [Google Scholar]
- 17.Ward ES. Immunoglobulin-like domains with increased half-lives. US006277375 B1. US Patent. 1997
- 18.Hill DM, Kasliwal T, Schwarz E, Hebert AM, Chen T, Gubina E, Zhang L, Kozlowski S. A dominant negative mutant beta 2-microglobulin blocks the extracellular folding of a major histocompatibility complex class I heavy chain. J. Biol. Chem. 2003;278:5630–5638. doi: 10.1074/jbc.M208381200. [DOI] [PubMed] [Google Scholar]
- 19.Zijlstra M, Bix M, Simister NE, Loring JM, Raulet DH, Jaenisch R. Beta 2-microglobulin deficient mice lack CD4-8+ cytolytic T cells. Nature. 1990;344:742–746. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki T, Ishii-Watabe A, Tada M, Kobayashi T, Kanayasu-Toyoda T, Kawanishi T, Yamaguchi T. Importance of neonatal FcR in regulating the serum half-life of therapeutic proteins containing the Fc domain of human IgG1: a comparative study of the affinity of monoclonal antibodies and Fc-fusion proteins to human neonatal FcR. J. Immunol. 2010;184:1968–1976. doi: 10.4049/jimmunol.0903296. [DOI] [PubMed] [Google Scholar]
- 21.Dall'Acqua WF, Kiener PA, Wu H. Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn) J. Biol. Chem. 2006;281:23514–23524. doi: 10.1074/jbc.M604292200. [DOI] [PubMed] [Google Scholar]
- 22.Vaccaro C, Zhou J, Ober RJ, Ward ES. Engineering the Fc region of immunoglobulin G to modulate in vivo antibody levels. Nat. Biotechnol. 2005;23:1283–1288. doi: 10.1038/nbt1143. [DOI] [PubMed] [Google Scholar]
- 23.Dall'Acqua WF, Woods RM, Ward ES, Palaszynski SR, Patel NK, Brewah YA, Wu H, Kiener PA, Langermann S. Increasing the affinity of a human IgG1 for the neonatal Fc receptor: biological consequences. J. Immunol. 2002;169:5171–5180. doi: 10.4049/jimmunol.169.9.5171. [DOI] [PubMed] [Google Scholar]